A Composite Substrate of Ag Nanoparticle-Decorated Inverse Opal Polydimethylsiloxane for Surface Raman Fluorescence Dual Enhancement
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of PS Nanoparticles
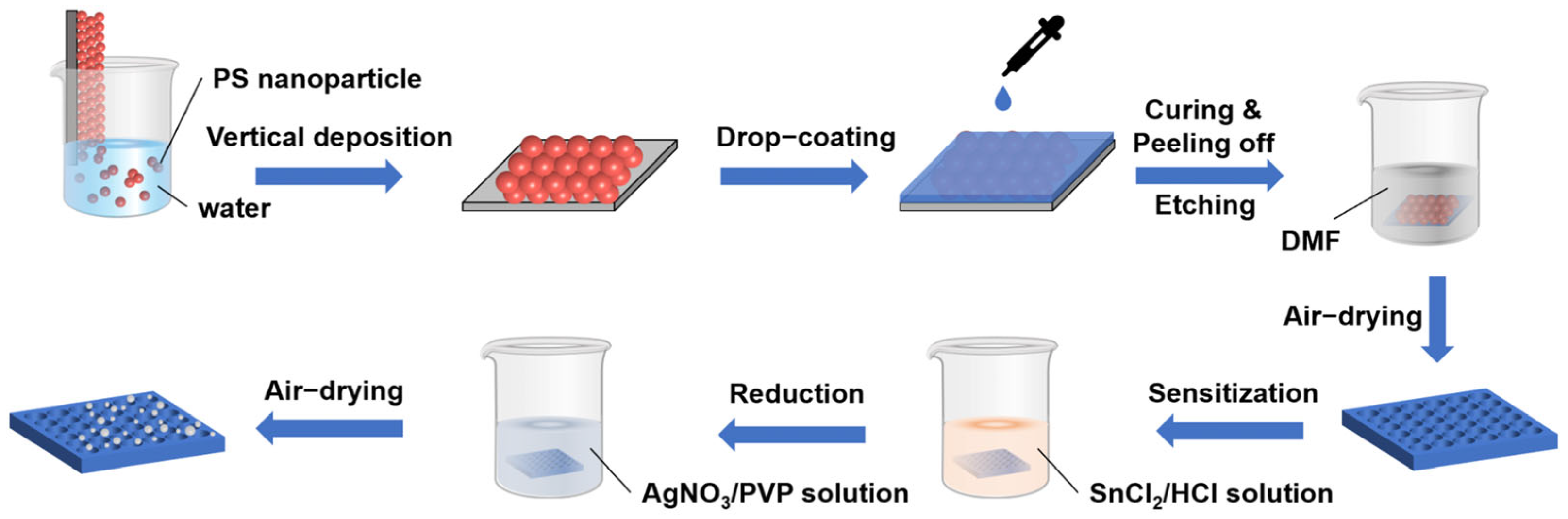
2.3. Preparation of PS/PDMS Composite Film
2.4. Fabrication of AIOP Composite Substrate
2.5. Characterization
2.6. SERS and SEF Measurement
2.7. EM Field Simulation
3. Results and Discussion
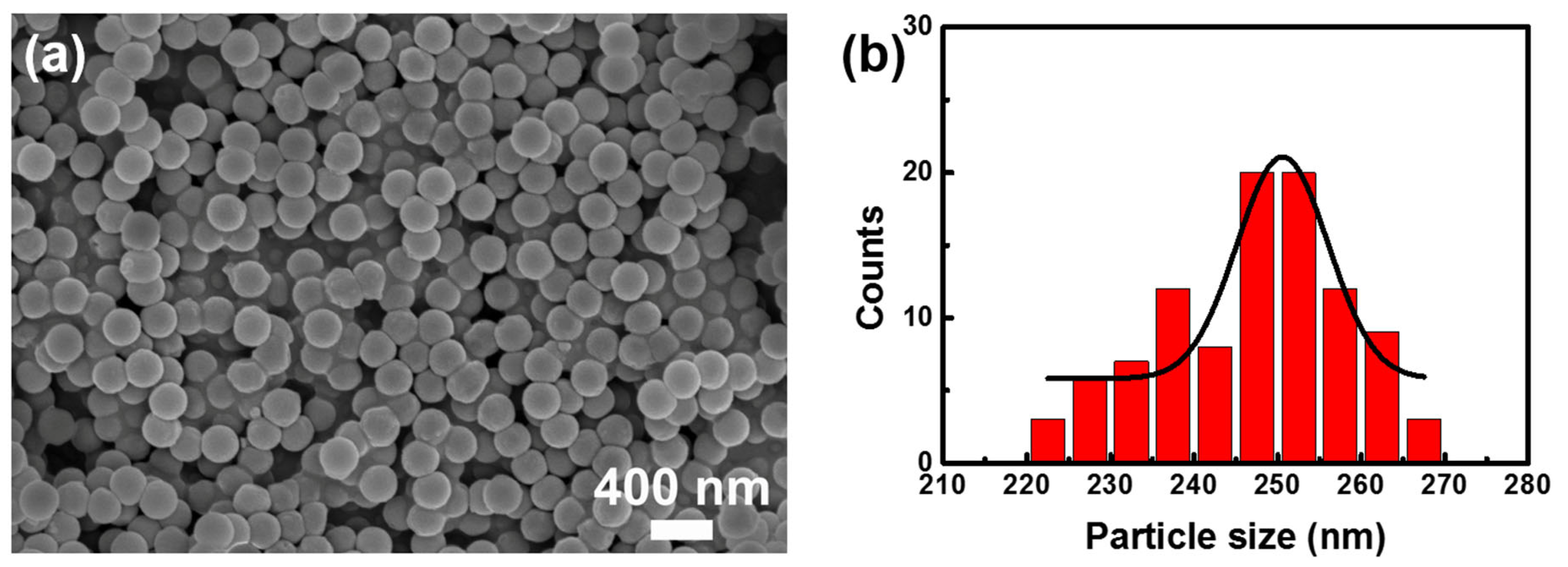
3.1. Characterization of PS Nanoparticles
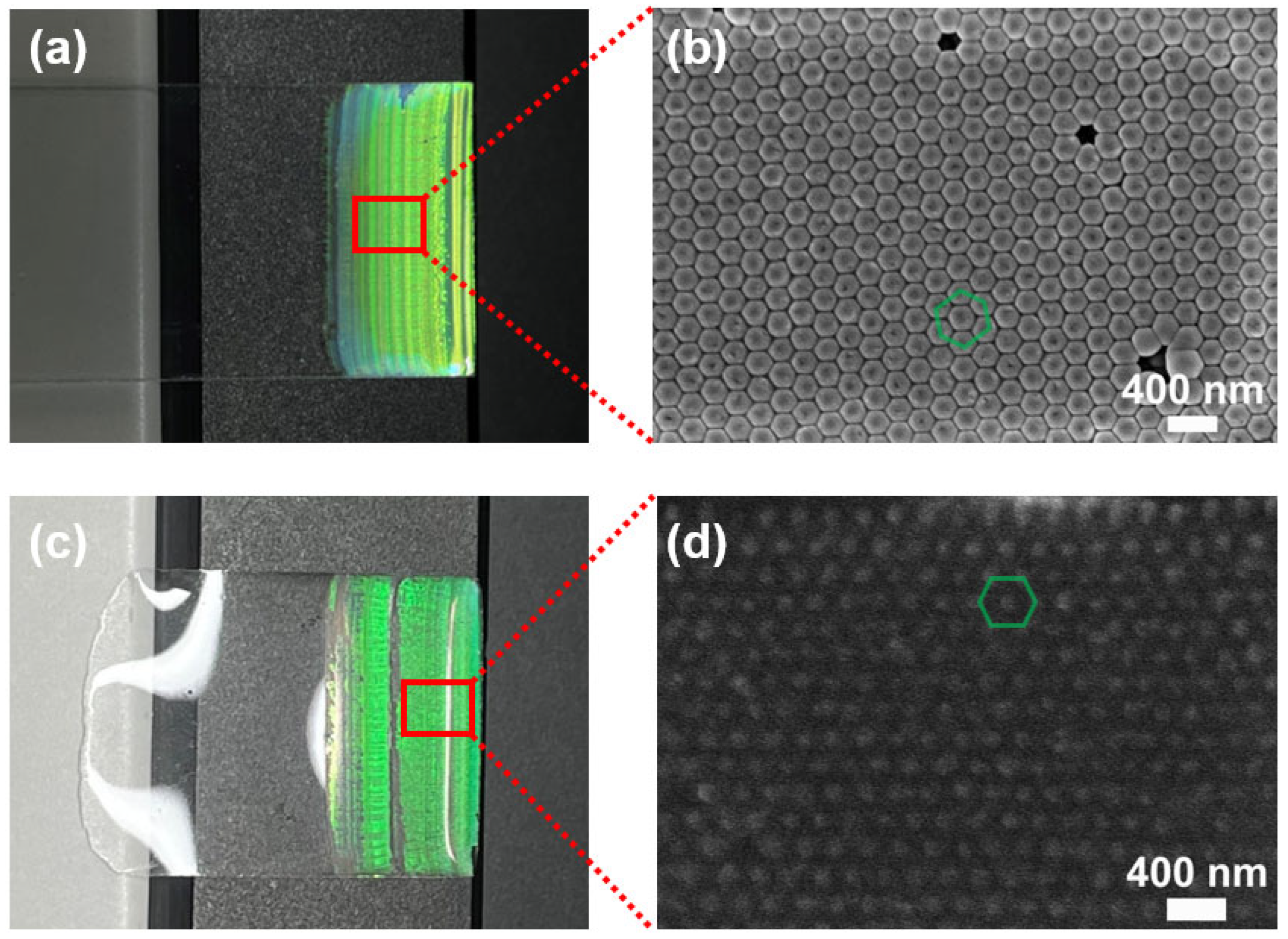
3.2. Self-Assembly and Transfer of PS Nanoparticles on PDMS
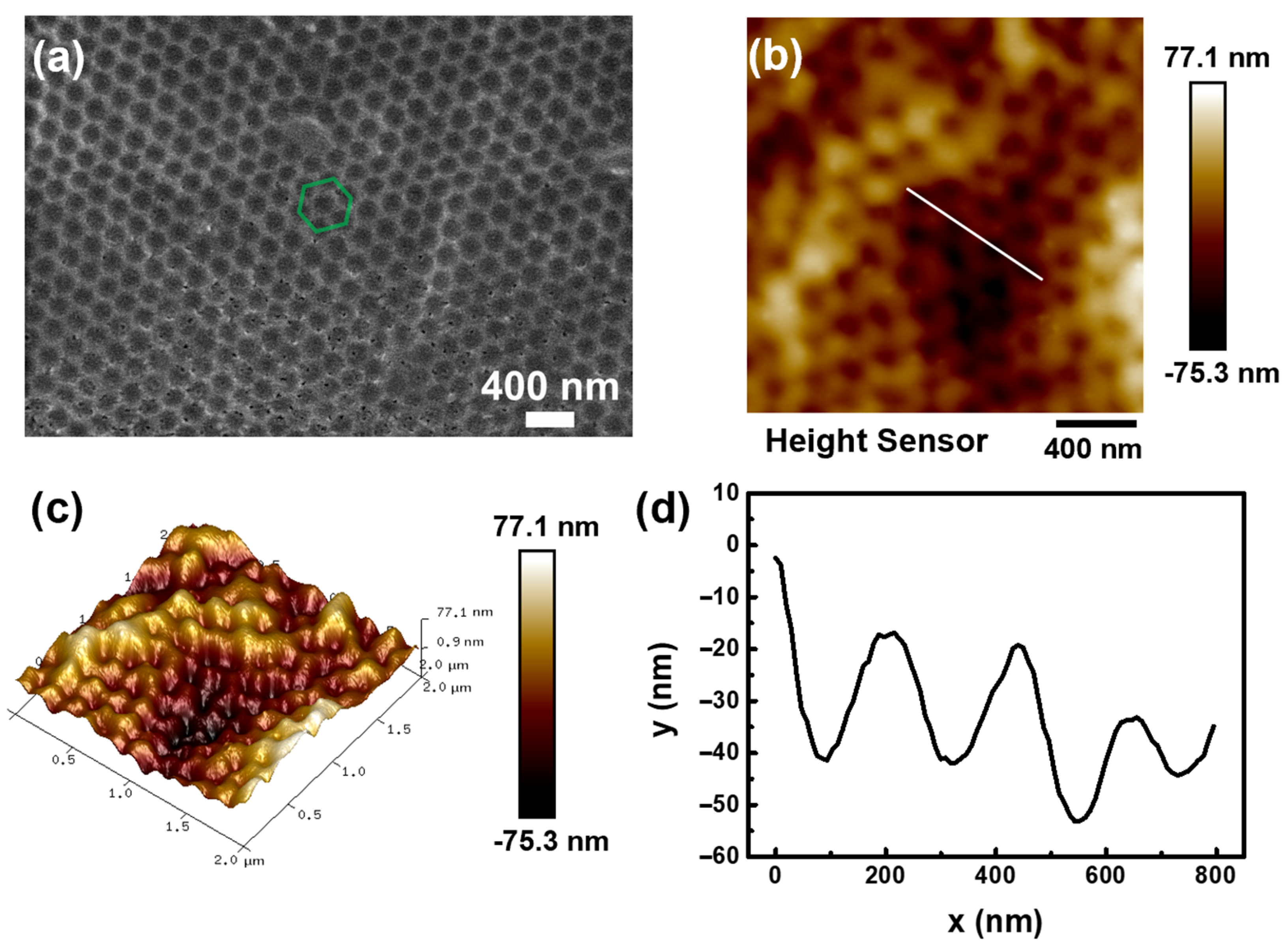
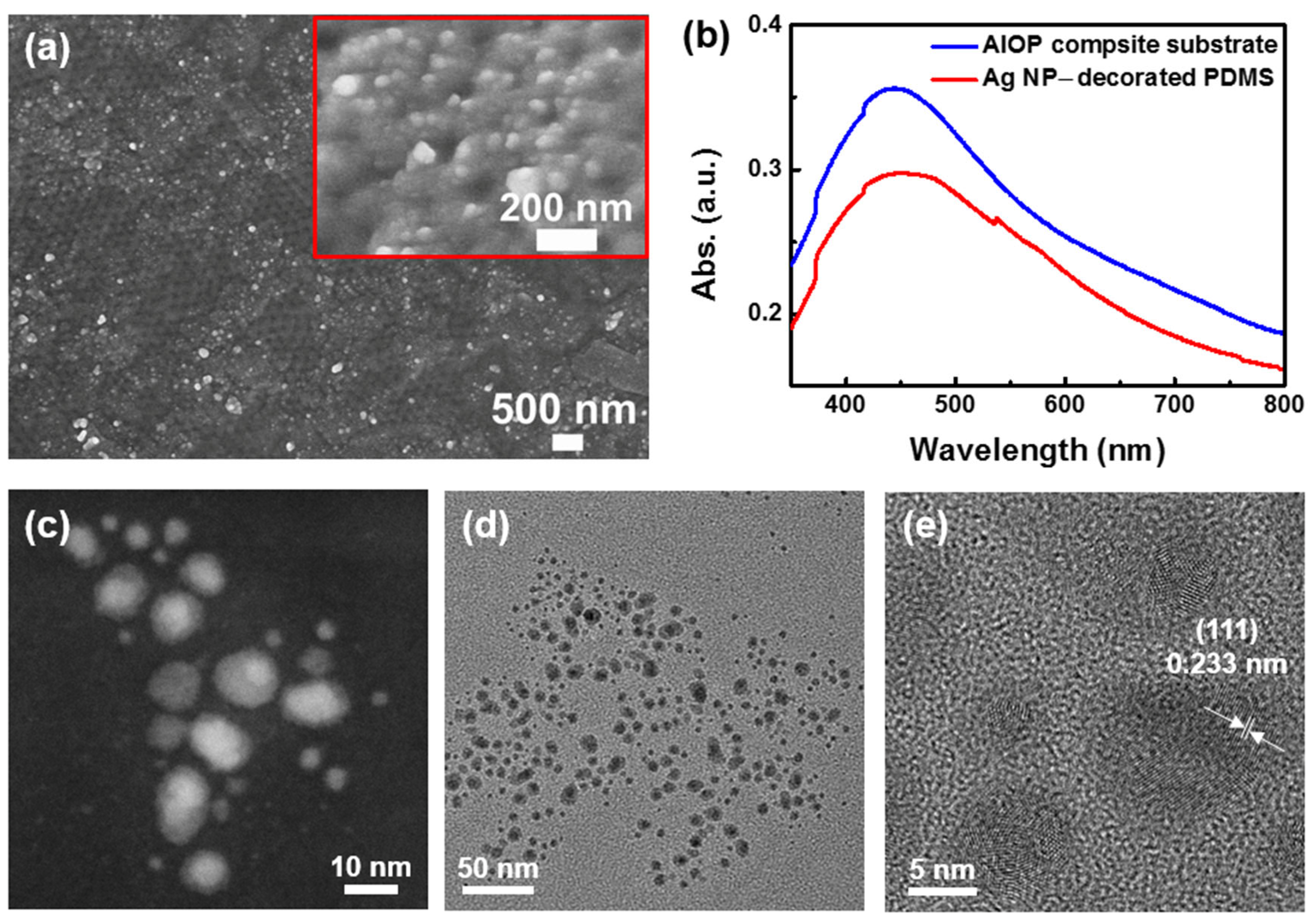
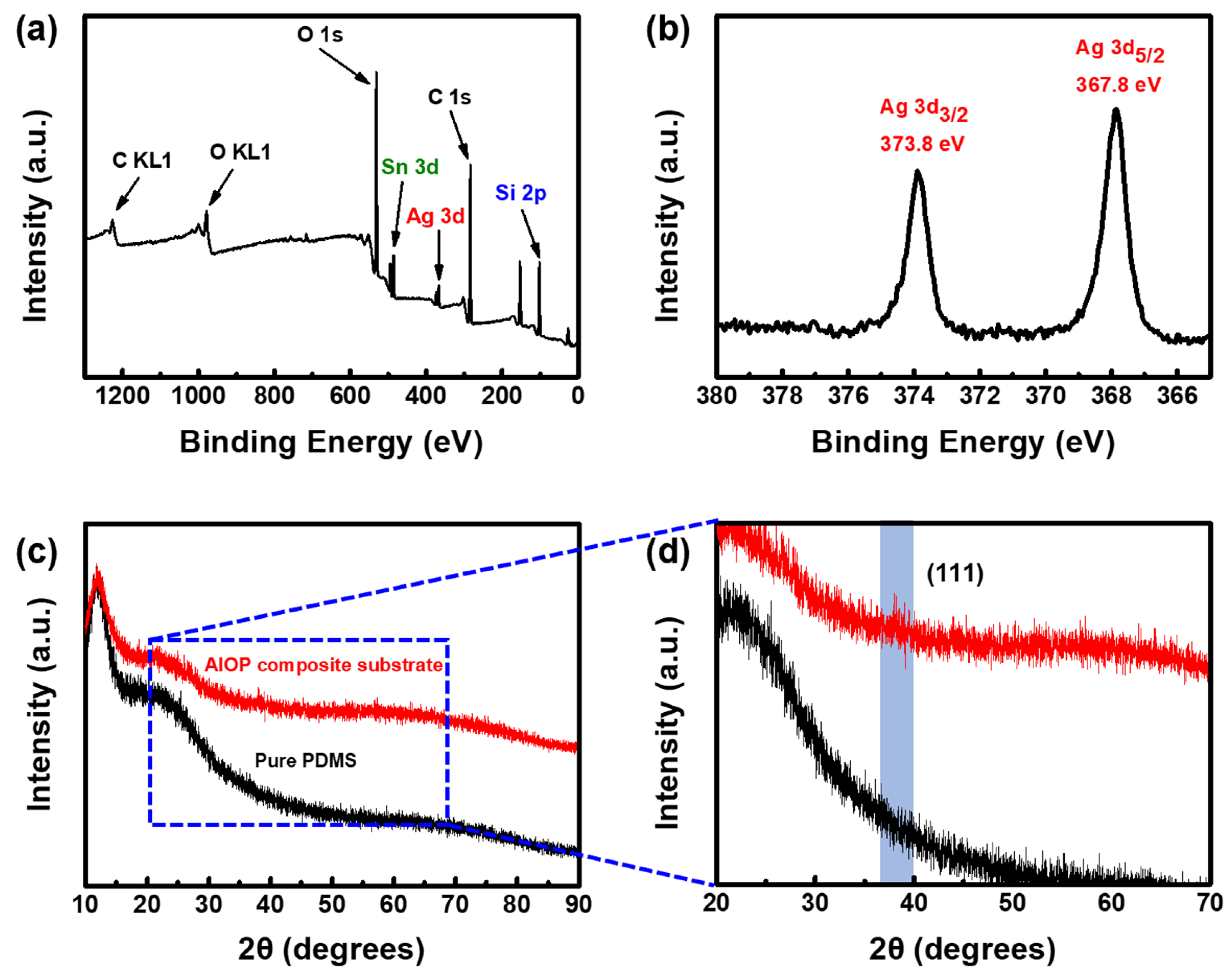
3.3. Characterization and Surface Analysis of AIOP Composite Substrate
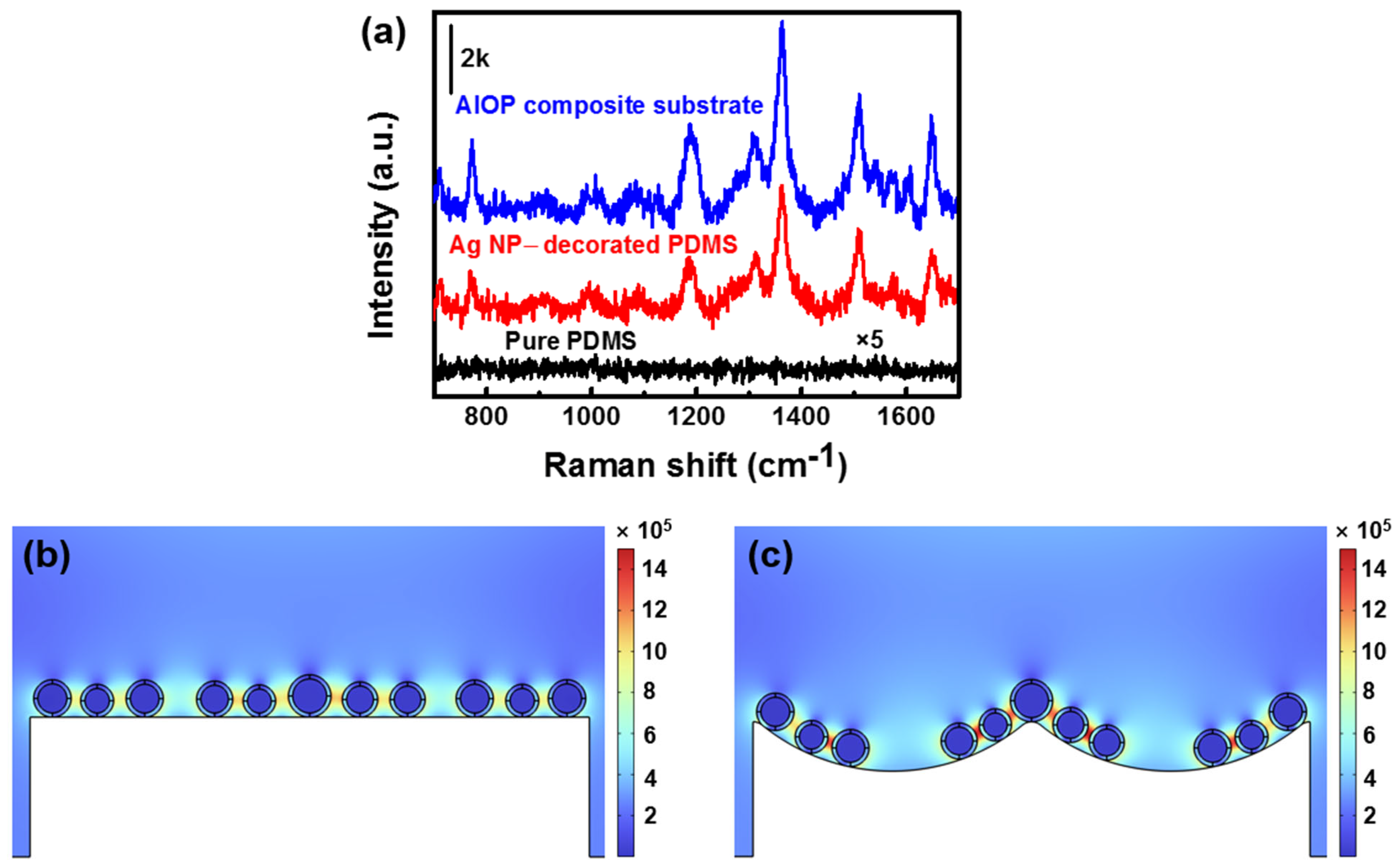
3.4. SERS Activity of AIOP Composite Substrate
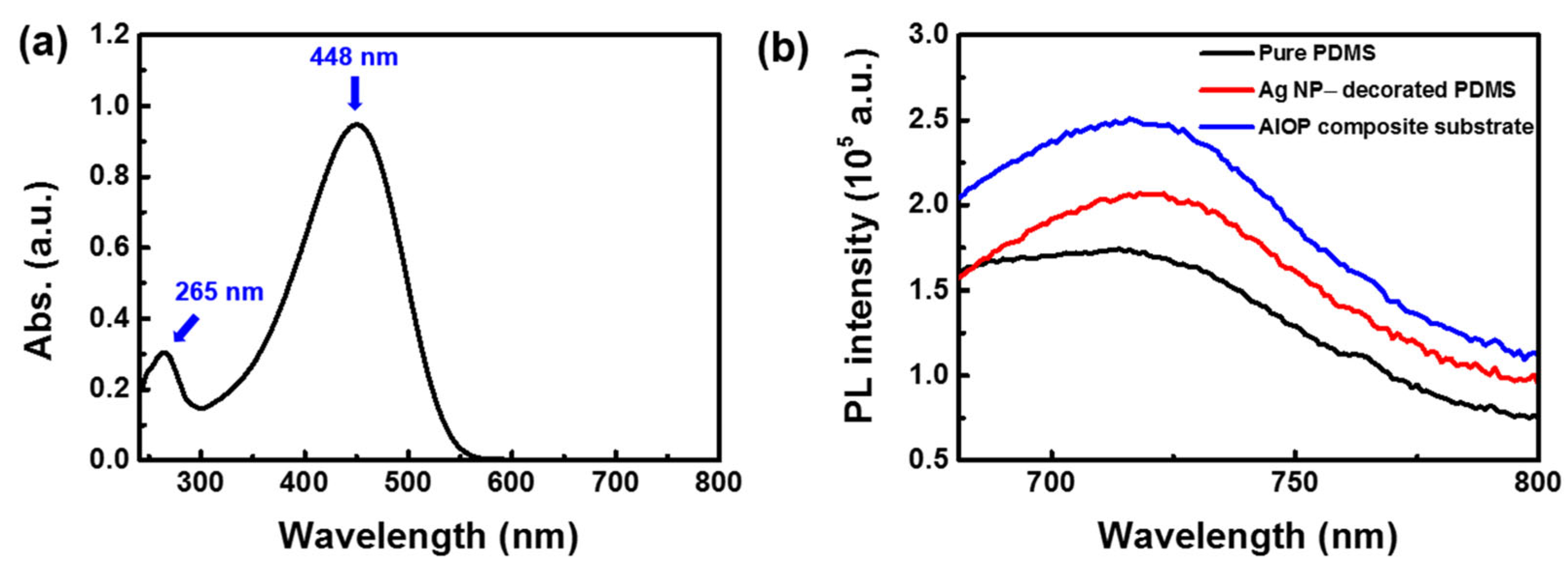
3.5. SEF Performance of AIOP Composite Substrate
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Li, D.; Wu, X.; Chen, Z.; Liu, T.; Mu, X. Surface-enhanced spectroscopy technology based on metamaterials. Microsyst. Nanoeng. 2025, 11, 60. [Google Scholar] [CrossRef]
- Srivastava, S.; Wang, W.; Zhou, W.; Jin, M.; Vikesland, P.J. Machine learning-assisted surface-enhanced Raman spectroscopy detection for environmental applications: A review. Environ. Sci. Technol. 2024, 58, 20830–20848. [Google Scholar] [CrossRef]
- Lu, Y.; Lin, L.; Ye, J. Human metabolite detection by surface-enhanced Raman spectroscopy. Mater. Today Bio 2022, 13, 100205. [Google Scholar] [CrossRef]
- Li, Z.; Chen, M.; Liang, Q.-B.; Pan, X.-H.; Wang, J.-D.; Weng, Y.-H.; Cao, S.-H.; Li, Y.-Q. Covalent organic framework-based surface plasmon-enhanced fluorescence sensing for real-time monitoring of cell apoptosis. Biosens. Bioelectron. 2025, 284, 117569. [Google Scholar] [CrossRef]
- Berger, L.M.; Duportal, M.; Menezes, L.d.S.; Cortés, E.; Maier, S.A.; Tittl, A.; Krischer, K. Improved In Situ Characterization of Electrochemical Interfaces Using Metasurface-Driven Surface-Enhanced IR Absorption Spectroscopy. Adv. Funct. Mater. 2023, 33, 2300411. [Google Scholar] [CrossRef]
- Wu, T.; Mao, Y.; Wang, T.; Ma, L.; Cao, J.; Xie, B.; Weia, J.; Lia, P. Evolving trends in nanozyme-based SERS systems for food contaminant monitoring: A review. Food Chem. 2025, 486, 144621. [Google Scholar] [CrossRef] [PubMed]
- Logan, N.; Cao, C.; Freitag, S.; Haughey, S.A.; Krska, R.; Elliott, C.T. Advancing Mycotoxin Detection in Food and Feed: Novel Insights from Surface-Enhanced Raman Spectroscopy (SERS). Adv. Mater. 2024, 36, 2309625. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Chen, S.; Sun, W.; Xu, R.; Yin, X.; Guo, D.; Yang, Y. Plasmon-enhanced fluorescence sensor based on Au nanocages for sensitive detection of norepinephrine. Anal. Chim. Acta 2025, 1354, 343995. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Wang, H.; Li, Q.; Li, Q.; Yang, Y.; Zhu, R.; Yang, J.; Liu, X.; Zhang, Q.; Wang, Y. Rapid and Scalable Preparation of Highly Uniform, Atomically Thin MSe2 (M = Ti, Nb, Ta) Nanosheets as Ultra-Sensitive SERS Substrates for Lateral Flow Immunoassay. Adv. Funct. Mater. 2025, 35, 2420786. [Google Scholar] [CrossRef]
- Huang, X.; Tian, H.; Huang, L.; Chen, Q.; Yang, Y.; Zeng, R.; Xu, J.; Chen, S.; Zhou, X.; Liu, G. Well-ordered Au nanoarray for sensitive and reproducible detection of hepatocellular carcinoma-associated miRNA via CHA-assisted SERS/fluorescence dual-mode sensing. Anal. Chem. 2023, 95, 5955–5966. [Google Scholar] [CrossRef]
- Hang, Y.; Boryczka, J.; Wu, N. Visible-light and near-infrared fluorescence and surface-enhanced Raman scattering point-of-care sensing and bio-imaging: A review. Chem. Soc. Rev. 2022, 51, 329–375. [Google Scholar] [CrossRef]
- Apostolaki, M.-A.; Sakellis, E.; Gardelis, S.; Likodimos, V. Interplay of plasmonic and charge transfer effects for ultrasensitive Ag–WO 3/TiO 2 photonic crystal SERS sensors. Mater. Adv. 2025, 6, 388–399. [Google Scholar] [CrossRef]
- Wang, J.; Pinkse, P.W.; Segerink, L.I.; Eijkel, J.C. Bottom-up assembled photonic crystals for structure-enabled label-free sensing. ACS Nano 2021, 15, 9299–9327. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Wu, J.; Yu, X.; Hong, R.; Zu, X.; Lin, X.; Luo, H.; Lin, W.; Yi, G. Fabrication of Au nanoparticle arrays on flexible substrate for tunable localized surface plasmon resonance. ACS Appl. Mater. Interfaces 2021, 13, 9281–9288. [Google Scholar] [CrossRef] [PubMed]
- Deka, T.; Nair, R.G. Recent advancements in surface plasmon resonance and Schottky junction assisted photocatalytic water splitting of noble metal decorated Titania: A review. Int. J. Hydrogen Energy 2024, 59, 322–342. [Google Scholar] [CrossRef]
- Cai, Y.Y.; Choi, Y.C.; Kagan, C.R. Chemical and Physical Properties of Photonic Noble-Metal Nanomaterials. Adv. Mater. 2023, 35, 2108104. [Google Scholar] [CrossRef]
- Choi, J.-H.; Choi, J.-W. Metal-enhanced fluorescence by bifunctional Au nanoparticles for highly sensitive and simple detection of proteolytic enzyme. Nano Lett. 2020, 20, 7100–7107. [Google Scholar] [CrossRef]
- Wang, D.; Xu, X.; Qiu, T.; Ren, N.; Liu, H.; Fang, G.; Liu, Z. Laser Construction TiOx-Ag heterostructure interface for high sensitive coupling resonance enhanced fluorescence in bacterial nucleic acid amplification sensor chip. Sens. Actuators B Chem. 2025, 423, 136727. [Google Scholar] [CrossRef]
- Lin, L.L.; Alvarez-Puebla, R.; Liz-Marzán, L.M.; Trau, M.; Wang, J.; Fabris, L.; Wang, X.; Liu, G.; Xu, S.; Han, X.X. Surface-enhanced Raman spectroscopy for biomedical applications: Recent advances and future challenges. ACS Appl. Mater. Interfaces 2025, 17, 16287–16379. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, Y.; Tang, Y.; Li, B.; Wang, Q.; Wang, J.; Gao, X.; Zhang, Y.; Wang, J.; Lei, Z. Tandem Dual-Ratiometric SERS Probe Enables Raman Imaging of Neurological pH Fluctuations in Epilepsy. Angew. Chem. Int. Ed. 2025, 64, e202504822. [Google Scholar] [CrossRef]
- Meng, T.; Shi, M.; Guo, Y.; Wang, H.; Fu, N.; Liu, Z.; Huang, B.; Lei, C.; Su, X.; Peng, B. Multifunctional Ag-coated CuO microbowl arrays for highly efficient, ultrasensitive, and recyclable surface-enhanced Raman scattering. Sens. Actuators B Chem. 2022, 354, 131097. [Google Scholar] [CrossRef]
- Hu, X.; Mu, Z.; Li, Y.; Bai, L.; Qing, M. Metal–organic frameworks-scaffold gold nanoclusters enabled aggregation-induced enhanced fluorescent sensor array for high-throughput detection of heavy metal ions. Microchem. J. 2025, 210, 113020. [Google Scholar] [CrossRef]
- Minamikawa, T.; Sakaguchi, R.; Harada, Y.; Tanioka, H.; Inoue, S.; Hase, H.; Mori, Y.; Takamatsu, T.; Yamasaki, Y.; Morimoto, Y. Long-range enhancement for fluorescence and Raman spectroscopy using Ag nanoislands protected with column-structured silica overlayer. Light Sci. Appl. 2024, 13, 299. [Google Scholar] [CrossRef]
- Luo, J.; Wang, Z.; Li, Y.; Wang, C.; Sun, J.; Ye, W.; Wang, X.; Shao, B. Durable and flexible Ag-nanowire-embedded PDMS films for the recyclable swabbing detection of malachite green residue in fruits and fingerprints. Sens. Actuators B Chem. 2021, 347, 130602. [Google Scholar] [CrossRef]
- Li, X.; Liu, H.; Gu, C.; Zhang, J.; Jiang, T. PDMS/TiO2/Ag hybrid substrate with intrinsic signal and clean surface for recyclable and quantitative SERS sensing. Sens. Actuators B Chem. 2022, 351, 130886. [Google Scholar] [CrossRef]
- Choudhary, R.; Srivastava, S.K. Templating assisted fabrication of flexible, highly stable and uniform plasmonic platform for ultrahigh enhancement of Raman and fluorescence signals: Model sensing of rhodamine-6G. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2025, 338, 126125. [Google Scholar] [CrossRef] [PubMed]
- Yi, K.; Fan, Z.; Ran, Q.; Jia, K.; Liu, X.; Wang, L. Scalable fabrication of silver covered polyurethane nanofibers as flexible SERS nanosensors for aflatoxin detection. Talanta 2023, 263, 124636. [Google Scholar] [CrossRef] [PubMed]
- Shao, T.; Xu, J.; Zhong, H.; Hu, Y.; Chen, J. A stable and flexible Au@ Ag NPs/PVA SERS platform for thiram residue detection on rough surface. Talanta 2024, 274, 126008. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.; Wang, T.; Liu, Q.; Tang, J.; Wu, D. Ag nanoparticles@ agar gel as a 3D flexible and stable SERS substrate with ultrahigh sensitivity. Langmuir 2022, 38, 13822–13832. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, L.; Lv, X.; Liu, J.; Liu, X.; Zhang, Y.; Zhang, D.; Li, S.; Wang, Q. Large-area fluorescence enhancement of R6G based on a uniform PVA-Au plasmonic substrate. Opt. Express 2022, 30, 43281–43292. [Google Scholar] [CrossRef]
- Ma, H.; Pan, S.-Q.; Wang, W.-L.; Yue, X.; Xi, X.-H.; Yan, S.; Wu, D.-Y.; Wang, X.; Liu, G.; Ren, B. Surface-enhanced Raman spectroscopy: Current understanding, challenges, and opportunities. ACS Nano 2024, 18, 14000–14019. [Google Scholar] [CrossRef]
- Tang, X.; Hao, Q.; Hou, X.; Lan, L.; Li, M.; Yao, L.; Zhao, X.; Ni, Z.; Fan, X.; Qiu, T. Exploring and engineering 2D transition metal dichalcogenides toward ultimate SERS performance. Adv. Mater. 2024, 36, 2312348. [Google Scholar] [CrossRef]
- Ma, Y.; Song, M.; Li, L.; Lao, X.; Liu, Y.; Wong, M.-c.; Yang, M.; Chen, H.; Hao, J. Attomolar-level detection of respiratory virus long-chain oligonucleotides based on FRET biosensor with upconversion nanoparticles and Au–Au dimer. Biosens. Bioelectron. 2024, 243, 115778. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Xiang, H.; Zhao, G.; Zhang, M.; Lin, J.; Yang, L.; Liu, H. A FRET assembly of fluorescent gold nanoclusters for ratiometric monitoring peroxynitrite in living cells and zebrafish. Sens. Actuators B Chem. 2023, 378, 133182. [Google Scholar] [CrossRef]
- Yang, N.; Kang, Y.; Cong, Y.; Wang, X.; Yao, C.; Wang, S.; Li, L. Controllable gold nanocluster–emulsion interface for direct cell penetration and photothermal killing. Adv. Mater. 2023, 35, 2208349. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Zhang, G.; Deng, S.; Zhang, G.; Xiao, X.; He, W.; Su, L.; Liu, C.; Lai, W. Triple strategy-enhanced immunochromatographic assay based on APCB and AIEFM for the ultrasensitive detection of AFM1. J. Hazard. Mater. 2023, 460, 132438. [Google Scholar] [CrossRef]
- Huang, K.; Le, N.; Wang, J.S.; Huang, L.; Zeng, L.; Xu, W.C.; Li, Z.; Li, Y.; Han, G. Designing next generation of persistent luminescence: Recent advances in uniform persistent luminescence nanoparticles. Adv. Mater. 2022, 34, 2107962. [Google Scholar] [CrossRef]
- Cai, Y.; Lu, M.; Qin, X.; Jin, D.; Zhou, J. Understanding shell coating effects to overcome quenching in single anisotropic upconversion nanoparticles. Nat. Commun. 2025, 16, 4927. [Google Scholar] [CrossRef]
- Qi, B.; Dai, W.; Lou, B.; Song, B.; Miao, Z.; Wei, Y.; Ma, C.; Wang, J. Amplifying Persistent Luminescence in Heavily Doped Nanopearls for Bioimaging and Solar-to-Chemical Synthesis. ACS Nano 2025, 19, 5818–5828. [Google Scholar] [CrossRef]
- Zhu, Y.-Z.; Zhou, R.-Y.; Hu, S.; Li, J.-F.; Tian, Z.-Q. Shell-Isolated Nanoparticle-Enhanced Raman Spectroscopy: Toward High Sensitivity and Broad Applicability. ACS Nano 2024, 18, 32287–32298. [Google Scholar] [CrossRef]
- Liu, Y.; Chui, K.K.; Fang, Y.; Wen, S.; Zhuo, X.; Wang, J. Metal–organic framework-enabled trapping of volatile organic compounds into plasmonic nanogaps for surface-enhanced raman scattering detection. ACS Nano 2024, 18, 11234–11244. [Google Scholar] [CrossRef]
- Wu, J.; Li, J.; Liu, X.; Gong, L.; Chen, J.; Tang, Z.; Lin, W.; Mu, Y.; Lin, X.; Hong, W. Unclonable photonic crystal hydrogels with controllable encoding capacity for anticounterfeiting. ACS Appl. Mater. Interfaces 2021, 14, 2369–2380. [Google Scholar] [CrossRef]
- Jiang, X.; Lai, Y.; Yang, M.; Yang, H.; Jiang, W.; Zhan, J. Silver nanoparticle aggregates on copper foil for reliable quantitative SERS analysis of polycyclic aromatic hydrocarbons with a portable Raman spectrometer. Analyst 2012, 137, 3995–4000. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Liao, T.; Liu, X.; Wu, J.; Tang, Z.; Pan, G.; Liu, H.; Lin, X.; Lin, W.; Li, Y. Modified ethylene/α-octene co-polymer composites based on dual toughening of hydrogen bonding network and nanospheres. Polym. Adv. Technol. 2022, 33, 2313–2324. [Google Scholar] [CrossRef]
- Truong, V.X.; Bachmann, J.; Unterreiner, A.N.; Blinco, J.P.; Barner-Kowollik, C. Wavelength-orthogonal stiffening of hydrogel networks with visible light. Angew. Chem. Int. Ed. 2022, 61, e202113076. [Google Scholar] [CrossRef] [PubMed]
- Stanton, M.M.; Ducker, R.E.; MacDonald, J.C.; Lambert, C.R.; McGimpsey, W.G. Super-hydrophobic, highly adhesive, polydimethylsiloxane (PDMS) surfaces. J. Colloid Interface Sci. 2012, 367, 502–508. [Google Scholar] [CrossRef]
- Ismail, A.E.; Grest, G.S.; Heine, D.R.; Stevens, M.J.; Tsige, M. Interfacial structure and dynamics of siloxane systems: PDMS− vapor and PDMS− water. Macromolecules 2009, 42, 3186–3194. [Google Scholar] [CrossRef]
- Chen, Y.; Cao, J.; Wei, H.; Wu, Z.; Wang, X.; Pei, Y. Synthesis of polyvinyl alcohol/Ag electrospun nanofibers as highly efficient flexible SERS substrates. Vib. Spectrosc. 2021, 114, 103246. [Google Scholar] [CrossRef]
- Wang, X.-Z.; Hu, Q.; Gao, M.; Liu, S.; Luo, J.-L. Tuning the subsurface oxygen of Ag2O-derived Ag nanoparticles to achieve efficient CO2 electroreduction to CO. Electrochim. Acta 2022, 403, 139656. [Google Scholar] [CrossRef]
- Cao, T.; Zhou, Y.; Zhang, J.; Zhao, B. Clean preparation of Ag@ Au nanoparticle-modified cotton fabric with durable antimicrobial properties by adsorption. Cellulose 2025, 32, 613–628. [Google Scholar] [CrossRef]
- Khan, J.; Naseem, I.; Bibi, S.; Ahmad, S.; Altaf, F.; Hafeez, M.; Almoneef, M.M.; Ahmad, K. Green synthesis of silver nanoparticles (Ag-NPs) using Debregeasia salicifolia for biological applications. Materials 2022, 16, 129. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Li, K.; Zhang, Y.; Du, J.; Ghafoor, S.; Lu, Y. A facile periodic porous Au nanoparticle array with high-density and built-in hotspots for SERS analysis. Appl. Surf. Sci. 2020, 527, 146807. [Google Scholar] [CrossRef]
- Yu, H.; Chen, Y.; Wen, Z.; Wang, R.; Jia, S.; Zhu, W.; Song, Y.; Sun, H.; Liu, B. Selective SERS Sensing of R6G Molecules Using MoS2 Nanoflowers under Pressure. Langmuir 2024, 40, 21804–21813. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Wu, J.; Liu, X.; Su, Q.; Yin, X.; Huang, Z.; Lin, X.; Lin, W.; Yi, G. Au-Nanoparticle-Array/Aligned-Ag-Nanowire-Based Flexible Dual Plasmonic Substrate for Sensitive Surface-Enhanced Raman Scattering Detection. Part. Part. Syst. Charact. 2021, 38, 2100160. [Google Scholar] [CrossRef]
- Yang, B.; Chen, G.; Ghafoor, A.; Zhang, Y.F.; Zhang, X.B.; Li, H.; Dong, X.R.; Wang, R.P.; Zhang, Y.; Zhang, Y. Chemical Enhancement and Quenching in Single-Molecule Tip-Enhanced Raman Spectroscopy. Angew. Chem. Int. Ed. 2023, 62, e202218799. [Google Scholar] [CrossRef]
- Yang, T.-H.; Ahn, J.; Shi, S.; Qin, D. Understanding the role of poly (vinylpyrrolidone) in stabilizing and capping colloidal silver nanocrystals. ACS Nano 2021, 15, 14242–14252. [Google Scholar] [CrossRef]
- Badawy, A.M.E.; Luxton, T.P.; Silva, R.G.; Scheckel, K.G.; Suidan, M.T.; Tolaymat, T.M. Impact of environmental conditions (pH, ionic strength, and electrolyte type) on the surface charge and aggregation of silver nanoparticles suspensions. Environ. Sci. Technol. 2010, 44, 1260–1266. [Google Scholar] [CrossRef]
- Dimitriev, O.P.; Blank, D.A.; Ganser, C.; Teichert, C. Effect of the polymer chain arrangement on exciton and polaron dynamics in P3HT and P3HT: PCBM films. J. Phys. Chem. C 2018, 122, 17096–17109. [Google Scholar] [CrossRef]
- Rekha, C.; Sameera, S.; Nayar, V.; Gopchandran, K. Simultaneous detection of different probe molecules using silver nanowires as SERS substrates. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 213, 150–158. [Google Scholar] [CrossRef]
- Xu, T.; Wang, X.; Huang, Y.; Lai, K.; Fan, Y. Rapid detection of trace methylene blue and malachite green in four fish tissues by ultra-sensitive surface-enhanced Raman spectroscopy coated with gold nanorods. Food Control 2019, 106, 106720. [Google Scholar] [CrossRef]
- Fateixa, S.; Nogueira, H.I.; Trindade, T. Surface-enhanced Raman scattering spectral imaging for the attomolar range detection of crystal violet in contaminated water. ACS Omega 2018, 3, 4331–4341. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Z.; Liang, H.; Chen, Z.; Li, J.; Wu, J.; Li, X.; Xiao, D. A Composite Substrate of Ag Nanoparticle-Decorated Inverse Opal Polydimethylsiloxane for Surface Raman Fluorescence Dual Enhancement. Polymers 2025, 17, 1995. https://doi.org/10.3390/polym17141995
Tang Z, Liang H, Chen Z, Li J, Wu J, Li X, Xiao D. A Composite Substrate of Ag Nanoparticle-Decorated Inverse Opal Polydimethylsiloxane for Surface Raman Fluorescence Dual Enhancement. Polymers. 2025; 17(14):1995. https://doi.org/10.3390/polym17141995
Chicago/Turabian StyleTang, Zilun, Hongping Liang, Zhangyang Chen, Jianpeng Li, Jianyu Wu, Xianfeng Li, and Dingshu Xiao. 2025. "A Composite Substrate of Ag Nanoparticle-Decorated Inverse Opal Polydimethylsiloxane for Surface Raman Fluorescence Dual Enhancement" Polymers 17, no. 14: 1995. https://doi.org/10.3390/polym17141995
APA StyleTang, Z., Liang, H., Chen, Z., Li, J., Wu, J., Li, X., & Xiao, D. (2025). A Composite Substrate of Ag Nanoparticle-Decorated Inverse Opal Polydimethylsiloxane for Surface Raman Fluorescence Dual Enhancement. Polymers, 17(14), 1995. https://doi.org/10.3390/polym17141995







