3.1. Experimental Characteristics of Adsorption Processes
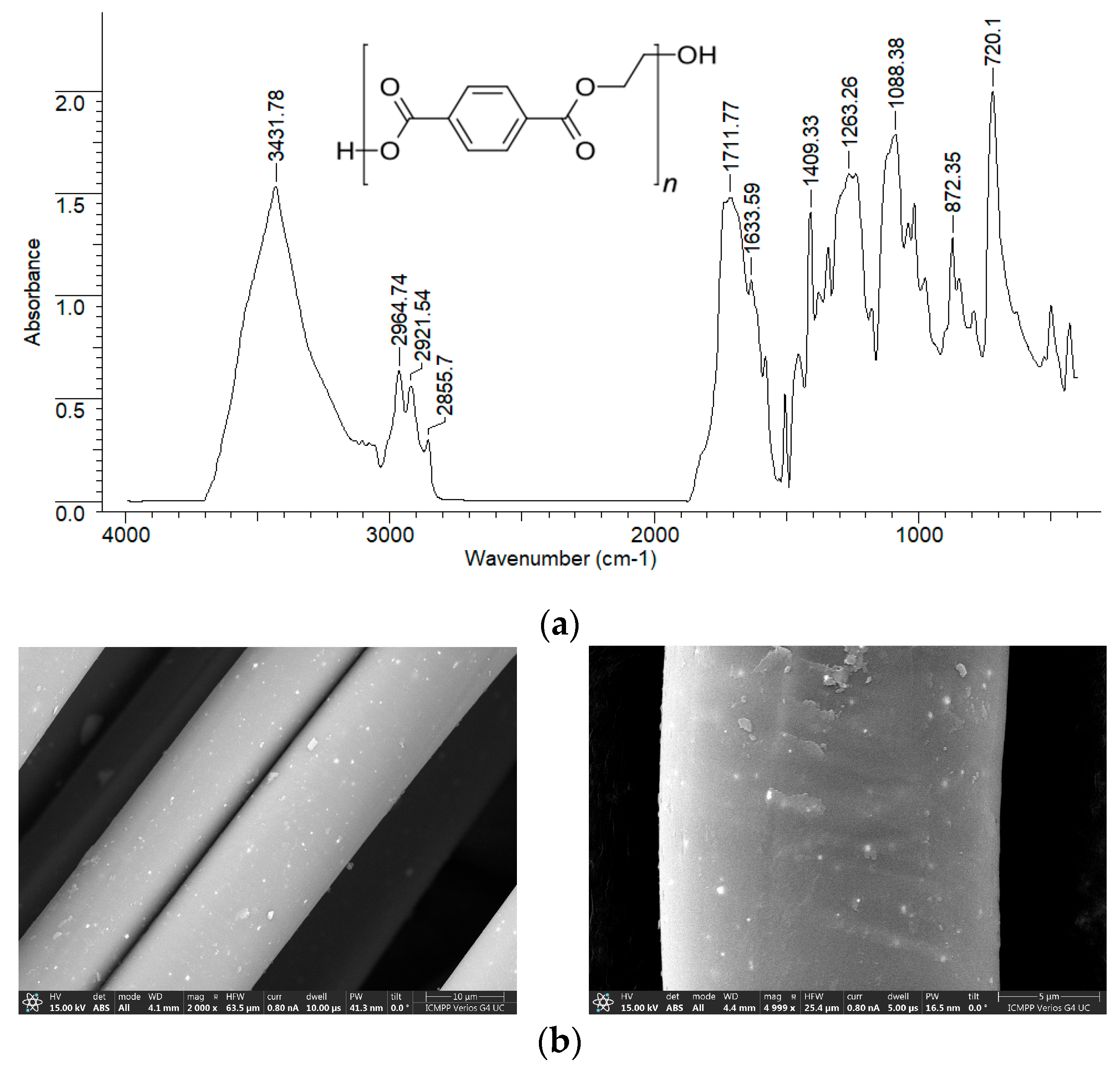
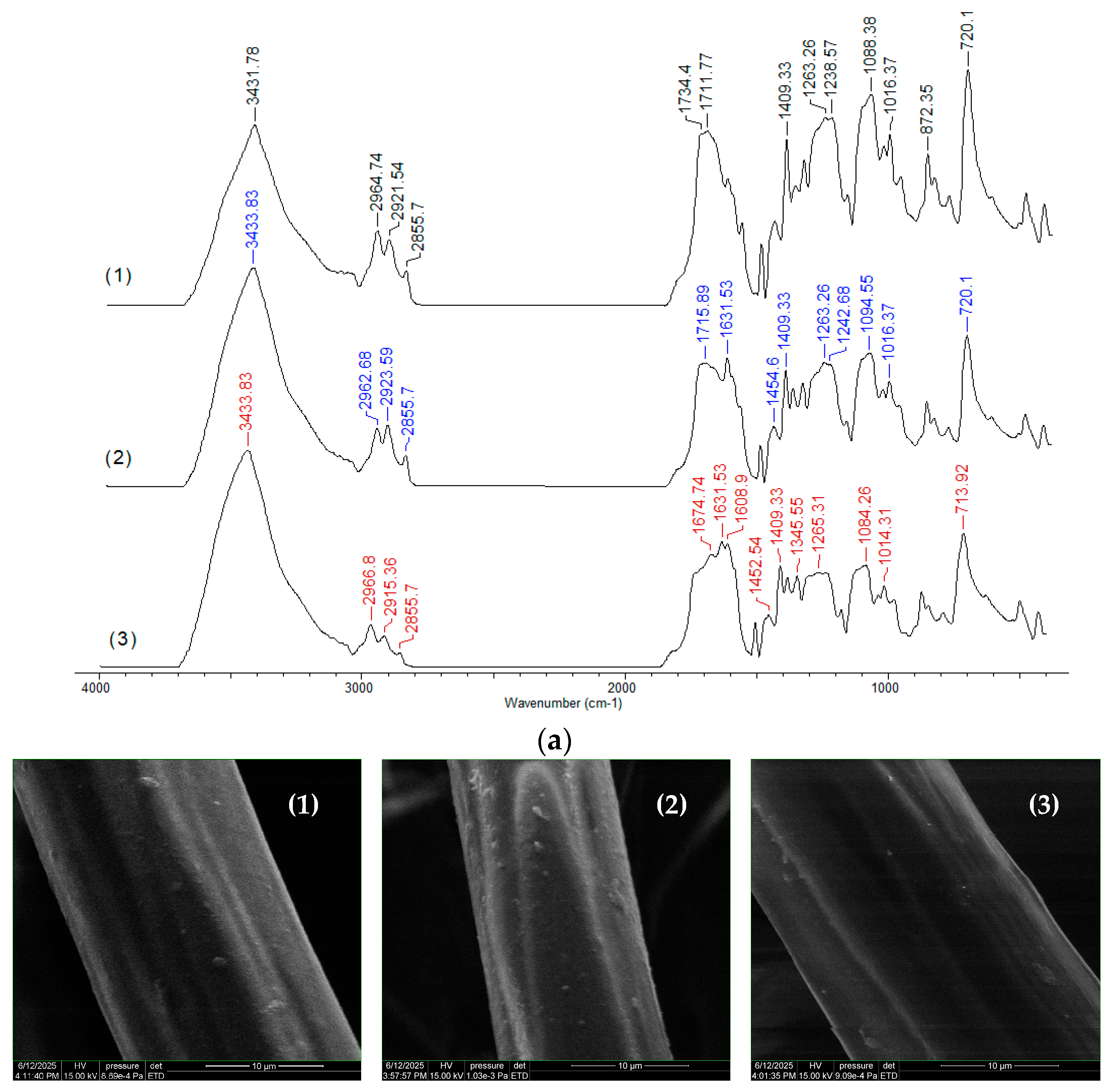
Due to the way they are obtained (mechanical processing of PET waste), PET fibers are expected to have a relatively smooth surface and a reduced number of superficial functional groups. These structural particularities are clearly highlighted by the FTIR spectrum and the SEM images, recorded experimentally (
Figure 3). However, the presence of hydroxyl groups (inter molecular hydrogen bonding) (3432 cm
−1), carbonyl groups (1711–1633 cm
−1), C-H bonds in saturated hydrocarbon radicals (2964–2855 cm
−1), C-O-C bonds (1088 cm
−1 cm), and the aromatic nucleus (872–720 cm
−1) can be easily observed in the FTIR spectrum due to the high intensity of the absorption bands (
Figure 3a).
Moreover, although the surface of PET fibers is smooth, increasing the magnification of SEM images reveals a series of irregularities (
Figure 3b) that may be important for the adsorption processes. All these structural features make PET fibers suitable as adsorbents for retaining large organic molecules from aqueous media.
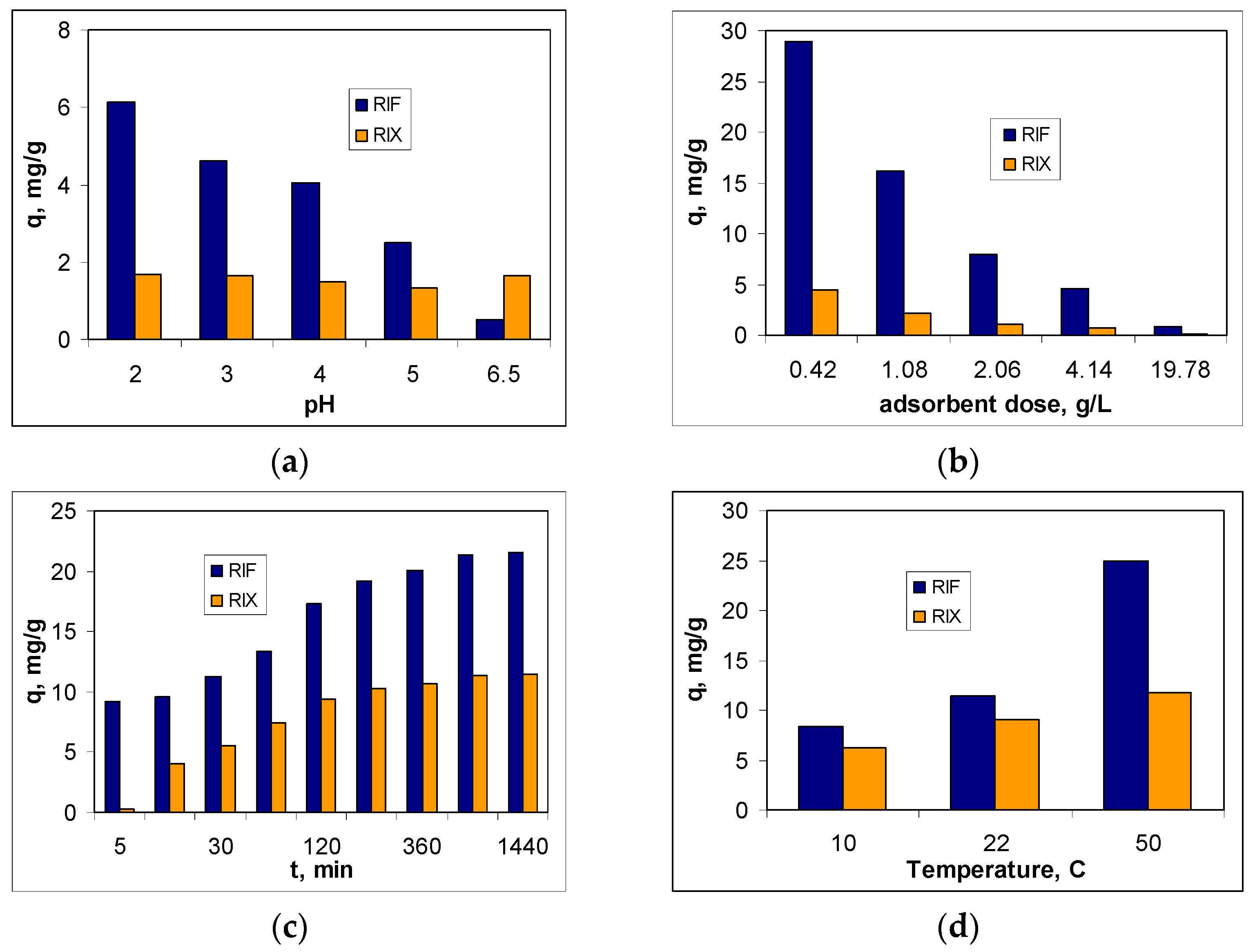
The next step in evaluating the feasibility of using PET fibers for the removal of RIF and RIX from aqueous environments is to examine the influence that experimental parameters (pH, adsorbent dose, contact time, and temperature) have on the efficiency of adsorption processes. The influence of each of these parameters on the adsorption capacity of PET fibers for the two studied antibiotics (RIF and RIX) is illustrated in
Figure 4.
The influence of pH on the efficiency of RIF and RIX retention on PET fibers was studied in the pH range between 2.0 and 6.5, and the adsorption capacity values are presented in
Figure 4a. The selection of this pH range was made considering the acidity constant values reported in the literature for RIF (1.8 and 7.9) [
36] and RIX (2.08, 3.02, 7.28, 9.32, and 12.55) [
37], and the other experimental conditions were adsorbent dose of 2.0 g/L, initial concentration of 21.1 mg/L, contact time of 3 h, and temperature of 22 °C. The obtained results show that at pH 2.0, the highest amount of RIF is retained (56.60%), and further increasing the pH leads to a decrease in the adsorption efficiency of this antibiotic. In the case of RIX, the highest values of q are obtained at both pH = 2.0 (1.68 mg/g) and pH = 6.5 (1.65 mg/g), although the variation in adsorption capacity across the entire pH range is quite small (0.30 mg/g).
It is well known that the variation in adsorption capacity with pH provides clear evidence that electrostatic interactions are involved in the adsorption process [
23]. Therefore, the ionic form of the antibiotics and the functional groups of PET fibers play an important role in facilitating such interactions. At pH 2.0 (strong acid media), RIF and RIX molecules are negatively charged (due to the ionization of the first functional group), while the carboxyl/carbonyl groups of PET fibers are undissociated/partially protonated (pKa1 for terephtalic acid (monomer) is 3.54 [
40]). Thus electrostatic interactions (rather weak) occur between the antibiotic molecules (RIF and RIX) and the functional groups on the PET fibers’ surface, interactions facilitated by the hydrogen ions (of marginal carboxyl groups or protonated carbonyl groups). Increasing the pH of the aqueous solution, although leading to an increase in the ionization degree of the antibiotic molecules, significantly reduces the formation of positive charges on the surface of the PET fibers. Consequently, the adsorption capacity decreases with increasing pH, and this is especially evident in the case of RIF adsorption. In the case of RIX molecules, increasing pH has a smaller influence on the efficiency of the adsorption process (
Figure 4a). This is because (i) the experimental values of the adsorption capacities are small, and the modification of the values at the first decimal place does not represent a significant variation, and (ii) unlike RIF (which has a single functional group dissociable in acidic media), RIX has two functional groups that can dissociate in acid media (pKa
1 = 2.08 and pKa
2 = 3.02), which leads to an increase in the number of negative charges that can be involved in electrostatic interactions. Based on these observations, the optimal pH for the adsorption of RIF and RIX on PET fibers was considered to be 2.0, and this value was used in all subsequent experiments.
The effect of adsorbent dose on the adsorption efficiency of RIF and RIX was examined in the range of 0.4–20 g/L, while the values of the other experimental parameters (pH = 2.0, initial concentration = 21.1 mg/L, contact time = 3 h, temperature = 22 ± 1 °C) were kept constant. As can be seen in
Figure 4b, as the amount of PET fibers increases, the adsorption capacity values (q, mg/g) decrease for both antibiotics (RIF and RIX). On the other hand, increasing the adsorbent dose within this range leads to an improvement in the removal percentage from 58.9 to 76.4% for RIF and from 8.9 to 12.7% for RIX (). This opposite variation in the parameters q and R with the increase in the amount of PET fibers is a consequence of the variation in the ratio between the number of active centers of the adsorbent and the number of antibiotic molecules in the aqueous solution. Increasing the adsorbent dose causes the antibiotic molecules present in the solution to be retained on an increasing amount of PET fibers, which means that the ratio between the number of antibiotic molecules and the number of active centers of the adsorbent will decrease. Under these conditions, considering the definition relationships (see Equations (1) and (2)), the adsorption capacity values decrease, while the removal percentage values increase. However, the decrease in q is not proportional to the increase in R. Thus, while the adsorption capacity values decrease by more than 36 times in the case of RIF and by more than 33 times in the case of RIX (
Figure 4b), the R values (%) increase by only 17% in the case of RIF and by approximately 4% in the case of RIX. Under these conditions, an adsorbent dose of 0.4 g/L can be considered optimal and was used for subsequent experiments.
The influence of contact time on the adsorption efficiency of RIF and RIX on PET fibers was examined over a fairly wide range (5–1440 min), under the following experimental conditions: pH = 2.0, adsorbent dose = 0.4 g/L, initial concentration = 21.1 mg/L, temperature = 22 ± 1 °C. The adsorption capacity values obtained for the adsorption of RIF and RIX on PET fibers are illustrated in
Figure 4c. According to experimental dependencies, the amount of RIF and RIX retained on PET fibers increases with contact time in the range of 5–180 min, after which it remains practically constant (320–1440 min). This variation in q as a function of contact time is valid for both antibiotics (RIF and RIX), although the adsorption capacity values differ significantly. This behavior suggests that, in the studied adsorption processes, the availability of RIF and RIX molecules to interact with the active centers of the adsorbent plays an important role. Initially, when most functional groups on the surface of PET fibers are free, the retention of RIF and RIX occurs fairly quickly. After the functional groups of PET fibers are occupied by antibiotic molecules, the adsorption process rate decreases significantly. The calculated adsorption capacity values remain practically constant, suggesting that equilibrium has been reached. Thus, for a contact time of 180 min, the retention percentage is 37.5% for RIF and 20.5% for RIX, while increasing the contact time to 1440 min leads to only a 6% increase for RIF and a 2.5% increase for RIX. Therefore, a contact time of 180 min can be considered sufficient for the studied adsorption processes to reach equilibrium.
Figure 4d shows the effect of temperature on the adsorption efficiency of RIF and RIX on PET fibers. The other experimental parameters were maintained at the previously established optimal values (pH = 2.0, initial concentration = 22.1 mg/L, adsorbent dose = 0.4 g/L, contact time = 180 min), while the temperature was adjusted at 10, 22, and 50 °C. It should be noted that both antibiotics (RIF and RIX) are thermally stable up to temperatures above 200 °C [
36,
37], and therefore, there is no problem with their decomposition during adsorption processes.
As can be seen from
Figure 4d, increasing temperature leads to an increase in adsorption capacities for both RIF and RIX, although this increase depends on the nature of the antibiotic in the aqueous solution. This, if in the case of RIF, the adsorption capacity increases by more than 3 times (from 8.30 to 24.91 mg/g) with the increase in temperature from 10 to 50 °C, in the case of RIX, the increase in adsorption capacity is more modest (1.8 times, from 6.24 to 11.73 mg/g) under the same experimental conditions. This variation in q values with increasing temperature suggests the endothermic nature of RIF and RIX adsorption on PET fibers and is most likely determined by the increased mobility of antibiotic molecules, which facilitates their retention on the adsorbent surface. However, increasing the temperature from 22 °C (ambient temperature) to 50 °C improves the removal percent values by only 27.12% in the case of RIF and by 3.74% in the case of RIX, which does not justify the energy consumption required to maintain the adsorption systems at a temperature of 50 °C. Therefore the temperature of 22 °C was considered to be much more suitable for the adsorption of RIF and RIX on PET fibers, both in terms of the efficiency of the adsorption process and from an economical point of view, and this value was selected as optimal.
3.2. Optimization of Adsorption Processes
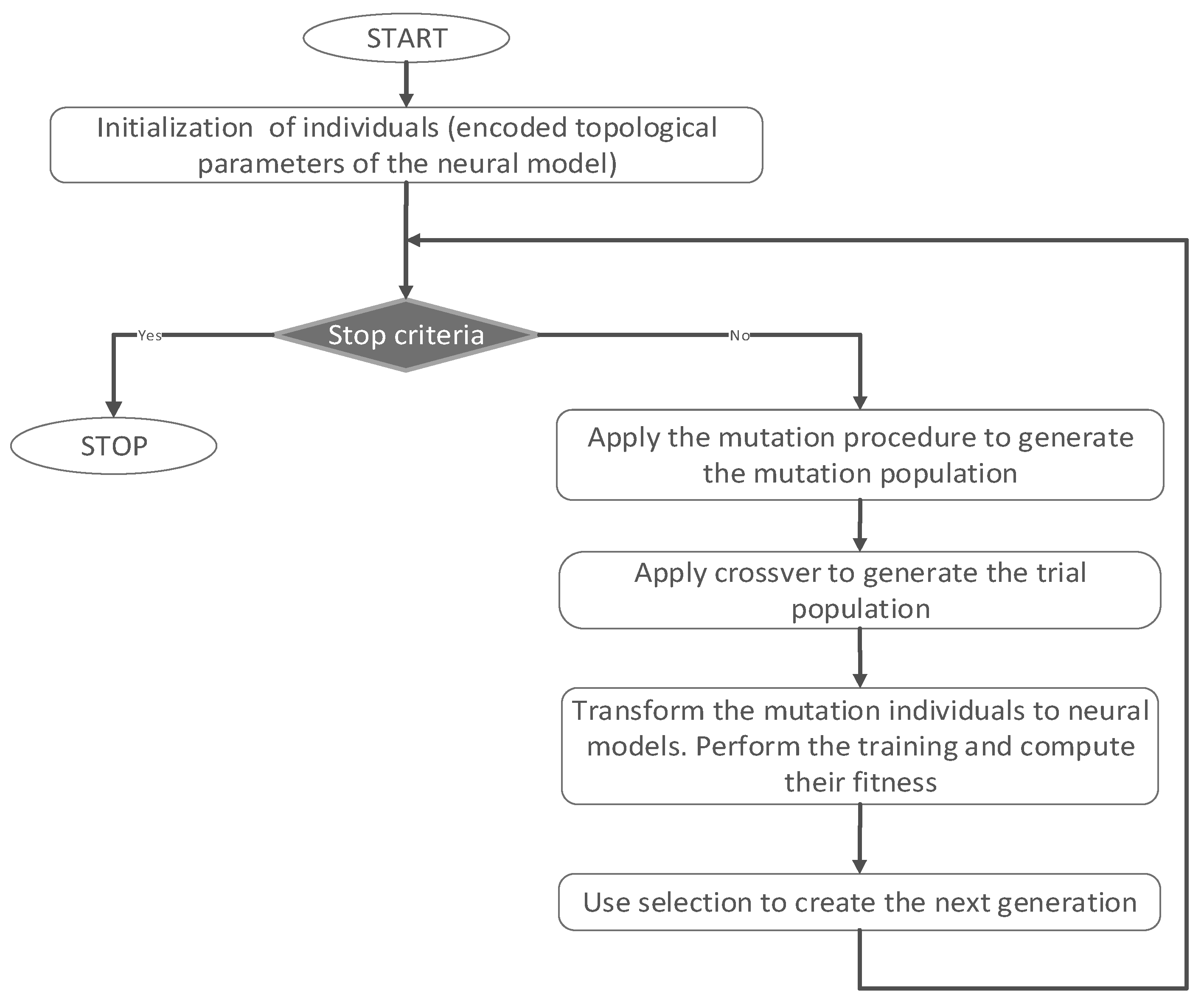
One of the main objectives of this study was to establish the experimental conditions for which the adsorption efficiency of the two antibiotics (RIF and RIX) on PET fibers is the highest possible. To achieve this objective, an optimization study was conducted. After collecting the experimental data, the obtained data set (comprising a total of 97 experiments) was normalized using the min–max approach [
35], and the data was randomly distributed into training and testing phases (70% for training and 30% for testing). Next, the DE algorithm was applied to determine the optimal model. The type of neural model considered in this study is the sequential model with dense layers. Since the DE variant used in this study uses fixed-length vectors due to the applied direct coding strategy, a limitation on the number of hidden layers and number of neurons in each hidden layer must be imposed. Therefore, a topology with a maximum of five hidden layers and 20 neurons in each hidden layer was considered. For the neurons in the hidden layers, the activation function is ReLU, and for the output neurons, it is set to linear. A L1 kernel regularization rate of 0.01 is considered, and for the Adam optimizer, a fixed learning rate of 0.05 is set based on a series of preliminary runs. To avoid over-training, the validation split in the training phase was set to 0.2. For the DE algorithm, the total number of generations was set to 30, and due to its stochastic nature, the optimal model was selected as the best one from 10 runs. The best model obtained has a topology with seven input features, four hidden layers with 19, 5, 6, and 3 neurons, respectively, and one output, corresponding to the adsorption efficiency (R, %).
Table 2 presents the statistical indicators that measure the performance of this model, and
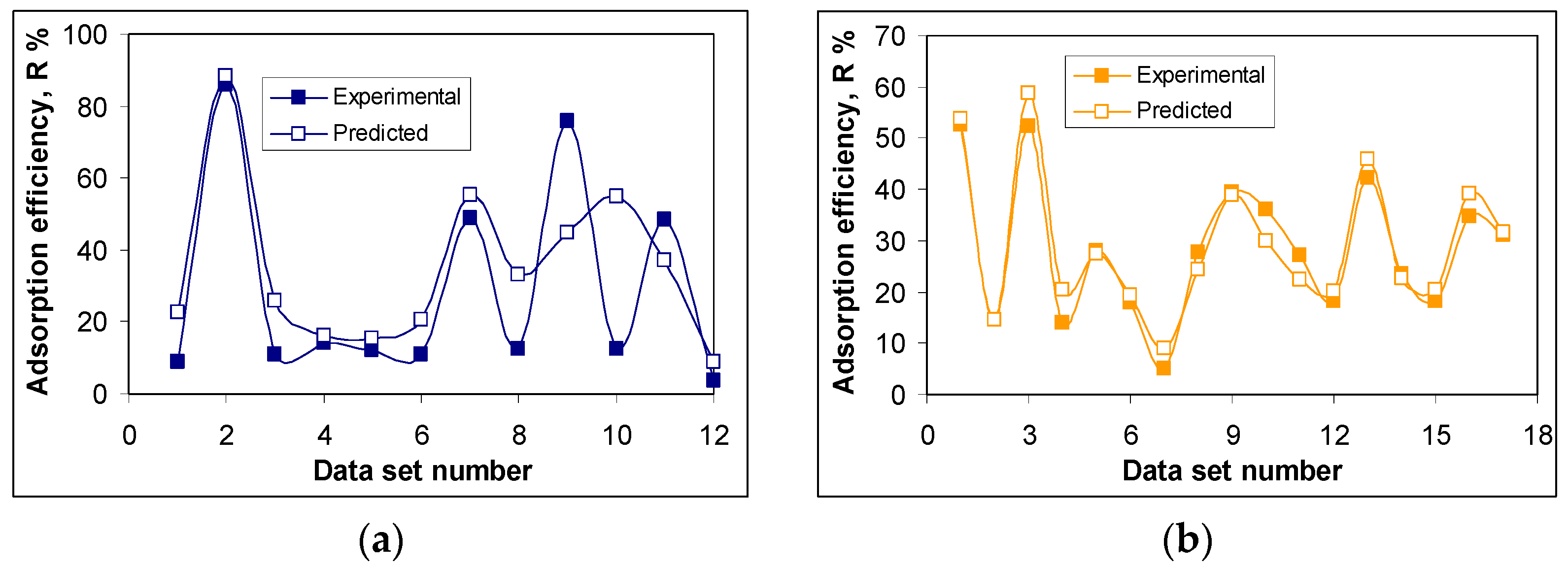
Figure 5 compares the predicted and the experimental values for the training sub-set.
As can be seen from
Figure 5, there is very good agreement between the values predicted by the ANN algorithm and the experimental ones, in terms of the adsorption efficiency (R, %), for both antibiotics (RIF and RIX). The highest values of the removal percent are obtained for the conditions of pH = 2.0, adsorbent dose = 0.4 ± 0.04 g/L, contact time = 180 min and temperature = 22 ± 1 °C. For an initial concentration of 21.1 mg RIF/L and 23.4 mg RIX/L, respectively, the removal percent calculated using the ANN algorithm is 88.13% (compared to 85.94%, experimental) for RIF, and 58.78% (compared to 52.42%, experimental) for RIX, respectively.
Therefore, these experimental conditions (pH = 2.0, adsorbent dose = 0.4 g/L, contact time = 180 min, and temperature = 22 ± 1 °C) allow for efficient adsorption of RIF and RIX onto PET fibers, at least in the range of low initial concentrations (below 25 mg/L).
However, there are also certain differences between the values predicted by the ANN algorithm and those obtained experimentally. The most notable are those obtained in the case of data sets number 9 and 10 for RIF adsorption (
Figure 5a). These data sets correspond to RIF adsorption at pH = 2.0, adsorbent dose = 19.9 g/L, contact time = 180 min, temperature = 22 °C (data set 9), and at pH = 2.0, adsorbent dose = 0.42 g/L, contact time = 1440 min, temperature = 22 °C (data set 10);the difference between the predicted R, % values, and those obtained experimentally is greater than 30% (+31.06% for data set 9, and −42.20% for data set 10). These differences may be caused by (i) the limitations of the ANN algorithm in predicting R% values—both cases (data sets 9 and 10) correspond to extreme limits of the experimental range, and the ANN model was unable to predict them efficiently—or (ii) the different contributions of each parameter (pH, adsorbent dose, contact time, temperature) on the performance of adsorption processes. If the first cause is quite unlikely (but not impossible), to check the second possible cause, the SHapley Additive exPlanations (SHAP) values [
39] were computed using the SHAP module. These values provide an objective way to explain how each parameter contributes to the adsorption processes, and the representation of SHAP values for the test data is shown in
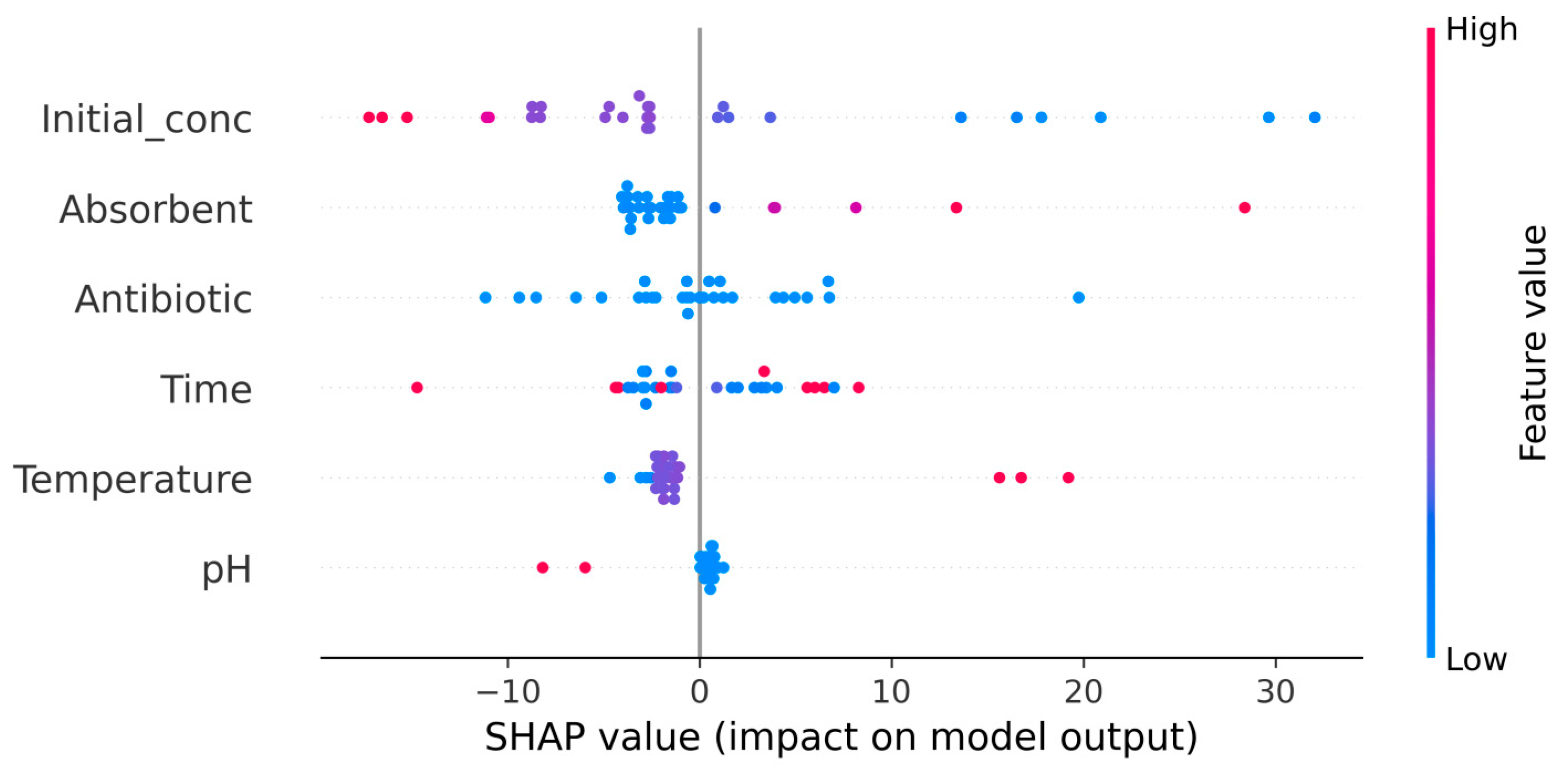
Figure 6.
The parameters on the Y-axis are ordered by their importance, while the SHAP values are represented on the X-axis. Positive SHAP values indicate a positive trend (an increase in these values leads to an increase in adsorption efficiency), whereas negative SHAP values indicate a negative trend (an increase in these values leads to a decrease in adsorption efficiency). The distance of SHAP values from 0 reflects the impact that each parameter has on influencing the efficiency of adsorption processes.
The SHAP values illustrated in
Figure 6 show that the initial concentration of the two antibiotics has the greatest contribution to achieving RIF and RIX adsorption on PET fibers, while pH has the smallest contribution. In addition, high initial antibiotic concentrations (indicated by red dots) lead to low adsorption efficiency (which decreases as the initial concentration increases). Increasing the adsorbent dose, contact time, and temperature can lead to improved adsorption process efficiency (
Figure 6), but economic considerations are also taken into account when determining the experimental values of these parameters. On the other hand, increasing the pH negatively affects the efficiency of adsorption processes, most likely due to changes in the dissociation state of antibiotic molecules (
Figure 6). In turn, the nature of the antibiotic influences the efficiency of the adsorption process. The blue color of the dots (
Figure 6) is due to the fact that only two antibiotics (RIF and RIX) were used in this study, but the distribution of these dots confirms the different efficiencies of RIF and RIX, observed experimentally.
3.3. Isotherm and Kinetic Modeling of Adsorption Processes
For the quantitative evaluation of RIF and RIX adsorption on PET fibers, it is necessary to model the adsorption isotherms and the kinetic curves obtained experimentally, under the conditions established as optimal. In this study, the adsorption isotherms were analyzed using the Langmuir, Freundlich, and Temkin models, while for the kinetic curves, the pseudo-first order, pseudo-second order, and intra-particle diffusion models were used.
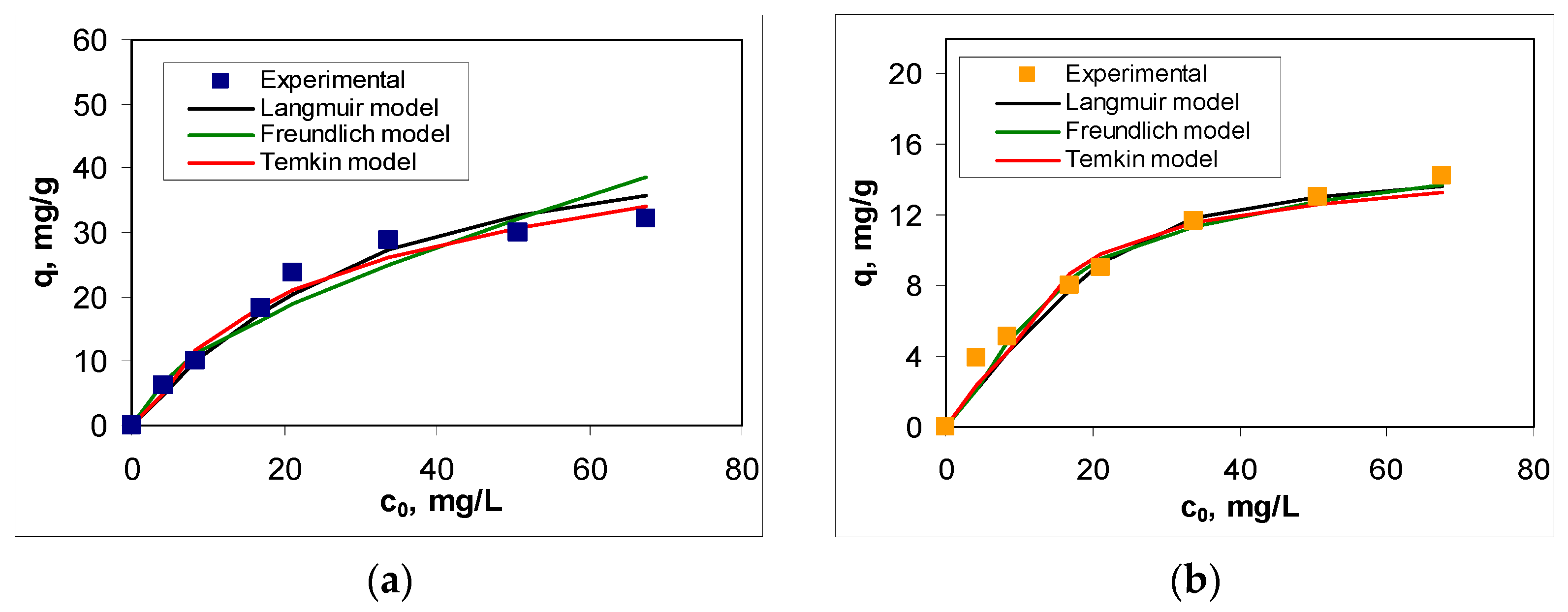
The experimental isotherms (
Figure 7) were obtained by varying the initial concentration of RIF and RIX within the range of 4.22–67.5 mg/L, while the other parameters were kept constant (at optimal values). As expected, increasing the initial antibiotic concentration leads to an increase in adsorption capacity over the entire concentration range. However, a detailed analysis of the obtained isotherms shows that (i) they are nonlinear—the increase in adsorption capacity with increasing initial concentration is more pronounced in the low concentration range (up to 25 mg/L) than in the high concentration range (above 25 mg/L)—and (ii) the efficiency of adsorption processes significantly depends on the nature of the antibiotic in the aqueous solution—the adsorption capacities obtained for RIF are more than 2 times higher than those obtained for RIX (
Figure 7).
These particularities suggest that the surface of PET fibers contains a limited number of active sites (functional groups) that can participate in adsorption processes (as evidenced by the FTIR spectrum—see
Figure 3). Moreover, the retention of antibiotic molecules involves specific interactions in which their chemical structure plays a crucial role. In other words, the ability of RIF and RIX molecules to interact with the functional groups of PET fibers represents the driving force behind the adsorption process. Once these functional groups on the adsorbent surface are occupied (through the binding of RIF and RIX molecules), an increase in the initial concentration leads to a much smaller variation in adsorption capacity (
Figure 7), suggesting that saturation has been reached. It should be noted that, according to experimental data (
Figure 7), the saturation of PET fibers can be observed at concentrations higher than 25 mg/L (for both studied antibiotics), highlighting the practical applicability of this adsorbent.
The selection of Langmuir, Freundlich, and Temkin models [
41] for the analysis of experimental data allows the determination of how the adsorption of RIF and RIX occurs on PET fibers (mono- or multilayer), as well as the predominant type of interactions responsible for binding antibiotic molecules to the adsorbent surface. The isotherms obtained for each model are also illustrated in
Figure 7, and the characteristic parameters of these models are summarized in
Table 3. The selection of the most suitable model was made using statistical parameters (R
2, RMSD, and Chi-square).
As can be seen from
Table 3, the values of statistical parameters do not differ significantly from one model to another. However, the careful analysis of these values shows that the Langmuir model is the most suitable for describing the experimental data, suggesting that the adsorption of RIF and RIX on PET fibers occurs in a single layer. The maximum adsorption capacity (q
max, mg/g) is almost 3 times higher in the case of RIF than in the case of RIX (
Table 3), which demonstrates that RIF has a stronger tendency to bind to the surface of PET fibers compared to RIX. Moreover, in both the case of RIF and RIX, the maximum adsorption capacity values calculated from the Langmuir model (44.84 mg RIF/g and 15.65 mg RIX/g) (
Table 3) are fairly close to those obtained experimentally (32.06 mg RIF/g and 14.23 mg RIX/g), which explains the flattening of the experimental adsorption isotherms (
Figure 7) at high concentrations of the two antibiotics. Most likely, the adsorption of RIF and RIX occurs only on the surface of PET fibers (in accordance with the Langmuir model), which involve physicochemical interactions between antibiotics and active sites of the adsorbent (according to the values of parameter
B from the Temkin model) (
Table 3); this behavior makes adsorption processes favorable events at high concentrations of antibiotics (according to the values of parameter
n from the Freundlich model) (
Table 3).
The maximum adsorption capacity values obtained for the retention of RIF and RIX on PET fibers are comparable to those reported in the literature for various adsorbents under similar experimental conditions. Several examples are presented in
Table 4.
All these aspects highlight the applicative potential of PET fibers in the removal of RIF and RIX from aqueous environments and encourage further research.
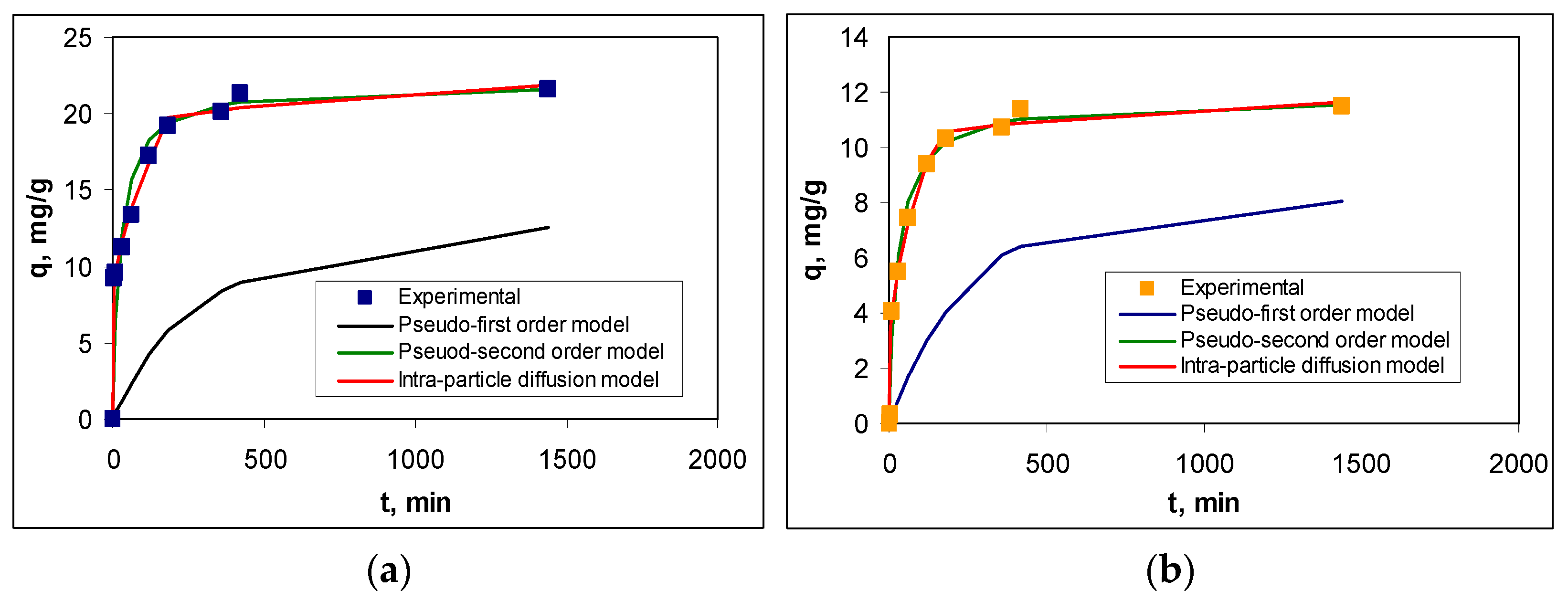
Figure 8 illustrates the experimental kinetic curves and those obtained using the pseudo-first order, pseudo-second order, and intra-particle diffusion kinetic models, while the kinetic parameters are summarized in
Table 5. And in this case, the selection of the most suitable model was made using statistical parameters (R
2, RMSD, and Chi-square).
The statistical parameters presented in
Table 5 show that the adsorption of RIF and RIX on PET fibers is best described by the pseudo-second order kinetic model. The agreement between the experimental data and this kinetic model is also supported by the calculated equilibrium adsorption capacity values (q
e,cal, mg/g), which are close to those obtained experimentally (q
e,exp, mg/g) for both antibiotics (
Table 5). This suggests that the adsorption processes of RIF and RIX on PET take place through physicochemical interactions between the functional groups of antibiotics and two active centers on the surface of the PET fibers.
The necessity for RIF and RIX molecules to interact with two functional groups (active centers) on the surface of PET fibers results in (i) a high initial adsorption rate when most functional groups on the adsorbent surface are free, followed by a decrease in this rate as the superficial functional groups become occupied, and the adsorption process reaching equilibrium in a short time (maximum 180 min); and (ii) a different adsorption efficiency for RIF and RIX under the same experimental conditions (
Figure 8), indicating the differing availability of functional groups in the two antibiotics to participate in such interactions, a factor closely linked to their chemical structure. However, as can be seen from
Table 5, the rate constants obtained for the pseudo-second order kinetic model (k
2, g/mg min) have the same order of magnitude, which demonstrates that the same types of interactions occur during the adsorption processes for both antibiotics.
Although the pseudo-first order kinetic model leads to adsorption capacity values (q
e,cal, mg/g) that differ significantly from those obtained experimentally (q
e,exp, mg/g), the regression coefficient (R
2) and the rate constants (k
1, 1/min) have comparable values to those obtained for the pseudo-second order model (
Table 5). These comparable values allow us to state that, most likely, the adsorption of RIF and RIX on PET fibers occurs in two stages: First, the antibiotic molecules interact with a single functional group (following pseudo-first order kinetics), after which the second interaction takes place and the kinetics transition to pseudo-second order. The second interaction serves to immobilize RIF and RIX on the surface of PET fibers, thus creating a second binding site for the large antibiotic molecules.
The contribution of elementary diffusion processes to the adsorption of RIF and RIX on PET fibers is highlighted by the intra-particle diffusion model (
Figure 8). According to this model, diffusion processes are not rate-determining steps (the linear dependency of q vs. t
1/
2 does not pass through the origin), and two regions can be identified (
Figure S1): Region I, which corresponds to the diffusion of RIF and RIX from the bulk solution to the surface of PET fibers, and Region II, which corresponds to the diffusion of antibiotics into the pores of PET fibers [
47,
48]. Moreover, the values of the kinetic parameters calculated for this model (
Table 5) show that the rate constants corresponding to Region I are an order of magnitude higher than those corresponding to Region II, while the antibiotic concentration (c, mg/L) is higher in Region II than in Region I. These differences suggest that the diffusion of antibiotic molecules from the bulk solution to the surface of the PET fibers occurs much more easily compared to their diffusion into the pores of the adsorbent. This is mainly due to the smooth surface of the PET fibers (as demonstrated by the SEM images—
Figure 3b), which allows RIF and RIX to bind only to the surface of the adsorbent (in accordance with the Langmuir model—
Table 3). It should also be noted that, compared to RIX, in the case of RIF, the elementary diffusion processes are faster, which results in both the rate constants and the c values (mg/L) being higher for both regions (
Table 5). These variations are most likely determined by the chemical structure of the two antibiotics. Although RIF has a higher molecular weight (822.405 g/mol) compared to RIX (782.879 g/mol), the presence of an additional heterocycle in the RIX molecule (
Table 1) alters the mobility of these molecules. Therefore, RIF molecules move more easily through the aqueous solution than RIX, and this influences the efficiency of the adsorption processes.
3.4. Thermodynamic Parameters
Experiments on the influence of temperature (
Figure 4d) showed that increasing the temperature from 10 to 50 °C causes an increase in the adsorption capacity by 74.27% in the case of RIF and 43.67% in the case of RIX. This behavior suggests that, regardless of the nature of the antibiotic, the adsorption process onto PET fibers is heat-absorbing. To obtain a quantitative evaluation of the thermodynamic behavior of RIF and RIX adsorption onto PET fibers, the thermodynamic parameters (∆G
0, ∆H
0, and ∆S
0) were calculated using the van’t Hoff equations [
49]. The values obtained for these parameters are presented in
Table 6, while the linear representations required for the calculation of ∆H
0 are illustrated in
Figure S2.
As can be seen from
Table 6, the Gibbs free energy values (ΔG
0) are negative for all temperature values, both for RIF and RIX, which indicates that the adsorption of the two antibiotics onto PET fibers is a spontaneous process. Moreover, the ΔG
0 values do not differ significantly depending on the nature of the antibiotic, which suggests that the adsorption of RIF and RIX onto PET fibers occurs through a similar mechanism. The enthalpy variation values (ΔH
0) are positive (
Table 6), which indicates that the adsorption processes of RIF and RIX onto PET fibers are endothermic and are favored by increasing temperature. This observation is consistent with the experimental results (
Figure 4d), which showed that raising the temperature from 10 to 50 °C leads to an increase in the adsorption capacity for both RIF and RIX. However, two observations must be made, namely (i) low values of this parameter indicate that the binding of antibiotic molecules to the surface of PET fibers does not involve breaking or forming chemical bonds, most likely, the interactions that occur in the adsorption processes are physicochemical (electrostatic, hydrogen bonds, π-π interactions), and (ii) the difference of one order of magnitude between the ΔH
0 value in the case of RIF compared to that obtained for RIX (
Table 6) suggests that in the adsorption of RIX, the share of physical interactions (such as hydrogen bonds or π-π interactions) is greater than in the case of RIF. This hypothesis is supported by the experimental results presented in the previous sections, which clearly highlight the higher efficiency of RIF adsorption on PET fibers compared to RIX. The positive values of the entropy variation (ΔS
0) (
Table 6) indicate an increase in the degree of disorder at the adsorbent–aqueous solution interface during the adsorption processes. This increase in disorder is most likely due to the water molecules that hydrate the antibiotic molecules, which are released as RIF and RIX bind to the adsorbent surface. The similar values of this parameter obtained for the two antibiotics suggest that their adsorption onto the surface of PET fibers occurs through a similar mechanism, involving the same type of interactions. All these observations show that the adsorption of RIF and RIX onto PET fibers is a spontaneous, endothermic, and thermodynamically feasible process, which may have practical applications.
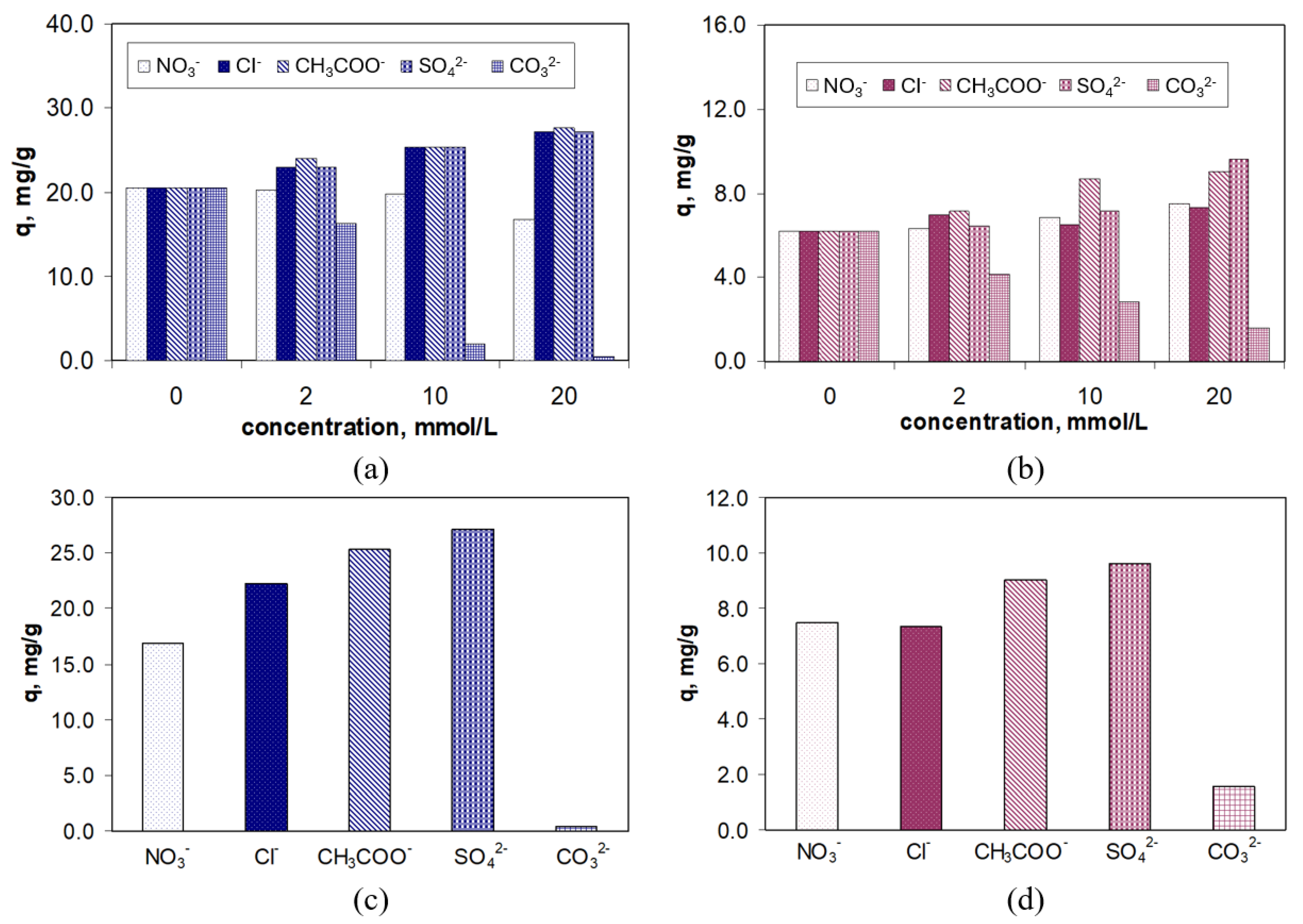
3.5. Effect of Co-Existing Ions
As was shown in the previous section (
Section 3.1), in a strongly acidic environment (pH = 2), established as optimal for these adsorption processes, the RIF and RIX molecules are negatively charged (due to the ionization of the first functional group), while the functional groups of the PET fibers are undissociated/partially protonated. Under these conditions, anions are expected to have a more pronounced influence on the efficiency of the adsorption processes than cations; this is the reason why only anions were selected for the experimental studies.
The experiments were carried out under optimal conditions (pH = 2.0; adsorbent dose = 0.4 g/L, contact time = 180 min, temperature = 22 ± 1 °C), for an initial antibiotic concentration of 21.1 mg/L, while the anion concentration (Cl
−, NO
3−, CH
3COO
−, SO
42−, and CO
32−) varied between 2.0 and 20.0 mmol/L. The results obtained are illustrated in
Figure 9.
As can be seen in
Figure 9, the presence of inorganic anions in the aqueous solution leads to an increase in the adsorption capacity for both RIF and RIX, with this increase becoming more pronounced as the concentration of anions rises. Moreover, the increase in adsorption capacity is more pronounced for RIF (maximum 7.6 mg/g) than for RIX (maximum 3.6 mg/g) (
Figure 9a,b), but regardless of the type of antibiotic, it follows the order of NO
3− < Cl
− < CH
3COO
− < SO
42− (
Figure 9c,d). This order corresponds to the Hofmeister series, which orders anions based on their hydration energy [
50]. Thus, anions with high hydration energies (e.g., SO
42−; ∆H
0hydr. = −1025 kJ/mol) lead to a greater increase in the adsorption capacity of RIF and RIX on PET fibers compared to anions with low hydration energies (e.g., NO
3−, ∆H
0hydr. = −380 kJ/mol) (
Figure 9c,d).
Based on these experimental results, it can be said that the presence of anions leads in the ordering of water molecules in the aqueous solution, an effect that becomes more significant as the hydration energy of the anion increases. As a result, the number of free water molecules or those hydrating RIF and RIX decreases, and consequently, the antibiotic molecules are “pushed” toward the surface of the PET fibers (which are also slightly hydrated). This “pushing” effect of antibiotic molecules toward the surface of the PET fibers also depends on their water solubility (
Table 1), and is more pronounced in the case of RIF (which is more readily soluble in water, thus binding a larger number of water molecules upon dissolution) compared to RIX (which is less soluble in water and therefore the number of water molecules bound upon dissolution is lower) (
Figure 9c,d). However, even the presence of high concentrations of inorganic salts does not significantly improve the removal percentages of RIF and RIX on PET fibers, with the increase in this parameter being a maximum of 14% for RIF and 6% for RIX.
An exception is the CO
32− ion. As shown in
Figure 9a,b, the presence of the CO
32− ion leads to a decrease in the adsorption capacity for both RIF and RIX, and this decrease becomes more significant as the concentration of the CO
32− ion increases. This behavior may be due to (i) the strongly acidic media used in the experimental studies (pH = 2.0), which changes the speciation form of the carbonate ions, or (ii) the direct interaction between the superficial functional groups of PET fibers and the carbonate ions, which prevents the adsorption of RIF and RIX. The first assumption is rather unlikely. Although at pH = 2.0 the predominant speciation form is CO
2 (according to the speciation diagram [
51]), no gas bubble formation indicating CO
2 release was observed experimentally. It is much more likely that the CO
32− ions in the aqueous solution interact directly with the functional groups on the surface of the PET fibers (due to structural similarities [
52,
53]), thereby blocking some functional groups of the adsorbent, which can no longer be involved in the adsorption process. The possibility of such interactions has already been reported in the literature for various materials with functional groups containing O-donor atoms in the presence of carbonate ions [
52,
53].
All these observations provide a more comprehensive understanding of the adsorption process of RIF and RIX onto PET fibers and are valuable both for the formulation of an adsorption mechanism and for practical applications.
3.6. Mechanism of RIF and RIX Adsorption on PET Fibers
The experimental results presented in the previous sections showed that (i) the adsorption of RIF and RIX onto PET fibers occurs with maximum efficiency when pH = 2.0, adsorbent dose = 0.4 g/L, contact time = 180 min, and temperature = 22 °C (see
Section 3.1); (ii) the adsorption takes place in a monolayer, is described by the Langmuir model, and involves the interaction between RIF and RIX with two functional groups on the adsorbent surface (see
Section 3.3); and (iii) the adsorption processes are spontaneous and endothermic (see
Section 3.4).
At this pH value, RIF and RIX are negatively charged due to the dissociation of hydroxyl-type groups (pKa
1 < 2.0) (
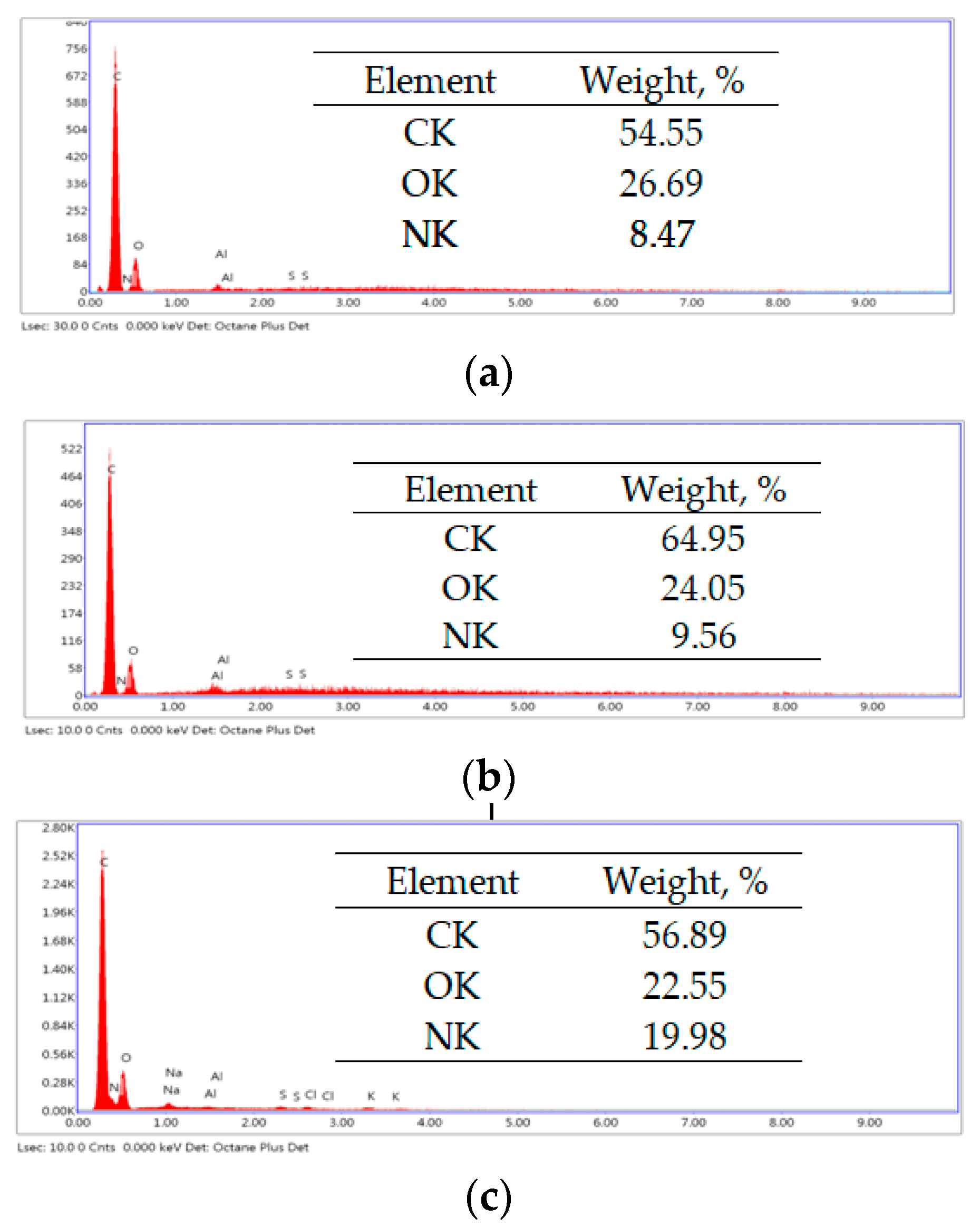
Table 1), while the functional groups of the adsorbent are protonated (positively charged). These conditions promote favorable electrostatic interactions between RIF, RIX, and the adsorbent surface. Consequently, the conditions are favorable for facilitating electrostatic interactions between the two antibiotics and the adsorbent. The spontaneous retention of RIF and RIX on PET fibers is demonstrated by the EDX spectra (
Figure 10).
The increase in carbon and nitrogen content after the adsorption of RIF and RIX (as shown in
Figure 10) provides evidence that both antibiotics are retained on the surface of the PET fibers. Furthermore, the “binding” of antibiotic molecules to the functional groups of the adsorbent predominantly through electrostatic interactions is evidenced by the FTIR spectra (
Figure 11a).
Comparison of the FTIR spectra of PET fibers before and after the adsorption of RIF and RIX (
Figure 11a) reveals that (i) no additional new bands appear in the spectra, indicating that the formation of highly covalent bonds is negligible; and (ii) the most significant shifts in absorption maxima correspond to C=O (1734–1711 cm
−1 in spectrum 1→1715–1631 cm
−1 and 1674–1631 cm
−1 in spectra 2 and 3) and C–O–C bonds (1088 cm
−1 in spectrum 1→1094 cm
−1 and 1084 cm
−1 in spectra 2 and 3), suggesting that these functional groups are most likely involved in the adsorption processes.
The changes in the maxima of the other absorption bands in the FTIR spectra are insignificant (within the range of ±4–6 cm
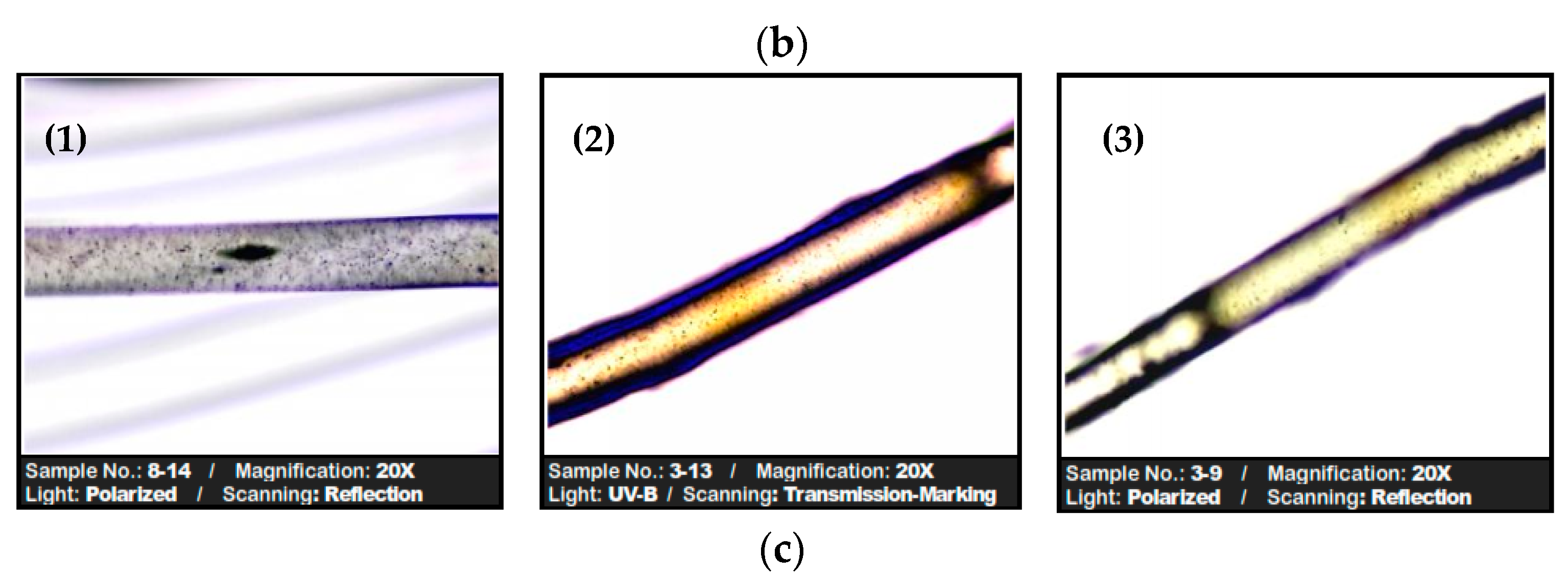
−1, determined by the resolution of the spectral recordings), which shows that the adsorption of RIF and RIX onto the PET fibers does not even alter the chemical proximity of the functional groups on the surface. This observation is also supported by microscopic images (SEM and optical) (
Figure 11b,c), where it can be seen that the structure of the PET fibers is not affected after antibiotic adsorption, but they only “cover” the surface of the adsorbent.
Taking all these aspects into account, it can be said that the adsorption of RIF and RIX onto PET fibers involves two stages (
Figure 12), namely (1) electrostatic interactions between the ionized functional groups of RIF and RIX and the superficial functional groups of the adsorbent (these occur rapidly and explain the need for a strongly acidic media (pH = 2) and the spontaneity of the adsorption processes), followed by (2) the “stabilization” of the antibiotic molecule on the surface of the PET fibers through physical interactions such as hydrogen bonding or π–π interactions (these require a longer period of time (180 min) and explain the correspondence between the experimental data and the Langmuir and pseudo-second order models).
The retention of RIF and RIX on PET fibers through these types of interactions has particularly important practical implications. One of the most significant is that desorption of the antibiotics can be achieved even by treatment with inorganic salt solutions (strong electrolytes), which are capable of breaking the electrostatic bonds formed during the adsorption processes. Our preliminary studies have shown that when PET fibers loaded with RIF or RIX are treated with a medical-grade polyelectrolyte solution (hydration salt solution, purchased from Biomega Natural Nutrients S.L., Madrid, Spain), desorption percent can reach approximately 40% in 30 min of contact time. However, the selection of the most suitable desorption agent, but especially its solution volume and concentration, requires much more rigorous experiments that must be discussed in detail. Therefore, these results will be presented in a subsequent study.