Recent Research Progress on Polyurethane Solid–Solid Phase Change Materials
Abstract
1. Introduction
2. Preparation of PUSSPCMs
2.1. Overview of PUSSPCMs
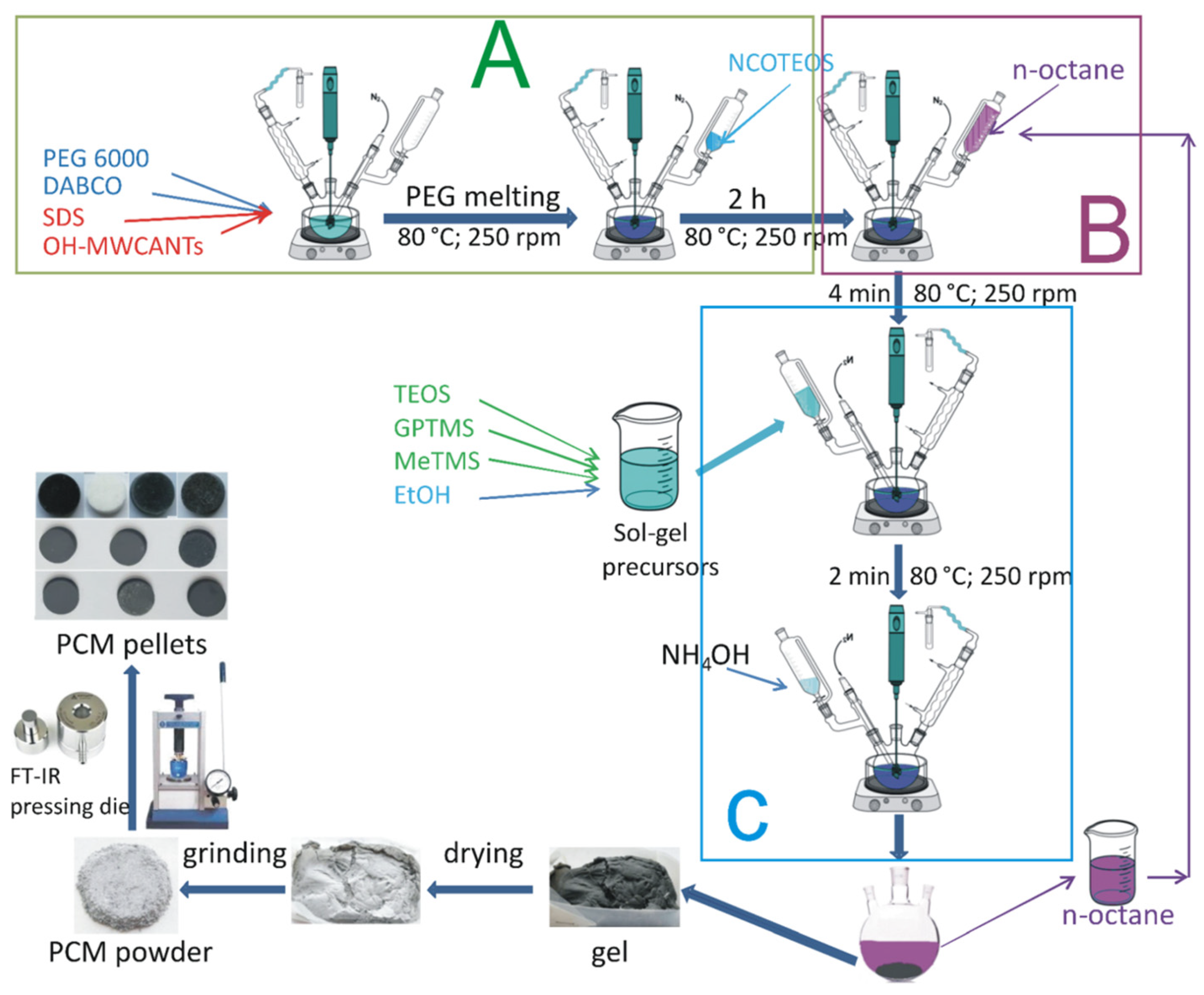
2.2. Materials
2.3. Preparation Methods
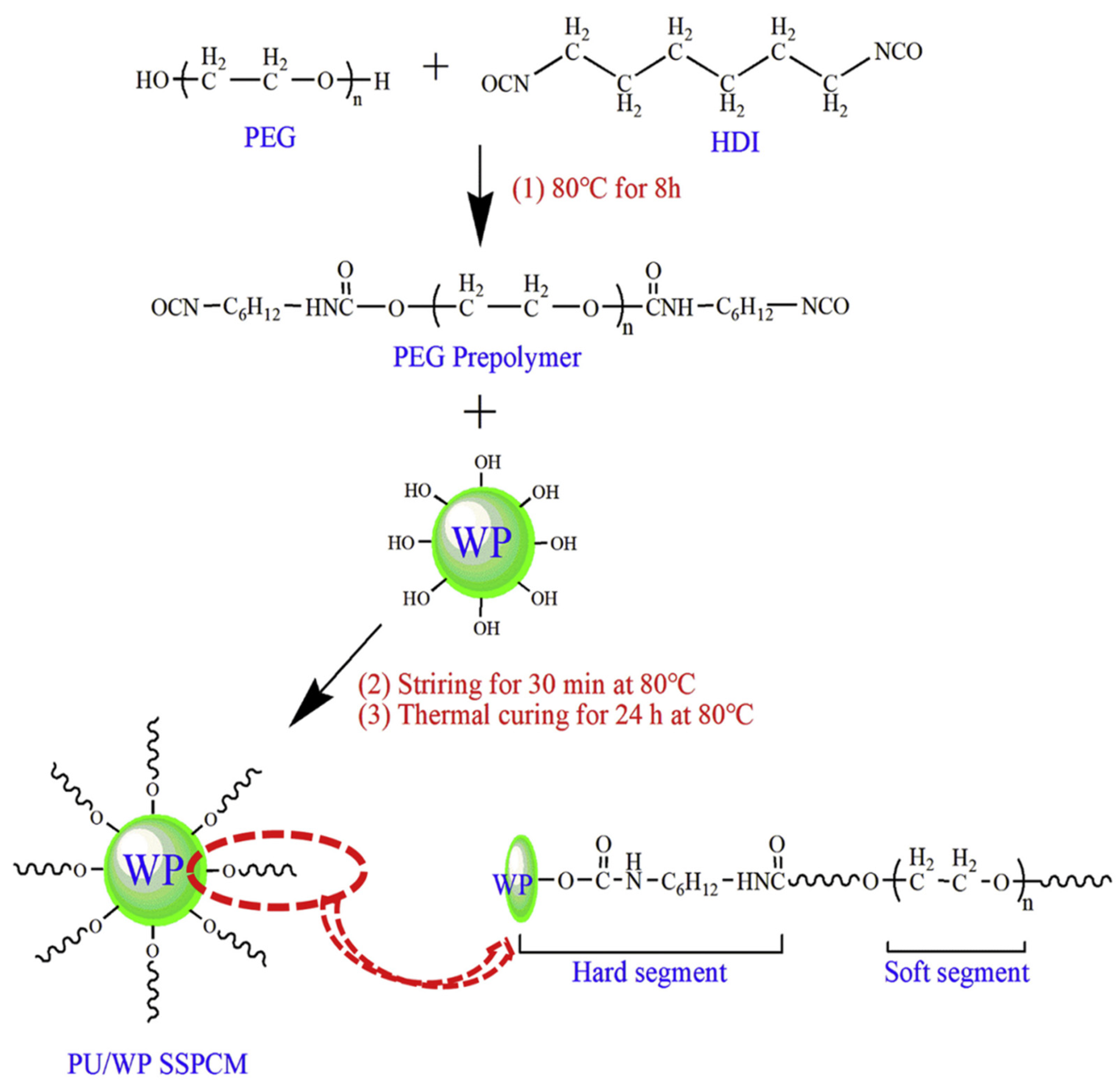
2.3.1. Two-Step Solution Polymerization Method
2.3.2. One-Step and Ontology Polymerization Methods
2.3.3. Evaluation of the Three Preparation Methods
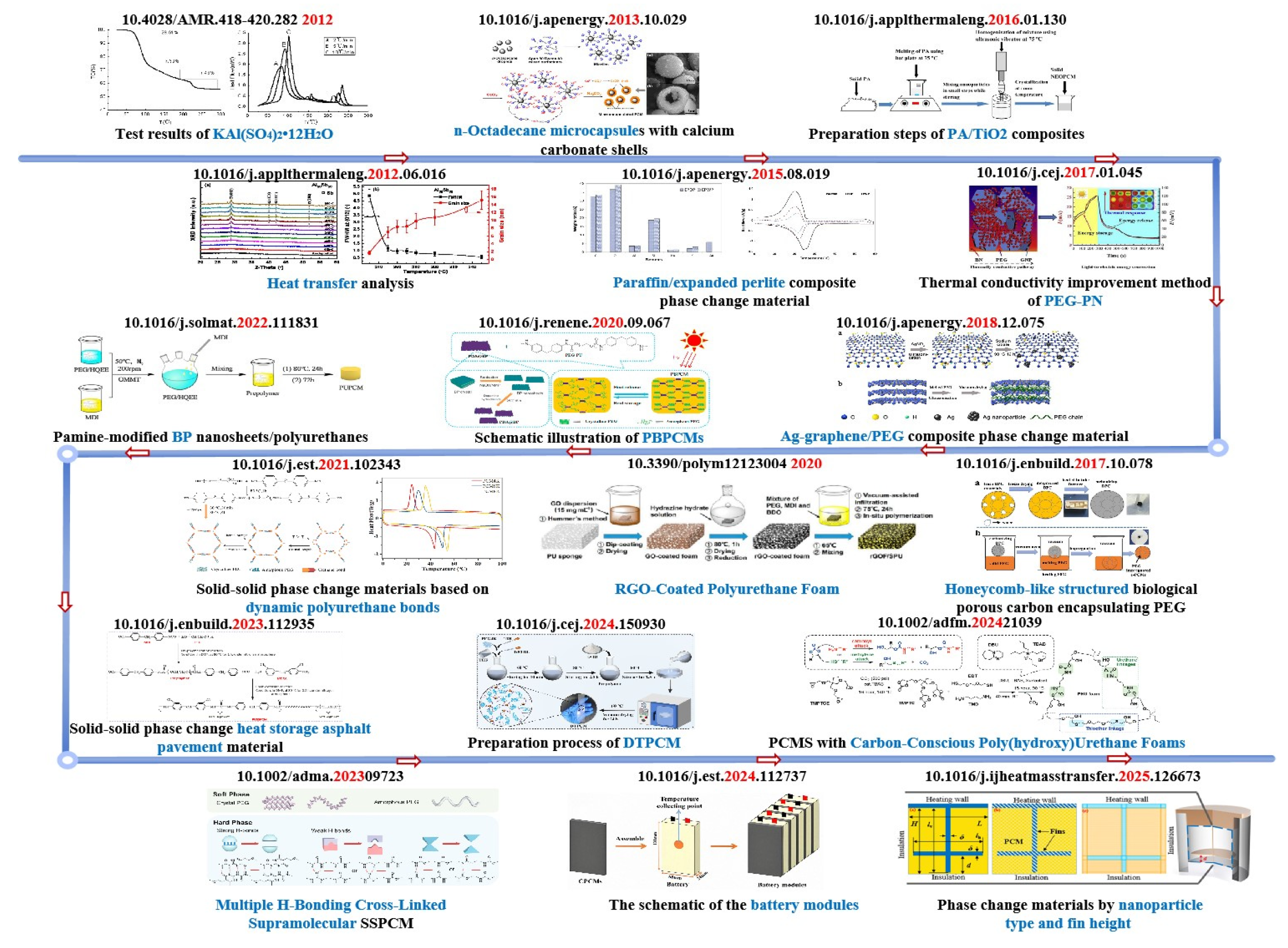
3. Properties of PUSSPCMs
3.1. Phase Change Temperature
3.2. Enthalpy of a Phase Transition
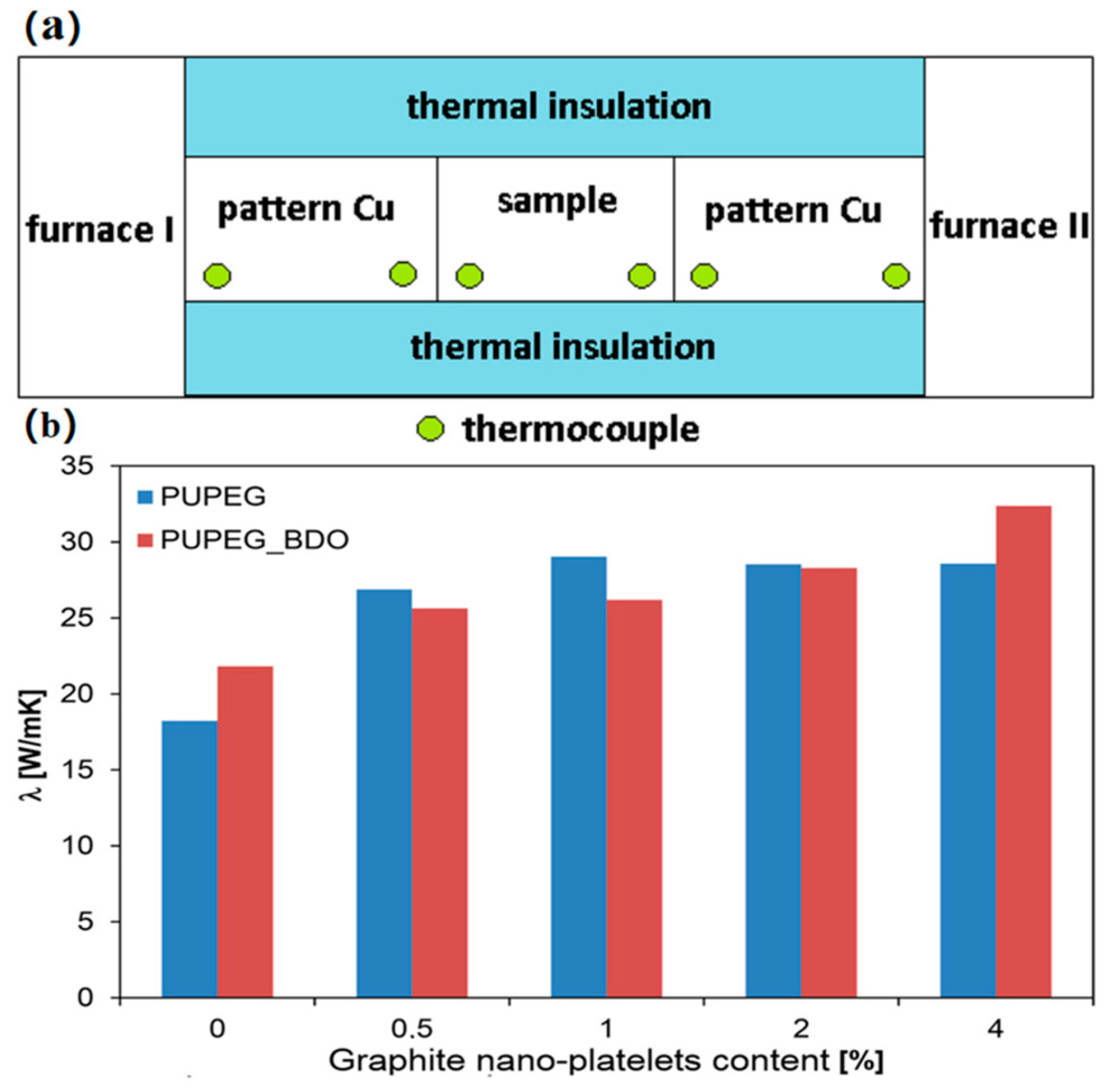
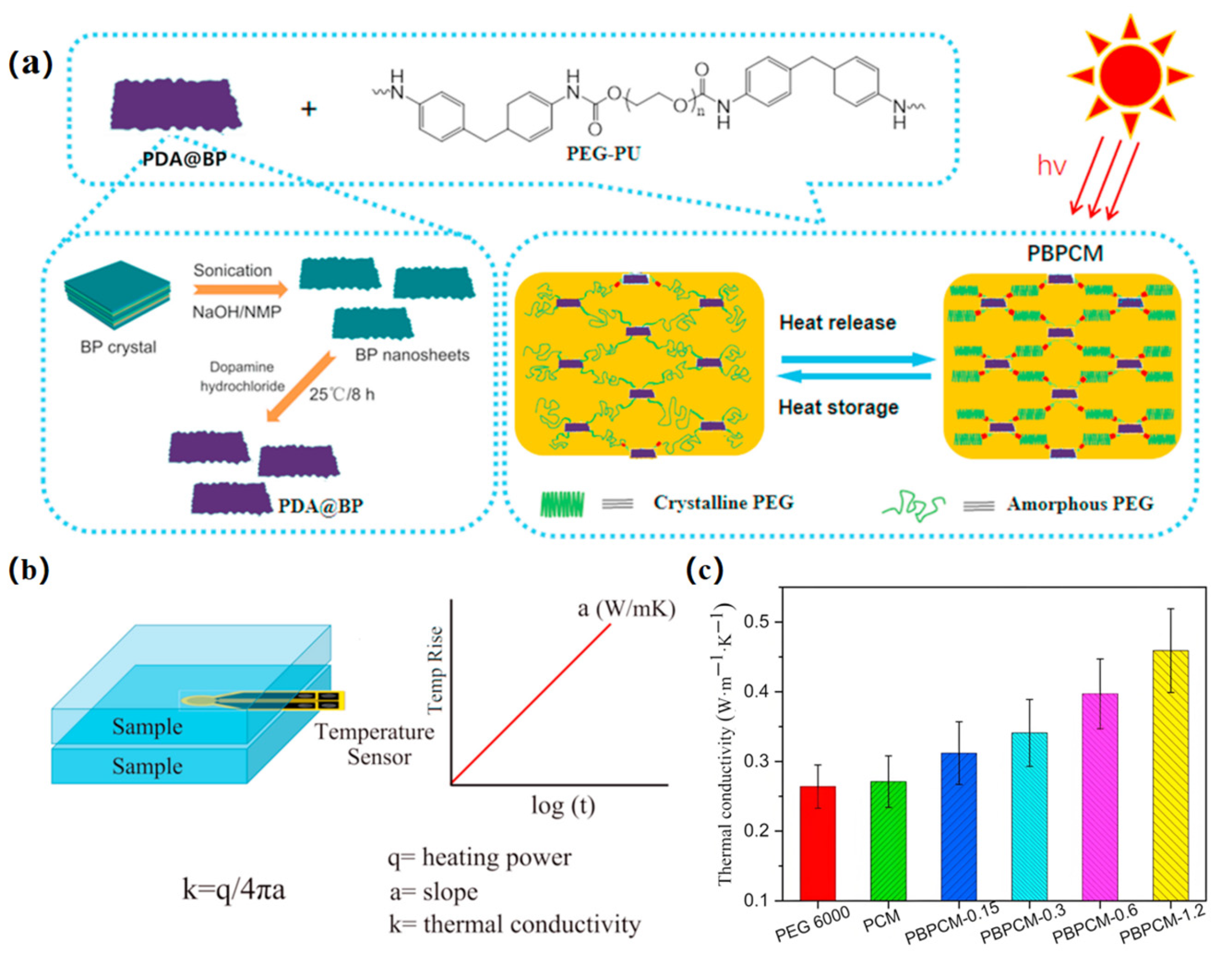
3.3. Thermal Conductivity
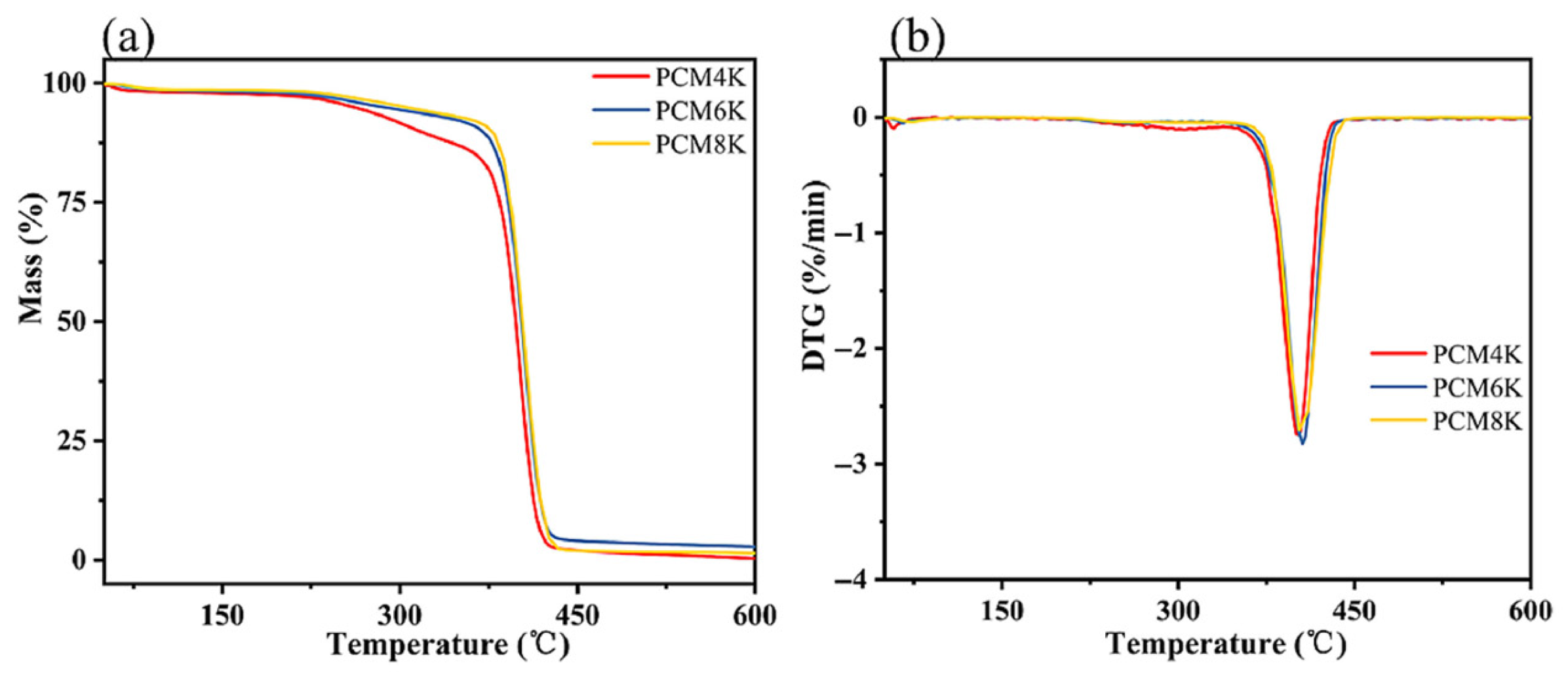
3.4. Thermal Stability
3.5. Phase Transition Cyclic Stability
4. Design Strategies for Polyurethane-Based Solid–Solid Phase Change Composites
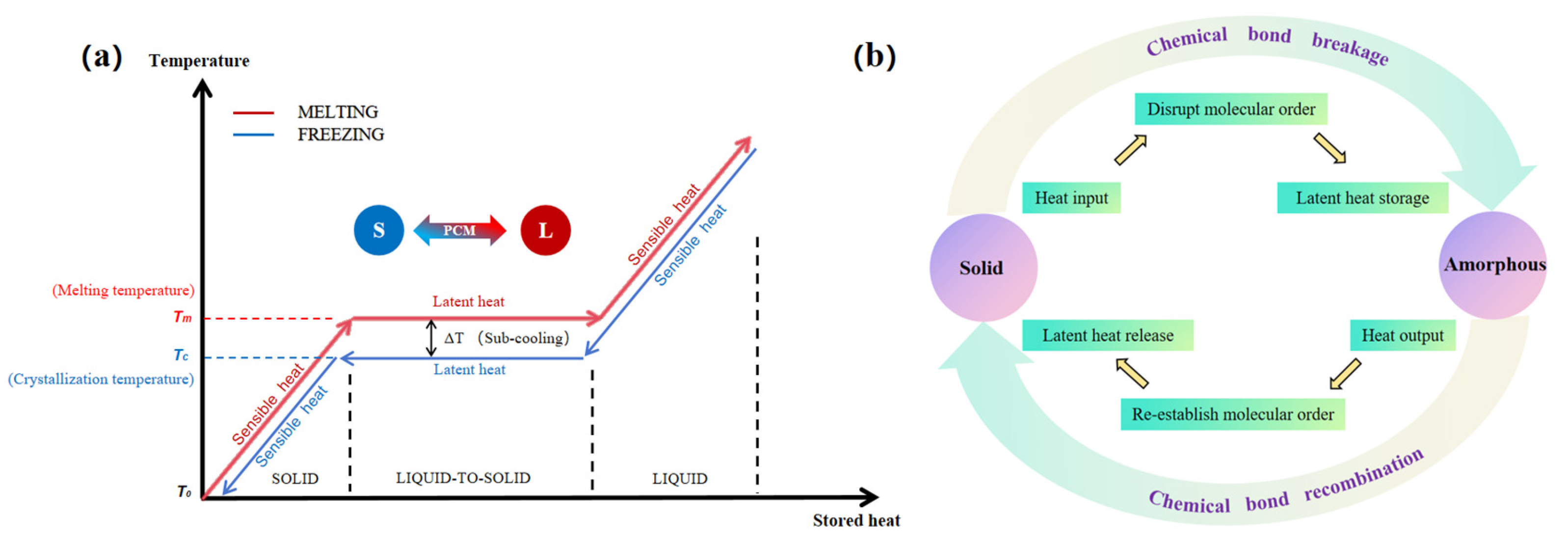
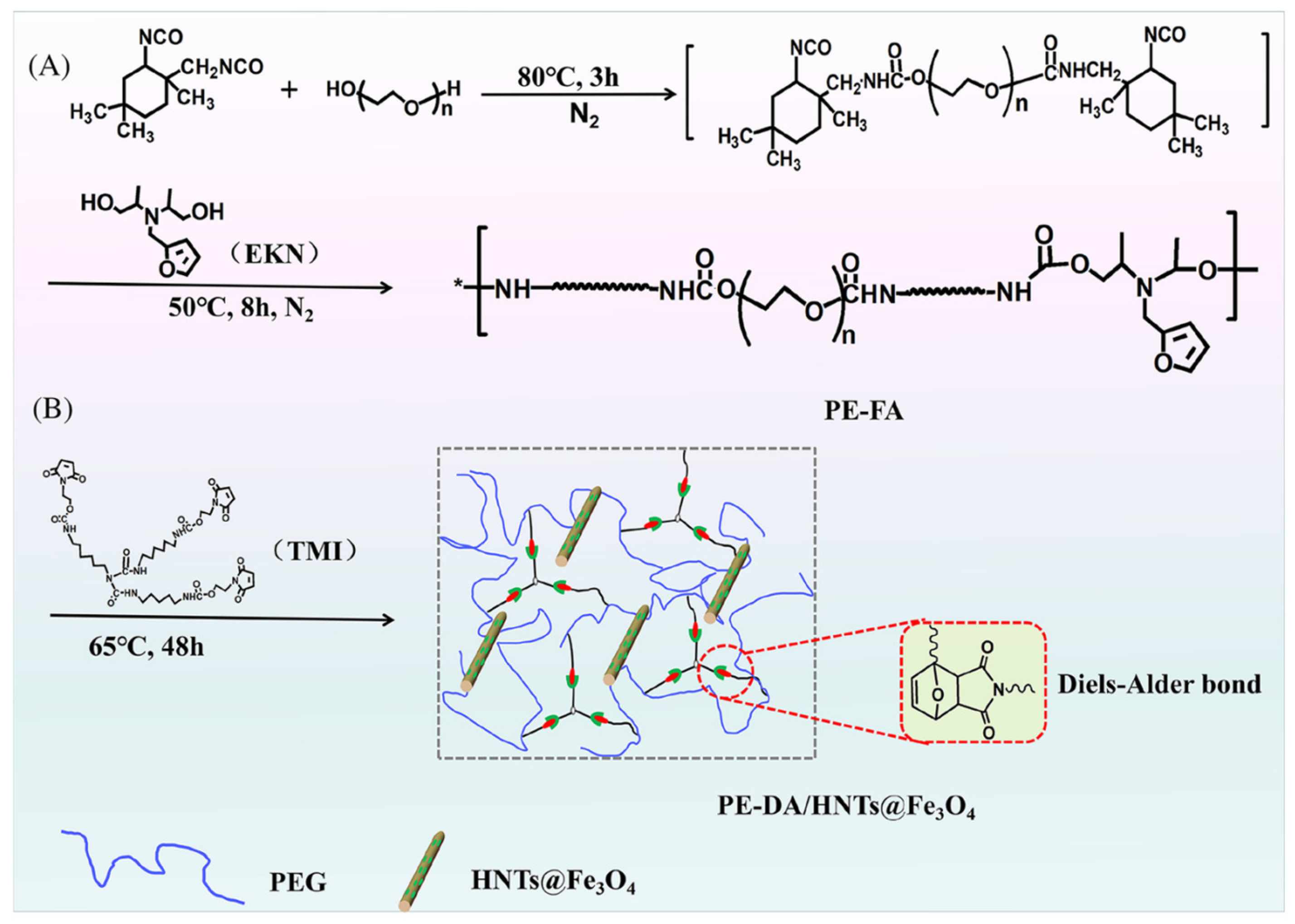
4.1. Introduction of Dynamic Covalent Bonds
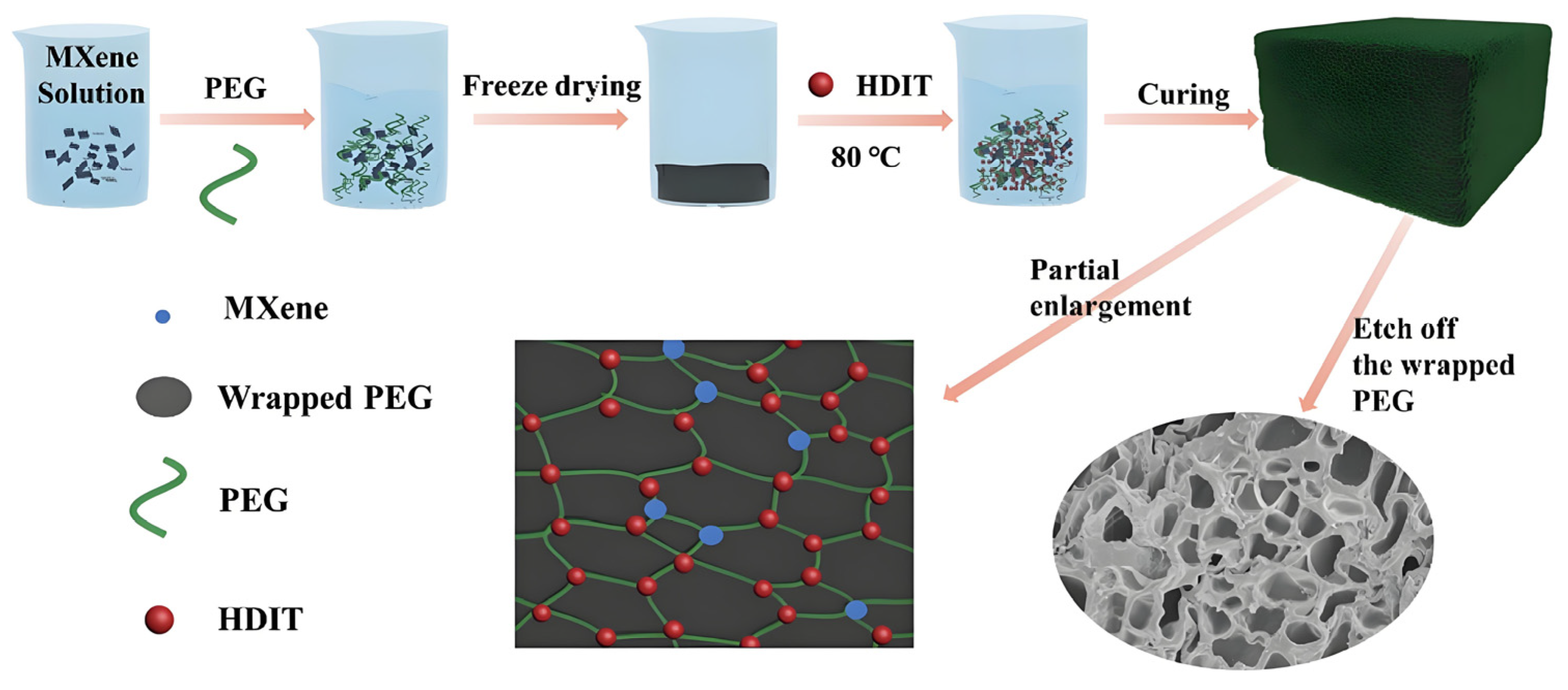
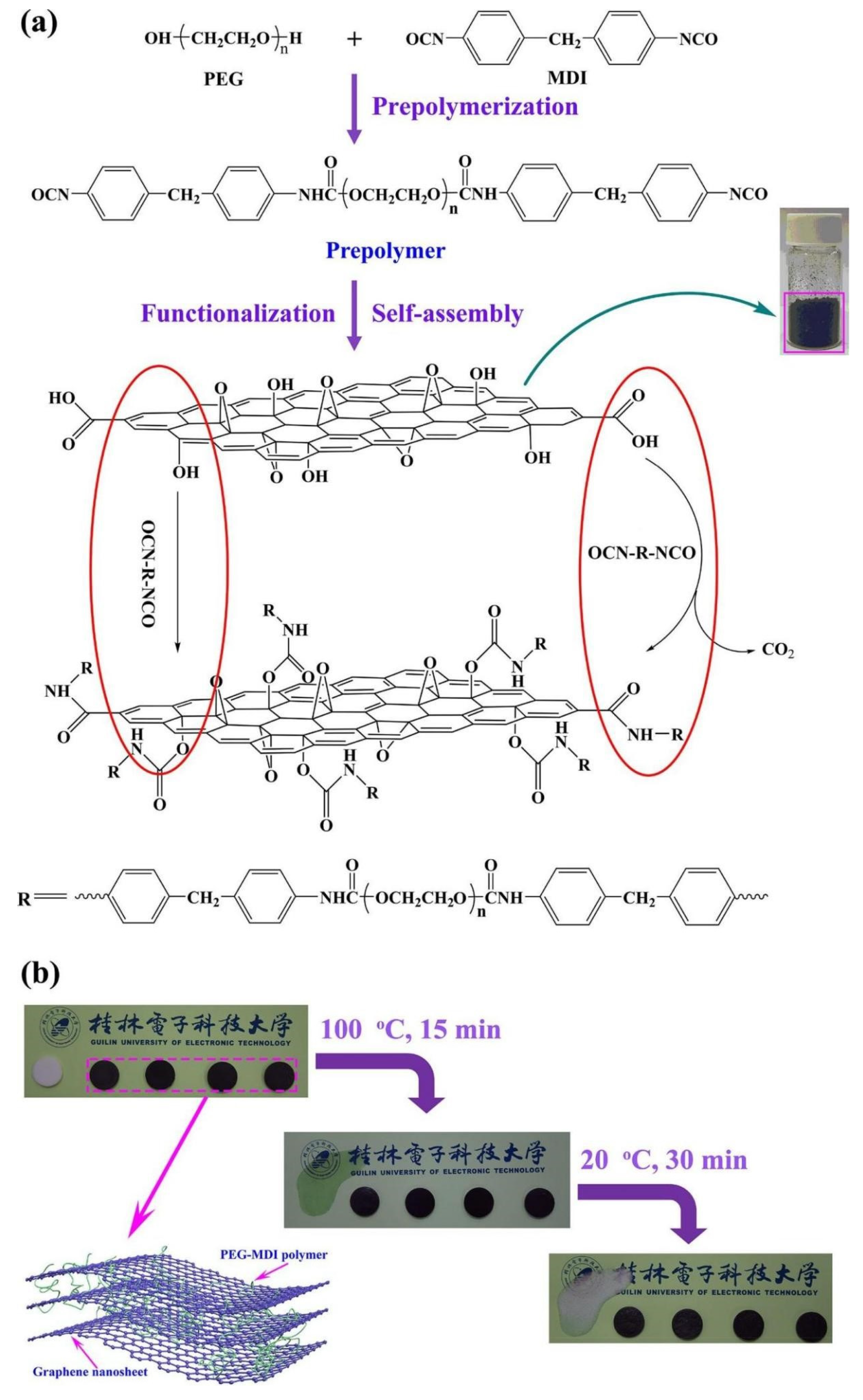
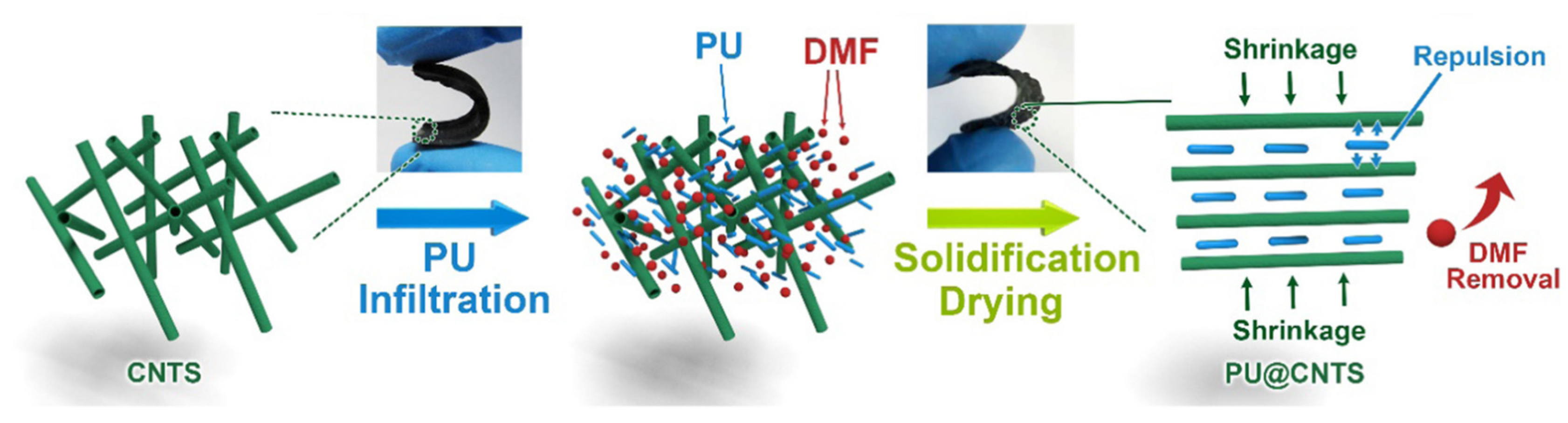
4.2. Introduction of Nanofillers
5. Applications of PUSSPCMs
5.1. Building Energy Efficiency
5.2. Electronics Thermal Management
5.3. Wearable Device Thermal Management
5.4. Solar Energy Storage
5.5. Other Areas
6. Green Development of PUSSPCMs
7. Conclusions and Recommendations
Funding
Conflicts of Interest
References
- Du, K.; Calautit, J.; Wang, Z.H.; Wu, Y.P.; Liu, H. A review of the applications of phase change materials in cooling, heating and power generation in different temperature ranges. Appl. Energy 2018, 220, 242–273. [Google Scholar] [CrossRef]
- Jiang, K.; Liao, G.L.; E, J.Q.; Zhang, F.; Chen, J.W.; Leng, E.W. Thermal management technology of power lithium-ion batteries based on the phase transition of materials: A review. J. Energy Storage 2020, 32, 101816. [Google Scholar] [CrossRef]
- Usman, A.; Xiong, F.; Aftab, W.; Qin, M.L.; Zou, R.Q. Emerging Solid-to-Solid Phase-Change Materials for Thermal-Energy Harvesting, Storage, and Utilization. Adv. Mater. 2022, 34, 2202457. [Google Scholar] [CrossRef]
- Mishra, R.K.; Verma, K.; Mishra, V.; Chaudhary, B. A review on carbon-based phase change materials for thermal energy storage. J. Energy Storage 2022, 50, 104166. [Google Scholar] [CrossRef]
- Tao, J.L.; Luan, J.D.; Liu, Y.; Qu, D.Y.; Yan, Z.; Ke, X. Technology development and application prospects of organic-based phase change materials: An overview. Renew. Sustain. Energy Rev. 2022, 159, 112175. [Google Scholar] [CrossRef]
- Zhang, S.; Feng, D.L.; Shi, L.; Wang, L.; Jin, Y.G.; Tian, L.M.; Li, Z.Y.; Wang, G.Y.; Zhao, L.; Yan, Y.Y. A review of phase change heat transfer in shape-stabilized phase change materials (ss-PCMs) based on porous supports for thermal energy storage. Renew. Sustain. Energy Rev. 2021, 135, 110127. [Google Scholar] [CrossRef]
- da Cunha, S.R.L.; de Aguiar, J.L.B. Phase change materials and energy efficiency of buildings: A review of knowledge. J. Energy Storage 2020, 27, 101083. [Google Scholar] [CrossRef]
- Reddy, V.J.; Ghazali, M.F.; Kumarasamy, S. Advancements in phase change materials for energy-efficient building construction: A comprehensive review. J. Energy Storage 2024, 81, 110494. [Google Scholar] [CrossRef]
- Li, Z.C.; Zhang, Y.; Zhang, S.F.; Tang, B.T. Phase change materials for lithium-ion battery thermal management systems: A review. J. Energy Storage 2024, 80, 110259. [Google Scholar] [CrossRef]
- Jia, M.; Sha, A.M.; Jiang, W.; Wang, W.T.; Yuan, D.D. Adhesion, rheology and temperature-adjusting performance of polyurethane-based solid-solid phase change asphalt mastics subjected to laboratory aging. Mater. Struct. 2023, 56, 57. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Zheng, S.L.; Zhu, S.Q.; Ma, J.N.; Sun, Z.M.; Farid, M. Evaluation of paraffin infiltrated in various porous silica matrices as shape-stabilized phase change materials for thermal energy storage. Energy Convers. Manag. 2018, 171, 361–370. [Google Scholar] [CrossRef]
- Lamrani, B.; Johannes, K.; Kuznik, F. Phase change materials integrated into building walls: An updated review. Renew. Sustain. Energy Rev. 2021, 140, 110751. [Google Scholar] [CrossRef]
- Pielichowska, K.; Pielichowski, K. Phase change materials for thermal energy storage. Prog. Mater. Sci. 2014, 65, 67–123. [Google Scholar] [CrossRef]
- Sundararajan, S.; Samui, A.B.; Kulkarni, P.S. Versatility of polyethylene glycol (PEG) in designing solid-solid phase change materials (PCMs) for thermal management and their application to innovative technologies. J. Mater. Chem. A 2017, 5, 18379–18396. [Google Scholar] [CrossRef]
- Li, S.W.; He, L.H.; Lu, H.L.; Hao, J.Z.; Wang, D.K.; Shen, F.R.; Song, C.; Liu, G.J.; Du, P.F.; Wang, Y.D.; et al. Ultrahigh-performance solid-solid phase change material for efficient, high-temperature thermal energy storage. Acta Mater. 2023, 249, 118852. [Google Scholar] [CrossRef]
- Xiao, Q.Q.; Xu, Y.; Li, X.Q.; Tang, H.D.; Li, H.; Zhang, L.H. Enhanced solar-thermal and electro-thermal storage performance of solid-solid composite phase change material. Compos. Commun. 2024, 45, 101818. [Google Scholar] [CrossRef]
- Wang, C.Y.; Geng, X.; Chen, J.; Wang, H.L.; Wei, Z.K.; Huang, B.X.; Liu, W.; Wu, X.D.; Hu, L.Y.; Su, G.H.; et al. Multiple H-Bonding Cross-Linked Supramolecular Solid-Solid Phase Change Materials for Thermal Energy Storage and Management. Adv. Mater. 2024, 36, 309723. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.S.; Huang, R.; Jiang, Z.N.; Li, Y.S.; He, F.F.; Zhang, Q.P.; Zhou, Y.L.; Wang, P.; He, G.S.; Yang, W.B. Recyclable Solid-Solid Phase Change Materials with Excellent Reprocessing Properties Based on Dynamic Disulfide Bonds. ACS Appl. Polym. Mater. 2023, 5, 1499–1508. [Google Scholar] [CrossRef]
- Wang, R.; Xiao, Y.; Lei, J.X. A solid-solid phase change material based on dynamic ion cross-linking with reprocessability at room temperature. Chem. Eng. J. 2020, 390, 124586. [Google Scholar] [CrossRef]
- Maghrabie, H.M.; Mohamed, A.S.A.; Fahmy, A.M.; Samee, A.A.A. Performance enhancement of PV panels using phase change material (PCM): An experimental implementation. Case Stud. Therm. Eng. 2023, 42, 102741. [Google Scholar] [CrossRef]
- Chai, Z.C.; Fang, M.H.; Min, X. Composite phase-change materials for photo-thermal conversion and energy storage:A review. Nano Energy 2024, 124, 109437. [Google Scholar] [CrossRef]
- Wang, H.C.; Guo, Y.H.; Ren, Y.; Yeboah, S.; Wang, J.; Long, F.; Zhang, Z.Y.; Jiang, R. Investigation of the thermal management potential of phase change material for lithium-ion battery. Appl. Therm. Eng. 2024, 236, 121590. [Google Scholar] [CrossRef]
- Javadi, F.S.; Metselaar, H.S.C.; Ganesan, P. Performance improvement of solar thermal systems integrated with phase change materials (PCM), a review. Sol. Energy 2020, 206, 330–352. [Google Scholar] [CrossRef]
- Singh, P.; Sharma, R.K.; Ansu, A.K.; Goyal, R.; Sari, A.; Tyagi, V.V. A comprehensive review on development of eutectic organic phase change materials and their composites for low and medium range thermal energy storage applications. Sol. Energy Mater. Sol. Cells 2021, 223, 110955. [Google Scholar] [CrossRef]
- Faraj, K.; Khaled, M.; Faraj, J.; Hachem, F.; Castelain, C. Phase change material thermal energy storage systems for cooling applications in buildings: A review. Renew. Sustain. Energy Rev. 2020, 119, 109579. [Google Scholar] [CrossRef]
- Liu, Y.S.; Yang, Y.Z. Preparation and thermal properties of Na2CO3·10H2O-Na2HPO4·12H2O eutectic hydrate salt as a novel phase change material for energy storage. Appl. Therm. Eng. 2017, 112, 606–609. [Google Scholar] [CrossRef]
- Huang, H.Y.; Li, H.F.; Li, Y.T.; Xia, Y.P.; Chu, H.L.; Zou, Y.J.; Xu, E.Y.; Zhang, H.Z.; Xu, F.; Sun, L.X. Three-dimensional hierarchical porous carbon enhanced thermal properties of polyurethane-based phase change materials for energy conversion and storage. J. Energy Storage 2024, 103, 114367. [Google Scholar] [CrossRef]
- Yin, Q.; Zhu, Z.G.; Li, W.; Guo, M.L.; Wang, Y.; Wang, J.P.; Zhang, X.X. Fabrication and Performance of Composite Microencapsulated Phase Change Materials with Palmitic Acid Ethyl Ester as Core. Polymers 2018, 10, 726. [Google Scholar] [CrossRef]
- Zhang, C.; Shi, Z.; Li, A.; Zhang, Y.F. RGO-Coated Polyurethane Foam/Segmented Polyurethane Composites as Solid-Solid Phase Change Thermal Interface Material. Polymers 2020, 12, 3004. [Google Scholar] [CrossRef]
- Lin, W.; Liang, C.C.; Zeng, J.L.; Cheng, J.J.; Wang, Y.P.; Zhang, F.; Li, S.X. Preparation and characterization of double-shell phase change material microcapsules with flame retardancy and temperature regulation capability as low-emission building fillers. J. Build. Eng. 2024, 89, 109257. [Google Scholar] [CrossRef]
- Wang, K.; Liu, C.; Xie, W.X.; Ke, Y.H.; You, X.Y.; Jing, B.H.; Shi, Y.Q. Effects of Ammonium Polyphosphate and Organic Modified Montmorillonite on Flame Retardancy of Polyethylene Glycol/Wood-Flour-Based Phase Change Composites. Molecules 2023, 28, 3464. [Google Scholar] [CrossRef]
- Dai, J.F.; Ling, R.Q.; Wang, K.Z. Thermal Performance and Dehydration Kinetics of KAl(SO4)2·12H2O as Phase Change Material. Mater. Process. Technol. 2012, 418–420, 282–285. [Google Scholar]
- Zhao, W.H.; Neti, S.; Oztekin, A. Heat transfer analysis of encapsulated phase change materials. Appl. Therm. Eng. 2013, 50, 143–151. [Google Scholar] [CrossRef]
- Yu, S.Y.; Wang, X.D.; Wu, D.Z. Microencapsulation of n-octadecane phase change material with calcium carbonate shell for enhancement of thermal conductivity and serving durability: Synthesis, microstructure, and performance evaluation. Appl. Energy 2014, 114, 632–643. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Sanjayan, J.; Wang, X.M.; Alam, M.; Wilson, J. A novel paraffin/expanded perlite composite phase change material for prevention of PCM leakage in cementitious composites. Appl. Energy 2015, 157, 85–94. [Google Scholar] [CrossRef]
- Sharma, R.K.; Ganesan, P.; Tyagi, V.V.; Metselaar, H.S.C.; Sandaran, S.C. Thermal properties and heat storage analysis of palmitic acid-TiO2 composite as nano-enhanced organic phase change material (NEOPCM). Appl. Therm. Eng. 2016, 99, 1254–1262. [Google Scholar] [CrossRef]
- Yang, J.; Tang, L.S.; Bao, R.Y.; Bai, L.; Liu, Z.Y.; Yang, W.; Xie, B.H.; Yang, M.B. Largely enhanced thermal conductivity of poly (ethylene glycol)/boron nitride composite phase change materials for solar-thermal-electric energy conversion and storage with very low content of graphene nanoplatelets. Chem. Eng. J. 2017, 315, 481–490. [Google Scholar] [CrossRef]
- Zhao, Y.J.; Min, X.; Huang, Z.H.; Liu, Y.G.; Wu, X.W.; Fang, M.H. Honeycomb-like structured biological porous carbon encapsulating PEG: A shape-stable phase change material with enhanced thermal conductivity for thermal energy storage. Energy Build. 2018, 158, 1049–1062. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.S.; Qiu, J.J.; Jin, X.; Umair, M.M.; Lu, R.W.; Zhang, S.F.; Tang, B.T. Ag-graphene/PEG composite phase change materials for enhancing solar-thermal energy conversion and storage capacity. Appl. Energy 2019, 237, 83–90. [Google Scholar] [CrossRef]
- Du, X.S.; Qiu, J.H.; Deng, S.; Du, Z.L.; Cheng, X.; Wang, H.B. Flame-retardant and solid-solid phase change composites based on dopamine-decorated BP nanosheets/Polyurethane for efficient solar-to-thermal energy storage. Renew. Energy 2021, 164, 1–10. [Google Scholar] [CrossRef]
- Huang, L.; Yang, Y.Y.; Yuan, D.D.; Cai, X.F. Solid-solid phase-change materials with excellent mechanical property and solid state plasticity based on dynamic urethane bonds for Thermal Energy Storage. J. Energy Storage 2021, 36, 102343. [Google Scholar] [CrossRef]
- Gao, N.; Tang, T.; Xiang, H.X.; Zhang, W.L.; Li, Y.B.; Yang, C.L.; Xia, T.; Liu, X.L. Preparation and structure-properties of crosslinking organic montmorillonite/polyurethane as solid-solid phase change materials for thermal energy storage. Sol. Energy Mater. Sol. Cells 2022, 244, 111831. [Google Scholar] [CrossRef]
- Jia, M.; Sha, A.M.; Jiang, W.; Li, X.Z.; Jiao, W.X. Developing a solid-solid phase change heat storage asphalt pavement material and its application as functional filler for cooling asphalt pavement. Energy Build. 2023, 285, 112935. [Google Scholar] [CrossRef]
- Xu, W.H.; Yang, W.S.; Su, J.T.; Huang, J.T.; Min, Y.G.; Yu, Y.S.; Zeng, Y.Y.; Chen, P.H.; Wang, Y.Z.; Li, X.X. Diatom-based biomass composites phase change materials with high thermal conductivity for battery thermal management. J. Energy Storage 2024, 96, 112737. [Google Scholar] [CrossRef]
- Zhu, G.Y.; Zou, M.M.; Luo, W.X.; Huang, Y.F.; Chen, W.J.; Hu, X.W.; Jiang, X.X.; Li, Q.L. A polyurethane solid-solid phase change material for flexible use in thermal management. Chem. Eng. J. 2024, 488, 150930. [Google Scholar] [CrossRef]
- Lee, M.; Dahlhauser, S.D.; Lucci, C.; Donohoe, B.S.; Allen, R.D.; Rorrer, N.A. Shape-Stabilization of Phase Change Materials with Carbon-Conscious Poly(hydroxy)Urethane Foams. Adv. Funct. Mater. 2025, 35, 2421039. [Google Scholar] [CrossRef]
- Xu, X.Y.; Peng, B.L.; Qiu, M.Z.T.; Li, F.X.; Su, F.M. Charging characteristic optimization of phase change materials by nanoparticle type and fin height. Int. J. Heat Mass Transf. 2025, 240, 126673. [Google Scholar] [CrossRef]
- Du, X.S.; Wang, H.B.; Du, Z.L.; Cheng, X. Synthesis and thermal properties of novel solid-solid phase change materials with comb-polyurethane block copolymer structure for thermal energy storage. Thermochim. Acta 2017, 651, 58–64. [Google Scholar] [CrossRef]
- Chen, C.Z.; Chen, J.; Jia, Y.F.; Topham, P.D.; Wang, L.G. Binary shape-stabilized phase change materials based on poly(ethylene glycol)/polyurethane composite with dual-phase transition. J. Mater. Sci. 2018, 53, 16539–16556. [Google Scholar] [CrossRef]
- Fan, X.; Pu, Z.; Zhu, M.; Jiang, Z.L.; Xu, J.L. Solvent-free synthesis of PEG modified polyurethane solid-solid phase change materials with different Mw for thermal energy storage. Colloid Polym. Sci. 2021, 299, 835–843. [Google Scholar] [CrossRef]
- Cao, H.W.; Qi, F.X.Y.; Liu, R.W.; Wang, F.T.; Zhang, C.X.; Zhang, X.N.; Chai, Y.Y.; Zhai, L.L. The influence of hydrogen bonding on N-methyldiethanolamine-extended polyurethane solid-solid phase change materials for energy storage. RSC Adv. 2017, 7, 11244–11252. [Google Scholar] [CrossRef]
- Huang, R.; Yang, A.S.; Ma, R.; Yang, H.X.; Tang, X.H.; Zhao, F.; He, R.; Zhang, K.; Yang, W.B. Polyurethane solid-solid phase change materials based on triple dynamic bonds with excellent mechanical and self-healing properties for sustainable thermal energy storage. J. Energy Storage 2025, 111, 115447. [Google Scholar] [CrossRef]
- Arshad, A.; Jabbal, M.; Yan, Y.Y.; Darkwa, J. The micro-/nano-PCMs for thermal energy storage systems: A state of art review. Int. J. Energy Res. 2019, 43, 5572–5620. [Google Scholar] [CrossRef]
- Harlé, T.; Nguyen, G.T.M.; Ledesert, B.; Mélinge, Y.; Hebert, R.L. Cross-linked polyurethane as solid-solid phase change material for low temperature thermal energy storage. Thermochim. Acta 2020, 685, 178191. [Google Scholar] [CrossRef]
- Fu, X.W.; Kong, W.B.; Zhang, Y.Y.; Jiang, L.; Wang, J.L.; Lei, J.X. Novel solid-solid phase change materials with biodegradable trihydroxy surfactants for thermal energy storage. RSC Adv. 2015, 5, 68881–68889. [Google Scholar] [CrossRef]
- Du, X.S.; Wang, H.B.; Wu, Y.; Du, Z.L.; Cheng, X. Solid-solid phase-change materials based on hyperbranched polyurethane for thermal energy storage. J. Appl. Polym. Sci. 2017, 134, 45014. [Google Scholar] [CrossRef]
- Fu, X.W.; Lei, Y.; Xiao, Y.; Wang, J.L.; Zhou, S.Y.; Lei, J.X. Graft poly(ethylene glycol)-based thermosetting phase change materials networks with ultrahigh encapsulation fraction and latent heat efficiency. Renew. Energy 2021, 179, 1076–1084. [Google Scholar] [CrossRef]
- Yang, Y.Y.; Cai, X.F.; Kong, W.B. A novel intrinsic photothermal and flexible solid-solid phase change materials with super mechanical toughness and multi-recyclability. Appl. Energy 2023, 332, 120564. [Google Scholar] [CrossRef]
- Lin, C.H.; Ge, H.; Ying, P.Y.; Wang, T.L.; Huang, M.; Zhang, P.; Yang, T.; Wu, J.B.; Levchenko, V. Synthesis and Properties of Dynamic Crosslinking Polyurethane/PEG Shape-Stable Phase Change Materials Based on the Diels-Alder Reaction. ACS Appl. Polym. Mater. 2023, 5, 4190–4198. [Google Scholar] [CrossRef]
- Wang, J.W.; Wu, Z.H.; Xie, H.Q.; Wang, T.T.; Wang, Y.Y.; Huang, Y.M.; Dong, L. Graphene oxide/polyurethane-based composite solid-solid phase change materials with enhanced energy storage capacity and photothermal performance. Int. J. Energy Res. 2022, 46, 22744–22756. [Google Scholar] [CrossRef]
- Zhang, T.T.; Zhang, C.C.; Xu, B.; Ren, W.J.; Chen, Z.Q. Investigation of Polyurethane-Based Halloysite Nanotube as Solid-Solid Phase Change Material for Thermal Energy Storage. Heat Transf. Res. 2022, 53, 61–73. [Google Scholar] [CrossRef]
- Wang, S.S.; Gu, X.B.; Kar, T.; Sari, A.; Çakir, E.; Varol, T.; Akçay, S.B.; Hekimoglu, G.; Gencel, O.; Tyagi, V.V. Improved form stability, thermal storage capacity and thermal conductivity of polyurethane based waste sponge carbon/expanded graphite/organic phase change materials coated by Ag nanoparticles. Sustain. Mater. Technol. 2025, 43, e01245. [Google Scholar] [CrossRef]
- Du, X.S.; Jin, L.Z.; Deng, S.; Zhou, M.; Du, Z.L.; Cheng, X.; Wang, H.B. Recyclable, Self-Healing, and Flame-Retardant Solid-Solid Phase Change Materials Based on Thermally Reversible Cross-Links for Sustainable Thermal Energy Storage. ACS Appl. Mater. Interfaces 2021, 13, 42991–43001. [Google Scholar] [CrossRef]
- Zhao, P.P.; Lu, P.; Zhao, Z.Y.; Chen, S.W.; Li, X.Y.; Deng, C.; Wang, Y.Z. Aromatic Schiff Base-Based polymeric phase change materials for Safe, Leak-Free, and efficient thermal energy management. Chem. Eng. J. 2022, 437, 135461. [Google Scholar] [CrossRef]
- Gong, S.; Ding, Y.; Li, X.L.; Liu, S.; Wu, H.; Lu, X.; Qu, J.P. Novel flexible polyurethane/MXene composites with sensitive solar thermal energy storage behavior. Compos. Part A Appl. Sci. Manuf. 2021, 149, 106505. [Google Scholar] [CrossRef]
- Sundararajan, S.; Samui, A.B.; Kulkarni, P.S. Thermal Energy Storage Using Poly(ethylene glycol) Incorporated Hyperbranched Polyurethane as Solid-Solid Phase Change Material. Ind. Eng. Chem. Res. 2017, 56, 14401–14409. [Google Scholar] [CrossRef]
- Lu, X.; Huang, J.T.; Wong, W.Y.; Qu, J.P. A novel bio-based polyurethane/wood powder composite as shape-stable phase change material with high relative enthalpy efficiency for solar thermal energy storage. Sol. Energy Mater. Sol. Cells 2019, 200, 109987. [Google Scholar] [CrossRef]
- Wei, K.; Wang, X.Q.; Ma, B.; Shi, W.S.; Duan, S.Y.; Liu, F.S. Study on rheological properties and phase-change temperature control of asphalt modified by polyurethane solid-solid phase change material. Sol. Energy 2019, 194, 893–902. [Google Scholar] [CrossRef]
- Shi, W.S.; Wei, K.; Fan, Y.Z.; Wang, X.Q.; Shi, J.H.; Wang, S.F.; Cheng, P. A flexible method for changing the transition temperature of polyurethane solid-solid phase change materials. Polym. Bull. 2023, 80, 1873–1891. [Google Scholar] [CrossRef]
- Huang, X.L.; Guo, J.; Gong, X.Y.; Li, S.L.; Gong, Y.M.; An, Q.D.; Zhang, S.; Guan, F.C. Preparation and characterization of pentaerythritol/butane tetracarboxylic acid/polyethylene glycol crosslinking copolymers as solid-solid phase change materials. J. Macromol. Sci. Part A Pure Appl. Chem. 2016, 53, 500–506. [Google Scholar] [CrossRef]
- Oktay, B.; Kayaman-Apohan, N. Biodegradable Polyurethane Solid-Solid Phase Change Materials. Chemistryselect 2021, 6, 6280–6285. [Google Scholar] [CrossRef]
- Zhou, J.H.; Liu, G.; Niu, Z.L.; Li, X.Y.; Zhao, J.J.; Li, X. Hyperbranched Waterborne Polyurethane Solid-Solid Phase Change Material for Thermal Energy Storage in Thermal Management Fabric. Fibers Polym. 2023, 24, 413–422. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, X.D.; Sheng, D.K.; Ji, F.; Dong, L.; Xu, S.B.; Wu, H.H.; Yang, Y.M. Graphene size-dependent phase change behaviors of in situ reduced grapehene oxide/polyurethane-based solid-solid phase change composites. Chem. Phys. Lett. 2018, 709, 52–59. [Google Scholar] [CrossRef]
- Pielichowska, K.; Bieda, J.; Szatkowski, P. Polyurethane/graphite nano-platelet composites for thermal energy storage. Renew. Energy 2016, 91, 456–465. [Google Scholar] [CrossRef]
- Kong, W.B.; Fu, X.W.; Liu, Z.M.; Zhou, C.L.; Lei, J.X. A facile synthesis of solid-solid phase change material for thermal energy storage. Appl. Therm. Eng. 2017, 117, 622–628. [Google Scholar] [CrossRef]
- Jiao, W.X.; Zhang, J.X.; Zhang, Z.Y.; Sha, A.M.; Jia, M. Development of PTMEG/MDI-based solid-solid phase change materials for asphalt pavements deicing. Constr. Build. Mater. 2025, 467, 140301. [Google Scholar] [CrossRef]
- Chen, M.Y.; Gong, Y.; Zhao, L.Y.; Chen, Y. Phase change material with outstanding thermal stability and mechanical strength for battery thermal management. J. Energy Storage 2024, 104, 114565. [Google Scholar] [CrossRef]
- Lin, C.H.; Ying, P.Y.; Huang, M.; Zhang, P.; Yang, T.; Liu, G.; Wu, J.B.; Levchenko, V. Processable and recyclable polyurethane/HNTs@Fe3O4 solid-solid phase change materials with excellent thermal conductivity for thermal energy storage. Polym. Compos. 2021, 42, 6816–6826. [Google Scholar] [CrossRef]
- Wang, X.Q.; Ma, B.; Wei, K.; Si, W.; Kang, X.X.; Fang, Y.F.; Zhang, H.F.; Shi, J.X.; Zhou, X.Y. Thermal storage properties of polyurethane solid-solid phase-change material with low phase-change temperature and its effects on performance of asphalt binders. J. Energy Storage 2022, 55, 105686. [Google Scholar] [CrossRef]
- Du, X.S.; Wang, H.B.; Cheng, X.; Du, Z.L. Synthesis and thermal energy storage properties of a solid-solid phase change material with a novel comb-polyurethane block copolymer structure. RSC Adv. 2016, 6, 42643–42648. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, S.H. Synthesis and characterization of biopolyurethane crosslinked with castor oil-based hyperbranched polyols as polymeric solid-solid phase change materials. Sci. Rep. 2022, 12, 14646. [Google Scholar] [CrossRef]
- Chen, R.J.; Yao, R.M.; Xia, W.; Zou, R.Q. Electro/photo to heat conversion system based on polyurethane embedded graphite foam. Appl. Energy 2015, 152, 183–188. [Google Scholar] [CrossRef]
- Liu, Z.M.; Wu, B.; Fu, X.W.; Yan, P.Y.; Yuan, Y.; Zhou, C.L.; Lei, J.X. Two components based polyethylene glycol/thermosetting solid-solid phase change material composites as novel form stable phase change materials for flexible thermal energy storage application. Sol. Energy Mater. Sol. Cells 2017, 170, 197–204. [Google Scholar] [CrossRef]
- Fang, S.M.; Li, C.; Gao, L.J.; Sun, Q.W.; Cui, J.; Zhou, L.M.; Yang, C.X.; Yu, H.F. Nanotemplate Fabrication of PEG-Containing Thermosetting Polyurethane Nanoarray toward Enhanced Thermal Storage. ACS Appl. Polym. Mater. 2019, 1, 2924–2932. [Google Scholar] [CrossRef]
- Wu, W.H.; Huang, X.Y.; Li, K.; Yao, R.M.; Chen, R.J.; Zou, R.Q. A functional form-stable phase change composite with high efficiency electro-to-thermal energy conversion. Appl. Energy 2017, 190, 474–480. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, X.J.; Liu, X.D.; Sheng, D.K.; Ji, F.C.; Dong, L.; Xu, S.B.; Wu, H.H.; Yang, Y.M. Polyurethane-based solid-solid phase change materials with halloysite nanotubes-hybrid graphene aerogels for efficient light- and electro-thermal conversion and storage. Carbon 2019, 142, 558–566. [Google Scholar] [CrossRef]
- Yuan, Y.; Wu, B.; Jiang, L.; Liu, Z.M.; Wang, Y.; Fu, X.W.; Kong, W.B.; Lei, J.X. Nonconstant enthalpy of thermosetting solid-solid phase change materials controlled by light. Energy Build. 2020, 214, 109894. [Google Scholar] [CrossRef]
- Yang, S.W.; Du, X.S.; Deng, S.; Qiu, J.H.; Du, Z.L.; Cheng, X.; Wang, H.B. Recyclable and self-healing polyurethane composites based on Diels-Alder reaction for efficient solar-to-thermal energy storage. Chem. Eng. J. 2020, 398, 125654. [Google Scholar] [CrossRef]
- Lu, X.; Fang, C.; Sheng, X.X.; Zhang, L.; Qu, J.P. One-Step and Solvent-Free Synthesis of Polyethylene Glycol-Based Polyurethane As Solid-Solid Phase Change Materials for Solar Thermal Energy Storage. Ind. Eng. Chem. Res. 2019, 58, 3024–3032. [Google Scholar] [CrossRef]
- Chen, K.P.; Yu, X.J.; Tian, C.R.; Wang, J.H. Preparation and characterization of form-stable paraffin/polyurethane composites as phase change materials for thermal energy storage. Energy Convers. Manag. 2014, 77, 13–21. [Google Scholar] [CrossRef]
- Ke, H.Z. Morphology and thermal performance of quaternary fatty acid eutectics/polyurethane/Ag form-stable phase change composite fibrous membranes. J. Therm. Anal. Calorim. 2017, 129, 1533–1545. [Google Scholar] [CrossRef]
- Wei, K.; Ma, B.; Duan, S.Y. Preparation and Properties of Bitumen-Modified Polyurethane Solid-Solid Phase Change Materials. J. Mater. Civ. Eng. 2019, 31, 04019139. [Google Scholar] [CrossRef]
- Chang, J.M.; Lu, H.Y.; Xiao, Y.L.; Wu, S.; Xiao, Y.; Wang, Y.C. A solid-solid phase change material with wide service temperature range based on polyethylene glycols with different molecular weights. J. Appl. Polym. Sci. 2024, 141, e54950. [Google Scholar] [CrossRef]
- Bidiyasar, R.; Kumar, R.; Jakhar, N. A critical review of polymer support-based shape-stabilized phase change materials for thermal energy storage applications. Energy Storage 2024, 6, e639. [Google Scholar] [CrossRef]
- Tian, H.; Yi, T.T.; Gong, Y.J. Analysis of Thermally Activated Sacrificial Micro Soft Layers for Reduced Surface-Ice Interface Strength. J. Mar. Sci. Eng. 2023, 11, 1186. [Google Scholar] [CrossRef]
- Strutton, J.W.; Moore, L.M.J.; Marcischak, J.C.; Alabada, C.D.; Ghiassi, K.B.; McCollum, J.M. Early Cure Analysis to Inform Direct Ink Writing of HTPB Polyurethane: Insights from Spectroscopy, Rheology, and Molecular Simulations. ACS Appl. Polym. Mater. 2023, 5, 8693–8703. [Google Scholar] [CrossRef]
- Zhang, Y.; Jia, Z.Y.; Hai, A.M.; Zhang, S.F.; Tang, B.T. Shape-stabilization micromechanisms of form-stable phase change materials—A review. Compos. Part A Appl. Sci. Manuf. 2022, 160, 107047. [Google Scholar] [CrossRef]
- Mnasri, T.; Abbessi, A.; Ben Younes, R.; Mazioud, A. Optimization of thermal conductivity in composites loaded with the solid-solid phase-change materials. Sci. Eng. Compos. Mater. 2018, 25, 1157–1165. [Google Scholar] [CrossRef]
- Mu, B.Y.; Li, M. Fabrication and characterization of polyurethane-grafted reduced graphene oxide as solid-solid phase change materials for solar energy conversion and storage. Sol. Energy 2019, 188, 230–238. [Google Scholar] [CrossRef]
- Wang, J.W.; Wu, Z.H.; Xie, H.Q.; Wang, T.T.; Wang, Y.Y.; Huang, Y.M.; Dong, L. Effect of Different Soft Segment Contents on the Energy Storage Capacity and Photo-Thermal Performance of Polyurethane-Based/Graphene Oxide Composite Solid-Solid Phase Change Materials. Polymers 2022, 14, 5156. [Google Scholar] [CrossRef]
- Xia, T.F.; Wu, B.J.; Zhang, H.Z.; Xu, F.; Sun, L.X.; Lin, X.C.; Liang, C.H.; Ma, L.; Peng, H.L.; Li, B.; et al. Titanium dioxide/graphene oxide synergetic reinforced composite phase change materials with excellent thermal energy storage and photo-thermal performances. J. Mater. Res. Technol.-JMRT 2024, 32, 4019–4027. [Google Scholar] [CrossRef]
- Wang, Y.H.; Xiao, Y.; Wu, B.; Lei, J.X. Solid-solid phase-change materials cross-linked by carbon quantum dots: Address leakage and high super-cooling degree simultaneously. J. Appl. Polym. Sci. 2021, 138, 50241. [Google Scholar] [CrossRef]
- Sari, A.; Biçer, A.; Alkan, C. Thermal energy storage characteristics of poly(styrene-co-maleic anhydride)-graft-PEG as polymeric solid-solid phase change materials. Sol. Energy Mater. Sol. Cells 2017, 161, 219–225. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, X.D.; Sheng, D.K.; Lin, C.H.; Ji, F.C.; Dong, L.; Xu, S.B.; Wu, H.H.; Yang, Y.M. Polyurethane-based solid-solid phase change materials with in situ reduced graphene oxide for light-thermal energy conversion and storage. Chem. Eng. J. 2018, 338, 117–125. [Google Scholar] [CrossRef]
- Bai, S.J.; Zhang, K.X.; Zhang, Q.; Zhu, Y.L.; Wang, W.Q.; Zhang, J.; Li, X.; Zhang, X.Q.; Wang, R. Intrinsic Flame Retardancy and Flexible Solid-Solid Phase Change Materials with Self-Healing and Recyclability. ACS Appl. Mater. Interfaces 2023, 15, 48613–48622. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.N.; Li, J.; Li, S.W.; Yang, X. Super-elastic and shape-stable solid-solid phase change materials for thermal management of electronics. J. Energy Storage 2022, 52, 104751. [Google Scholar] [CrossRef]
- Cui, M.L.; Tian, C.; Yang, Y.Y.; Huang, L.; Liu, Q.; Yang, N.; Zhao, F.Q.; Cai, X.F.; Kong, W.B. Intrinsic photothermal phase change materials with enhanced toughness and flexibility for thermal management in extreme environments. Chem. Eng. J. 2023, 475, 146091. [Google Scholar] [CrossRef]
- Lv, S.S.; Liu, X.L.; Wang, J.G.; Xu, Q.; Song, C.; Xuan, Y.M. Flexible highly thermally conductive biphasic composite films for multifunctional solar/electro-thermal conversion energy storage and thermal management. J. Clean. Prod. 2023, 426, 139004. [Google Scholar] [CrossRef]
- Yang, Y.Y.; Liu, C.H.; Shi, Y.L.; Wang, P.Y.; Xu, Y.; Kong, W.B. Intrinsic photothermal block polyurethane solid-solid phase-change materials with high mechanical toughness and multi-recyclability controlled by crosslinking density. J. Energy Storage 2025, 113, 115583. [Google Scholar] [CrossRef]
- Yuan, A.Q.; Wu, B.; Wang, Y.; Zhao, Y.Y.; Liu, Q.F.; Lei, J.X. Recyclable solid-solid phase-change materials cross-linked by reversible oxime-carbamate bonds for solar energy storage. Int. J. Energy Res. 2020, 44, 9185–9193. [Google Scholar] [CrossRef]
- Geng, X.Y.; Qin, M.L.; Shen, Z.H.; Xiong, F.; Di, J.T.; Yang, C.X.; Wang, Y.G.; Gao, S.; Gao, S.Y.; Wang, Q.N.; et al. Muscle-Inspired Super-Flexible Phase Change Materials with Programmable Deformation for Photothermal Actuation. Adv. Funct. Mater. 2024, 35, 2418848. [Google Scholar] [CrossRef]
- Wei, Z.K.; Liao, Y.S.; Liu, T.R.; Yuan, A.Q.; Wu, X.D.; Jiang, L.; Lei, J.X.; Fu, X.W. Design of Sustainable Self-Healing Phase Change Materials by Dynamic Semi-Interpenetrating Network Structure. Adv. Funct. Mater. 2024, 34, 2312019. [Google Scholar] [CrossRef]
- Tareq, A.Z.; Hyder, M.; Merino, D.H.; Mohan, S.D.; Cooper, J.A.; Hayes, W. Design and synthesis of aliphatic supramolecular polymers featuring amide, urethane, and urea hydrogen bonding units. Eur. Polym. J. 2025, 228, 113782. [Google Scholar] [CrossRef]
- Sarier, N.; Arat, R.; Menceloglu, Y.; Onder, E.; Boz, E.C.; Oguz, O. Production of PEG grafted PAN copolymers and their electrospun nanowebs as novel thermal energy storage materials. Thermochim. Acta 2016, 643, 83–93. [Google Scholar] [CrossRef]
- Nikpourian, H.; Bahramian, A.R.; Abdollahi, M. On the thermal performance of a novel PCM nanocapsule: The effect of core/shell. Renew. Energy 2020, 151, 322–331. [Google Scholar] [CrossRef]
- Shen, J.F.; Ma, Y.Q.; Zhou, F.; Sheng, X.X.; Chen, Y. Thermophysical properties investigation of phase change microcapsules with low supercooling and high energy storage capability: Potential for efficient solar energy thermal management. J. Mater. Sci. Technol. 2024, 191, 199–208. [Google Scholar] [CrossRef]
- Jelita, M.; Saleh, H. Comparative analysis of heat transfer performance in cavities with varied wall conditions and nano encapsulated phase change material-water. J. Energy Storage 2024, 95, 112532. [Google Scholar] [CrossRef]
- Cai, J.; Yang, B.; Akbarzadeh, A. Origami Metamaterials Enable Low-Stress-Driven Giant Elastocaloric Effect. ACS Nano 2023, 18, 894–908. [Google Scholar] [CrossRef]
- Alhashash, A.; Saleh, H. Impact of Surface Undulation on Flow and Heat Transfer Characteristics in an Enclosure Filled with Nanoencapsulated Phase Change Materials (NEPCMs). Math. Probl. Eng. 2021, 2021, 8899995. [Google Scholar] [CrossRef]
- Sadr, A.N.; Shekaramiz, M.; Zarinfar, M.; Esmaily, A.; Khoshtarash, H.; Toghraie, D. Simulation of mixed-convection of water and nano-encapsulated phase change material inside a square cavity with a rotating hot cylinder. J. Energy Storage 2022, 47, 103606. [Google Scholar] [CrossRef]
- Yang, G.; Liu, X.Y.; Lipik, V. Evaluation of silica aerogel-reinforced polyurethane foams for footwear applications. J. Mater. Sci. 2018, 53, 9463–9472. [Google Scholar] [CrossRef]
- Xia, Y.P.; Zhang, H.Z.; Huang, P.R.; Huang, C.W.; Xu, F.; Zou, Y.J.; Chu, H.L.; Yan, E.H.; Sun, L.X. Graphene-oxide-induced lamellar structures used to fabricate novel composite solid-solid phase change materials for thermal energy storage. Chem. Eng. J. 2019, 362, 909–920. [Google Scholar] [CrossRef]
- Abdeali, G.; Bahramian, A.R.; Abdollahi, M. Review on Nanostructure Supporting Material Strategies in Shape -stabilized Phase Change Materials. J. Energy Storage 2020, 29, 101299. [Google Scholar] [CrossRef]
- Aftab, W.; Mahmood, A.; Guo, W.H.; Yousaf, M.; Tabassum, H.; Huang, X.Y.; Liang, Z.B.; Cao, A.Y.; Zou, R.Q. Polyurethane-based flexible and conductive phase change composites for energy conversion and storage. Energy Storage Mater. 2019, 20, 401–409. [Google Scholar] [CrossRef]
- Xiong, F.; Yuan, K.J.; Aftab, W.; Jiang, H.Y.; Shi, J.M.; Liang, Z.B.; Gao, S.; Zhong, R.Q.; Wang, H.L.; Zou, R.Q. Copper Sulfide Nanodisk-Doped Solid-Solid Phase Change Materials for Full Spectrum Solar-Thermal Energy Harvesting and Storage. ACS Appl. Mater. Interfaces 2021, 13, 1377–1385. [Google Scholar] [CrossRef]
- Nistor, C.L.; Gifu, I.C.; Anghel, E.M.; Ianchis, R.; Cirstea, C.D.; Nicolae, C.A.; Gabor, A.R.; Atkinson, I.; Petcu, C. Novel PEG6000-Silica-MWCNTs Shape-Stabilized Composite Phase-Change Materials (ssCPCMs) for Thermal-Energy Storage. Polymers 2023, 15, 3022. [Google Scholar] [CrossRef]
- Zhi, M.Y.; Yue, S.; Zheng, L.L.; Su, B.J.; Fu, J.; Sun, Q. Recent developments in solid-solid phase change materials for thermal energy storage applications. J. Energy Storage 2024, 89, 111570. [Google Scholar] [CrossRef]
- Atmaca, N.; Atmaca, A.; Özçetin, A.I. The impacts of restoration and reconstruction of a heritage building on life cycle energy consumption and related carbon dioxide emissions. Energy Build. 2021, 253, 111507. [Google Scholar] [CrossRef]
- Zingre, K.T.; Kumar, D.; Wan, M.P. Analysing the Effect of Substrate Properties on Building Envelope Thermal Performance in Various Climates. Energies 2020, 13, 5119. [Google Scholar] [CrossRef]
- Arumugam, C.; Shaik, S.; Roy, A.; Kontoleon, K.J.; Cuce, E.; Shaik, A.H.; Chakraborty, S.; Alwetaishi, M.; Cuce, P.M.; Gupta, M. Analysis of the benefits of adopting roof sandwich panels integrated with PCM versus PUR to mitigate energy costs and carbon dioxide emissions. J. Energy Storage 2024, 77, 109947. [Google Scholar] [CrossRef]
- Harle, T.; Hebert, R.L.; Nguyen, G.T.M.; Ledesert, B.A. A composite of cross-linked polyurethane as solid-solid phase change material and plaster for building application. Energy Build. 2022, 262, 111945. [Google Scholar] [CrossRef]
- Liu, J.Y.; Jia, S.F.; Lin, X.X.; Cao, H.M.; Wang, W.B.; Guo, X.; Sun, W.S. Fabrication of thermal energy storage wood composite based on shape-stable phase change material. Mater. Res. Express 2021, 8, 055304. [Google Scholar] [CrossRef]
- Li, Z.L.; Liu, Z.G.; Fang, Y.; Wu, H.; Niu, R.; Wu, L.D.; Li, Y.; Sui, M.; Wang, H.; Lu, X.; et al. Bio-based Polylactic acid/Polyurethane blends with good recyclability and excellent shape stability for solar thermal energy storage. Sol. Energy Mater. Sol. Cells 2023, 258, 112406. [Google Scholar] [CrossRef]
- Li, H.Z.Y.; Xing, C.W.; Zhu, B.H.; Zhang, X.; Gao, Y.; Tang, S.X.; Cheng, H.L. Comparative analysis of four styrene-butadiene-styrene (SBS) structure repair agents in the rejuvenation of aged SBS-modified bitumen. Constr. Build. Mater. 2025, 476, 141232. [Google Scholar] [CrossRef]
- Xing, C.W.; Tang, S.X.; Chang, Z.B.; Han, Z.C.; Li, H.Z.Y.; Zhu, B.H. A comprehensive review on the plant-mixed cold recycling technology of emulsified asphalt: Raw materials and factors affecting performances. Constr. Build. Mater. 2024, 439, 137344. [Google Scholar] [CrossRef]
- Wei, K.; Liu, Z.; Wang, L.; Ma, B.A.; Fan, Y.Z.; Shi, J.H.; Cheng, P. Preparation of polyurethane solid-solid low temperature PCMs granular asphalt mixes and study of phase change temperature control behavior. Sol. Energy 2022, 231, 149–157. [Google Scholar] [CrossRef]
- Shi, J.H.; Wei, K.; Fan, Y.Z.; Cheng, P.; Wang, S.F.; Shi, W.S.; Ma, B. Influence of low-temperature polyurethane solid-solid PCMs particles on the pavement performance of asphalt mixtures. Constr. Build. Mater. 2022, 357, 129386. [Google Scholar] [CrossRef]
- Jiao, W.X.; Sha, A.M.; Zhang, J.; Jia, M.; Jiang, W.; Hu, L.Q. Design and properties of polyurethane solid-solid phase-change granular temperature regulation asphalt mixtures. Sol. Energy 2023, 253, 47–57. [Google Scholar] [CrossRef]
- Jia, M.; Sha, A.M.; Jiang, W.; Wang, W.T.; Yuan, D.D.; Li, J.G.; Dai, J.H.; Jiao, W.X. A solid-solid phase change filler with enhanced thermal properties for cooling asphalt mastic. Sol. Energy 2022, 242, 105–118. [Google Scholar] [CrossRef]
- Liu, T.; Guo, N.S.; Jin, X.; Tan, Y.Q.; You, Z.P.; Cui, S.C.; Chu, Z.Y.; Fang, C.Z. Thermoregulation, rheological properties and modification mechanism of asphalt modified with PUSSPCMs. Constr. Build. Mater. 2023, 372, 130763. [Google Scholar] [CrossRef]
- Liu, T.; Guo, N.S.; You, Z.P.; Tan, Y.Q.; Fang, C.Z. Thermal and mechanical properties of PEG4000 and PUSSPCMs for thermoregulation of asphalt. Constr. Build. Mater. 2024, 412, 134776. [Google Scholar] [CrossRef]
- Sari, A.; Biçer, A.; Alkan, C. Thermal energy storage properties of polyethylene glycol grafted styrenic copolymer as novel solid-solid phase change materials. Int. J. Energy Res. 2020, 44, 3976–3989. [Google Scholar] [CrossRef]
- Wu, T.T.; Wang, C.H.; Hu, Y.X.; Zeng, X.X.; Song, M.J. Flexible solid-solid phase change materials with high stability for thermal management. Int. J. Heat Mass Transf. 2023, 211, 124202. [Google Scholar] [CrossRef]
- Luo, Y.J.; Zhou, D.Q.; Yang, W.S.; Li, C.B.; Liu, H.J.; Qiang, W.; Yang, X.X.; Zhao, G.F.; Bi, C.X.; Wang, T.Y.; et al. Phosphorus-nitrogen based flame retardant polyurethane composite phase change materials for battery thermal safety system. Appl. Therm. Eng. 2025, 258, 124763. [Google Scholar] [CrossRef]
- Salgado-Pizarro, R.; Puigjaner, C.; García, J.; Fernández, A.I.; Barreneche, C. Copper- and manganese-based layered hybrid organic-inorganic compounds with polymorphic transitions as energy storage materials. J. Mater. Chem. A 2024, 12, 18544–18553. [Google Scholar] [CrossRef]
- Liu, M.Q.; Huang, Z.W.; Bashir, A.; Zhang, Y.C.; Qian, H.X.; Ouyang, X.; Hu, Y.; Chen, H.B.; Chen, D.Z. A novel photocurable polymeric solid-solid phase change material for intelligent temperature regulators. Chem. Eng. J. 2024, 500, 157322. [Google Scholar] [CrossRef]
- Shi, J.M.; Aftab, W.; Liang, Z.B.; Yuan, K.J.; Maqbool, M.; Jiang, H.Y.; Xiong, F.; Qin, M.L.; Gao, S.; Zou, R.Q. Tuning the flexibility and thermal storage capacity of solid-solid phase change materials towards wearable applications. J. Mater. Chem. A 2020, 8, 20133–20140. [Google Scholar] [CrossRef]
- Tian, C.; Ning, J.Y.; Yang, Y.Y.; Zeng, F.H.; Huang, L.; Liu, Q.; Lv, J.H.; Zhao, F.Q.; Kong, W.B.; Cai, X.F. Super tough and stable solid-solid phase change material based on π-π stacking. Chem. Eng. J. 2022, 429, 132447. [Google Scholar] [CrossRef]
- Zhao, Z.; Tong, N.N.; Song, H.; Guo, Y.; Wang, J.M. Preparation and characterization of phase-change energy storage nonwoven fabric. J. Ind. Text. 2022, 51, 7089S–7103S. [Google Scholar] [CrossRef]
- Kong, M.; Guo, X.Y.; Zhang, S.F.; Zhang, Y.; Tang, B.T. Structural colored textiles with high color visibility and stability for intelligent thermoregulating performance. Chem. Eng. J. 2023, 473, 145332. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Ma, H.S.; He, Y.; Shi, B.R.; Zhong, J.H.; Zhou, Y.; Liu, X.D.; Yang, Y.M. High reflectivity and latent heat synergetic TiO2/Cs0.33WO3-PU double-layer materials with zero transmittance. Chem. Eng. J. 2024, 496, 145332. [Google Scholar] [CrossRef]
- Wu, H.Y.; Yu, Y.T.; Yu, Y.L.; Shi, J.; Morikawa, H.; Zhu, C.H. A facile method for continuous production of temperature-adaptive hyperthermal management core-sheath polyurethane nanofiber yarns based on vanadium dioxide toward commercialization. J. Energy Storage 2024, 86, 111311. [Google Scholar] [CrossRef]
- Alva, G.; Lin, Y.X.; Fang, G.Y. Synthesis and characterization of chain-extended and branched polyurethane copolymers as form stable phase change materials for solar thermal conversion storage. Sol. Energy Mater. Sol. Cells 2018, 186, 14–28. [Google Scholar] [CrossRef]
- Zhou, Y.; Sheng, D.K.; Liu, X.D.; Lin, C.H.; Ji, F.; Dong, L.; Xu, S.B.; Yang, Y.M. Synthesis and properties of crosslinking halloysite nanotubes/polyurethanebased solid-solid phase change materials. Sol. Energy Mater. Sol. Cells 2018, 174, 84–93. [Google Scholar] [CrossRef]
- Du, X.S.; Qu, J.H.; Deng, S.; Du, Z.L.; Cheng, X.; Wang, H.B. Ti3C2Tx@PDA-Integrated Polyurethane Phase Change Composites with Superior Solar-Thermal Conversion Efficiency and Improved Thermal Conductivity. ACS Sustain. Chem. Eng. 2020, 8, 5799–5806. [Google Scholar] [CrossRef]
- Bao, L.H.; Liu, Z.H. Near-infrared absorption photothermal conversion polyurethane film for energy storage. J. Polym. Res. 2021, 28, 21. [Google Scholar] [CrossRef]
- Li, L.X.; Peng, J.; Wang, L.; Lai, J.J.; Zhao, C.X.; Xiang, D.; Li, H.; Yan, G.L.; Li, Z.Y.; Wu, Y.P. Highly flexible GO-polyurethane solid-solid phase change composite materials for efficient photothermal conversion and thermal energy storage. J. Mater. Chem. A 2025, 13, 3073–3083. [Google Scholar] [CrossRef]
- Wang, L.; Ye, L.; Cheng, X.; Huang, C.G.; Zhang, Y.L.; Situ, Y.; Huang, H. Shape-stabilized polyethylene glycol composite phase change materials based on dendritic mesoporous silica sphere support for thermal energy storage. Sol. Energy Mater. Sol. Cells 2025, 282, 113425. [Google Scholar] [CrossRef]
- Cobos, M.; Pagalday, E.; Puyadena, M.; Cabido, X.; Martin, L.; Múgica, A.; Irusta, L.; González, A. Waterborne hybrid polyurethane coatings containing Casein as sustainable green flame retardant through different synthesis approaches. Prog. Org. Coat. 2023, 174, 107278. [Google Scholar] [CrossRef]
- Sarkar, S.; Mestry, S.; Mhaske, S.T. Developments in phase change material (PCM) doped energy efficient polyurethane (PU) foam for perishable food cold-storage applications: A review. J. Energy Storage 2022, 50, 104620. [Google Scholar] [CrossRef]
- Kumar, A.; Hsieh, P.Y.; Shaikh, M.O.; Kumar, R.K.R.; Chuang, C.H. Flexible Temperature Sensor Utilizing MWCNT Doped PEG-PU Copolymer Nanocomposites. Micromachines 2022, 13, 197. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.W.; Shi, X.Y.; Gao, M.H.; Huang, C.H.; Huang, T.; Zhang, N.; Yang, J.H.; Qi, X.D.; Wang, Y. Light-actuated shape memory and self-healing phase change composites supported by MXene/waterborne polyurethane aerogel for superior solar-thermal energy storage. Compos. Commun. 2021, 28, 100980. [Google Scholar] [CrossRef]
- Amaral, C.; Vicente, R.; Ferreira, V.M.; Silva, T. Polyurethane foams with microencapsulated phase change material: Comparative analysis of thermal conductivity characterization approaches. Energy Build. 2017, 153, 392–402. [Google Scholar] [CrossRef]
- Sha, A.M.; Zhang, J.; Jia, M.; Jiang, W.; Jiao, W.X. Development of polyurethane-based solid-solid phase change materials for cooling asphalt pavements. Energy Build. 2022, 259, 111873. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, A.Q.; Zhao, Y.Y.; Liu, Q.F.; Lei, J.X. Phase change material with flexible crosslinking for thermal energy storage. J. Appl. Polym. Sci. 2020, 137, 48497. [Google Scholar] [CrossRef]


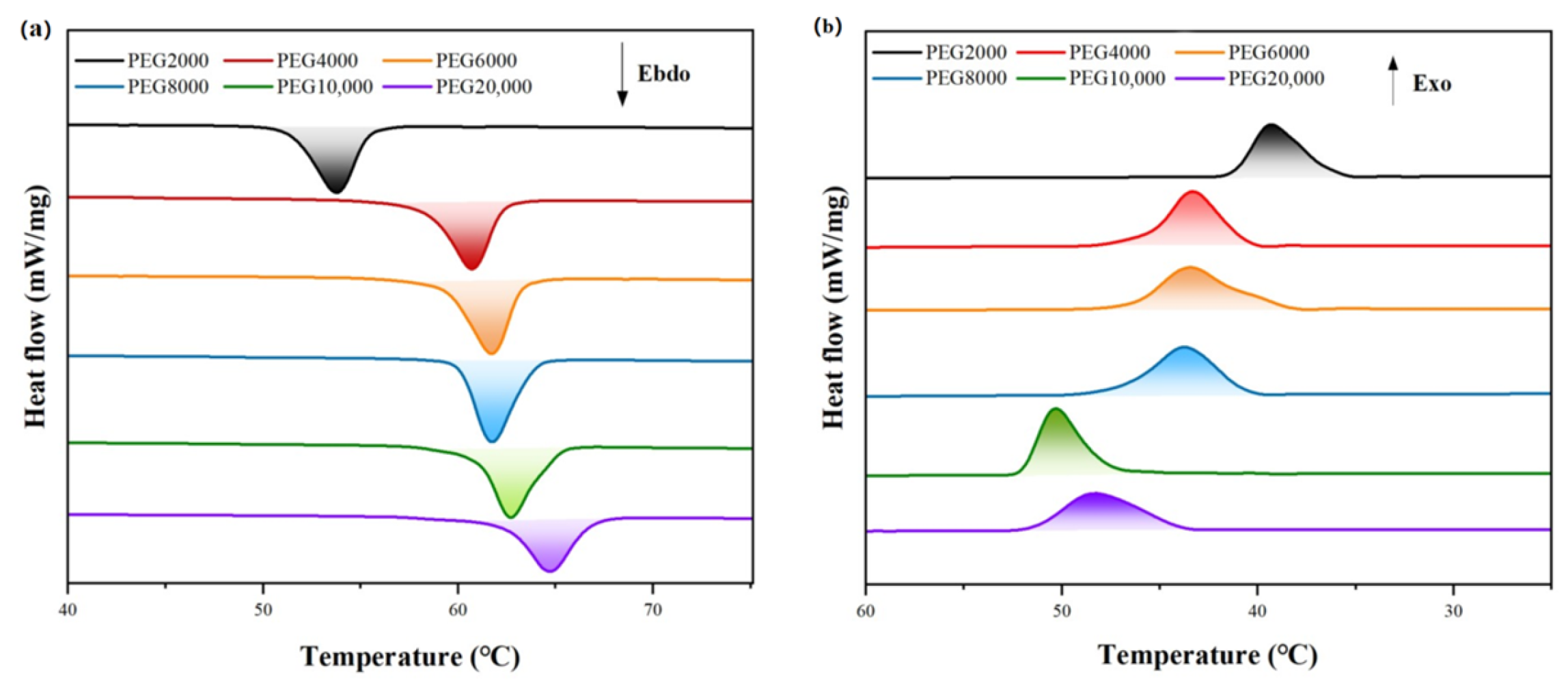









| Molecular Weight of PEG | Tm (°C) | ΔHm (J/g) | Tc (°C) | ΔHc (J/g) |
|---|---|---|---|---|
| 2000 | 50.8 | 191.2 | 41.0 | 191.8 |
| 4000 | 57.9 | 201.7 | 45.4 | 200.3 |
| 6000 | 58.2 | 207.1 | 46.2 | 205.9 |
| 8000 | 60.0 | 212.1 | 47.3 | 211.2 |
| 10,000 | 61.3 | 207.4 | 52.1 | 204.2 |
| 20,000 | 62.5 | 184.7 | 51.9 | 183.6 |
| Category | Abbreviation | Molecular Mass | Chemical Structure |
|---|---|---|---|
| Aromatic isocyanates | MDI | 250.26 | 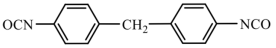 |
| TDI | 174.16 | 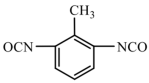 | |
| Aliphatic isocyanates | HDI | 168.19 | 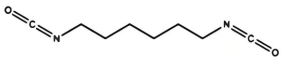 |
| IPDI | 222.32 | 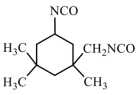 | |
| HMDI | 262.35 | 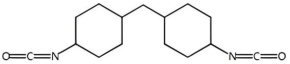 | |
| TMXDI | 244.30 |  |
| Category | Abbreviation | Molecular Mass | Chemical Structure |
|---|---|---|---|
| Aliphatic compounds | EG | 62.07 | 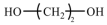 |
| Glycerol | 92.09 |  | |
| BDO | 90.12 | 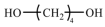 | |
| PE/PE-T | 136.15 | 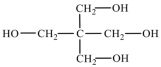 | |
| Aromatic compounds | HQEE | 198.22 | 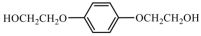 |
| MOCA | 267.15 | 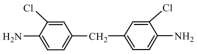 |
| Evaluation Dimensions | Two-Step Solution Polymerization Method | One-Step Method | Bulk-Polymerization |
|---|---|---|---|
| Molecular Weight Control | Excellent: Precise control over prepolymer and final product MW; Narrow MW distribution. | Poor: Typically broader MW distribution; Lower control precision. | Poor: Difficult control due to high viscosity and mixing issues; Broader MW distribution. |
| Structural Regularity | Excellent: Forms well-defined soft/hard segment microphase separation; Clear block structure. | Moderate: Generally lower degree of microphase separation and structural regularity compared to two-step. | Moderate: Mixing inhomogeneity may reduce regularity and affect microphase separation. |
| Solvent Use/Environmental Impact | Poor: Requires significant organic solvents. Issues with volatilization, recovery, and residue; Poor eco-friendliness; Residual solvent may reduce ΔH. | Variable: Can be solution-based (solvent issues similar to two-step) or bulk/solvent-free (Excellent eco-friendliness for bulk). | Excellent: Solvent-free. No issues with volatilization, recovery, or residue; Best eco-friendliness. |
| Process Complexity | High: Multiple steps (prepolymer synthesis, chain extension/crosslinking, solvent removal); Laborious and time-consuming. | Low: All reactants mixed and reacted in one step; Simplest process; Highly convenient. | Moderate: No solvent handling, but requires solving challenges of mixing, heat transfer, and degassing under high viscosity; Higher equipment demands. |
| Phase Change Enthalpy (ΔH) | High: High solvent and recovery costs; Higher energy consumption (solvent removal); Longer process time. | Low: Fewer steps, high efficiency; Lower solvent use (if used) than two-step; Lowest cost (especially bulk method). | Moderate: No solvent costs; Potentially higher equipment investment, but operational costs (energy, time) typically lower than two-step. |
| Cost | Typically Highest: Good structural regularity favors crystallization of soft segments; ΔH approaches theoretical value. | Moderate: Can achieve high values, but usually slightly lower than highly regular two-step products. | Variable: Depends on mixing homogeneity and structural regularity; Typically between one-step and two-step or slightly lower. |
| Thermal Stability | Excellent: Mild reaction conditions minimize side products; Well-defined structure generally ensures good stability. | Good: Generally sufficient for applications, depends on formulation and reaction control. | Good: Higher reaction temperatures may promote side reactions but can also form stable bonds; Absence of solvent residue benefits stability. |
| Author | PUSSPCM | Melting Temperature (°C) | Crystallization Temperature (°C) | Latent Heat (J/g) | Thermal Conductivity (W/(m·K)) | Thermal Stability (°C) | Phase Change Cyclic Stability | Ref. |
|---|---|---|---|---|---|---|---|---|
| Gao et al. | PEG + MDI + OMMT | 54.97 | 27.35 | 106.8 | 0.3035 | 368 °C | - | [42] |
| Wei et al. | PTMEG2000 + MDI + MOCA | 13.6 | −3.5 | 49.49 | 0.2212 | - | - | [68] |
| Zhang et al. | PEG6000 + MDI + BDO + SPU | 42.6 | 41.2 | 61.12 | 0.44 | - | - | [29] |
| Huang et al. | PEG4000 + HDI + DMG + CUCI2 + PTOL | 49.8 | 19.5 | 86.67 | - | 325 | - | [52] |
| Shi et al. | PEG4000 + PEG2000 + MDI + MOCA | 38.45 | 37.53 | 69.38 | - | 230 | - | [69] |
| Huang et al. | PEG1000 + MDI + NPG | 37.32 | - | 120.45 | - | 400.1 | - | [70] |
| Fan et al. | PEG8000 + MDI + TS200 | 59.08 | 28.47 | 129.59 | - | 300 (1.78%) | - | [50] |
| Oktay et al. | PEG + IPDI + n-Octadecyl alcohol | 57 | 29 | 126 | - | 293 | - | [71] |
| Zhou et al. | PEG8000 + IPDI + DMBA | 61.34 | 31.66 | 161.57 | - | >70 | ≥500 | [72] |
| Zhou et al. | PEG4000 + HDIBDO | 48.7–58.4 | 24.5–38.1 | 139.2 | - | 280–334 (5%) | - | [73] |
| Kinga et al. | PEG8000 + MDI + BDO | 50.2–56.6 | 118.0–164.4 | 42.8 | - | 372–383 (5%) | - | [74] |
| Jia et al. | PEG8000 MDI + MOCA | 50.2 | 111.2 | 32.3 | - | - | - | [43] |
| Kong et al. | PEG8000 + PAPI | 50.48 | 41.38 | 111.7 | - | 360 | - | [75] |
| Jiao et al. | PEG + PTMEG + MDI + MOCA | 13.6 | 4.1 | 37.1 | - | - | - | [76] |
| Chen et al. | PEG + HDI + DBTDL | 56.51 | 29.66 | 123.5 | 0.61 | 250 | - | [77] |
| Lin et al. | PEG4000 + IPDI + EKN + TMI + FeCl3·6H2O | 48.39 | 16.59 | 91.3 | 0.223 | >250 | - | [78] |
| Wang et al. | PTMEG + MDI + MOCA | 42.8 | 6.1 | 50.7 | - | >260 | - | [79] |
| Du et al. | EPTG + IPDI + BDO | 59.0 | 29.1 | 121.9 | - | 320–415 | 100 (2%) | [80] |
| LI et al. | PEG + MDI + CO | 51.67 ± 0.20 | 27.04 | 117.9 | - | >250 | 100 (3.3%) | [81] |
| Chen et al. | PEG + IPDI | 46.1 | 40.0 | 80.3 | 3.5 | 280 | - | [82] |
| Liu et al. | PEG8000 + HEA + HDDA + MDI + DBTDI | 57.4 | 46.2 | 157.4 | - | >200 | 100 (1.2%) | [83] |
| Wang et al. | PEG10000 + MDI + BDO + GO | 57.9 | 40.9 | 152.0 | 0.972 | 379 (5%) | 50 (3.8%) | [60] |
| Fang et al. | PEG + IPDI + DBTI + HEMA + AAO | 25 | - | 6.49 | - | - | - | [84] |
| Wu et al. | PEG6000 + HDI + PGF | 43.8 | 38.9 | 60.3 | 10.86 | 300 | - | [85] |
| Haung et al. | PEG8000 + HDI + HPMC | 56.68 | 42.20 | 158.59 | - | - | >100 | [27] |
| Zhou et al. | PEG4000 + HDIB + I-AA + HNT + GO | 57.4 | 33.7 | 103.3 | - | 316 (5%) | - | [86] |
| Yuan et al. | PEG8000 + MDI + EA + MR + DBTDI + BAH | 47.9 | - | 87.42 | - | - | - | [87] |
| Yang et al. | PEG6000 + IPDI + DBTDI + BDO | 40.2 | 24.9 | 71.1 | 0.370 | 250 | - | [88] |
| Lu et al. | PEG6000 + HDIT + DBTDI | 63.9 | 33.9 | 89.7 | - | 422.9 | >200 | [89] |
| Chen et al. | PEG + MDI + BDO + n-Eicosane | 57.43 | - | 141.2 | - | - | - | [90] |
| Cao et al. | PEG + MDEA + MDI + BDO | 55.7 | 33.6 | 137.5 | - | - | - | [51] |
| Types of Dynamic Covalent Bonds | Reversible Conditions | Chemical Structure |
|---|---|---|
| Diels–Alder | Thermally reversible | [4 + 2] cycloaddition products |
| Disulfide bond | Thermal, redox, or mechanical force | –S–S– |
| Imine bond (Schiff base) | pH or humidity | –C=N– |
| Transesterification bond | Thermal or catalytic | –COO– |
| Hydrazone bond | pH-responsive | –NH–N=C– |
| Borate ester bond | pH or saccharide-triggered | –B–O– |
| Nanofiller Type | Specific Material | Function | Modification Strategies |
|---|---|---|---|
| Carbon-based Fillers | GO | Enhance thermal conductivity Improve mechanical strength | Amination (APTES) Carboxylation |
| CNTs | Directional thermal conduction Suppress supercooling | Acid treatment (HNO3/H2SO4) PEG grafting | |
| Ceramic Fillers | BN | High thermal conductivity Electrical insulation | Hydroxylation (H2O2) Silane coupling (KH550) |
| SiO2 | Inhibit phase separation Reduce supercooling | Hollow structure Stearic acid grafting | |
| Metal Oxides | Al2O3 | Improve thermal stability Reduce thermal expansion | Nanowire morphology Phosphate treatment |
| ZnO | Photothermal conversion | Nanorod arrays Ag nanoparticle loading | |
| Bio-based Fillers | CNC/CNF | Mechanical reinforcement Flame retardancy | TEMPO oxidation Acetylation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Xiao, J.; Yao, T.; Wang, M. Recent Research Progress on Polyurethane Solid–Solid Phase Change Materials. Polymers 2025, 17, 1933. https://doi.org/10.3390/polym17141933
Wang Z, Xiao J, Yao T, Wang M. Recent Research Progress on Polyurethane Solid–Solid Phase Change Materials. Polymers. 2025; 17(14):1933. https://doi.org/10.3390/polym17141933
Chicago/Turabian StyleWang, Ziqiang, Jingjing Xiao, Tengkun Yao, and Menghao Wang. 2025. "Recent Research Progress on Polyurethane Solid–Solid Phase Change Materials" Polymers 17, no. 14: 1933. https://doi.org/10.3390/polym17141933
APA StyleWang, Z., Xiao, J., Yao, T., & Wang, M. (2025). Recent Research Progress on Polyurethane Solid–Solid Phase Change Materials. Polymers, 17(14), 1933. https://doi.org/10.3390/polym17141933