Surfactant-Free Electrosprayed Alginate Beads for Oral Delivery of Hydrophobic Compounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Ultrasonication
2.3. Alginate Hydrogel Bead Formation via Electrospraying
2.4. Microscopy and Morphological Analysis
3. Results
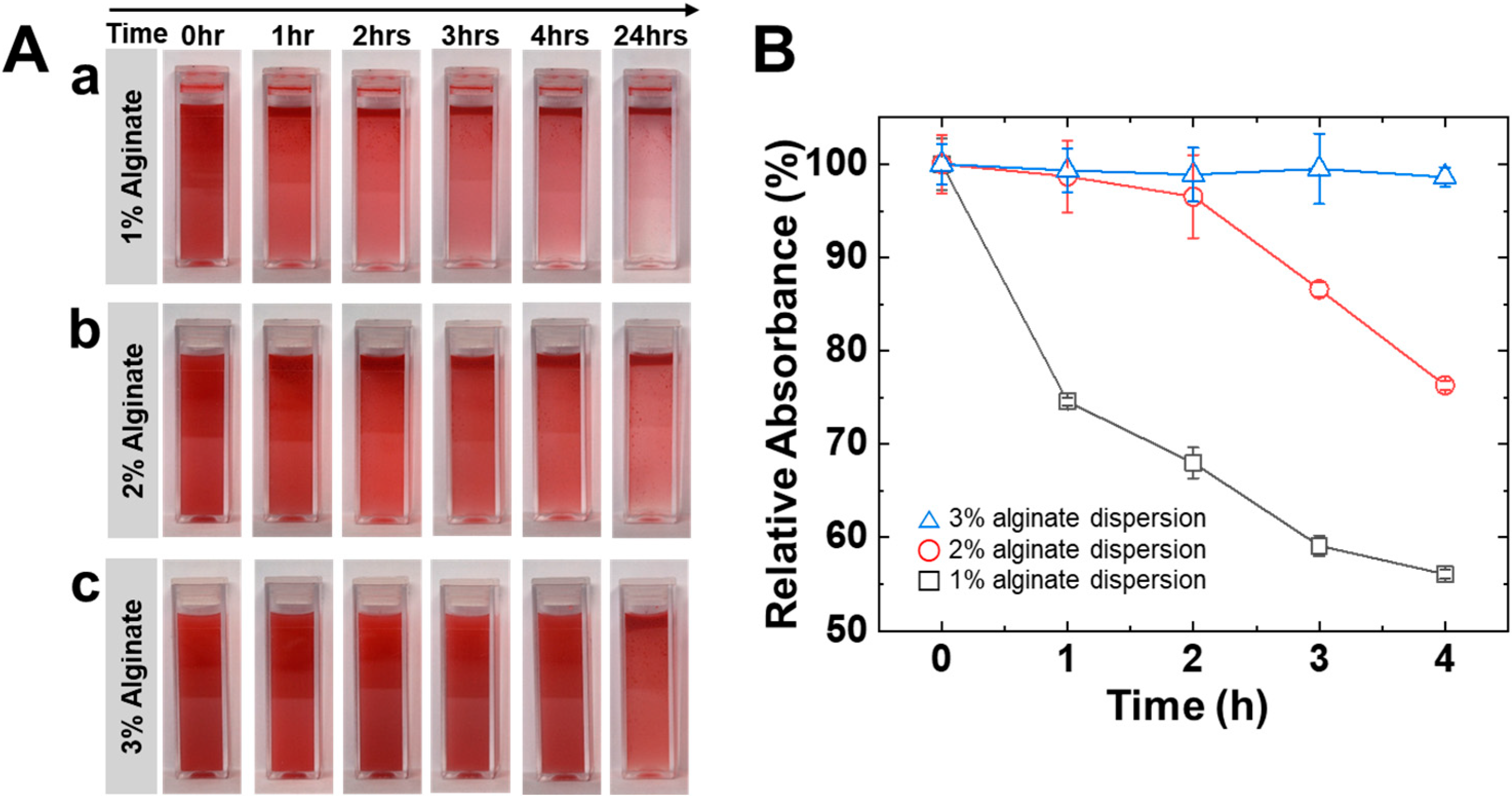
3.1. Preparation of Oil Dispersion via High-Viscosity Alginate Solution
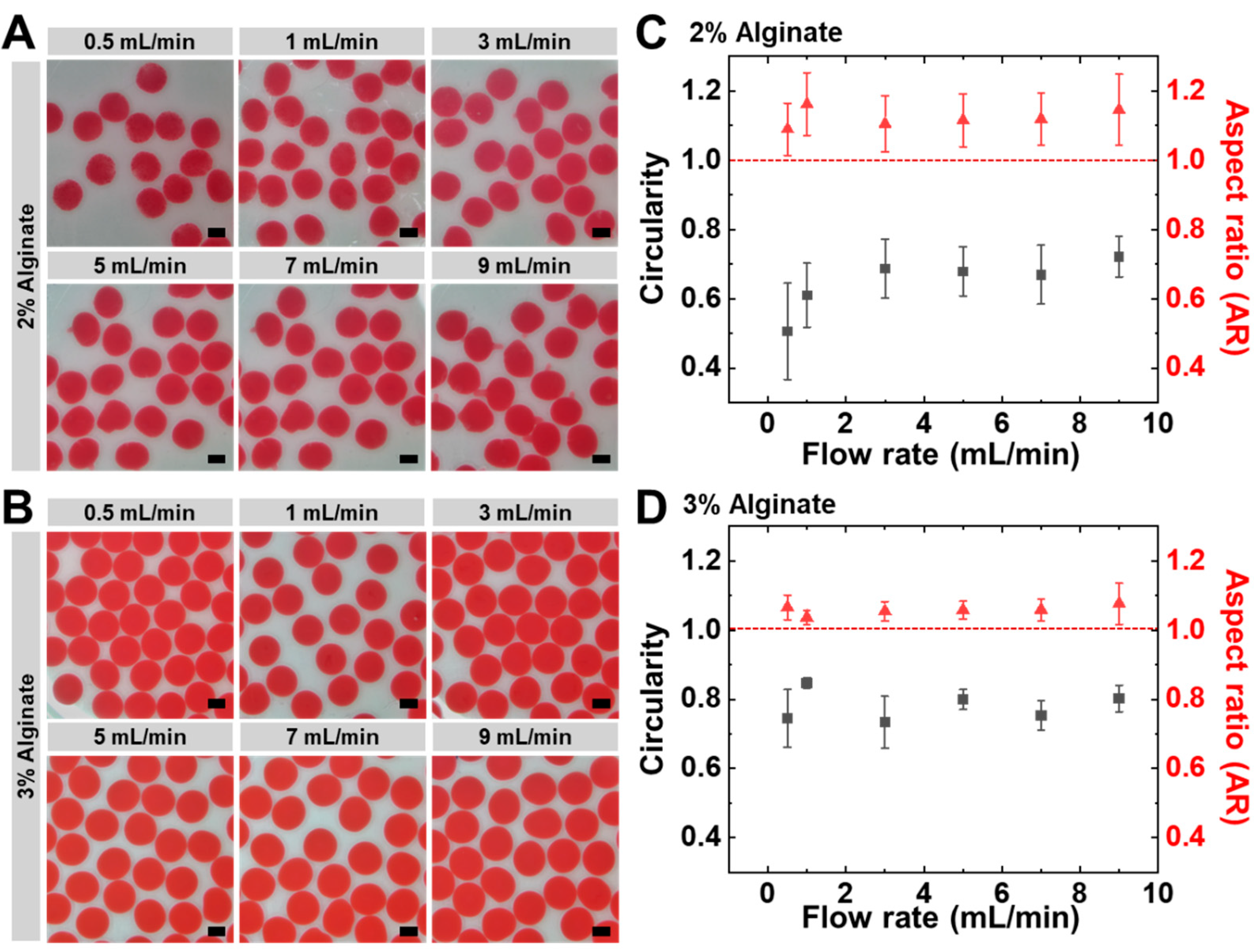
3.2. Electrospray-Assisted Fabrication of Uniform Alginate Hydrogel Beads
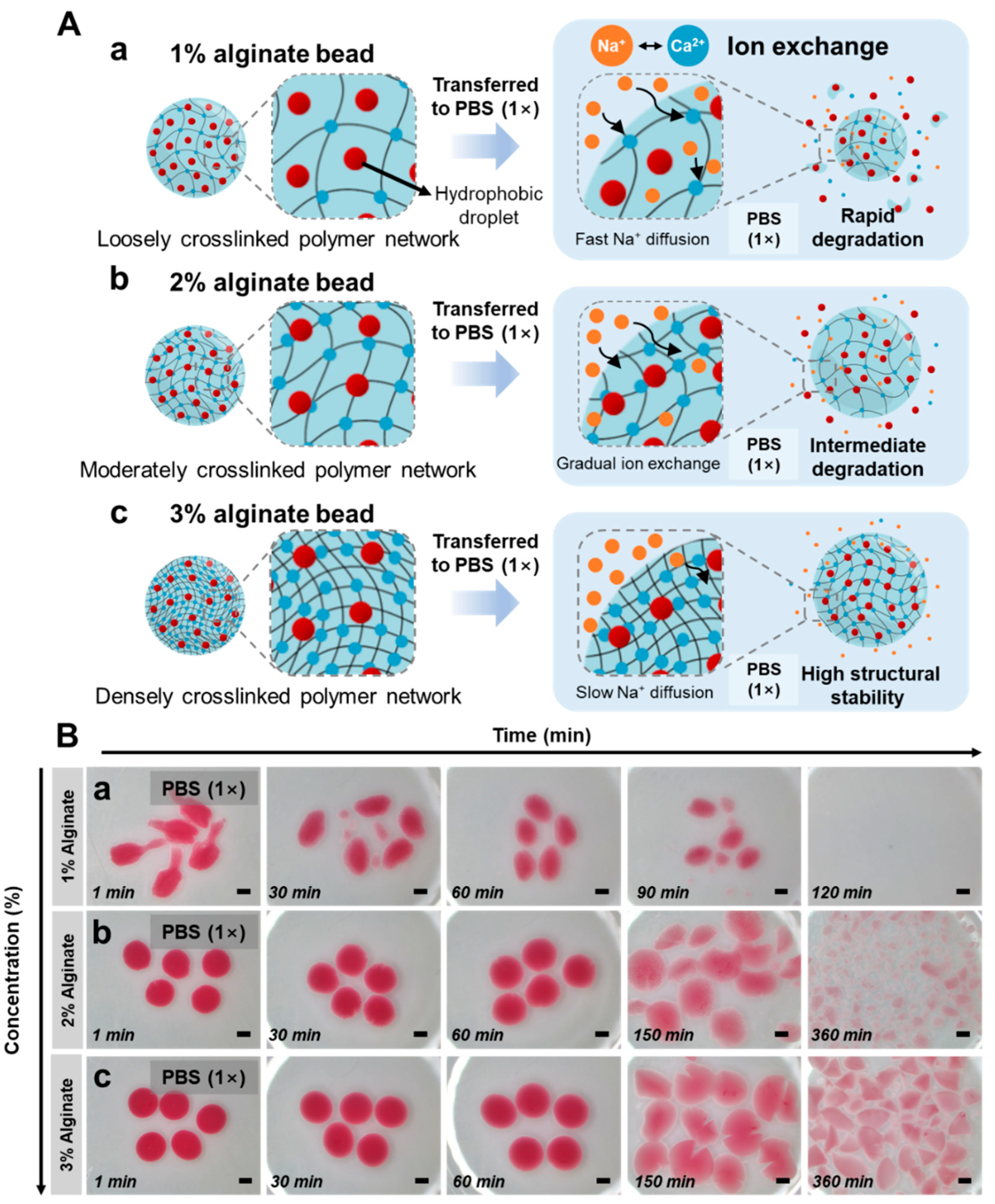
3.3. Degradation-Driven Triggered Release from Tunable Alginate Hydrogel Networks
3.4. Stimuli-Responsive Degradation and Release from Alginate Hydrogel Beads Triggered by Ionic Environment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Park, K. Controlled drug delivery systems: Past forward and future back. J. Control. Release 2014, 190, 3–8. [Google Scholar] [CrossRef]
- Abramson, A.; Caffarel-Salvador, E.; Khang, M.; Dellal, D.; Silverstein, D.; Gao, Y.; Frederiksen, M.R.; Vegge, A.; Hubálek, F.; Water, J.J.; et al. An ingestible self-orienting system for oral delivery of macromolecules. Science 2019, 363, 611–615. [Google Scholar] [CrossRef]
- Kirkpatrick, P. Pressures in the pipeline. Nat. Rev. Drug Discov. 2003, 2, 337. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Kalepu, S.; Nekkanti, V. Insoluble drug delivery strategies: Review of recent advances and business prospects. Acta Pharm. Sin. B 2015, 5, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-H.; Cheng, L.-C.; Doyle, P.S. Nanoemulsion-Loaded Capsules for Controlled Delivery of Lipophilic Active Ingredients. Adv. Sci. 2020, 7, 2001677. [Google Scholar] [CrossRef]
- Feeney, O.M.; Crum, M.F.; McEvoy, C.L.; Trevaskis, N.L.; Williams, H.D.; Pouton, C.W.; Charman, W.N.; Bergström, C.A.S.; Porter, C.J.H. 50 years of oral lipid-based formulations: Provenance, progress and future perspectives. Adv. Drug Deliv. Rev. 2016, 101, 167–194. [Google Scholar] [CrossRef] [PubMed]
- Kohli, K.; Chopra, S.; Dhar, D.; Arora, S.; Khar, R.K. Self-emulsifying drug delivery systems: An approach to enhance oral bioavailability. Drug Discov. Today 2010, 15, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Chatzidaki, M.D.; Mitsou, E. Advancements in Nanoemulsion-Based Drug Delivery Across Different Administration Routes. Pharmaceutics 2025, 17, 337. [Google Scholar] [CrossRef]
- Subramaniam, S.; Elz, A.; Wignall, A.; Kamath, S.; Ariaee, A.; Hunter, A.; Newblack, T.; Wardill, H.R.; Prestidge, C.A.; Joyce, P. Self-emulsifying drug delivery systems (SEDDS) disrupt the gut microbiota and trigger an intestinal inflammatory response in rats. Int. J. Pharm. 2023, 648, 123614. [Google Scholar] [CrossRef]
- Maher, S.; Geoghegan, C.; Brayden, D.J. Safety of surfactant excipients in oral drug formulations. Adv. Drug Deliv. Rev. 2023, 202, 115086. [Google Scholar] [CrossRef] [PubMed]
- Ezike, T.C.; Okpala, U.S.; Onoja, U.L.; Nwike, C.P.; Ezeako, E.C.; Okpara, O.J.; Okoroafor, C.C.; Eze, S.C.; Kalu, O.L.; Odoh, E.C.; et al. Advances in drug delivery systems, challenges and future directions. Heliyon 2023, 9, e17488. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, J.; Chen, Z.; Zhu, X.; Möller, M. Hybrid nanostructured particles via surfactant-free double miniemulsion polymerization. Nat. Commun. 2018, 9, 1918. [Google Scholar] [CrossRef]
- Wu, S.; Zhou, Y.; Asakawa, N.; Wen, M.; Sun, Y.; Ming, Y.; Song, T.; Chen, W.; Ma, G.; Xia, Y. Engineering CaP-Pickering emulsion for enhanced mRNA cancer vaccines via dual DC and NK activations. J. Control. Release 2024, 373, 837–852. [Google Scholar] [CrossRef]
- Chou, I.C.; Chen, S.-I.; Chiu, W.-Y. Surfactant-free dispersion polymerization as an efficient synthesis route to a successful encapsulation of nanoparticles. RSC Adv. 2014, 4, 47436–47447. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Josef, E.; Zilberman, M.; Bianco-Peled, H. Composite alginate hydrogels: An innovative approach for the controlled release of hydrophobic drugs. Acta Biomater. 2010, 6, 4642–4649. [Google Scholar] [CrossRef]
- Gombotz, W.R.; Wee, S. Protein release from alginate matrices. Adv. Drug Deliv. Rev. 1998, 31, 267–285. [Google Scholar] [CrossRef]
- Massana Roquero, D.; Othman, A.; Melman, A.; Katz, E. Iron(iii)-cross-linked alginate hydrogels: A critical review. Mater. Adv. 2022, 3, 1849–1873. [Google Scholar] [CrossRef]
- Håti, A.G.; Bassett, D.C.; Ribe, J.M.; Sikorski, P.; Weitz, D.A.; Stokke, B.T. Versatile, cell and chip friendly method to gel alginate in microfluidic devices. Lab A Chip 2016, 16, 3718–3727. [Google Scholar] [CrossRef]
- Axpe, E.; Oyen, M.L. Applications of Alginate-Based Bioinks in 3D Bioprinting. Int. J. Mol. Sci. 2016, 17, 1976. [Google Scholar] [CrossRef]
- Mazutis, L.; Vasiliauskas, R.; Weitz, D.A. Microfluidic Production of Alginate Hydrogel Particles for Antibody Encapsulation and Release. Macromol. Biosci. 2015, 15, 1641–1646. [Google Scholar] [CrossRef] [PubMed]
- Sattari, A.; Janfaza, S.; Mashhadi Keshtiban, M.; Tasnim, N.; Hanafizadeh, P.; Hoorfar, M. Microfluidic On-Chip Production of Alginate Hydrogels Using Double Coflow Geometry. ACS Omega 2021, 6, 25964–25971. [Google Scholar] [CrossRef]
- Shieh, H.; Saadatmand, M.; Eskandari, M.; Bastani, D. Microfluidic on-chip production of microgels using combined geometries. Sci. Rep. 2021, 11, 1565. [Google Scholar] [CrossRef]
- Utech, S.; Prodanovic, R.; Mao, A.S.; Ostafe, R.; Mooney, D.J.; Weitz, D.A. Microfluidic Generation of Monodisperse, Structurally Homogeneous Alginate Microgels for Cell Encapsulation and 3D Cell Culture. Adv. Healthc. Mater. 2015, 4, 1628–1633. [Google Scholar] [CrossRef]
- Soares, R.M.D.; Siqueira, N.M.; Prabhakaram, M.P.; Ramakrishna, S. Electrospinning and electrospray of bio-based and natural polymers for biomaterials development. Mater. Sci. Eng. C 2018, 92, 969–982. [Google Scholar] [CrossRef]
- Yeo, L.Y.; Gagnon, Z.; Chang, H.-C. AC electrospray biomaterials synthesis. Biomaterials 2005, 26, 6122–6128. [Google Scholar] [CrossRef]
- Doméjean, H.; de la Motte Saint Pierre, M.; Funfak, A.; Atrux-Tallau, N.; Alessandri, K.; Nassoy, P.; Bibette, J.; Bremond, N. Controlled production of sub-millimeter liquid core hydrogel capsules for parallelized 3D cell culture. Lab A Chip 2017, 17, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, Y.; Chen, Y.; Ma, X.; Chen, W. Droplet-Based Microfluidic Preparation of Shape-Variable Alginate Hydrogel Magnetic Micromotors. Nanomaterials 2022, 12, 115. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, S.K.; Sharma, S. Investigation of swelling/degradation behaviour of alginate beads crosslinked with Ca2+ and Ba2+ ions. React. Funct. Polym. 2004, 59, 129–140. [Google Scholar] [CrossRef]
- Berardi, A.; Bauhuber, S.; Sawafta, O.; Warnke, G. Alginates as tablet disintegrants: Understanding disintegration mechanisms and defining ranges of applications. Int. J. Pharm. 2021, 601, 120512. [Google Scholar] [CrossRef] [PubMed]


| Alginate Concentration (% w/v) | Oil Content (% v/v) | Surfactant | Total Volume (mL) | Sonication Power (W) | Cycle (%) | Sonication Time (min) | Actual Duration (min) |
|---|---|---|---|---|---|---|---|
| 1 | 1 | None | 100 | 500 | 80 | 15 | 30 |
| 2 | 1 | None | 100 | 500 | 80 | 15 | 30 |
| 3 | 1 | None | 100 | 500 | 80 | 15 | 30 |
| Alginate (%) | Nozzle (μm) | Voltage (kV) | Frequency (Hz) | Flow Rate (mL/min) | Bead Shape |
|---|---|---|---|---|---|
| 1 | 1000 | 1.0 | 100 | 0.5~9 | Irregular |
| 2 | 1000 | 1.0 | 100 | 0.5~9 | Spherical |
| 3 | 1000 | 1.0 | 100 | 0.5~9 | Spherical |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, H.-S.; Kim, H.-J.; Kang, S.-M.; Choi, C.-H. Surfactant-Free Electrosprayed Alginate Beads for Oral Delivery of Hydrophobic Compounds. Polymers 2025, 17, 2098. https://doi.org/10.3390/polym17152098
Jeong H-S, Kim H-J, Kang S-M, Choi C-H. Surfactant-Free Electrosprayed Alginate Beads for Oral Delivery of Hydrophobic Compounds. Polymers. 2025; 17(15):2098. https://doi.org/10.3390/polym17152098
Chicago/Turabian StyleJeong, Hye-Seon, Hyo-Jin Kim, Sung-Min Kang, and Chang-Hyung Choi. 2025. "Surfactant-Free Electrosprayed Alginate Beads for Oral Delivery of Hydrophobic Compounds" Polymers 17, no. 15: 2098. https://doi.org/10.3390/polym17152098
APA StyleJeong, H.-S., Kim, H.-J., Kang, S.-M., & Choi, C.-H. (2025). Surfactant-Free Electrosprayed Alginate Beads for Oral Delivery of Hydrophobic Compounds. Polymers, 17(15), 2098. https://doi.org/10.3390/polym17152098







