Enhancing Biodegradation of Poly(lactic acid) in Compost at Room Temperature by Compounding Jade Particles
Abstract
1. Introduction
1.1. Challenges with PLA
1.2. Methods to Enhance Biodegradation Speed
1.3. Methods to Monitor Biodegradation
1.4. New Approach
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Screening NZ Jade Particles
2.2.2. Composite Preparation (Extrusion Compounding)
2.2.3. Fabricating Filament for 3D Printing
2.2.4. Three-Dimensional-Printing Strips
2.2.5. Compression-Molding Strips
2.2.6. Moisture Content of Compost
2.2.7. Degree of Crystallinity
2.2.8. Placing Samples in Compost and Mass Measurements
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mazlan, N.; Lin, L.; Park, H.E. Microplastics in the New Zealand Environment. Processes 2022, 10, 265. [Google Scholar] [CrossRef]
- Rosenboom, J.-G.; Langer, R.; Traverso, G. Bioplastics for a circular economy. Nat. Rev. Mater. 2022, 7, 117–137. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, W.; Siraj, S.; Al-Marzouqi, A.H. 3D Printing PLA Waste to Produce Ceramic Based Particulate Reinforced Composite Using Abundant Silica-Sand: Mechanical Properties Characterization. Polymers 2020, 12, 2579. [Google Scholar] [CrossRef] [PubMed]
- Muthe, L.P.; Pickering, K.; Gauss, C. A Review of 3D/4D Printing of Poly-Lactic Acid Composites with Bio-Derived Reinforcements. Compos. Part C Open Access 2022, 8, 100271. [Google Scholar] [CrossRef]
- Fouly, A.; Alnaser, I.; Assaifan, A.; Abdo, H. Evaluating the Performance of 3D-Printed PLA Reinforced with Date Pit Particles for Its Suitability as an Acetabular Liner in Artificial Hip Joints. Polymers 2022, 14, 3321. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, A.; Peltzer, M.; Ruseckaite, R. Poly(lactic acid) Science and Technology; RCS: Cambridge, UK, 2014. [Google Scholar]
- Sin, L.T.; Tueen, B.S. Poly(lactic acid): A Practical Guide for the Processing, Manufacturing, and Applications of PLA, 2nd ed.; Elsevier: Oxford, UK, 2019. [Google Scholar]
- Kalb, B.; Pennings, A.J. General crystallization behaviour of poly(l-lactic acid). Polymer 1980, 21, 607–612. [Google Scholar] [CrossRef]
- Ikada, Y.; Jamshidi, K.; Tsuji, H.; Hyon, S.H. Stereocomplex formation between enantiomeric poly(lactides). Macromolecules 1987, 20, 904–906. [Google Scholar] [CrossRef]
- Saeidlou, S.; Huneault, M.A.; Li, H.; Sammut, P.; Park, C.B. Evidence of a dual network/spherulitic crystalline morphology in PLA stereocomplexes. Polymer 2012, 53, 5816–5824. [Google Scholar] [CrossRef]
- Michalski, A.; Makowski, T.; Biedroń, T.; Brzeziński, M.; Biela, T. Controlling polylactide stereocomplex (sc-PLA) self-assembly: From microspheres to nanoparticles. Polymer 2016, 90, 242–248. [Google Scholar] [CrossRef]
- Lehermeier, H.J.; Dorgan, J.R. Melt rheology of poly(lactic acid): Consequences of blending chain architectures. Polym. Eng. Sci. 2001, 41, 2172–2184. [Google Scholar] [CrossRef]
- Dorgan, J.R.; Janzen, J.; Clayton, M.P.; Hait, S.B.; Knauss, D.M. Melt rheology of variableL-content poly(lactic acid). J. Rheol. 2005, 49, 607–619. [Google Scholar] [CrossRef]
- Othman, N.; Xu, C.; Mehrkhodavandi, P.; Hatzikiriakos, S.G. Thermorheological and mechanical behavior of polylactide and its enantiomeric diblock copolymers and blends. Polymer 2012, 53, 2443–2452. [Google Scholar] [CrossRef]
- Nofar, M.; Salehiyan, R.; Sinha Ray, S. Rheology of poly (lactic acid)-based systems. Polym. Rev. 2019, 59, 465–509. [Google Scholar] [CrossRef]
- Lin, L.; Lee, Y.; Park, H.E. Recycling and rheology of poly(lactic acid) (PLA) to make foams using supercritical fluid. Phys. Fluids 2021, 33, 067119. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, W.; Zhang, H.; Park, C.B. Continuous processing of low-density, microcellular poly(lactic acid) foams with controlled cell morphology and crystallinity. Chem. Eng. Sci. 2012, 75, 390–399. [Google Scholar] [CrossRef]
- Nofar, M.; Park, C.B. Poly (lactic acid) foaming. Prog. Polym. Sci. 2014, 39, 1721–1741. [Google Scholar] [CrossRef]
- Julien, J.M.; Quantin, J.C.; Bénézet, J.C.; Bergeret, A.; Lacrampe, M.F.; Krawczak, P. Chemical foaming extrusion of poly(lactic acid) with chain-extenders: Physical and morphological characterizations. Eur. Polym. J. 2015, 67, 40–49. [Google Scholar] [CrossRef]
- Tabatabaei, A.; Park, C.B. In-situ visualization of PLA crystallization and crystal effects on foaming in extrusion. Eur. Polym. J. 2017, 96, 505–519. [Google Scholar] [CrossRef]
- Yang, Y.; Li, X.; Zhang, Q.; Xia, C.; Chen, C.; Chen, X.; Yu, P. Foaming of poly(lactic acid) with supercritical CO2: The combined effect of crystallinity and crystalline morphology on cellular structure. J. Supercrit. Fluids 2019, 145, 122–132. [Google Scholar] [CrossRef]
- Cosate de Andrade, M.F.; Souza, P.M.S.; Cavalett, O.; Morales, A.R. Life Cycle Assessment of Poly(Lactic Acid) (PLA): Comparison Between Chemical Recycling, Mechanical Recycling and Composting. J. Polym. Environ. 2016, 24, 372–384. [Google Scholar] [CrossRef]
- McKeown, P.; Jones, M.D. The Chemical Recycling of PLA: A Review. Sustain. Chem. 2020, 1, 1. [Google Scholar] [CrossRef]
- Cisneros-López, E.O.; Pal, A.K.; Rodriguez, A.U.; Wu, F.; Misra, M.; Mielewski, D.F.; Kiziltas, A.; Mohanty, A.K. Recycled poly(lactic acid)–based 3D printed sustainable biocomposites: A comparative study with injection molding. Mater. Today Sustain. 2020, 7–8, 100027. [Google Scholar] [CrossRef]
- Hedjazi, L.; Guessasma, S.; Belhabib, S.; Stephant, N. On the Mechanical Performance of Polylactic Material Reinforced by Ceramic in Fused Filament Fabrication. Polymers 2022, 14, 2924. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lin, Y.; Liu, M.; Meng, L.; Li, C. A review of research and application of poly(lactic acid) composites. J. Appl. Polym. Sci. 2022, 140, e53477. [Google Scholar] [CrossRef]
- Lin, L.; Dang, Q.A.; Park, H.E. Enhanced Degradability, Mechanical Properties, and Flame Retardation of Poly(Lactic Acid) Composite with New Zealand Jade (Pounamu) Particles. Polymers 2023, 15, 3270. [Google Scholar] [CrossRef] [PubMed]
- Garlotta, D. A Literature Review of Poly(Lactic Acid). J. Polym. Environ. 2001, 9, 63–84. [Google Scholar] [CrossRef]
- Cristea, M.; Ionita, D.; Iftime, M.M. Dynamic Mechanical Analysis Investigations of PLA-Based Renewable Materials: How Are They Useful? Materials 2020, 13, 5302. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, M.L.; Androsch, R. Industrial Applications of Poly(lactic acid); Springer: Cham, Switzerland, 2018. [Google Scholar]
- Madhavan Nampoothiri, K.; Nair, N.R.; John, R.P. An overview of the recent developments in polylactide (PLA) research. Bioresour. Technol. 2010, 101, 8493–8501. [Google Scholar] [CrossRef] [PubMed]
- Ren, J. Biodegradable Poly(lactic acid): Synthesis, Modification, Processing and Applications; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Auras, R.; Harte, B.; Selke, S. An Overview of Polylactides as Packaging Materials. Macromol. Biosci. 2004, 4, 835–864. [Google Scholar] [CrossRef] [PubMed]
- Casalini, T.; Rossi, F.; Castrovinci, A.; Perale, G. A Perspective on Poly(lactic acid)-Based Polymers Use for Nanoparticles Synthesis and Applications. Front. Bioeng. Biotechnol. 2019, 7, 259. [Google Scholar] [CrossRef] [PubMed]
- Murariu, M.; Dubois, P. PLA composites: From production to properties. Adv. Drug Deliv. Rev. 2016, 107, 17–46. [Google Scholar] [CrossRef] [PubMed]
- Henton, D.E.; Gruber, P.; Lunt, J.; Randall, J. Poly(lactic acid) Technology. In Natural Fibers, Biopolymers, and Biocomposites; Mohanty, A.K., Misra, M., Drzal, L.T., Eds.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Auras, R.; Lim, L.-T.; Selke, S.E.M.; Tsuji, H. Poly(lactic acid); John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010. [Google Scholar]
- Nofar, M.; Park, C.B. Polylactide Foams: Fundamentals, Manufacturing, and Applications; Elsevier: Oxford, UK, 2018; pp. 17–34. [Google Scholar]
- Lim, L.T.; Auras, R.; Rubino, M. Processing technologies for poly(lactic acid). Prog. Polym. Sci. 2008, 33, 820–852. [Google Scholar] [CrossRef]
- Di Lorenzo, M.L.; Androsch, R. Synthesis, Structure and Properties of Poly(lactic acid); Springer: Cham, Switzerland, 2018. [Google Scholar]
- Yao, X.; Yang, X.; Lu, Y.; Qiu, Y.; Zeng, Q. Review of the Synthesis and Degradation Mechanisms of Some Biodegradable Polymers in Natural Environments. Polymers 2024, 17, 66. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, S.; Eblagon, K.M.; Miranda, F.; R. Pereira, M.F.; Figueiredo, J.L. Towards Controlled Degradation of Poly(lactic) Acid in Technical Applications. C 2021, 7, 42. [Google Scholar] [CrossRef]
- Bher, A.; Mayekar, P.C.; Auras, R.A.; Schvezov, C.E. Biodegradation of Biodegradable Polymers in Mesophilic Aerobic Environments. Int. J. Mol. Sci. 2022, 23, 12165. [Google Scholar] [CrossRef] [PubMed]
- Hajilou, N.; Mostafayi, S.S.; Yarin, A.L.; Shokuhfar, T. A Comparative Review on Biodegradation of Poly(Lactic Acid) in Soil, Compost, Water, and Wastewater Environments: Incorporating Mathematical Modeling Perspectives. AppliedChem 2024, 5, 1. [Google Scholar] [CrossRef]
- Ghosh, K.; Jones, B.H. Roadmap to Biodegradable Plastics—Current State and Research Needs. ACS Sustain. Chem. Eng. 2021, 9, 6170–6187. [Google Scholar] [CrossRef]
- Ahsan, W.A.; Hussain, A.; Lin, C.; Nguyen, M.K. Biodegradation of Different Types of Bioplastics through Composting—A Recent Trend in Green Recycling. Catalysts 2023, 13, 294. [Google Scholar] [CrossRef]
- Wu, Y.; Gao, X.; Wu, J.; Zhou, T.; Nguyen, T.T.; Wang, Y. Biodegradable Poly(lactic acid) and Its Composites: Characteristics, Processing, and Sustainable Applications in Sports. Polymers 2023, 15, 3096. [Google Scholar] [CrossRef]
- Qi, X.; Ren, Y.; Wang, X. New advances in the biodegradation of Poly(lactic) acid. Int. Biodeterior. Biodegrad. 2017, 117, 215–223. [Google Scholar] [CrossRef]
- Mat Yasin, N.; Akkermans, S.; Van Impe, J.F.M. Enhancing the biodegradation of (bio)plastic through pretreatments: A critical review. Waste Manag. 2022, 150, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yasin, N.M.; Polanska, M.; Verbeken, K.; Van Impe, J.F.M.; Akkermans, S. Optimizing thermal and thermal-alkaline pretreatments for poly(lactic acid) biodegradation by Amycolatopsis orientalis and Amycolatopsis thailandensis. Bioresour. Technol. 2025, 420, 132114. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Venegas, M.; Friščić, T.; Auclair, K. Efficient Mechano-Enzymatic Hydrolysis of Poly(lactic acid) under Moist-Solid Conditions. ACS Sustain. Chem. Eng. 2023, 11, 9924–9931. [Google Scholar] [CrossRef]
- Brown, M.H.; Badzinski, T.D.; Pardoe, E.; Ehlebracht, M.; Maurer-Jones, M.A. UV Light Degradation of Poly(lactic acid) Kickstarts Enzymatic Hydrolysis. ACS Mater. Au 2023, 4, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Gorrasi, G.; Pantani, R. Hydrolysis and Biodegradation of Poly(lactic acid). In Synthesis, Structure and Properties of Poly(lactic acid); Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 2017; pp. 119–151. [Google Scholar]
- Polidar, M.; Metzsch-Zilligen, E.; Pfaendner, R. Controlled and Accelerated Hydrolysis of Polylactide (PLA) through Pentaerythritol Phosphites with Acid Scavengers. Polymers 2022, 14, 4237. [Google Scholar] [CrossRef] [PubMed]
- D’Amario, J.; Limsukon, W.; Bher, A.; Auras, R. Impact of hydrolysis pretreatment on the compostability of biodegradable poly(caprolactone) and poly(lactic acid) films. RSC Appl. Polym. 2025, 3, 711–721. [Google Scholar] [CrossRef]
- Bule Možar, K.; Miloloža, M.; Martinjak, V.; Cvetnić, M.; Kušić, H.; Bolanča, T.; Kučić Grgić, D.; Ukić, Š. Potential of Advanced Oxidation as Pretreatment for Microplastics Biodegradation. Separations 2023, 10, 132. [Google Scholar] [CrossRef]
- Mayekar, P.C.; Auras, R. Speeding it up: Dual effects of biostimulants and iron on the biodegradation of poly(lactic acid) at mesophilic conditions. Environ. Sci. Process. Impacts 2024, 26, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Mayekar, P.C.; Auras, R. Accelerating Biodegradation: Enhancing Poly(lactic acid) Breakdown at Mesophilic Environmental Conditions with Biostimulants. Macromol. Rapid Commun. 2024, 45, e2300641. [Google Scholar] [CrossRef] [PubMed]
- Satti, S.M.; Shah, A.A.; Marsh, T.L.; Auras, R. Biodegradation of Poly(lactic acid) in Soil Microcosms at Ambient Temperature: Evaluation of Natural Attenuation, Bio-augmentation and Bio-stimulation. J. Polym. Environ. 2018, 26, 3848–3857. [Google Scholar] [CrossRef]
- Mayekar, P.C.; Limsukon, W.; Bher, A.; Auras, R. Breaking It Down: How Thermoplastic Starch Enhances Poly(lactic acid) Biodegradation in Compost─A Comparative Analysis of Reactive Blends. ACS Sustain. Chem. Eng. 2023, 11, 9729–9737. [Google Scholar] [CrossRef]
- Momeni, S.; Craplewe, K.; Safder, M.; Luz, S.; Sauvageau, D.; Elias, A. Accelerating the Biodegradation of Poly(lactic acid) through the Inclusion of Plant Fibers: A Review of Recent Advances. ACS Sustain. Chem. Eng. 2023, 11, 15146–15170. [Google Scholar] [CrossRef] [PubMed]
- Ndazi, B.S.; Karlsson, S. Characterization of hydrolytic degradation of poly(lactic acid)/rice hulls composites in water at different temperatures. Express Polym. Lett. 2011, 5, 119–131. [Google Scholar] [CrossRef]
- Hendrick, E.; Frey, M. Increasing Surface Hydrophilicity in Poly(Lactic Acid) Electrospun Fibers by Addition of Pla-b-Peg Co-Polymers. J. Eng. Fibers Fabr. 2014, 9, 153–164. [Google Scholar] [CrossRef]
- Colli-Gongora, P.E.; Moo-Tun, N.M.; Herrera-Franco, P.J.; Valadez-Gonzalez, A. Assessing the Effect of Cellulose Nanocrystal Content on the Biodegradation Kinetics of Multiscale Poly(lactic acid) Composites under Controlled Thermophilic Composting Conditions. Polymers 2023, 15, 3093. [Google Scholar] [CrossRef] [PubMed]
- Van de Perre, D.; Serbruyns, L.; Coltelli, M.-B.; Gigante, V.; Aliotta, L.; Lazzeri, A.; Geerinck, R.; Verstichel, S. Tuning Biodegradation of Poly (lactic acid) (PLA) at Mild Temperature by Blending with Poly (butylene succinate-co-adipate) (PBSA) or Polycaprolactone (PCL). Materials 2024, 17, 5436. [Google Scholar] [CrossRef] [PubMed]
- Dou, T.; Zhou, B.; Hu, S.; Zhang, P. Evolution of the structural polymorphs of poly(l-lactic acid) during the in vitro mineralization of its hydroxyapatite nanocomposites by attenuated total reflection fourier transform infrared mapping coupled with principal component analysis. Polymer 2021, 236, 124318. [Google Scholar] [CrossRef]
- Luo, Y.; Lin, Z.; Guo, G. Biodegradation Assessment of Poly (Lactic Acid) Filled with Functionalized Titania Nanoparticles (PLA/TiO2) under Compost Conditions. Nanoscale Res. Lett. 2019, 14, 56. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.R.A.; Marques, C.S.; Arruda, T.R.; Teixeira, S.C.; de Oliveira, T.V. Biodegradation of Polymers: Stages, Measurement, Standards and Prospects. Macromol 2023, 3, 371–399. [Google Scholar] [CrossRef]
- Strotmann, U.; Thouand, G.; Pagga, U.; Gartiser, S.; Heipieper, H.J. Toward the future of OECD/ISO biodegradability testing-new approaches and developments. Appl. Microbiol. Biotechnol. 2023, 107, 2073–2095. [Google Scholar] [CrossRef] [PubMed]
- Rossetti, I.; Conte, F.; Ramis, G. Kinetic Modelling of Biodegradability Data of Commercial Polymers Obtained under Aerobic Composting Conditions. Eng 2021, 2, 54–68. [Google Scholar] [CrossRef]
- ISO 20200-2023; Plastics—Determination of the Degree of Disintegration of Plastic Materials Under Composting conditions in a Laboratory-Scale Test. ISO: Geneva, Switzerland, 2023.
- ISO 21701-2019; Textiles—Test Method for Accelerated Hydrolysis of Textile Materials and Biodegradation Under Controlled Composting Conditions of the Resulting Hydrolysate. ISO: Geneva, Switzerland, 2019.
- Dragomir, T.-L.; Pană, A.-M.; Ordodi, V.; Gherman, V.; Dumitrel, G.-A.; Nanu, S. An Empirical Model for Predicting Biodegradation Profiles of Glycopolymers. Polymers 2021, 13, 1819. [Google Scholar] [CrossRef] [PubMed]
- Baldera-Moreno, Y.; Pino, V.; Farres, A.; Banerjee, A.; Gordillo, F.; Andler, R. Biotechnological Aspects and Mathematical Modeling of the Biodegradation of Plastics under Controlled Conditions. Polymers 2022, 14, 375. [Google Scholar] [CrossRef] [PubMed]
- Strotmann, U.; Reuschenbach, P.; Schwarz, H.; Pagga, U. Development and Evaluation of an Online CO2 Evolution Test and a Multicomponent Biodegradation Test System. Appl. Environ. Microbiol. 2004, 70, 4621–4628. [Google Scholar] [CrossRef] [PubMed]
- Fortunati, E.; Luzi, F.; Puglia, D.; Dominici, F.; Santulli, C.; Kenny, J.M.; Torre, L. Investigation of thermo-mechanical, chemical and degradative properties of PLA-limonene films reinforced with cellulose nanocrystals extracted from Phormium tenax leaves. Eur. Polym. J. 2014, 56, 77–91. [Google Scholar] [CrossRef]
- Fernandes, I.P.; Barbosa, M.; Amaral, J.S.; Pinto, V.; Rodrigues, J.L.; Ferreira, M.J.; Barreiro, M.F. Biobased Additives as Biodegradability Enhancers with Application in TPU-Based Footwear Components. J. Renew. Mater. 2016, 4, 47–56. [Google Scholar] [CrossRef]
- Wu, N.; Zhang, H. Mechanical properties and phase morphology of super-tough PLA/PBAT/EMA-GMA multicomponent blends. Mater. Lett. 2017, 192, 17–20. [Google Scholar] [CrossRef]
- Zhou, H.; Zhao, M.; Qu, Z.; Mi, J.; Wang, X.; Deng, Y. Thermal and Rheological Properties of Poly(lactic acid)/Low-Density Polyethylene Blends and Their Supercritical CO2 Foaming Behavior. J. Polym. Environ. 2018, 26, 3564–3573. [Google Scholar] [CrossRef]
- Wu, D.; Huang, A.; Fan, J.; Xu, R.; Liu, P.; Li, G.; Yang, S. Effect of blending procedures and reactive compatibilizers on the properties of biodegradable poly(butylene adipate-co-terephthalate)/poly(lactic acid) blends. J. Polym. Eng. 2021, 41, 95–108. [Google Scholar] [CrossRef]
- Grapes, R.H.; Yun, S.T. Geochemistry of a New Zealand nephrite weathering rind. N. Z. J. Geol. Geophys. 2010, 53, 413–426. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, S.; Yan, W.; He, M.; Qin, J.; Qin, S.; Yu, J. Crystallization Morphology Regulation on Enhancing Heat Resistance of Poly(lactic acid). Polymers 2020, 12, 1563. [Google Scholar] [CrossRef]
- Maragkaki, A.; Malliaros, N.G.; Sampathianakis, I.; Lolos, T.; Tsompanidis, C.; Manios, T. Evaluation of Biodegradability of Poly(lactic acid) and Compostable Bags from Food Waste under Industrial Composting. Sustainability 2023, 15, 15963. [Google Scholar] [CrossRef]
- Kale, G.; Auras, R.; Singh, S.P.; Narayan, R. Biodegradability of polylactide bottles in real and simulated composting conditions. Polym. Test. 2007, 26, 1049–1061. [Google Scholar] [CrossRef]
- ISO 14855-2:2018; Determination of the Ultimate Aerobic Biodegradability of Plastic Materials Under Controlled Composting Conditions—Method by Analysis of Evolved Carbon Dioxide. ISO: Geneva, Switzerland, 2018.
- Bilo, F.; Pandini, S.; Sartore, L.; Depero, L.E.; Gargiulo, G.; Bonassi, A.; Federici, S.; Bontempi, E. A sustainable bioplastic obtained from rice straw. J. Clean. Prod. 2018, 200, 357–368. [Google Scholar] [CrossRef]
- Knudsmark Sjøholm, K.; Birch, H.; Hammershøj, R.; Saunders, D.M.V.; Dechesne, A.; Loibner, A.P.; Mayer, P. Determining the Temperature Dependency of Biodegradation Kinetics for 34 Hydrocarbons while Avoiding Chemical and Microbial Confounding Factors. Environ. Sci. Technol. 2021, 55, 11091–11101. [Google Scholar] [CrossRef] [PubMed]
- Klimczuk, B.; Rudnicka, A.; Owczarek, O.; Puszkarz, A.K.; Szparaga, G.; Puchalski, M. Investigation of the Hydrolytic Degradation Kinetics of 3D-Printed PLA Structures under a Thermally Accelerated Regime. Materials 2024, 17, 1043. [Google Scholar] [CrossRef] [PubMed]
- Trofimchuk, E.; Ostrikova, V.; Ivanova, O.; Moskvina, M.; Plutalova, A.; Grokhovskaya, T.; Shchelushkina, A.; Efimov, A.; Chernikova, E.; Zhang, S.; et al. Degradation of Structurally Modified Polylactide under the Controlled Composting of Food Waste. Polymers 2023, 15, 4017. [Google Scholar] [CrossRef] [PubMed]
- Xochitl, Q.-P.; María del Consuelo, H.-B.; María del Consuelo, M.-S.; Rosa María, E.-V.; Alethia, V.-M. Degradation of Plastics in Simulated Landfill Conditions. Polymers 2021, 13, 1014. [Google Scholar] [CrossRef] [PubMed]
- Mejia, E.; Cherupurakal, N.; Mourad, A.-H.I.; Al Hassanieh, S.; Rabia, M. Effect of Processing Techniques on the Microstructure and Mechanical Performance of High-Density Polyethylene. Polymers 2021, 13, 3346. [Google Scholar] [CrossRef] [PubMed]


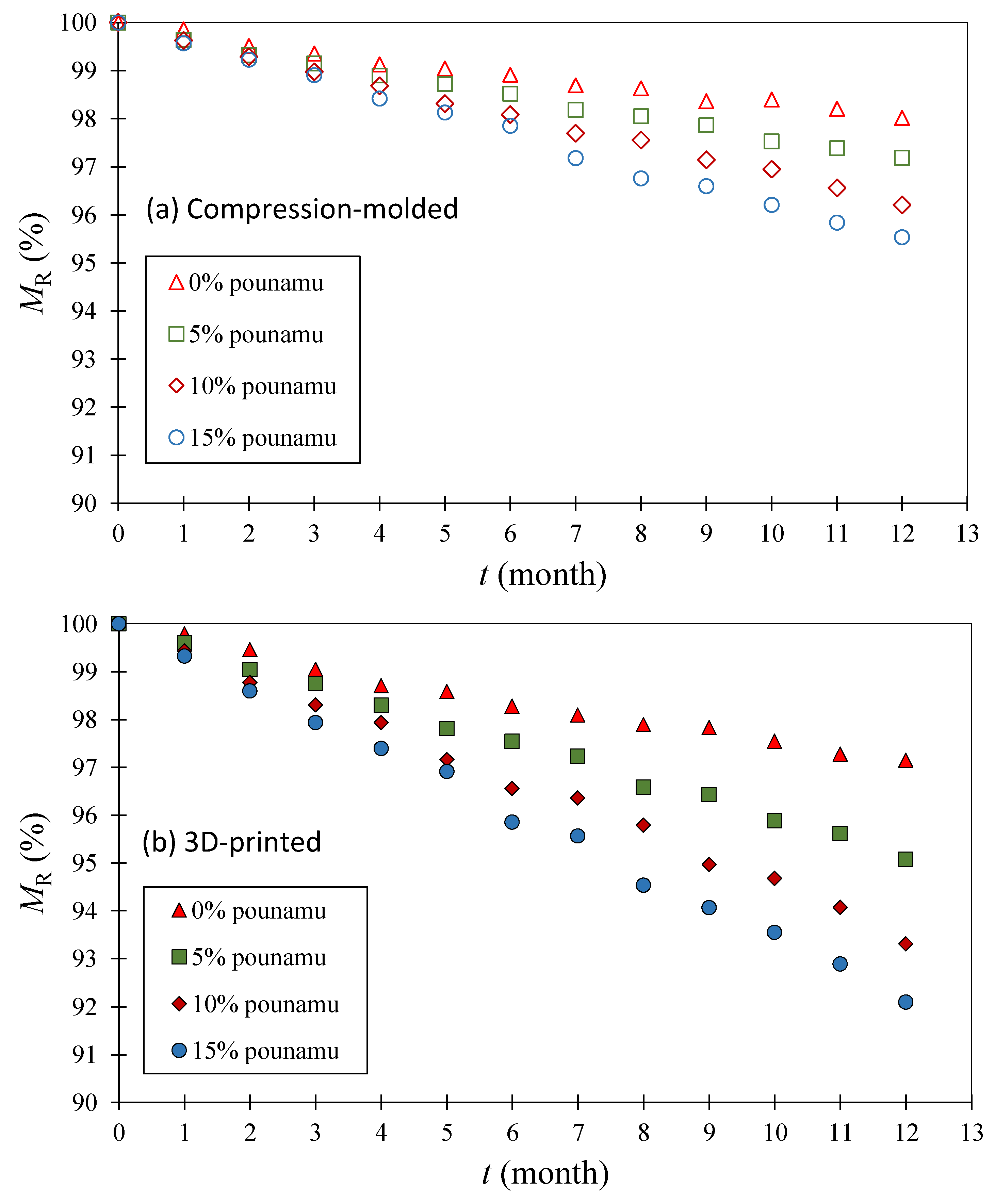
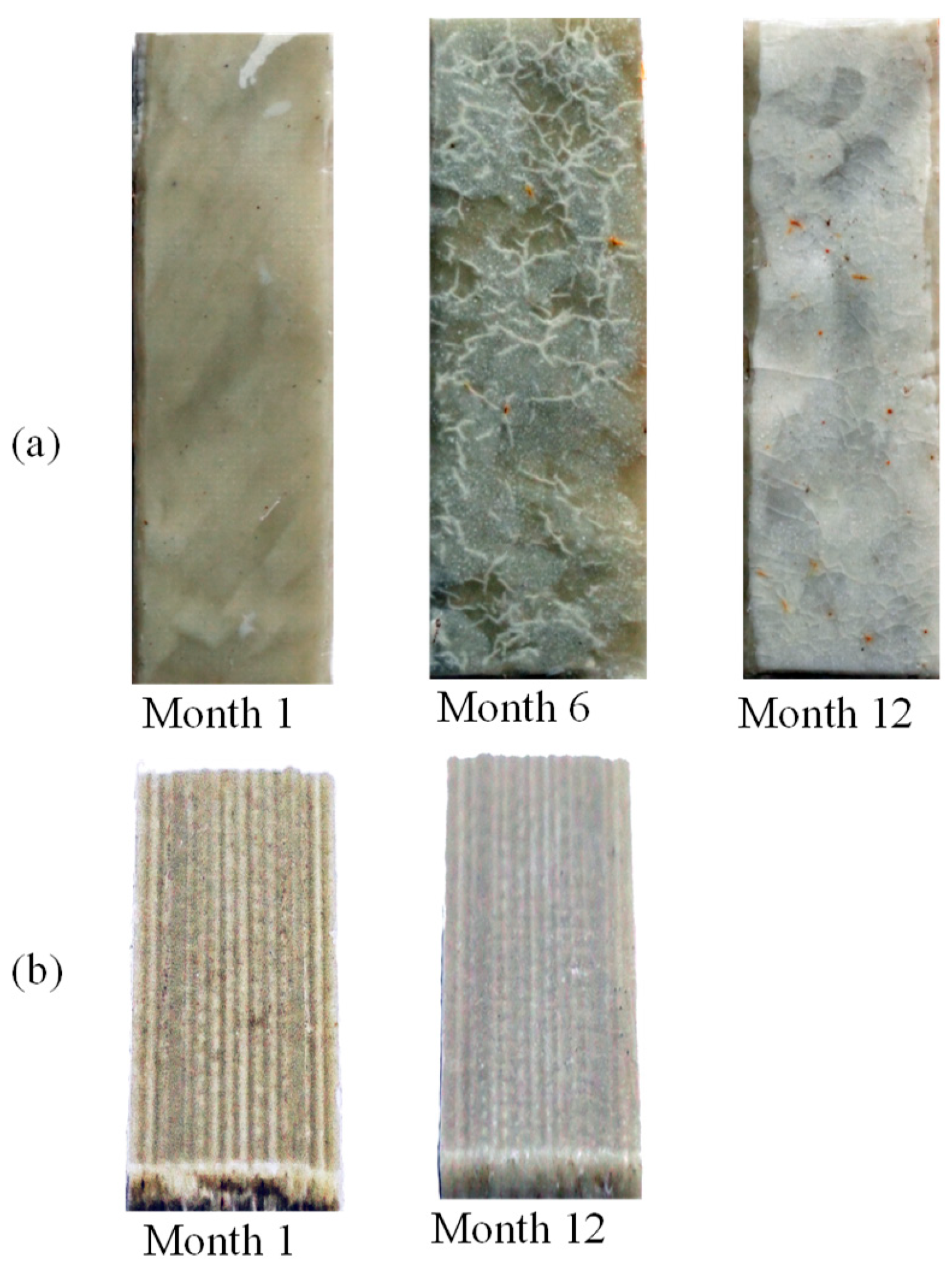
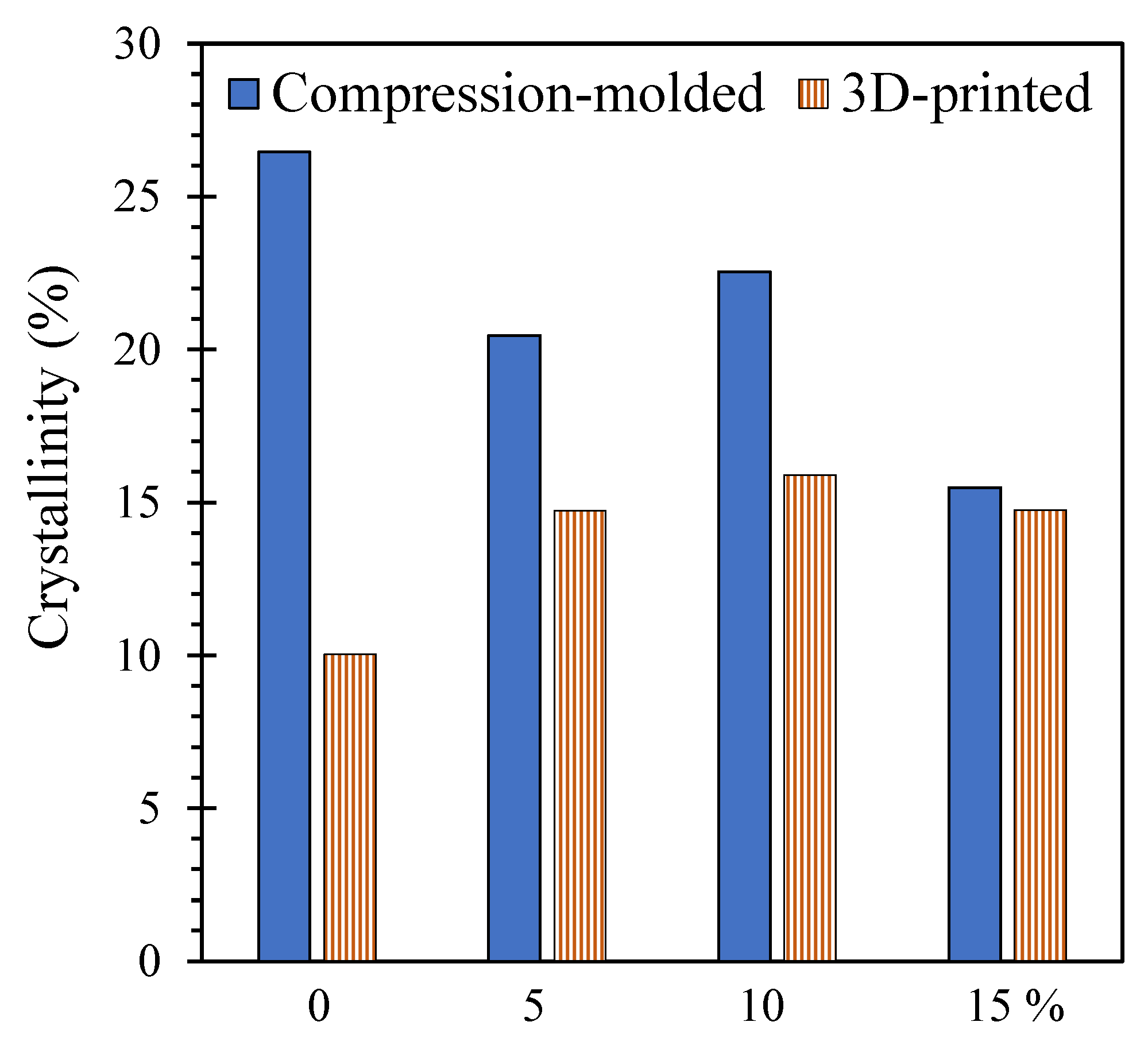
| Screen Size (µm) | wt% |
|---|---|
| >212 | 0.12 |
| 106–212 | 0.30 |
| 75–106 | 1.09 |
| 38–75 | 2.46 |
| 0–38 | 96.04 |
| Code | PLA (wt%) | NZ Jade Particles (wt%) |
|---|---|---|
| 0 wt% | 100.0 | 0.0 |
| 5 wt% | 95.0 | 5.0 |
| 10 wt% | 90.0 | 10.0 |
| 15 wt% | 85.0 | 15.0 |
| Temperatures (Die to Feed) (°C) | 185–190 |
|---|---|
| Screw speed (rpm) | 180 |
| Feed speed (rpm) | 25–30 depending on the formulation |
| Pelletizer speed (m/min) | 17.5 |
| Pellet diameter (mm) | 2.5 |
| Running pressure (bar) | 30–45 depending on the formulation |
| Temperatures (Die to Feed) (°C) | 150–190 |
|---|---|
| Screw speed (rpm) | 150 |
| Feed speed (rpm) | 20–30 depending on the formulation |
| Caterpillar speed (m/min) | 25 |
| Running pressure (bar) | 50–70 depending on the formulation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, L.; Joe, M.; Dang, Q.A.; Park, H.E. Enhancing Biodegradation of Poly(lactic acid) in Compost at Room Temperature by Compounding Jade Particles. Polymers 2025, 17, 2037. https://doi.org/10.3390/polym17152037
Lin L, Joe M, Dang QA, Park HE. Enhancing Biodegradation of Poly(lactic acid) in Compost at Room Temperature by Compounding Jade Particles. Polymers. 2025; 17(15):2037. https://doi.org/10.3390/polym17152037
Chicago/Turabian StyleLin, Lilian, Matthew Joe, Quang A. Dang, and Heon E. Park. 2025. "Enhancing Biodegradation of Poly(lactic acid) in Compost at Room Temperature by Compounding Jade Particles" Polymers 17, no. 15: 2037. https://doi.org/10.3390/polym17152037
APA StyleLin, L., Joe, M., Dang, Q. A., & Park, H. E. (2025). Enhancing Biodegradation of Poly(lactic acid) in Compost at Room Temperature by Compounding Jade Particles. Polymers, 17(15), 2037. https://doi.org/10.3390/polym17152037







