Electrospun Molecularly Imprinted Polymers for Environmental Remediation: A Mini Review
Abstract
1. Introduction
1.1. Molecularly Imprinted Polymers
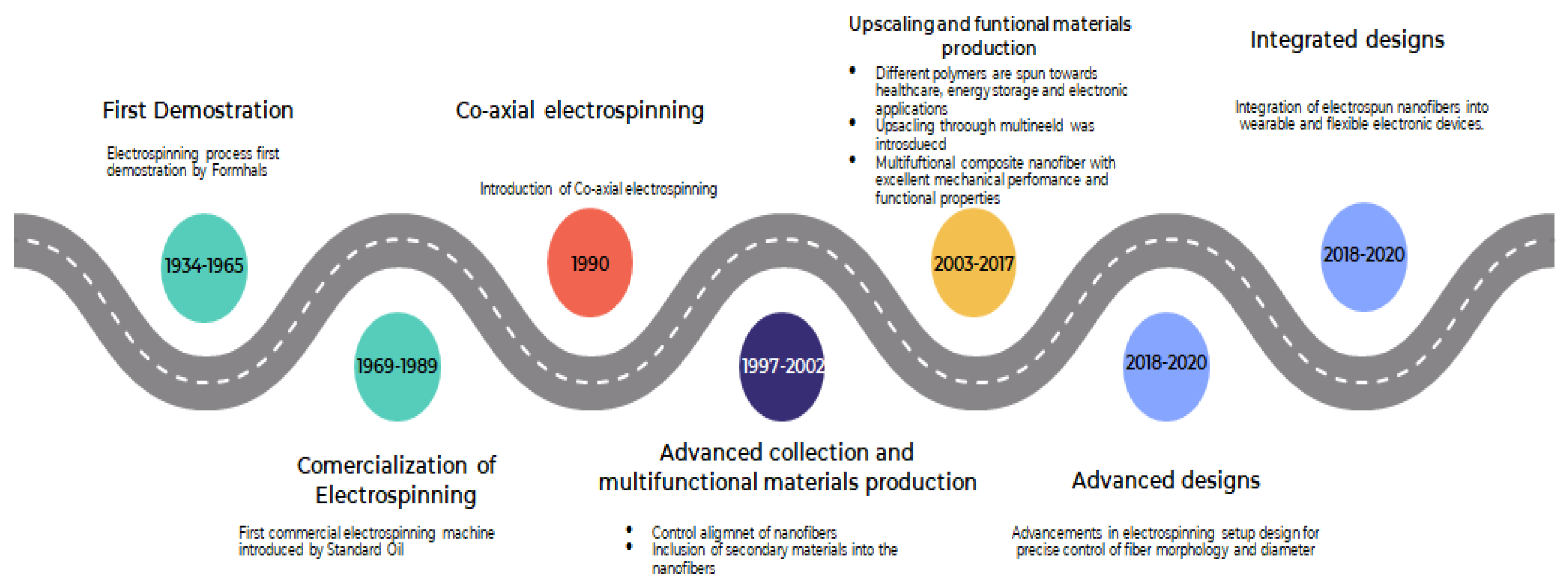
1.2. Electrospinning
2. Remediations
2.1. Dyes
2.1.1. Occurrence and Quantification of Dyes in Water
2.1.2. Electrospun MIMs and Dye Removal Techniques
2.2. Heavy Metals
2.2.1. Occurrence and Quantification of Heavy Metals in Water
2.2.2. Electrospun MIMs and Heavy Metal Removal Techniques
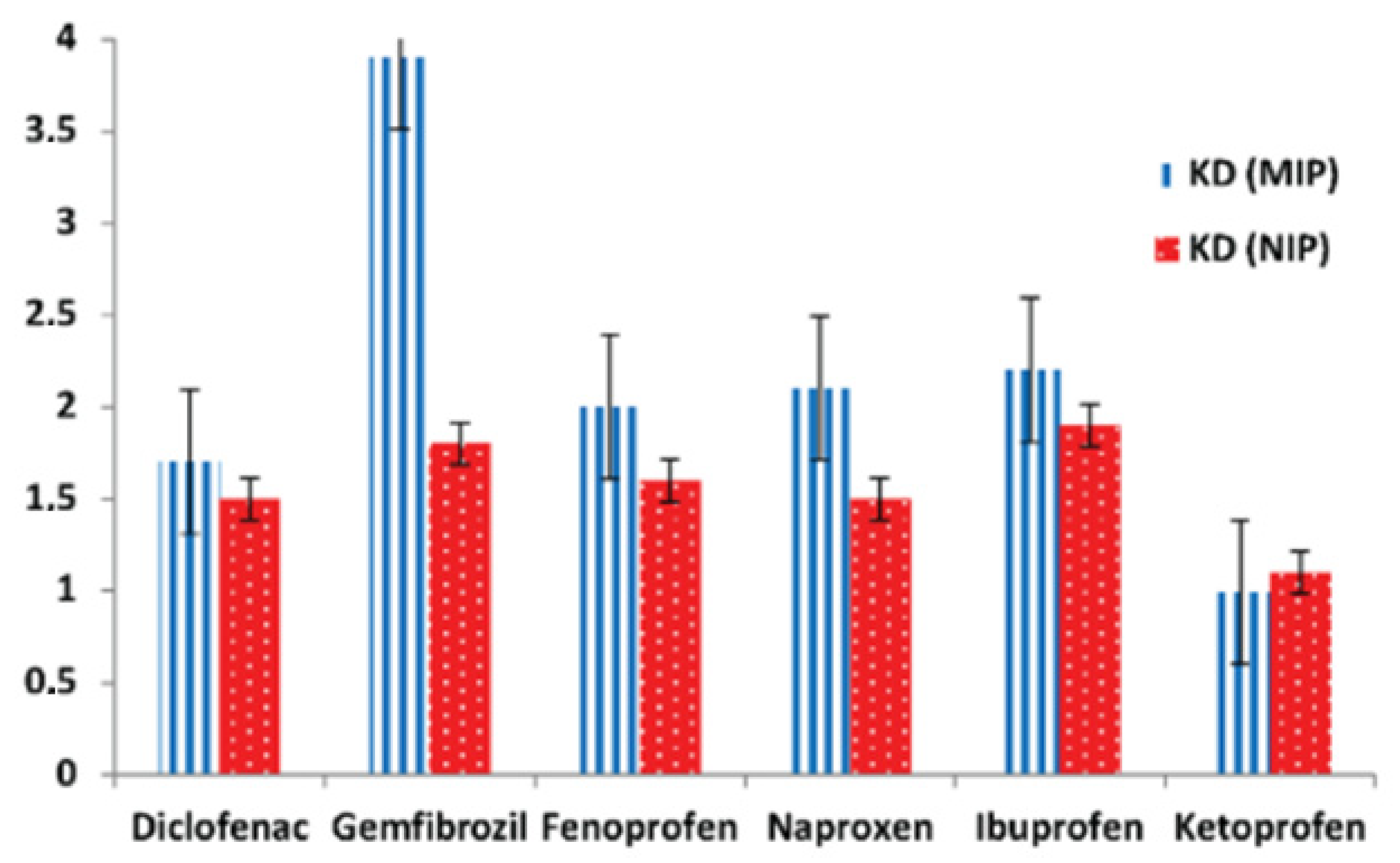
2.3. Emerging Pollutants (NSAIDs and ARVs)
2.3.1. Occurrence and Quantification of NSAIDs and ARVs in Water
2.3.2. Removal Techniques for NSAIDs and ARVs
3. Other Pollutants Removal Using MIMs
4. Limitations
5. Future Outlook and Conclusions
Funding
Conflicts of Interest
References
- Sigonya, S.; Mokhothu, T.H.; Mokhena, T.C.; Makhanya, T.R. Mitigation of Non-Steroidal Anti-Inflammatory and Antiretroviral Drugs as Environmental Pollutants by Adsorption Using Nanomaterials as Viable Solution—A Critical Review. Appl. Sci. 2023, 13, 772. [Google Scholar] [CrossRef]
- Mlunguza, N.Y.; Ncube, S.; Nokwethemba Mahlambi, P.; Chimuka, L.; Madikizela, L.M. Adsorbents and removal strategies of non-steroidal anti-inflammatory drugs from contaminated water bodies. J. Environ. Chem. Eng. 2019, 7, 103142. [Google Scholar] [CrossRef]
- Nkosi, S.M.; Mahlambi, P.N.; Chimuka, L. Synthesis, characterisation and optimisation of bulk molecularly imprinted polymers from nonsteroidal anti-inflammatory drugs. S. Afr. J. Chem. 2022, 76, 56–64. [Google Scholar] [CrossRef]
- Mlunguza, N.Y.; Mdluli, P.S.; Zunngu, S.S.; Madikizela, L.M.; Tavengwa, N.T.; Chimuka, L. Application of molecularly imprinted polymer designed for the selective extraction of ketoprofen from wastewater. Water SA 2018, 44, 406. [Google Scholar] [CrossRef]
- Fan, J.P.; Luo, J.J.; Zhang, X.H.; Zhen, B.; Dong, C.Y.; Li, Y.C.; Shen, J.; Cheng, Y.T.; Chen, H.P. A novel electrospun Β-CD/CS/PVA nanofiber membrane for simultaneous and rapid removal of organic micropollutants and heavy metal ions from water. Chem. Eng. J. 2019, 378, 122232. [Google Scholar] [CrossRef]
- Van Tran, T.; Nguyen, D.T.C.; Le, H.T.N.; Vo, D.V.N.; Nanda, S.; Nguyen, T.D. Optimization, equilibrium, adsorption behavior and role of surface functional groups on graphene oxide-based nanocomposite towards diclofenac drug. J. Environ. Sci. 2020, 93, 137–150. [Google Scholar] [CrossRef]
- Larsson, E.; Al-Hamimi, S.; Jönsson, J.Å. Behaviour of nonsteroidal anti-inflammatory drugs and eight of their metabolites during wastewater treatment studied by hollow fibre liquid phase microextraction and liquid chromatography mass spectrometry. Sci. Total Environ. 2014, 485–486, 300–308. [Google Scholar] [CrossRef]
- Megahed, S.H.; Abdel-Halim, M.; El-shabrawy, Y.I.; Saad, E.M.; Hefnawy, A.; Handoussa, H.; Mizaikoff, B.; El Gohary, N.A. Design, synthesis and medical prospects of electrospun molecularly imprinted fibers. Sci. Rep. 2025, 15, 26082. [Google Scholar] [CrossRef]
- Patel, K.D.; Kim, H.W.; Knowles, J.C.; Poma, A. Molecularly Imprinted Polymers and Electrospinning: Manufacturing Convergence for Next-Level Applications. Adv. Funct. Mater. 2020, 30, 2001955. [Google Scholar] [CrossRef]
- Crapnell, R.D.; Street, R.J.; Ferreira-Silva, V.; Down, M.P.; Peeters, M.; Banks, C.E. Electrospun Nylon Fibers with Integrated Polypyrrole Molecularly Imprinted Polymers for the Detection of Glucose. Anal. Chem. 2021, 93, 13235–13241. [Google Scholar] [CrossRef] [PubMed]
- Belbruno, J.J. Molecularly Imprinted Polymers. Chem. Rev. 2019, 119, 94–119. [Google Scholar] [CrossRef]
- Vasapollo, G.; Del Sole, R.; Mergola, L.; Lazzoi, M.R.; Scardino, A.; Scorrano, S.; Mele, G. Molecularly imprinted polymers: Present and future prospective. Int. J. Mol. Sci. 2011, 12, 5908–5945. [Google Scholar] [CrossRef]
- Boysen, R.I. Advances in the development of molecularly imprinted polymers for the separation and analysis of proteins with liquid chromatography. J. Sep. Sci. 2019, 42, 51–71. [Google Scholar] [CrossRef]
- Cheong, W.J.; Yang, S.H.; Ali, F. Molecular imprinted polymers for separation science: A review of reviews. J. Sep. Sci. 2013, 36, 609–628. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Chen, L.; Li, J.; Zhang, Z. Current status and challenges of ion imprinting. J. Mater. Chem. A 2015, 3, 13598–13627. [Google Scholar] [CrossRef]
- Zahedi, P.; Fallah-Darrehchi, M.; Nadoushan, S.A.; Aeinehvand, R.; Bagheri, L.; Najafi, M. Morphological, thermal and drug release studies of poly (methacrylic acid)-based molecularly imprinted polymer nanoparticles immobilized in electrospun poly (ε-caprolactone) nanofibers as dexamethasone delivery system. Korean J. Chem. Eng. 2017, 34, 2110–2118. [Google Scholar] [CrossRef]
- Piletsky, S.; Canfarotta, F.; Poma, A.; Bossi, A.M.; Piletsky, S. Molecularly Imprinted Polymers for Cell Recognition. Trends Biotechnol. 2020, 38, 368–387. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, X.; Zhang, L.; Gong, J. Electrospun template directed molecularly imprinted nanofibers incorporated with BiOI nanoflake arrays as photoactive electrode for photoelectrochemical detection of triphenyl phosphate. Biosens. Bioelectron. 2017, 92, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Cieplak, M.; Kutner, W. Artificial Biosensors: How Can Molecular Imprinting Mimic Biorecognition? Trends Biotechnol. 2016, 34, 922–941. [Google Scholar] [CrossRef]
- Gao, D.; Wang, D.D.; Zhang, Q.; Yang, F.Q.; Xia, Z.N.; Zhang, Q.H.; Yuan, C.S. In Vivo Selective Capture and Rapid Identification of Luteolin and Its Metabolites in Rat Livers by Molecularly Imprinted Solid-Phase Microextraction. J. Agric. Food Chem. 2017, 65, 1158–1166. [Google Scholar] [CrossRef]
- Peng, S.; Wen, Y.; Ming, Y.; Huang, T.; Xu, G.; Yan, J.; Huang, J.; Song, Z.; Wang, W.; Breadmore, M.C.; et al. Molecularly imprinted polymers based enrichment and separation for trace analysis in capillary electrophoresis. Talanta 2026, 297, 128549. [Google Scholar] [CrossRef]
- Demirkurt, M.; Olcer, Y.A.; Demir, M.M.; Eroglu, A.E. Electrospun polystyrene fibers knitted around imprinted acrylate microspheres as sorbent for paraben derivatives. Anal. Chim. Acta 2018, 1014, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ali, Z.; Ahmad, R. Nanotechnology for Water Treatment; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar] [CrossRef]
- Zhu, F.; Zheng, Y.M.; Zhang, B.G.; Dai, Y.R. A critical review on the electrospun nanofibrous membranes for the adsorption of heavy metals in water treatment. J. Hazard. Mater. 2021, 401, 123608. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Guan, G.; Wang, X.; Lou, T. Electrospun molecularly imprinted sodium alginate/polyethylene oxide nanofibrous membranes for selective adsorption of methylene blue. Int. J. Biol. Macromol. 2022, 207, 62–71. [Google Scholar] [CrossRef]
- Zhao, R.; Li, X.; Sun, B.; Li, Y.; Li, Y.; Wang, C. Preparation of molecularly imprinted sericin/poly(vinyl alcohol) electrospun fibers for selective removal of methylene blue. Chem. Res. Chin. Univ. 2017, 33, 986–994. [Google Scholar] [CrossRef]
- Kebede, T.G.; Dube, S.; Nindi, M.M. Biopolymer electrospun nanofibres for the adsorption of pharmaceuticals from water systems. J. Environ. Chem. Eng. 2019, 7, 103330. [Google Scholar] [CrossRef]
- Camiré, A.; Espinasse, J.; Chabot, B.; Lajeunesse, A. Development of electrospun lignin nanofibers for the adsorption of pharmaceutical contaminants in wastewater. Environ. Sci. Pollut. Res. 2020, 27, 3560–3573. [Google Scholar] [CrossRef]
- Ostovan, A.; Arabi, M.; Wang, Y.; Li, J.; Li, B.; Wang, X.; Chen, L. Greenificated Molecularly Imprinted Materials for Advanced Applications. Adv. Mater. 2022, 34, 2203154. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Tharpa, K.; Dima, Ş.O. Molecularly Imprinted Membranes: Past, Present, and Future. Chem. Rev. 2016, 116, 11500–11528. [Google Scholar] [CrossRef]
- Kempe, M.; Mosbach, K. Separation of amino acids, peptides and proteins on molecularly imprinted stationary phases. J. Chromatogr. A 1995, 691, 317–323. [Google Scholar] [CrossRef]
- Erdo, J. Trends in Analytical Chemistry Electrosynthesized molecularly imprinted polymers for protein recognition. TrAC Trends Anal. Chem. 2016, 79, 179–190. [Google Scholar] [CrossRef]
- Janiak, D.S.; Kofinas, P. Molecular imprinting of peptides and proteins in aqueous media. Anal. Bioanal. Chem. 2007, 389, 399–404. [Google Scholar] [CrossRef]
- Yang, H.; Liu, H.B.; Tang, Z.S.; Qiu, Z.D.; Zhu, H.X.; Song, Z.X.; Jia, A.L. Synthesis, performance, and application of molecularly imprinted membranes: A review. J. Environ. Chem. Eng. 2021, 9, 106352. [Google Scholar] [CrossRef]
- Kang, M.S.; Lee, J.H.; Kim, K.S. Small Toxic Molecule Detection and Elimination Using Molecularly Imprinted Polymers (MIPs). Biosensors 2025, 15, 393. [Google Scholar] [CrossRef]
- Rashid, S.; Rohit, J.V. Molecularly Imprinted Polymer Nanoparticles and Imprinted Nanocomposites Based Optical Sensors for the Detection of Chemical Pollutants. J. Inorg. Organomet. Polym. Mater. 2025. [Google Scholar] [CrossRef]
- Chen, W.; Ma, Y.; Pan, J.; Meng, Z.; Pan, G.; Sellergren, B. Molecularly imprinted polymers with stimuli-responsive affinity: Progress and perspectives. Polymers 2015, 7, 1689–1715. [Google Scholar] [CrossRef]
- Syed Yaacob, S.F.F.; Suwaibatu, M.; Raja Jamil, R.Z.; Mohamed Zain, N.N.; Raoov, M.; Mohd Suah, F.B. Review of molecular imprinting polymer: Basic characteristics and removal of phenolic contaminants based on the functionalized cyclodextrin monomer. J. Chem. Technol. Biotechnol. 2023, 98, 312–330. [Google Scholar] [CrossRef]
- Gao, J.; Chen, L.; Xing, W.; Yu, C.; Yan, Y.; Wu, Y. “Nanomagnet-inspired” design on molecularly imprinted nanofiber membrane: Mechanisms for improved transport selectivity of sufficient specific sites. J. Membr. Sci. 2023, 672, 121467. [Google Scholar] [CrossRef]
- Ahmed, Y.W.; Loukanov, A.; Tsai, H.C. State-of-the-Art Synthesis of Porous Polymer Materials and Their Several Fantastic Biomedical Applications: A Review. Adv. Healthc. Mater. 2024, e2403743. [Google Scholar] [CrossRef]
- Rahman, M.; Dip, T.M.; Nur, M.G.; Hossain, M.H.; Snow, F.; Hossain, N.B.; Mirabedini, A.; Quigley, A.; Padhye, R.; Houshyar, S. Nanomaterial-Integrated 3D Biofabricated Structures for Advanced Biomedical Applications. Macromol. Mater. Eng. 2025, 2500083. [Google Scholar] [CrossRef]
- Du, Y.; Yu, D.G.; Yi, T. Electrospun Nanofibers as Chemosensors for Detecting Environmental Pollutants: A Review. Chemosensors 2023, 11, 208. [Google Scholar] [CrossRef]
- Macagnano, A.; Molinari, F.N.; Papa, P.; Mancini, T.; Lupi, S.; D’Arco, A.; Taddei, A.R.; Serrecchia, S.; De Cesare, F. Nanofibrous Conductive Sensor for Limonene: One-Step Synthesis via Electrospinning and Molecular Imprinting. Nanomaterials 2024, 14, 1123. [Google Scholar] [CrossRef]
- Wang, S.; She, Y.; Hong, S.; Du, X.; Yan, M.; Wang, Y.; Qi, Y.; Wang, M.; Jiang, W.; Wang, J. Dual-template imprinted polymers for class-selective solid-phase extraction of seventeen triazine herbicides and metabolites in agro-products. J. Hazard. Mater. 2019, 367, 686–693. [Google Scholar] [CrossRef]
- Hasanah, A.N.; Safitri, N.; Zulfa, A.; Neli, N.; Rahayu, D. Factors affecting preparation of molecularly imprinted polymer and methods on finding template-monomer interaction as the key of selective properties of the materials. Molecules 2021, 26, 5612. [Google Scholar] [CrossRef]
- Chen, H.W.; Lin, M.F. Characterization, biocompatibility, and optimization of electrospun SF/PCL/CS composite nanofibers. Polymers 2020, 12, 1439. [Google Scholar] [CrossRef]
- Hou, Y.; Ofori, E.A.; Gbologah, L.; Xiong, Y.; Mensah-Darkwa, K.; Tawiah, B.; Fei, B.; Zhao, X. Electrochemical Fiber Electrode Fabrication by Spinning: State-of-the-Art and Perspectives. ACS Electrochem. 2025, 1, 774–804. [Google Scholar] [CrossRef]
- Yıldız, Ü.Y.; Hussain, C.G.; Keçili, R.; Hussain, C.M. Green approaches for the preparation of molecularly imprinted polymers. In Green Imprinted Materials from Design to Environmental and Food Applications; Elsevier: Amsterdam, The Netherlands, 2024; pp. 69–94. [Google Scholar] [CrossRef]
- Haghi, A.K.; Akbari, M. Trends in electrospinning of natural nanofibers. Phys. Status Solidi Appl. Mater. Sci. 2007, 204, 1830–1834. [Google Scholar] [CrossRef]
- Rai, M.; Biswas, J.K. Nanomaterials: Ecotoxicity, Safety, and Public Perception; Springer International Publishing: Cham, Switzerland, 2018. [Google Scholar]
- Li, D.; Xia, Y. Electrospinning of nanofibers: Reinventing the wheel? Adv. Mater. 2004, 16, 1151–1170. [Google Scholar] [CrossRef]
- Chua, C. Review The Design of Scaffolds for Use in Tissue Engineering. Part II. Rapid Prototyping Techniques. Tissue Eng. 2002, 8, 1–11. [Google Scholar]
- Mckee, M.G.; Layman, J.M.; Cashion, M.P.; Long, T.E. Supporting Online Material for Phospholipid Non-woven Electrospun Membranes. Science 2006, 311, 353–355. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, S.; Jose, R.; Archana, P.S.; Nair, A.S.; Balamurugan, R.; Venugopal, J.; Teo, W.E. Science and engineering of electrospun nanofibers for advances in clean energy, water filtration, and regenerative medicine. J. Mater. Sci. 2010, 45, 6283–6312. [Google Scholar] [CrossRef]
- Laudenslager, M.J.; Scheffler, R.H.; Sigmund, W.M. Electrospun materials for energy harvesting, conversion, and storage: A review. Pure Appl. Chem. 2010, 82, 2137–2156. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fi ber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef]
- Yong, C.; Wang, Z.; Zhang, X.; Shi, X.; Ni, Z.; Fu, H.; Ding, G.S.; Fu, Z.R.; Yin, H. The therapeutic effect of monocyte chemoattractant protein-1 delivered by an electrospun scaffold for hyperglycemia and nephrotic disorders. Int. J. Nanomed. 2014, 9, 985–993. [Google Scholar] [CrossRef]
- Wang, R.; Liu, Y.; Li, B.; Hsiao, B.S.; Chu, B. Electrospun nanofibrous membranes for high flux microfiltration. J. Membr. Sci. 2012, 392–393, 167–174. [Google Scholar] [CrossRef]
- Liu, X.; Yang, J.L.; Tong, L.Y.; Zhang, Q.; Li, X.W.; Chen, J.D. Preparation of Cu(II)-imprinted nanofibers from co-electrospinning PVA and imprinting complex. Chem. Res. Chin. Univ. 2015, 31, 1062–1065. [Google Scholar] [CrossRef]
- Li, W.; Li, Y.; Liu, J.; Chao, S.; Yang, T.; Li, L.; Wang, C.; Li, X. A Novel Hollow Carbon@MnO2 Electrospun Nanofiber Adsorbent for Efficient Removal of Pb2+ in Wastewater. Chem. Res. Chin. Univ. 2021, 37, 496–504. [Google Scholar] [CrossRef]
- Rammika, M.; Darko, G.; Torto, N. Incorporation of Ni(II)-dimethylglyoxime ion-imprinted polymer into electrospun polysulphone nanofibre for the determination of Ni(II) ions from aqueous samples. Water SA 2011, 37, 539–546. [Google Scholar] [CrossRef][Green Version]
- Yoshimatsu, K.; Ye, L.; Lindberg, J.; Chronakis, I.S. Selective molecular adsorption using electrospun nanofiber affinity membranes. Biosens. Bioelectron. 2008, 23, 1208–1215. [Google Scholar] [CrossRef]
- Li, Y.; Qiu, T.; Xu, X. Preparation of lead-ion imprinted crosslinked electro-spun chitosan nanofiber mats and application in lead ions removal from aqueous solutions. Eur. Polym. J. 2013, 49, 1487–1494. [Google Scholar] [CrossRef]
- Piperno, S.; Tse Sum Bui, B.; Haupt, K.; Gheber, L.A. Immobilization of molecularly imprinted polymer nanoparticles in electrospun poly(vinyl alcohol) nanofibers. Langmuir 2011, 27, 1547–1550. [Google Scholar] [CrossRef]
- Li, L.; Liu, H.; Lei, X.; Zhai, Y. Electrospun Nanofiber Membranes Containing Molecularly Imprinted Polymer (MIP) for Rhodamine B (RhB). Adv. Chem. Eng. Sci. 2012, 2, 266–274. [Google Scholar] [CrossRef]
- Alfikro, I.; Afrizal, N.; Jorena, J.; Saleh, K.; Satya, O.; Virgo, F.; Royani, I. Incorporation of Fe(III)-IIPs (Ion Imprinted Polymers) and PVA/Gelatine Nanofiber using Electrospinning Method: A Report; EAI: Gent, Belgium, 2024. [Google Scholar] [CrossRef]
- Bagbi, Y.; Pandey, A.; Solanki, P.R. Electrospun Nanofibrous Filtration Membranes for Heavy Metals and Dye Removal. In Nanoscale Materials in Water Purification; Elsevier: Amsterdam, The Netherlands, 2019; pp. 275–288. [Google Scholar] [CrossRef]
- Nguyen, C.H.; Juang, R.S. Efficient removal of cationic dyes from water by a combined adsorption-photocatalysis process using platinum-doped titanate nanomaterials. J. Taiwan Inst. Chem. Eng. 2019, 99, 166–179. [Google Scholar] [CrossRef]
- Wang, L.; Li, L.; Cao, D. A BODIPY-based dye with red fluorescence in solid state and used as a fluorescent and colorimetric probe for highly selective detection of cyanide. Sens. Actuators B Chem. 2017, 239, 1307–1317. [Google Scholar] [CrossRef]
- Ouakouak, A.; Abdelhamid, M.; Thouraya, B.; Chahinez, H. Development of a Novel Adsorbent Prepared from Dredging Sediment for Effective Removal of Dye in Aqueous Solutions. Appl. Sci. 2021, 11, 10722. [Google Scholar] [CrossRef]
- Shahadat, M. Regeneration performance of clay-based adsorbents for the removal of industrial dyes: A review. RSC Adv. 2018, 8, 24571–24587. [Google Scholar] [CrossRef] [PubMed]
- Shojaei, M.; Esmaeili, H. Ultrasonic-assisted synthesis of zeolite/activated carbon @ MnO 2 composite as a novel adsorbent for treatment of wastewater containing methylene blue and brilliant blue. Environ. Monit. Assess. 2022, 194, 279. [Google Scholar] [CrossRef] [PubMed]
- Abid, M.F.; Zablouk, M.A.; Abid-alameer, A.M. Experimental study of dye removal from industrial wastewater by membrane technologies of reverse osmosis and nanofiltration. Iran. J. Environ. Health Sci. Eng. 2012, 9, 17. [Google Scholar] [CrossRef]
- Peramune, D.; Manatunga, D.C.; Dassanayake, R.S.; Premalal, V.; Liyanage, R.N.; Gunathilake, C.; Abidi, N. Recent advances in biopolymer-based advanced oxidation processes for dye removal applications: A review. Environ. Res. 2022, 215, 114242. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.G.; Liu, G.H.; Qi, L.; Wang, H.C.; Ye, Z.F.; Zhao, Q.L. Heavy metal-contained wastewater in China: Discharge, management and treatment. Sci. Total Environ. 2022, 808, 152091. [Google Scholar] [CrossRef]
- Mahurpawar, M. Effects of Heavy Metals on Human Healtheffects of Heavy Metals on Human Health. Int. J. Res. Granthaalayah 2015, 530, 1–7. [Google Scholar] [CrossRef]
- Njoku, P.; Bennard, O.; Akudinobi, B. Potential health risk and levels of heavy metals in water resources of lead—Zinc mining communities of Abakaliki, southeast Nigeria. Appl. Water Sci. 2020, 10, 184. [Google Scholar] [CrossRef]
- Madikizela, L.M.; Chimuka, L.; Ncube, S. Metal pollution source apportionment in two important Rivers of Eastern Cape Province, South Africa: A case study of Bizana and Mthatha Rivers. Environ. Forensics 2021, 24, 71–84. [Google Scholar] [CrossRef]
- Esalah, J.; Husein, M.M. Removal of Heavy Metals from Aqueous Solutions by Precipitation-Filtration Using Novel Organo- Phosphorus Ligands. Sep. Sci. Technol. 2008, 43, 3461–3475. [Google Scholar] [CrossRef]
- Pepe, F.; De Gennaro, B.; Aprea, P.; Caputo, D. Natural zeolites for heavy metals removal from aqueous solutions: Modeling of the fixed bed Ba2+/Na+ ion-exchange process using a mixed phillipsite/chabazite-rich tuff. Chem. Eng. J. 2013, 219, 37–42. [Google Scholar] [CrossRef]
- Dong, L.; Wang, Z.; Gu, P.; Chen, G.; Jiang, R. A new function of spent activated carbon in BAC process: Removing heavy metals by ion exchange mechanism. J. Hazard. Mater. 2018, 359, 76–84. [Google Scholar] [CrossRef]
- Zhao, F.; Xiang, H.; Min, X.; Tang, C. Recent advances in membrane filtration for heavy metal removal from wastewater: A mini review. J. Water Process Eng. 2022, 49, 103023. [Google Scholar] [CrossRef]
- Shu, C.; Chiew, C.; Gourich, W.; Pasbakhsh, P.; Eong, P.; Tey, T.; Pin, C.; Chan, E. Life cycle assessment on alginate-based nanocomposite beads for the removal of lead (II) from aqueous solutions. J. Water Process Eng. 2022, 45, 102531. [Google Scholar] [CrossRef]
- Awokoya, K.N.; Moronkola, B.A.; Chigome, S.; Ondigo, D.A.; Tshentu, Z.; Torto, N. Molecularly imprinted electrospun nanofibers for adsorption of nickel-5,10,15,20-tetraphenylporphine (NTPP) in organic media. J. Polym. Res. 2013, 20, 148. [Google Scholar] [CrossRef]
- Sigonya, S.; Chibuzor, S.; Phumlani, O.; Mdluli, S.; Hendrica, T. Method optimisation and application based on solid phase extraction of non steroidal anti-inflammatory drugs, antiretroviral drugs, and a lipid regulator from coastal areas of Durban, South Africa. SN Appl. Sci. 2022, 4, 231. [Google Scholar] [CrossRef]
- Eggen, T.; Moeder, M.; Arukwe, A. Municipal landfill leachates: A significant source for new and emerging pollutants. Sci. Total Environ. 2010, 408, 5147–5157. [Google Scholar] [CrossRef]
- Aini, W.; Ibrahim, W.; Veni, K.; Marsin, M. Novel sol–gel hybrid methyltrimethoxysilane–tetraethoxysilane as solid phase extraction sorbent for organophosphorus pesticides. J. Chromatogr. A 2012, 1229, 55–62. [Google Scholar] [CrossRef]
- Andrade-Eiroa, A.; Canle, M.; Leroy-Cancellieri, V.; Cerdà, V. Solid-phase extraction of organic compounds: A critical review. part ii. TrAC Trends Anal. Chem. 2016, 80, 655–667. [Google Scholar] [CrossRef]
- Mbhele, Z.E.; Ncube, S.; Madikizela, L.M. Synthesis of a molecularly imprinted polymer and its application in selective extraction of fenoprofen from wastewater. Environ. Sci. Pollut. Res. 2018, 25, 36724–36735. [Google Scholar] [CrossRef] [PubMed]
- Lagha, A. A Molecularly Imprinted Polymer for the Selective Solid-Phase Extraction of Ibuprofen from Urine Samples. Open Chem. Biomed. Methods J. 2011, 4, 7–13. [Google Scholar] [CrossRef][Green Version]
- Zhang, L.; Guo, Y.; Chi, W.H.; Shi, H.G.; Ren, H.Q.; Guo, T.Y. Electrospun nanofibers containing p-nitrophenol imprinted nanoparticles for the hydrolysis of paraoxon. Chin. J. Polym. Sci. (Engl. Ed.) 2014, 32, 1469–1478. [Google Scholar] [CrossRef]
- Chang, K.L.; Teng, T.C.; Fu, C.K.; Liu, C.H. Improving biodegradation of Bisphenol A by immobilization and inducer. Process Saf. Environ. Prot. 2019, 128, 128–134. [Google Scholar] [CrossRef]
- Wu, Y.T.; Zhang, Y.H.; Zhang, M.; Liu, F.; Wan, Y.C.; Huang, Z.; Ye, L.; Zhou, Q.; Shi, Y.; Lu, B. Selective and simultaneous determination of trace bisphenol A and tebuconazole in vegetable and juice samples by membrane-based molecularly imprinted solid-phase extraction and HPLC. Food Chem. 2014, 164, 527–535. [Google Scholar] [CrossRef]
- Liu, F.; Liu, Q.; Zhang, Y.; Liu, Y.; Wan, Y.; Gao, K.; Huang, Y.; Xia, W.; Wang, H.; Shi, Y.; et al. Molecularly imprinted nanofiber membranes enhanced biodegradation of trace bisphenol A by Pseudomonas aeruginosa. Chem. Eng. J. 2015, 262, 989–998. [Google Scholar] [CrossRef]
- Gore, P.M.; Khurana, L.; Siddique, S.; Panicker, A.; Kandasubramanian, B. Ion-imprinted electrospun nanofibers of chitosan/1-butyl-3-methylimidazolium tetrafluoroborate for the dynamic expulsion of thorium (IV) ions from mimicked effluents. Environ. Sci. Pollut. Res. 2018, 25, 3320–3334. [Google Scholar] [CrossRef]
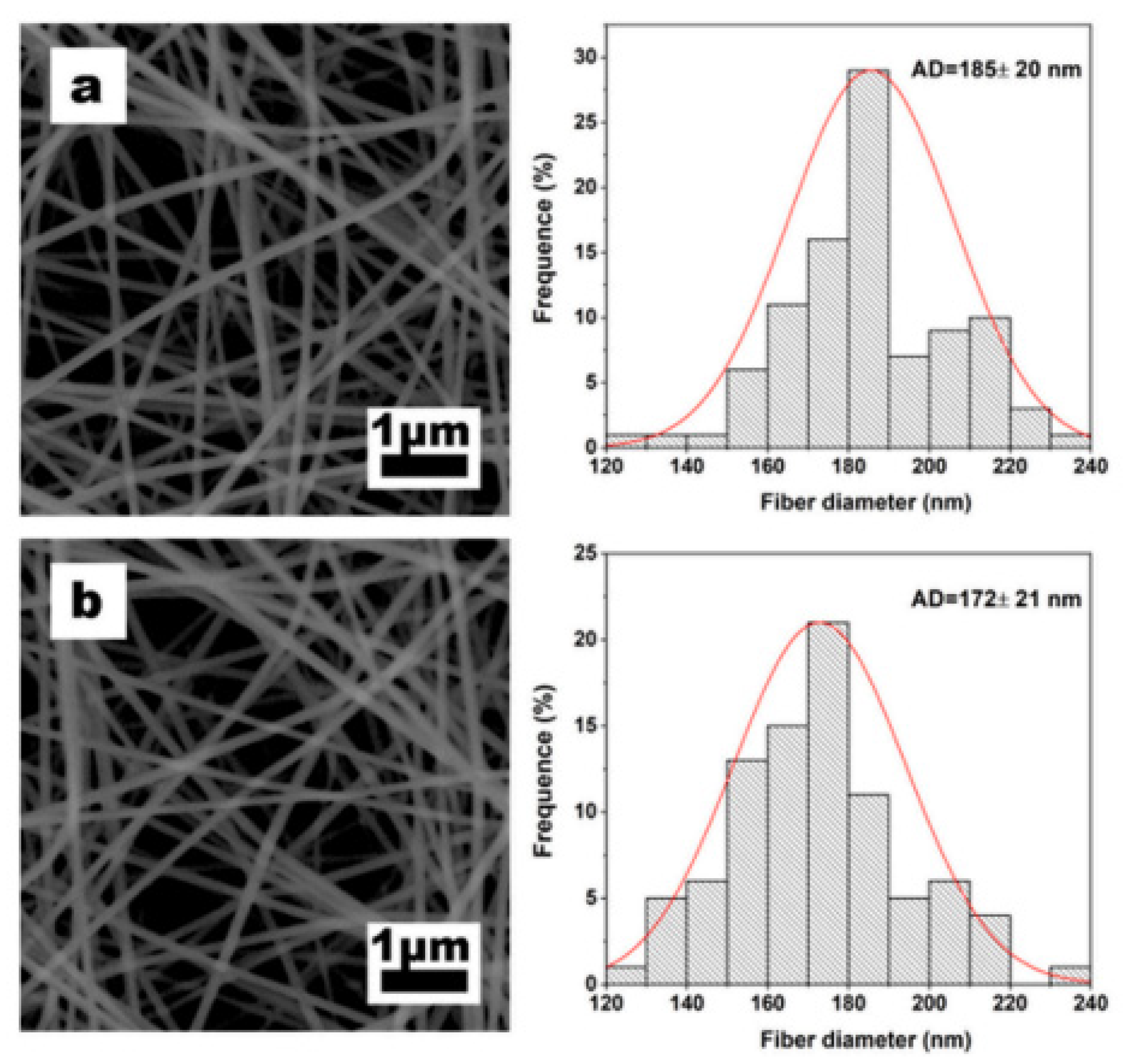

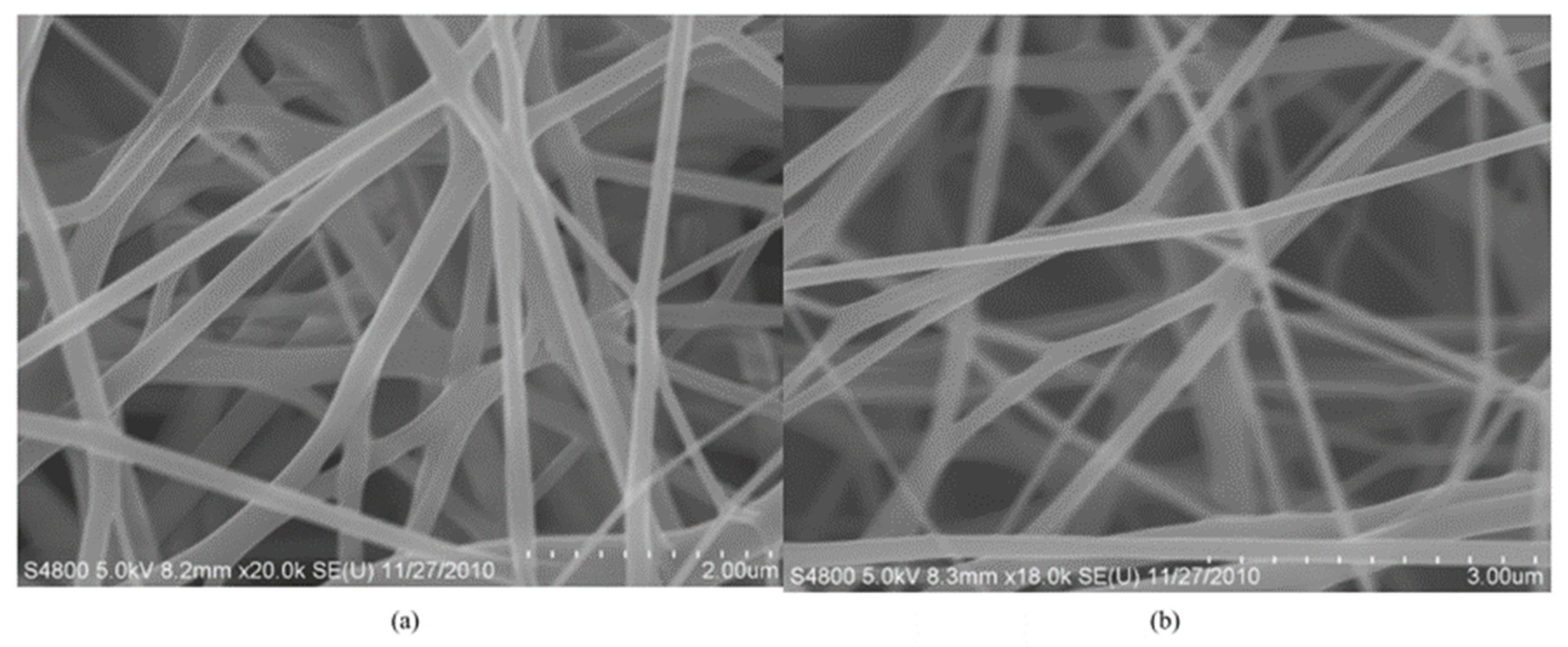
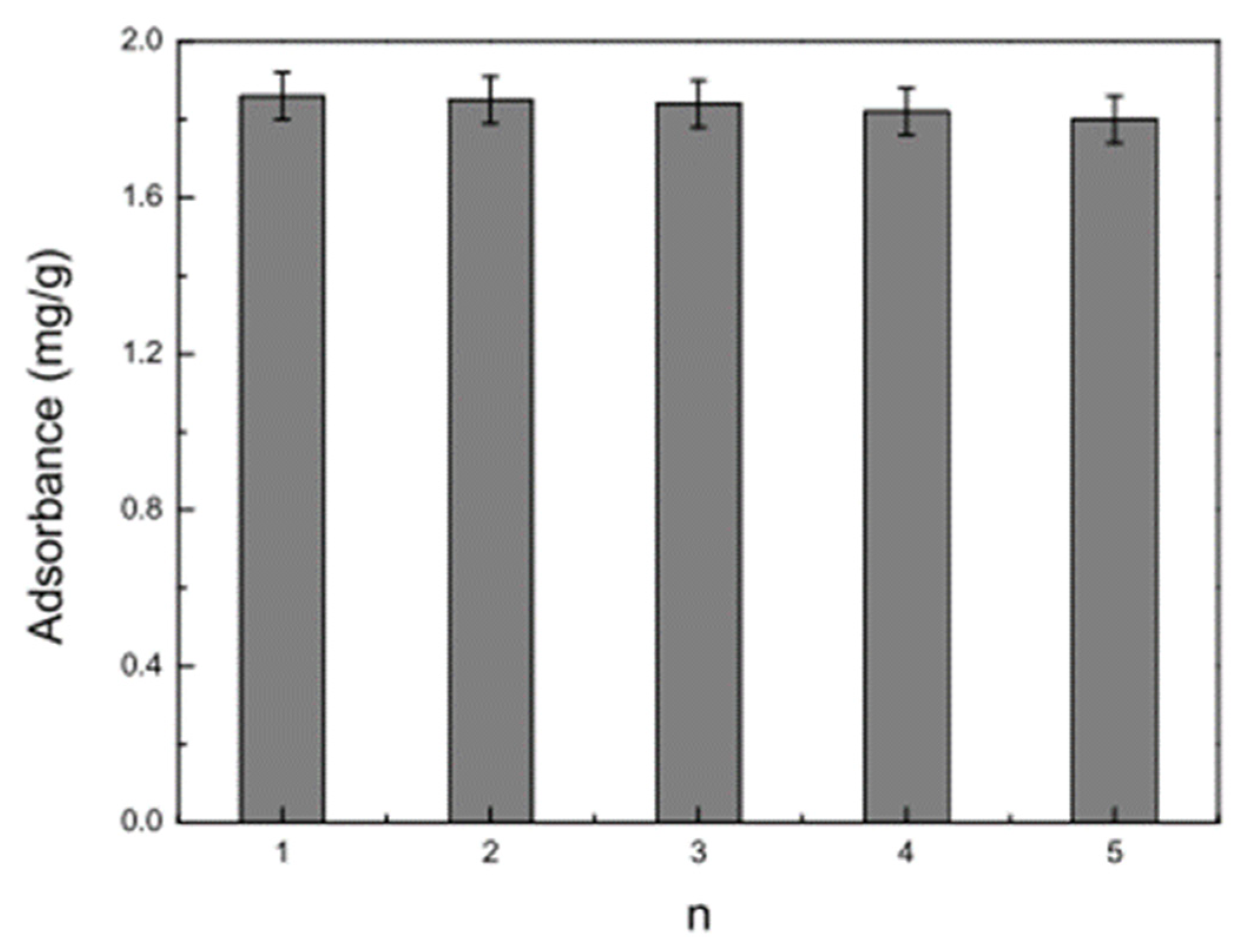
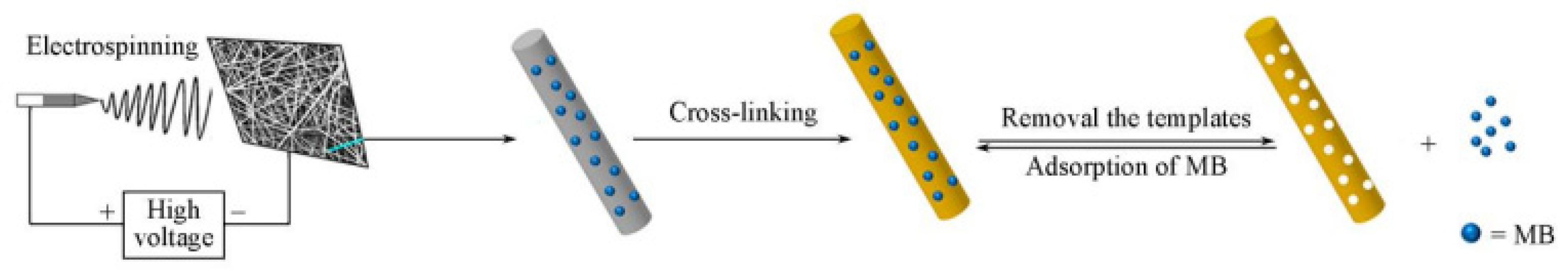

| Formulation | Electrospinning Technique | Optimal Processing Conditions | Highlights | Refs. |
|---|---|---|---|---|
| Polyethylene terephthalate (PET) | -Voltage = 18 kV -Tip-to-collector (TTC) = 15 cm -RH = 45% -Temperature = 25 °C | An optimal pH of 7 and an adsorption time of 80 min were identified for RhB uptake. The system achieved over 90% recovery across five consecutive cycles. | [65] | |
| Polyvinyl alcohol (PVA)/sercin-methylene blue (MB) | Classic electrospinning | -Voltage = 15 kV -Tip-to-collector (TTC) = 18 cm | The imprinted membrane was prepared by crosslinking with glutaraldehyde, followed by washing to remove the target molecule. The imprinted electrospun membrane demonstrated enhanced selectivity and superior adsorption capacity compared to the non-imprinted counterpart. | [26] |
| Polyvinyl alcohol (PVA)/Cu-L-histidine | Classic electrospinning | -Voltage = 26 kV -Feeding rate = 0.4 mL/h -TTC = 7 cm | After crosslinking using glutaraldehyde, the imprinted nanofibrous membrane was washed to remove the copper. The desorption capacity reached approximately 115 mg/g, maintaining about 88% of the initial adsorption capacity after five regeneration cycles. Selectivity coefficient of 52, 54 and 66 for Cu relative to Pb, Ni and Zn were attained. | [59] |
| Polyacrylonitrile (PAN) | Classic electrospinning | - | Following pre-oxidation and carbonization processes to convert PAN into carbon nanofibers, the fibers were immersed in a KMnO4 solution and subjected to a hydrothermal treatment to produce a hollow-structured membrane coated with manganese oxide nanosheets. The imprinted membrane exhibited its highest adsorption capacity at pH 6, reaching an optimal value of approximately 461 mg/g. After five adsorption–desorption cycles, the membrane retained about 81% of its initial adsorption capacity. | [60] |
| polysulphone (PSU)/nickel (II)-dimethyl glyoxime | Classic electrospinning | -Voltage = 15 kV -TTC = 12 cm | The imprinted membrane was employed for solid-phase extraction (SPE) and removal of nickel (Ni), achieving recovery rates above 90% even in the presence of interfering metal ions | [61] |
| Chitosan | Classic electrospinning | -Voltage = 18.5 kV -TTC = 9 cm | Chitosan combined with lead chloride was prepared via electrospinning, followed by crosslinking using glutaraldehyde vapor and washing with EDTA to remove the template. The imprinted fibers demonstrated an adsorption capacity of 577 mg/g at pH 6. | [63] |
| poly(ethyleneterephthalate) (PET)/propranolol | -Voltage = 20 kV -TTC = 20 cm | The resulting composite membrane exhibited a strong affinity for the target, effectively preventing any leakage from the membrane. | [62] | |
| PVA/gelatine | -Voltage = 15 kV -Feeding rate = 0.25 mL/h -RH = 55% -TTC = 12 cm | Fibers with an average diameter of 38 nm were produced, featuring spherical linkages (beads). However, no adsorption studies were conducted; the inclusion of the template was confirmed solely through XPS analysis. | [66] | |
| PVA | -TTC = 15 cm -Voltage = 20 kV | The fibers were crosslinked using butanediol diglycidyl ether, followed by washing steps. The nanofibers demonstrated approximately 100% recovery after the adsorption–desorption cycle. | [64] |
| Sample | Langmuir Isotherm | Freundlich Isotherm | ||||
|---|---|---|---|---|---|---|
| Qmax (mg/g) | B (L/mg) | R2 | Kf (mg/g) | n | R2 | |
| SA/PEO-MINM | 3186.74 | 2.11 × 10−2 | 0.997 | 384.13 | 2.90 | 0.945 |
| SA/PEO-NINM | 2551.02 | 3.40 × 10−3 | 0.990 | 39.05 | 1.65 | 0.969 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sigonya, S.; Mothudi, B.M.; Fakayode, O.J.; Mokhena, T.C.; Mayer, P.; Mokhothu, T.H.; Makhanya, T.R.; Shingange, K. Electrospun Molecularly Imprinted Polymers for Environmental Remediation: A Mini Review. Polymers 2025, 17, 2082. https://doi.org/10.3390/polym17152082
Sigonya S, Mothudi BM, Fakayode OJ, Mokhena TC, Mayer P, Mokhothu TH, Makhanya TR, Shingange K. Electrospun Molecularly Imprinted Polymers for Environmental Remediation: A Mini Review. Polymers. 2025; 17(15):2082. https://doi.org/10.3390/polym17152082
Chicago/Turabian StyleSigonya, Sisonke, Bakang Mo Mothudi, Olayemi J. Fakayode, Teboho C. Mokhena, Paul Mayer, Thabang H. Mokhothu, Talent R. Makhanya, and Katekani Shingange. 2025. "Electrospun Molecularly Imprinted Polymers for Environmental Remediation: A Mini Review" Polymers 17, no. 15: 2082. https://doi.org/10.3390/polym17152082
APA StyleSigonya, S., Mothudi, B. M., Fakayode, O. J., Mokhena, T. C., Mayer, P., Mokhothu, T. H., Makhanya, T. R., & Shingange, K. (2025). Electrospun Molecularly Imprinted Polymers for Environmental Remediation: A Mini Review. Polymers, 17(15), 2082. https://doi.org/10.3390/polym17152082








