Innovative Biobased Active Composites of Cellulose Acetate Propionate with Tween 80 and Cinnamic Acid for Blueberry Preservation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
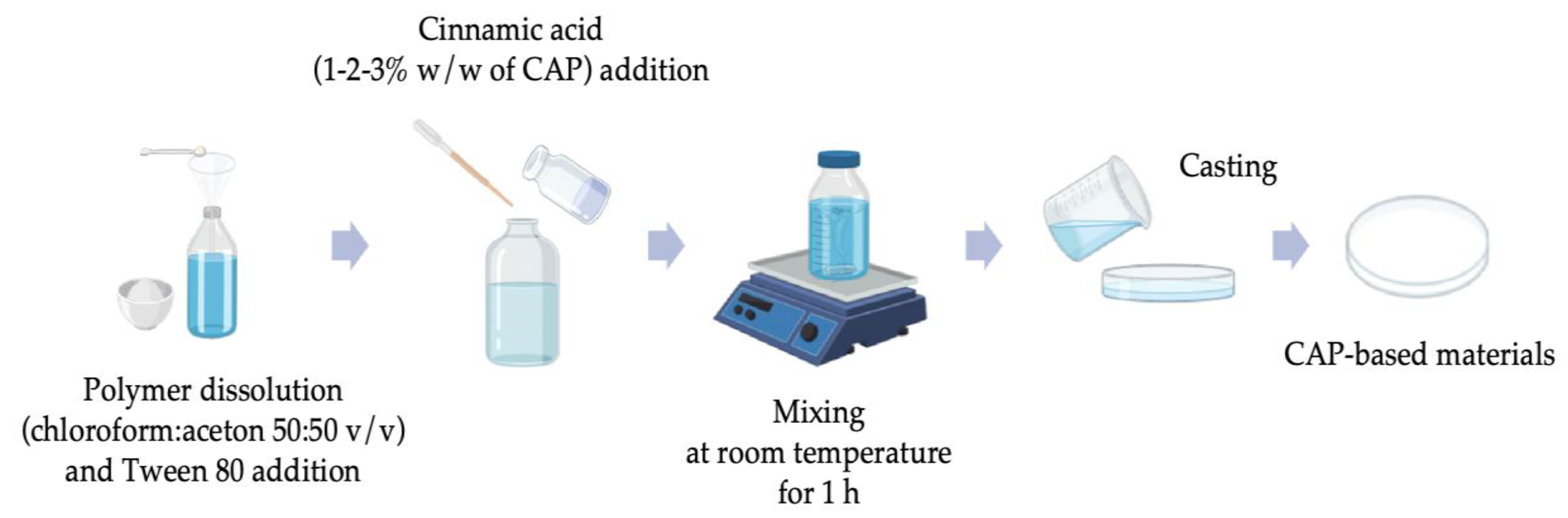
2.2. Fabrication of CAP-Based Materials
2.3. Characterization of CAP-Based Films
2.3.1. Fourier Transform Infrared Analysis
2.3.2. Morphology and Topography Study
2.3.3. Mechanical Properties
2.3.4. Water Vapor Transmission Rate
2.3.5. Transparency and UV Barrier Properties
2.3.6. Assessment of Antioxidative Properties
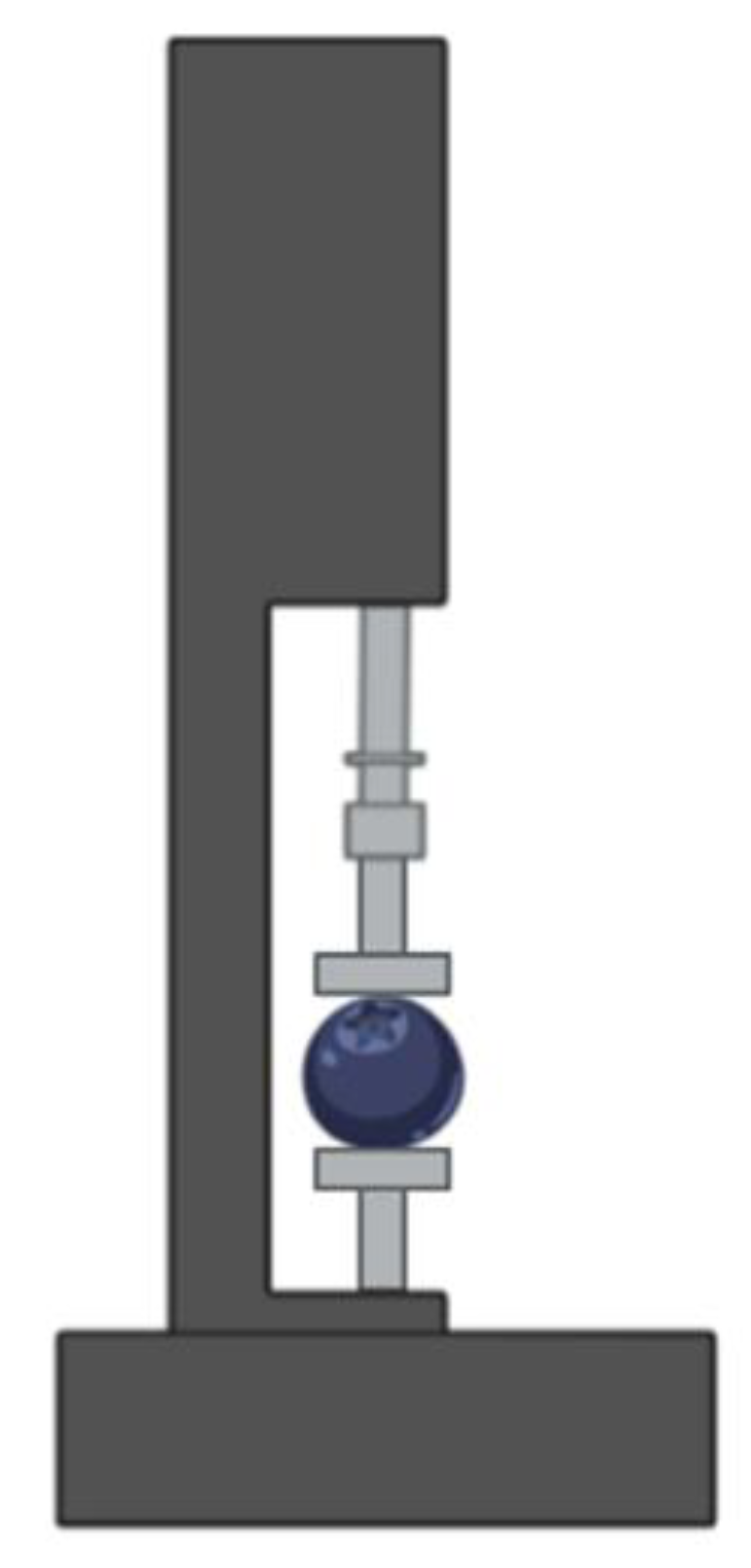
2.3.7. Blueberries Storage and Firmness
2.3.8. Determination of Bactericidal Properties
2.3.9. Statistical Analysis
3. Results and Discussion
3.1. FTIR Analysis of Studied Materials
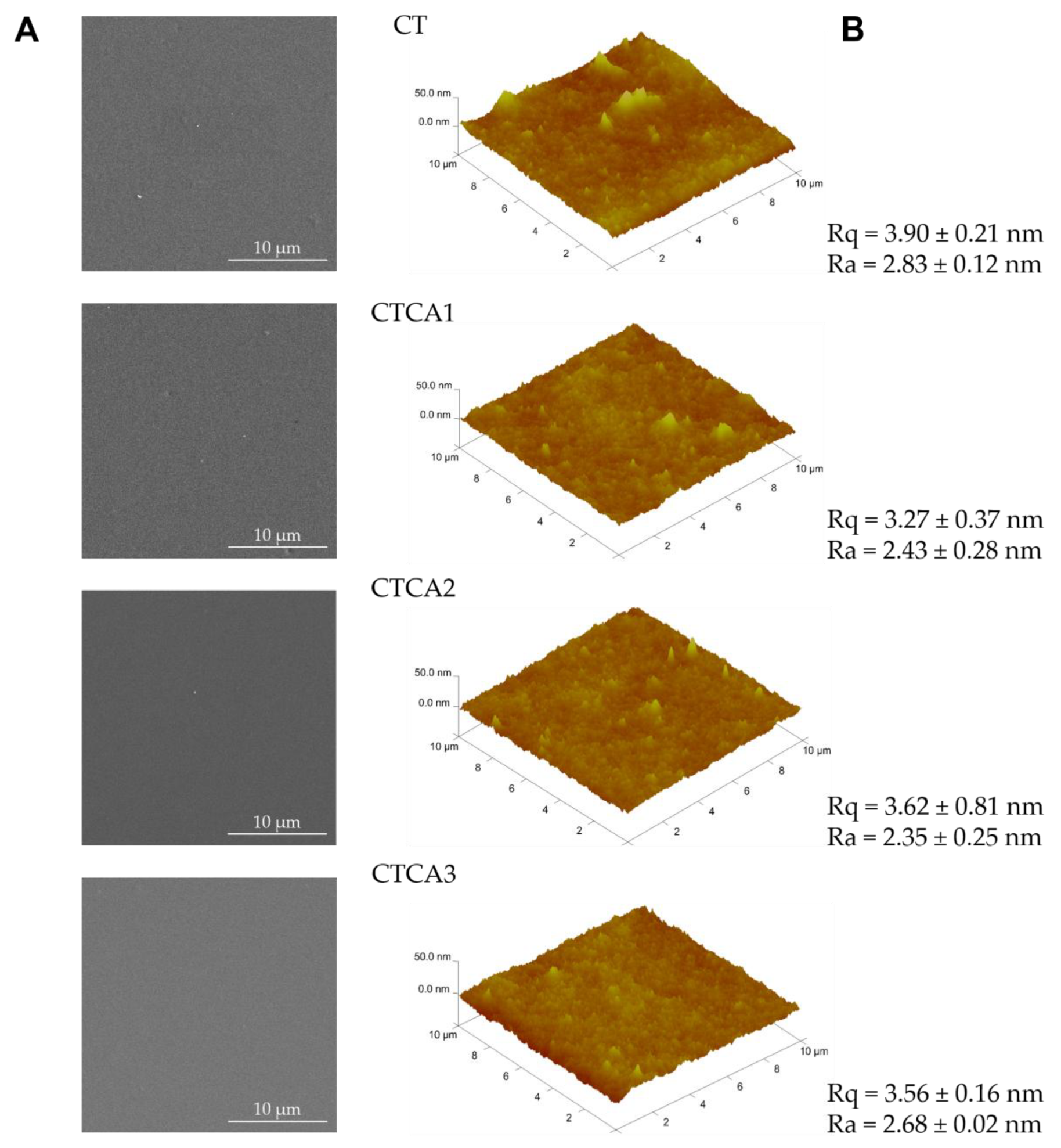
3.2. SEM and AFM Results and Discussion
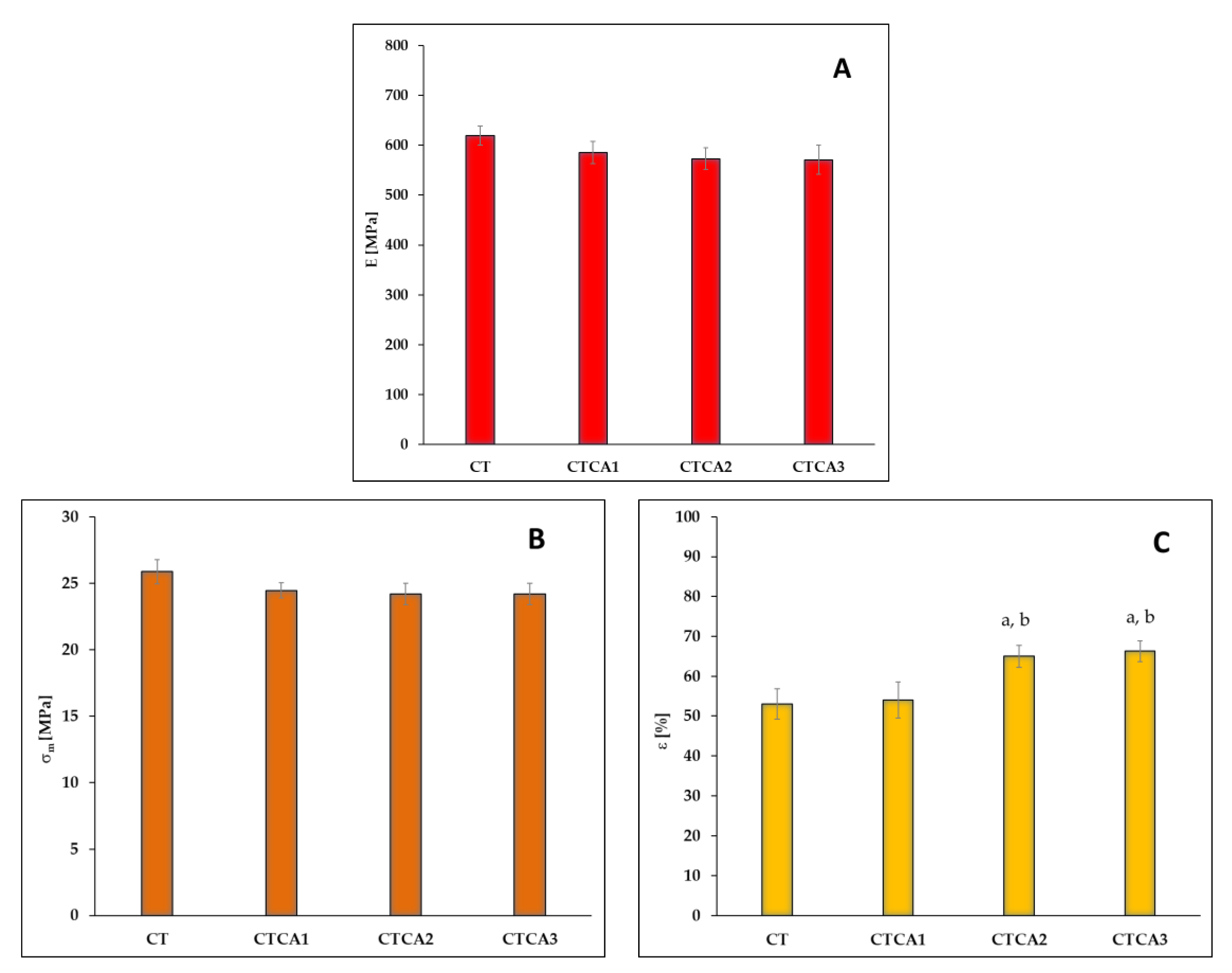
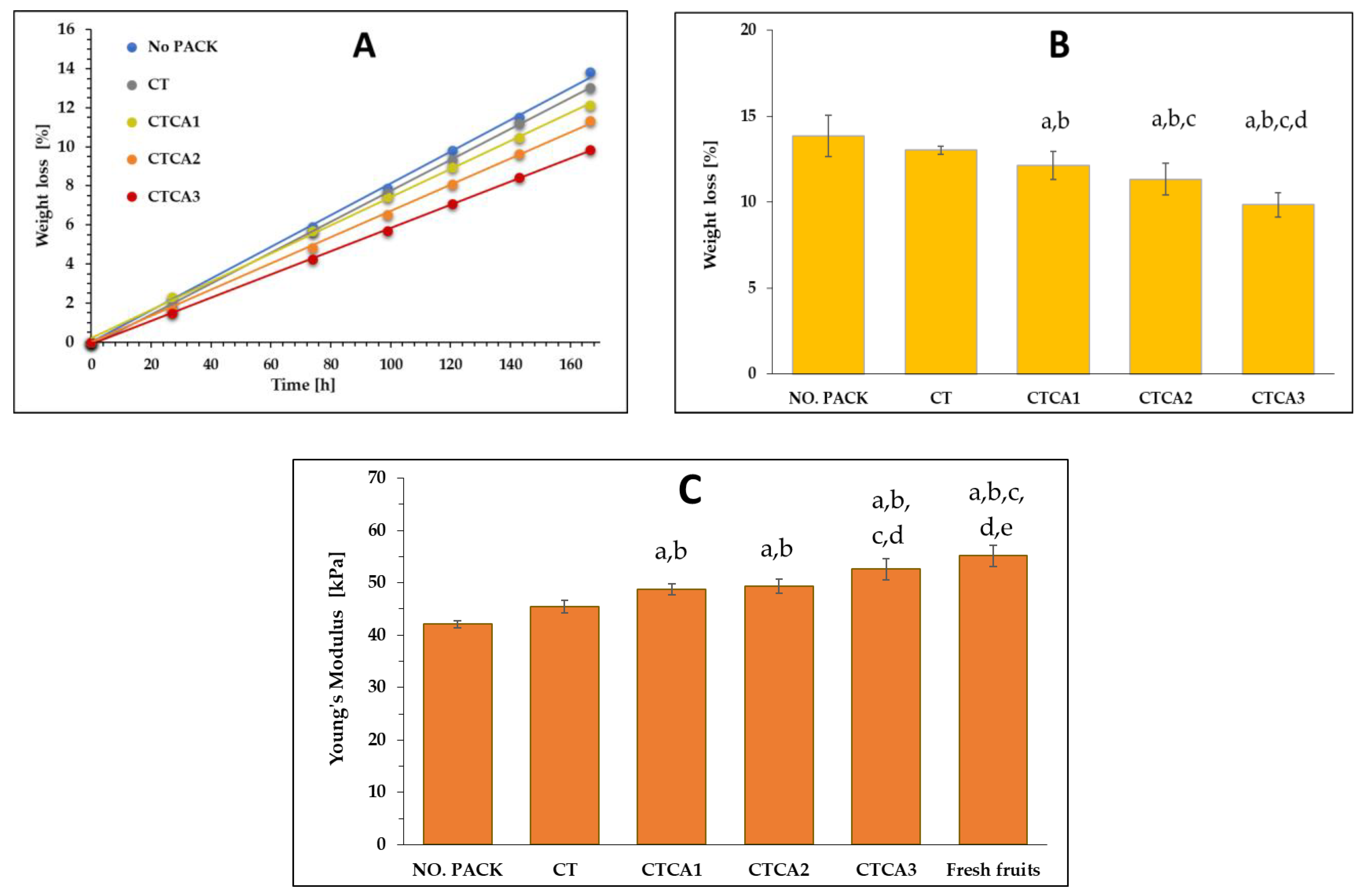
3.3. Changes in Mechanical Properties
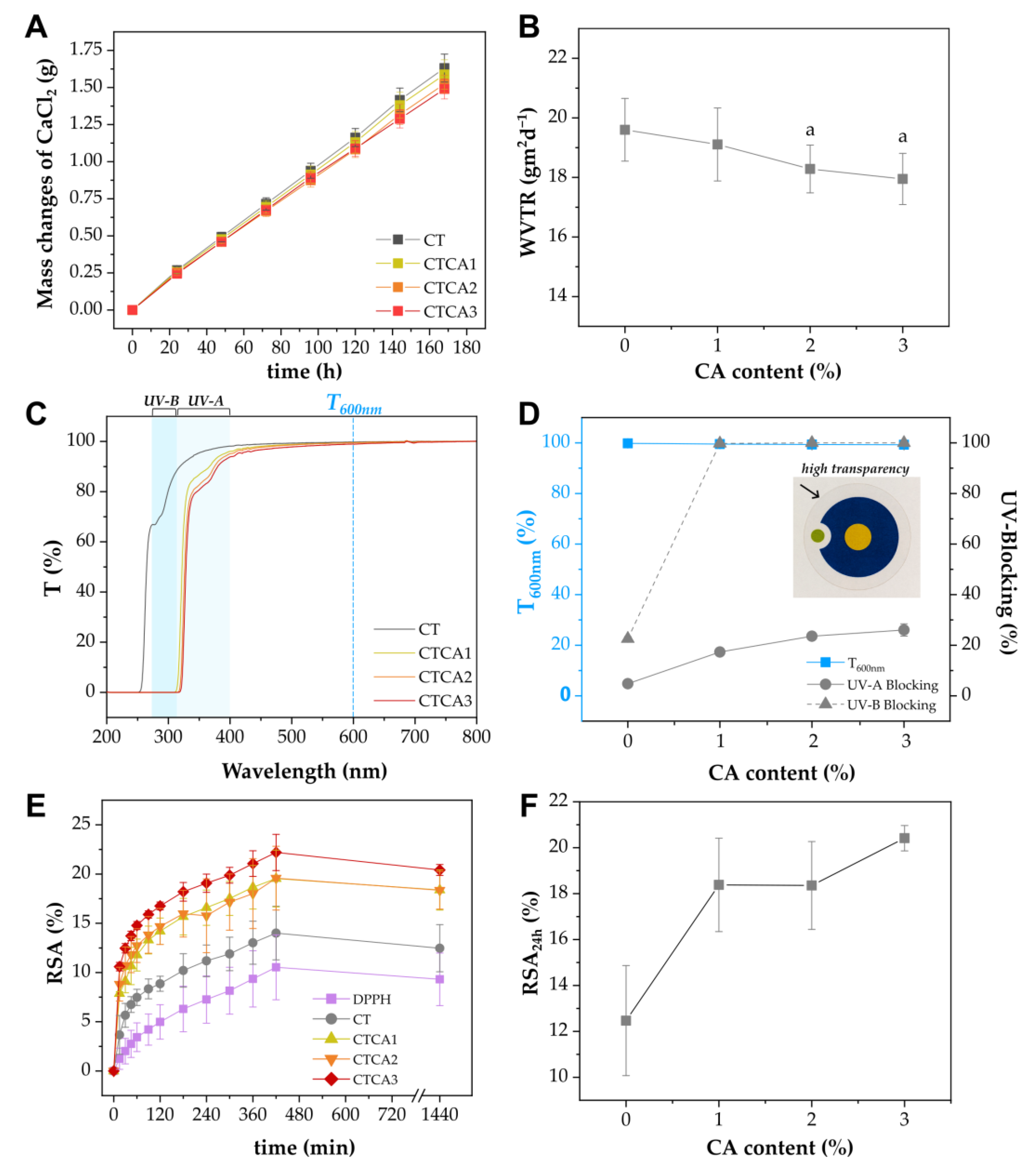
3.4. Barrier to Moisture
3.5. UV-Blocking Study
3.6. Antioxidative Activity
3.7. Blueberries Storage
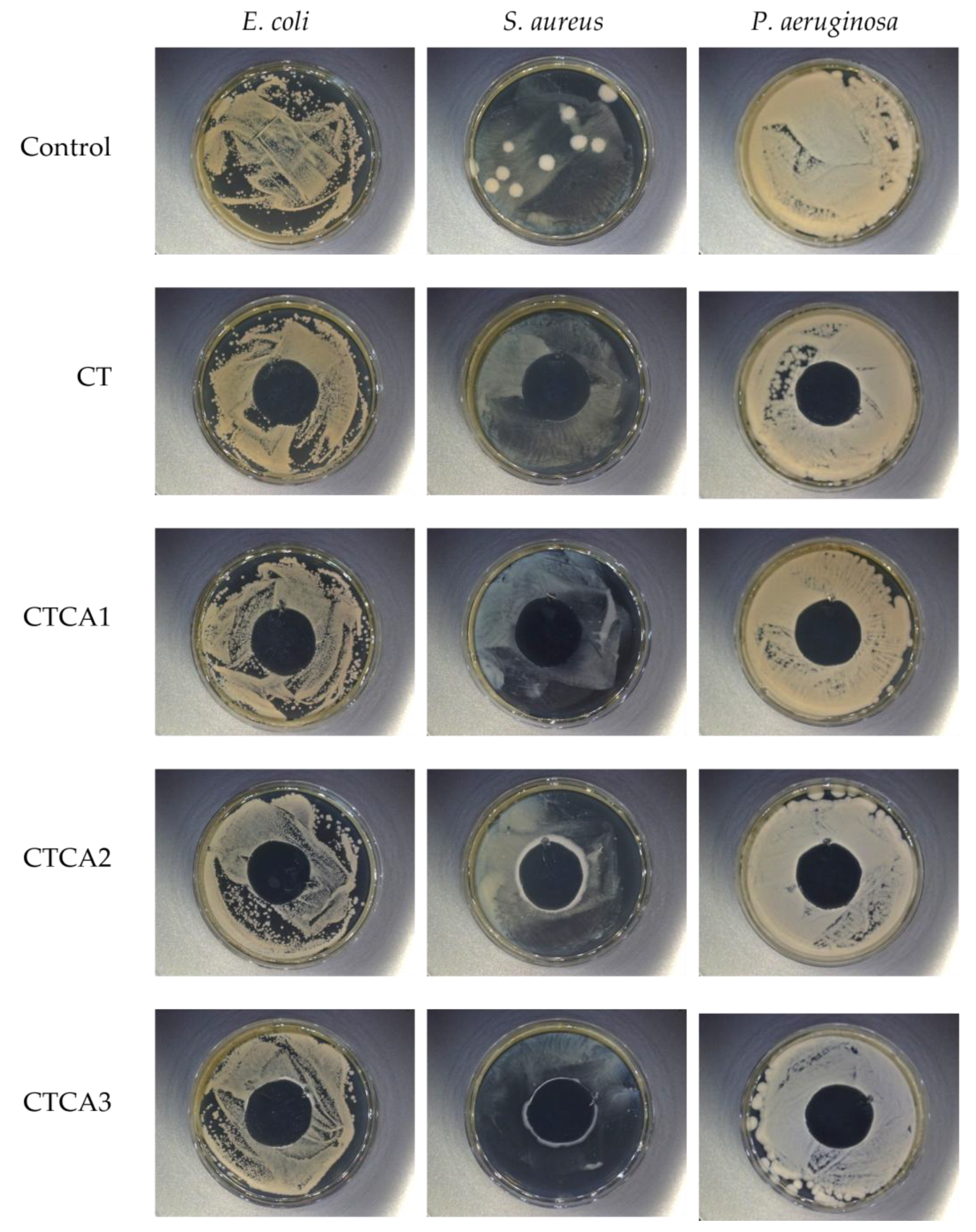
3.8. Antibacterial Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ferreira, P.S.; Ribeiro, S.M.; Pontes, R.; Nunes, J. Production Methods and Applications of Bioactive Polylactic Acid: A Review. Environ. Chem. Lett. 2024, 22, 1831–1859. [Google Scholar] [CrossRef]
- Lombardi, A.; Fochetti, A.; Vignolini, P.; Campo, M.; Durazzo, A.; Lucarini, M.; Puglia, D.; Luzi, F.; Papalini, M.; Renzi, M.; et al. Natural Active Ingredients for Poly (Lactic Acid)-Based Materials: State of the Art and Perspectives. Antioxidants 2022, 11, 2074. [Google Scholar] [CrossRef]
- Liu, X.; Xu, F.; Yong, H.; Chen, D.; Tang, C.; Kan, J.; Liu, J. Recent Advances in Chitosan-Based Active and Intelligent Packaging Films Incorporated with Flavonoids. Food Chem. X 2025, 25, 102200. [Google Scholar] [CrossRef]
- Kamau, P.G.; Cruz-Romero, M.C.; Alzate, P.C.; Morris, M.A.; Kerry, J.P. Active Packaging Containing Natural Antimicrobials as a Potential and Innovative Technology to Extend Shelf-Life of Fish Products—A Review. Food Packag. Shelf Life 2025, 49, 101500. [Google Scholar] [CrossRef]
- Jogaiah, S.; Mujtaba, A.G.; Mujtaba, M.; Archana; De Britto, S.; Geetha, N.; Belorkar, S.A.; Shetty, H.S. Chitosan-Metal and Metal Oxide Nanocomposites for Active and Intelligent Food Packaging; A Comprehensive Review of Emerging Trends and Associated Challenges. Carbohydr. Polym. 2025, 357, 123459. [Google Scholar] [CrossRef]
- Maky, M.A.; Sonomoto, K.; Zendo, T. Antimicrobial Activity of Carboxymethyl Cellulose Films Containing Plantaricin W and Enterocin F4-9 for Meat Preservation. Int. J. Mol. Sci. 2025, 26, 6083. [Google Scholar] [CrossRef] [PubMed]
- Olewnik-Kruszkowska, E.; Gierszewska, M.; Richert, A.; Grabska-Zielińska, S.; Rudawska, A.; Bouaziz, M. Antibacterial Films Based on Polylactide with the Addition of Quercetin and Poly(Ethylene Glycol). Materials 2021, 14, 1643. [Google Scholar] [CrossRef] [PubMed]
- Nasution, H.; Harahap, H.; Julianti, E.; Safitri, A.; Jaafar, M. Smart Packaging Based on Polylactic Acid: The Effects of Antibacterial and Antioxidant Agents from Natural Extracts on Physical–Mechanical Properties, Colony Reduction, Perishable Food Shelf Life, and Future Prospective. Polymers 2023, 15, 4103. [Google Scholar] [CrossRef]
- Celuppi, L.C.M.; Capelezzo, A.P.; Cima, L.B.; Zeferino, R.C.F.; Carniel, T.A.; Zanetti, M.; de Mello, J.M.M.; Fiori, M.A.; Riella, H.G. Microbiological, Thermal and Mechanical Performance of Cellulose Acetate Films with Geranyl Acetate. Int. J. Biol. Macromol. 2023, 228, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Jayakrishnan, A.; Shahana, S.; Ayswaria, R. Preparation and Evaluation of Bioactive Cellulose Acetate Films from Musa Acuminata. RSC Sustain. 2024, 2, 2335–2347. [Google Scholar] [CrossRef]
- Gemili, S.; Yemenicioğlu, A.; Altınkaya, S.A. Development of Cellulose Acetate Based Antimicrobial Food Packaging Materials for Controlled Release of Lysozyme. J. Food Eng. 2009, 90, 453–462. [Google Scholar] [CrossRef]
- Huang, K.; Wang, B.; Cao, Y.; Li, H.; Wang, J.; Lin, W.; Mu, C.; Liao, D. Homogeneous Preparation of Cellulose Acetate Propionate (CAP) and Cellulose Acetate Butyrate (CAB) from Sugarcane Bagasse Cellulose in Ionic Liquid. J. Agric. Food Chem. 2011, 59, 5376–5381. [Google Scholar] [CrossRef]
- Girdthep, S.; Punyodom, W.; Molloy, R.; Channuan, W. Effect of Tween 80 on the Mechanical and Thermal Properties of Solution-Cast Blends of Poly (Lactic Acid) and Cellulose Acetate Butyrate Films. Int. Conf. Chem. Chem. Process 2011, 10, 95–100. [Google Scholar]
- Brandelero, R.P.H.; Yamashita, F.; Grossmann, M.V.E. The Effect of Surfactant Tween 80 on the Hydrophilicity, Water Vapor Permeation, and the Mechanical Properties of Cassava Starch and Poly(Butylene Adipate-Co-Terephthalate) (PBAT) Blend Films. Carbohydr. Polym. 2010, 82, 1102–1109. [Google Scholar] [CrossRef]
- Song, X.; Zuo, G.; Chen, F. Effect of Essential Oil and Surfactant on the Physical and Antimicrobial Properties of Corn and Wheat Starch Films. Int. J. Biol. Macromol. 2018, 107, 1302–1309. [Google Scholar] [CrossRef]
- Han, Y.; Yu, M.; Wang, L. Physical and Antimicrobial Properties of Sodium Alginate/Carboxymethyl Cellulose Films Incorporated with Cinnamon Essential Oil. Food Packag. Shelf Life 2018, 15, 35–42. [Google Scholar] [CrossRef]
- Chu, Y.; Xu, T.; Gao, C.; Liu, X.; Zhang, N.; Feng, X.; Liu, X.; Shen, X.; Tang, X. Evaluations of Physicochemical and Biological Properties of Pullulan-Based Films Incorporated with Cinnamon Essential Oil and Tween 80. Int. J. Biol. Macromol. 2019, 122, 388–394. [Google Scholar] [CrossRef]
- Ordoñez, R.; Atarés, L.; Chiralt, A. Antibacterial Properties of Cinnamic and Ferulic Acids Incorporated to Starch and PLA Monolayer and Multilayer Films. Food Control 2022, 136, 108878. [Google Scholar] [CrossRef]
- Ordoñez, R.; Atarés, L.; Chiralt, A. Multilayer Antimicrobial Films Based on Starch and PLA with Superficially Incorporated Ferulic or Cinnamic Acids for Active Food Packaging Purposes. Food Chem. Adv. 2023, 2, 100250. [Google Scholar] [CrossRef]
- Tong, W.Y.; Ahmad Rafiee, A.R.; Leong, C.R.; Tan, W.-N.; Dailin, D.J.; Almarhoon, Z.M.; Shelkh, M.; Nawaz, A.; Chuah, L.F. Development of Sodium Alginate-Pectin Biodegradable Active Food Packaging Film Containing Cinnamic Acid. Chemosphere 2023, 336, 139212. [Google Scholar] [CrossRef] [PubMed]
- Zasada, L.; Swiontek Brzezinska, M.; Ciesielska, M.; Olewnik-Kruszkowska, E.; Kaczmarek-Szczepańska, B. Enhancement of Chitosan-Based Films for Blueberries Packaging- by Modification with Ellagic Acid and Cinnamic Acid. Polym. Degrad. Stab. 2025, 234, 111200. [Google Scholar] [CrossRef]
- Andrade, J.; González-Martínez, C.; Chiralt, A. Physical and Active Properties of Poly (Vinyl Alcohol) Films with Phenolic Acids as Affected by the Processing Method. Food Packag. Shelf Life 2022, 33, 100855. [Google Scholar] [CrossRef]
- Olewnik-Kruszkowska, E.; Ferri, M.; Cardeira, M.C.; Gierszewska, M.; Rudawska, A. Comparison of Polylactide-Based Active Films Containing Berberine and Quercetin as Systems for Maintaining the Quality and Safety of Blueberries. Polymers 2024, 16, 1577. [Google Scholar] [CrossRef] [PubMed]
- Ferri, M.; Ganzerli, F.; Portone, A.; Petrachi, T.; Veronesi, E.; Morselli, D.; Degli Esposti, M.; Fabbri, P. Skin Barrier Restoration by Waste-Derived Multifunctional Adhesive Hydrogel Based on Tannin-Modified Chitosan. ACS Appl. Mater. Interfaces 2025, 17, 35066–35079. [Google Scholar] [CrossRef] [PubMed]
- Pramod, K.; Suneesh, C.V.; Shanavas, S.; Ansari, S.H.; Ali, J. Unveiling the Compatibility of Eugenol with Formulation Excipients by Systematic Drug-Excipient Compatibility Studies. J. Anal. Sci. Technol. 2015, 6, 34. [Google Scholar] [CrossRef]
- Fu, X.; Kong, W.; Zhang, Y.; Jiang, L.; Wang, J.; Lei, J. Novel Solid-Solid Phase Change Materials with Biodegradable Trihydroxy Surfactants for Thermal Energy Storage. RSC Adv. 2015, 5, 68881–68889. [Google Scholar] [CrossRef]
- Kujawa, J.; Rynkowska, E.; Fatyeyeva, K.; Knozowska, K.; Wolan, A.; Dzieszkowski, K.; Li, G.; Kujawski, W. Preparation and Characterization of Cellulose Acetate Propionate Films Functionalized with Reactive Ionic Liquids. Polymers 2019, 11, 1217. [Google Scholar] [CrossRef]
- Lee, J.-E.; Shim, S.-B.; Park, J.-H.; Chung, I. Interfacial Properties and Melt Processability of Cellulose Acetate Propionate Composites by Melt Blending of Biofillers. Polymers 2022, 14, 4286. [Google Scholar] [CrossRef]
- Kalinowska, M.; Świsłocka, R.; Lewandowski, W. The Spectroscopic (FT-IR, FT-Raman and 1H, 13C NMR) and Theoretical Studies of Cinnamic Acid and Alkali Metal Cinnamates. J. Mol. Struct. 2007, 834–836, 572–580. [Google Scholar] [CrossRef]
- Schilling, M.; Bouchard, M.; Khanjian, H.; Learner, T.; Phenix, A.; Rivenc, R. Application of Chemical and Thermal Analysis Methods for Studying Cellulose Ester Plastics. Acc. Chem. Res. 2010, 43, 888–896. [Google Scholar] [CrossRef]
- Barandiaran, A.; Gomez-Caturla, J.; Ivorra-Martinez, J.; Lascano, D.; Selles, M.A.; Moreno, V.; Fenollar, O. Esters of Cinnamic Acid as Green Plasticizers for Polylactide Formulations with Improved Ductility. Macromol. Mater. Eng. 2023, 308, 2300022. [Google Scholar] [CrossRef]
- Barandiaran, A.; Montanes, N.; Sanchez-Nacher, L.; Balart, R.; Selles, M.A.; Moreno, V. Investigation of Cinnamic Acid Derivatives as Alternative Plasticizers for Improved Ductility of Polyvinyl Chloride Films. Polymers 2023, 15, 4265. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gardner, D.J.; Stark, N.M.; Bousfield, D.W.; Tajvidi, M.; Cai, Z. Moisture and Oxygen Barrier Properties of Cellulose Nanomaterial-Based Films. ACS Sustain. Chem. Eng. 2018, 6, 49–70. [Google Scholar] [CrossRef]
- Ordoñez, R.; Atarés, L.; Chiralt, A. Properties of PLA Films with Cinnamic Acid: Effect of the Processing Method. Food Bioprod. Process. 2022, 133, 25–33. [Google Scholar] [CrossRef]
- Ferri, M.; Lenzi, L.; Degli Esposti, M.; Martellosio, L.; Benítez, J.J.; Hierrezuelo, J.; Grifé-Ruiz, M.; Romero, D.; Guzmán-Puyol, S.; Heredia-Guerrero, J.A.; et al. Triphenyl Acetic Glyceroate as a Sustainable Multifunctional Additive for Developing Transparent, Biodegradable, and Flexible Polylactide Green Alternative to Polyethylene-Based Films for Food Packaging. Chem. Eng. J. 2025, 508, 160887. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Guo, X.; Wang, H.; Qian, F.; Lv, Y. Active Biodegradable Polyvinyl Alcohol-Hemicellulose/Tea Polyphenol Films with Excellent Moisture Resistance Prepared via Ultrasound Assistance for Food Packaging. Coatings 2021, 11, 219. [Google Scholar] [CrossRef]
- Kadirvel, V.; Palanisamy, Y.; Ganesan, N.D. Active Packaging System—An Overview of Recent Advances for Enhanced Food Quality and Safety. Packag. Technol. Sci. 2024, 38, 145–162. [Google Scholar] [CrossRef]
- Pontiki, E.; Hadjipavlou-Litina, D.; Litinas, K.; Geromichalos, G. Novel Cinnamic Acid Derivatives as Antioxidant and Anticancer Agents: Design, Synthesis and Modeling Studies. Molecules 2014, 19, 9655–9674. [Google Scholar] [CrossRef]
- Ferri, M.; Papchenko, K.; Degli Esposti, M.; Tondi, G.; De Angelis, M.G.; Morselli, D.; Fabbri, P. Fully Biobased Polyhydroxyalkanoate/Tannin Films as Multifunctional Materials for Smart Food Packaging Applications. ACS Appl. Mater. Interfaces 2023, 15, 28594–28605. [Google Scholar] [CrossRef]
- Chen, J.; Ran, M.; Wang, M.; Liu, X.; Liu, S.; Yu, Y. Structure-Activity Relationships of Antityrosinase and Antioxidant Activities of Cinnamic Acid and Its Derivatives. Biosci. Biotechnol. Biochem. 2021, 85, 1697–1705. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M. Antimicrobial Polymer-Based Materials for Food Packaging Applications. Polymers 2020, 12, 731. [Google Scholar] [CrossRef]
- Iturralde-García, R.D.; Cinco-Moroyoqui, F.J.; Martínez-Cruz, O.; Ruiz-Cruz, S.; Wong-Corral, F.J.; Borboa-Flores, J.; Cornejo-Ramírez, Y.I.; Bernal-Mercado, A.T.; Del-Toro-Sánchez, C.L. Emerging Technologies for Prolonging Fresh-Cut Fruits’ Quality and Safety during Storage. Horticulturae 2022, 8, 731. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Islam, M.; Johnson, J.; Xu, C.-Y.; Mazhar, M.; Naiker, M. Comparative Analysis of Shelf-Life, Antioxidant Activity, and Phytochemical Contents of Australian-Grown and Imported Dragon Fruit under Ambient Conditions. Horticulturae 2024, 10, 1048. [Google Scholar] [CrossRef]
- Zhang, R.; Lan, W.; Ding, J.; Ahmed, S.; Qin, W.; He, L.; Liu, Y. Effect of PLA/PBAT Antibacterial Film on Storage Quality of Passion Fruit during the Shelf-Life. Molecules 2019, 24, 3378. [Google Scholar] [CrossRef] [PubMed]
- Ashique, S.; Mukherjee, T.; Mohanty, S.; Garg, A.; Mishra, N.; Kaushik, M.; Bhowmick, M.; Chattaraj, B.; Mohanto, S.; Srivastava, S.; et al. Blueberries in Focus: Exploring the Phytochemical Potentials and Therapeutic Applications. J. Agric. Food Res. 2024, 18, 101300. [Google Scholar] [CrossRef]
- Pérez-Lavalle, L.; Carrasco, E.; Valero, A. Strategies for Microbial Decontamination of Fresh Blueberries and Derived Products. Foods 2020, 9, 1558. [Google Scholar] [CrossRef]
- De Morais, M.C.; de Oliveira Lima, E.; Perez-Castillo, Y.; de Sousa, D.P. Synthetic Cinnamides and Cinnamates: Antimicrobial Activity, Mechanism of Action, and In Silico Study. Molecules 2023, 28, 1918. [Google Scholar] [CrossRef]
- Cai, R.; Miao, M.; Yue, T.; Zhang, Y.; Cui, L.; Wang, Z.; Yuan, Y. Antibacterial Activity and Mechanism of Cinnamic Acid and Chlorogenic Acid against Alicyclobacillus Acidoterrestris Vegetative Cells in Apple Juice. Int. J. Food Sci. Technol. 2019, 54, 1697–1705. [Google Scholar] [CrossRef]
- Ruwizhi, N.; Aderibigbe, B.A. Cinnamic Acid Derivatives and Their Biological Efficacy. Int. J. Mol. Sci. 2020, 21, 5712. [Google Scholar] [CrossRef] [PubMed]
- Bułakowska, A.; Sławiński, J.; Hałasa, R.; Hering, A.; Gucwa, M.; Ochocka, J.R.; Stefanowicz-Hajduk, J. An In Vitro Antimicrobial, Anticancer and Antioxidant Activity of N–[(2–Arylmethylthio)Phenylsulfonyl]Cinnamamide Derivatives. Molecules 2023, 28, 3087. [Google Scholar] [CrossRef] [PubMed]
- Ordoñez, R.; Atarés, L.; Chiralt, A. Physicochemical and Antimicrobial Properties of Cassava Starch Films with Ferulic or Cinnamic Acid. LWT 2021, 144, 111242. [Google Scholar] [CrossRef]
- Skaro Bogojevic, S.; Perminova, D.; Jaksic, J.; Milcic, M.; Medakovic, V.; Milovanovic, J.; Nikodinovic-Runic, J.; Maslak, V. Novel Cinnamic Acid-Based PET Derivatives as Quorum Sensing Modulators. J. Mol. Struct. 2024, 1300, 137291. [Google Scholar] [CrossRef]

| Parameter | ISO 20645 | ISO 22196 |
|---|---|---|
| Type of assessment | Qualitative | Quantitative |
| Tested organisms | Staphylococcus aureus (ATCC 6538), Escherichia coli (ATCC 11229) | Staphylococcus aureus (ATCC 6538P) Escherichia coli (ATCC 8739) |
| Sample type | Non-leaching materials | Non-porous plastic or coated surfaces |
| Method principle | Agar diffusion (zone of inhibition and contact activity) | Direct inoculation and recovery of bacteria after contact |
| Evaluation criteria | Presence/absence and size of inhibition zone; Growth under sample | Logarithmic reduction in CFU after 24 h |
| Incubation conditions | 37 ± 1 °C, 18–24 h | 35 ± 1 °C, ≥ 90% RH, 24 h-120 h |
| Result expression | Zone diameter and qualitative classification | Log reduction value (e.g., ≥ 2 log = significant activity) |
| Interpretation of results | Zone around = leaching activity; Only under = contact activity | Numerical efficacy against baseline/control surface |
| Bacteria Strains | Samples | R | % Reduction | Antibacterial Efficacy |
|---|---|---|---|---|
| E. coli (ATCC 8739P) | CT | - | - | - |
| CTCA1 | 1.8 | >90.0 | satisfactory | |
| CTCA2 | 2.0 | >99.0 | very good | |
| CTCA3 | 2.3 | >99.0 | very good | |
| S. aureus (ATCC 65388) | CT | - | - | - |
| CTCA1 | 2.4 | >99.9 | very good | |
| CTCA2 | 2.5 | >99.9 | very good | |
| CTCA3 | 2.7 | >99.9 | very good | |
| P. aeruginosa (ATCC 8739) | CT | - | - | - |
| CTCA1 | 2.1 | >99.0 | very good | |
| CTCA2 | 2.3 | >99.0 | very good | |
| CTCA3 | 2.6 | >99.0 | very good |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olewnik-Kruszkowska, E.; Ferri, M.; Degli Esposti, M.; Richert, A.; Fabbri, P. Innovative Biobased Active Composites of Cellulose Acetate Propionate with Tween 80 and Cinnamic Acid for Blueberry Preservation. Polymers 2025, 17, 2072. https://doi.org/10.3390/polym17152072
Olewnik-Kruszkowska E, Ferri M, Degli Esposti M, Richert A, Fabbri P. Innovative Biobased Active Composites of Cellulose Acetate Propionate with Tween 80 and Cinnamic Acid for Blueberry Preservation. Polymers. 2025; 17(15):2072. https://doi.org/10.3390/polym17152072
Chicago/Turabian StyleOlewnik-Kruszkowska, Ewa, Martina Ferri, Micaela Degli Esposti, Agnieszka Richert, and Paola Fabbri. 2025. "Innovative Biobased Active Composites of Cellulose Acetate Propionate with Tween 80 and Cinnamic Acid for Blueberry Preservation" Polymers 17, no. 15: 2072. https://doi.org/10.3390/polym17152072
APA StyleOlewnik-Kruszkowska, E., Ferri, M., Degli Esposti, M., Richert, A., & Fabbri, P. (2025). Innovative Biobased Active Composites of Cellulose Acetate Propionate with Tween 80 and Cinnamic Acid for Blueberry Preservation. Polymers, 17(15), 2072. https://doi.org/10.3390/polym17152072











