Electroactive Poly(amic acid) Films Grafted with Pendant Aniline Tetramer for Hydrogen Sulfide Gas Sensing Applications
Abstract
1. Introduction
2. Experimental Section
2.1. Chemicals and Materials
2.2. Instruments
2.3. Synthesis of Pendant Electroactive Diamine Monomer (PEDA)
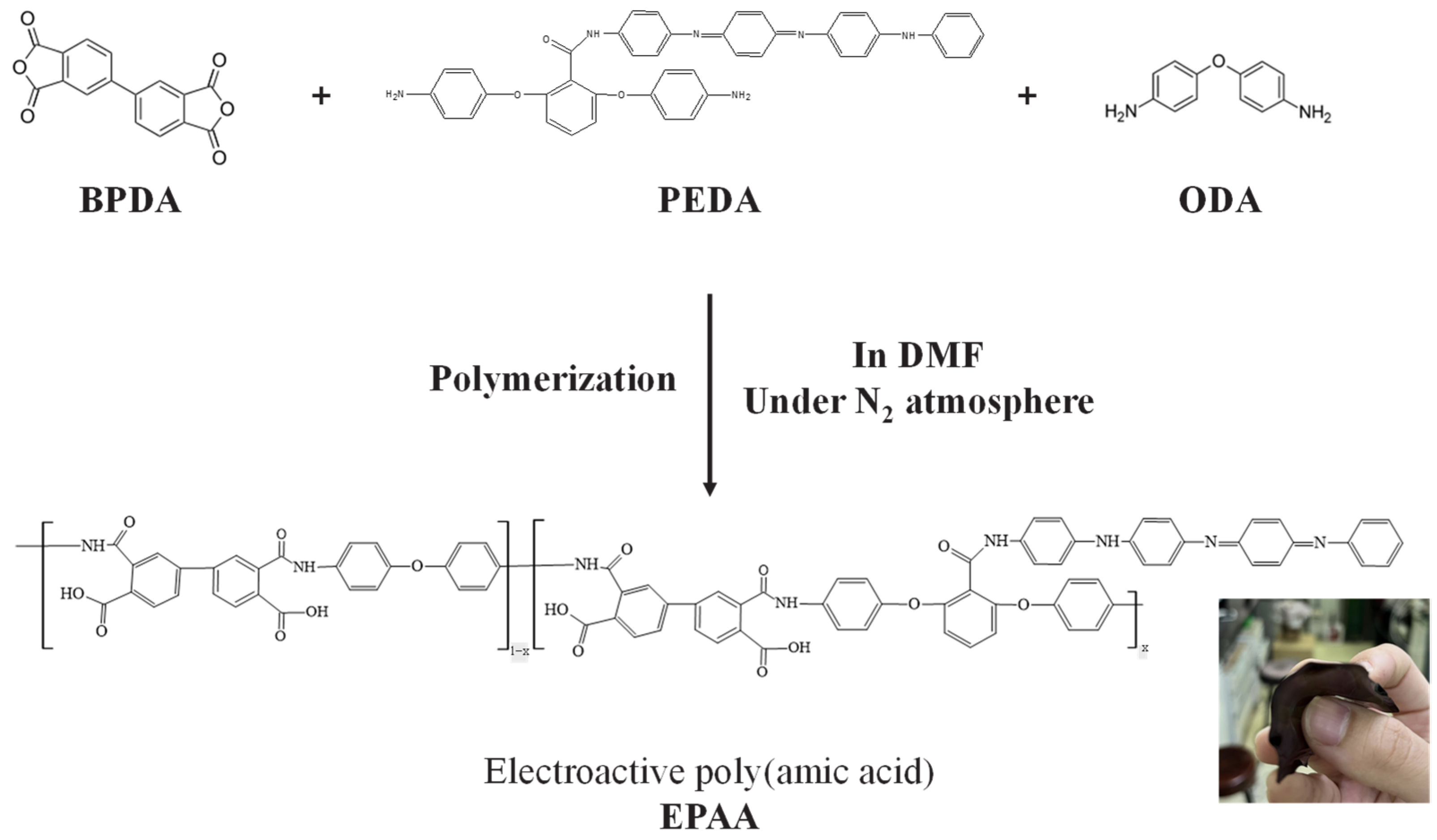
2.4. Synthesis of Electroactive Poly(amic acid) (EPAA)
2.5. Preparation of EPAA Composites-Coated Sensors
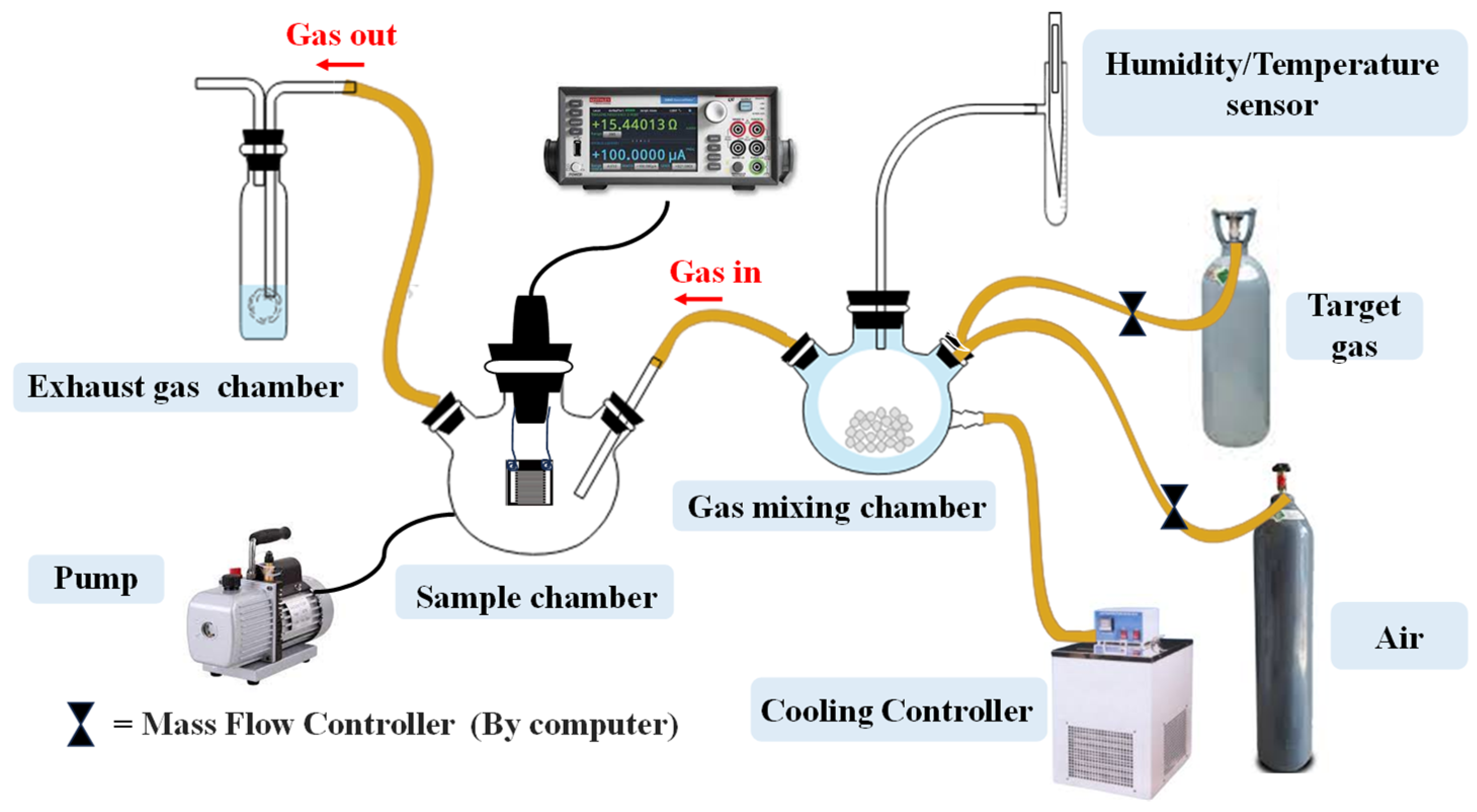
2.6. Gas Sensing Experiment
3. Results and Discussion
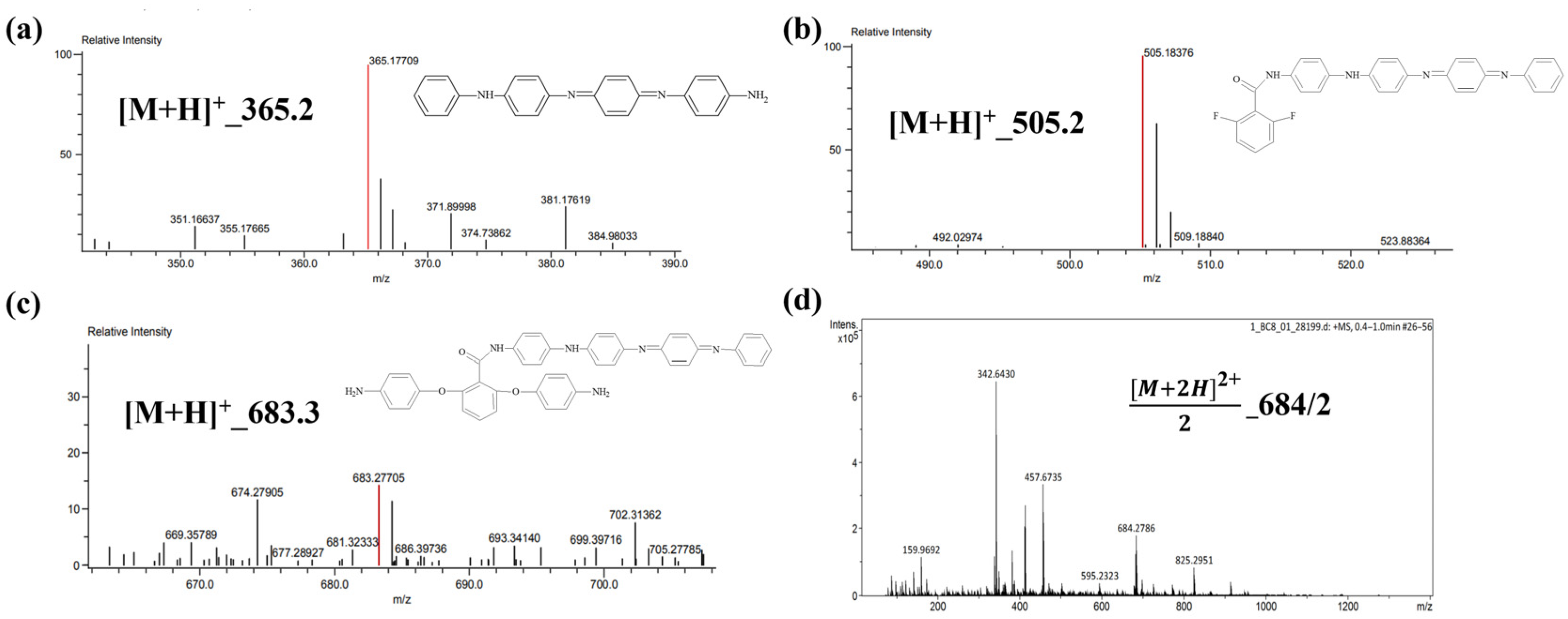
3.1. Mass Spectrum of Electroactive Monomers (AT and PEDA)
3.2. FTIR Analyses of Electroactive Monomers (AT and PEDA)
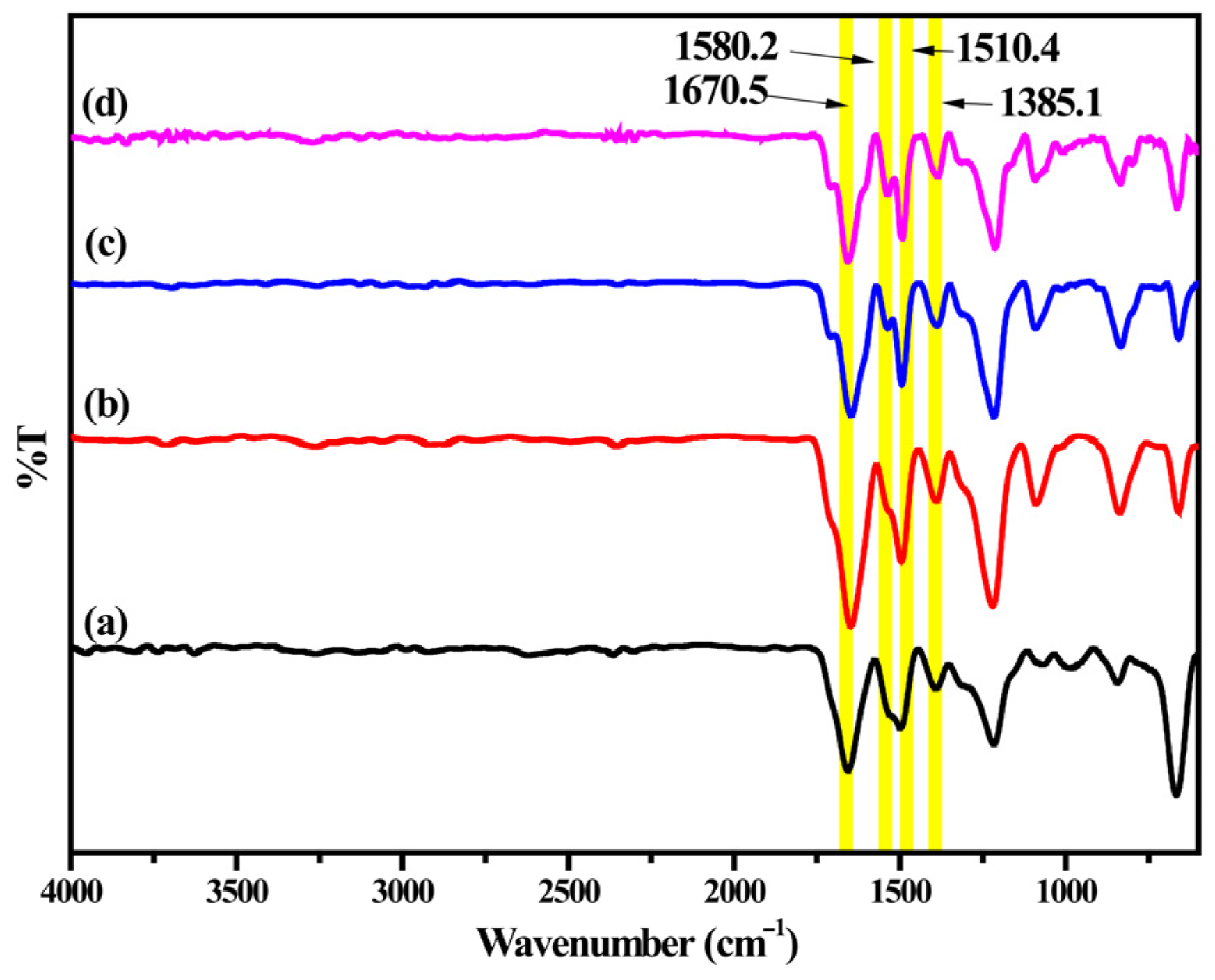
3.3. ATR-FTIR Analyses of EPAA
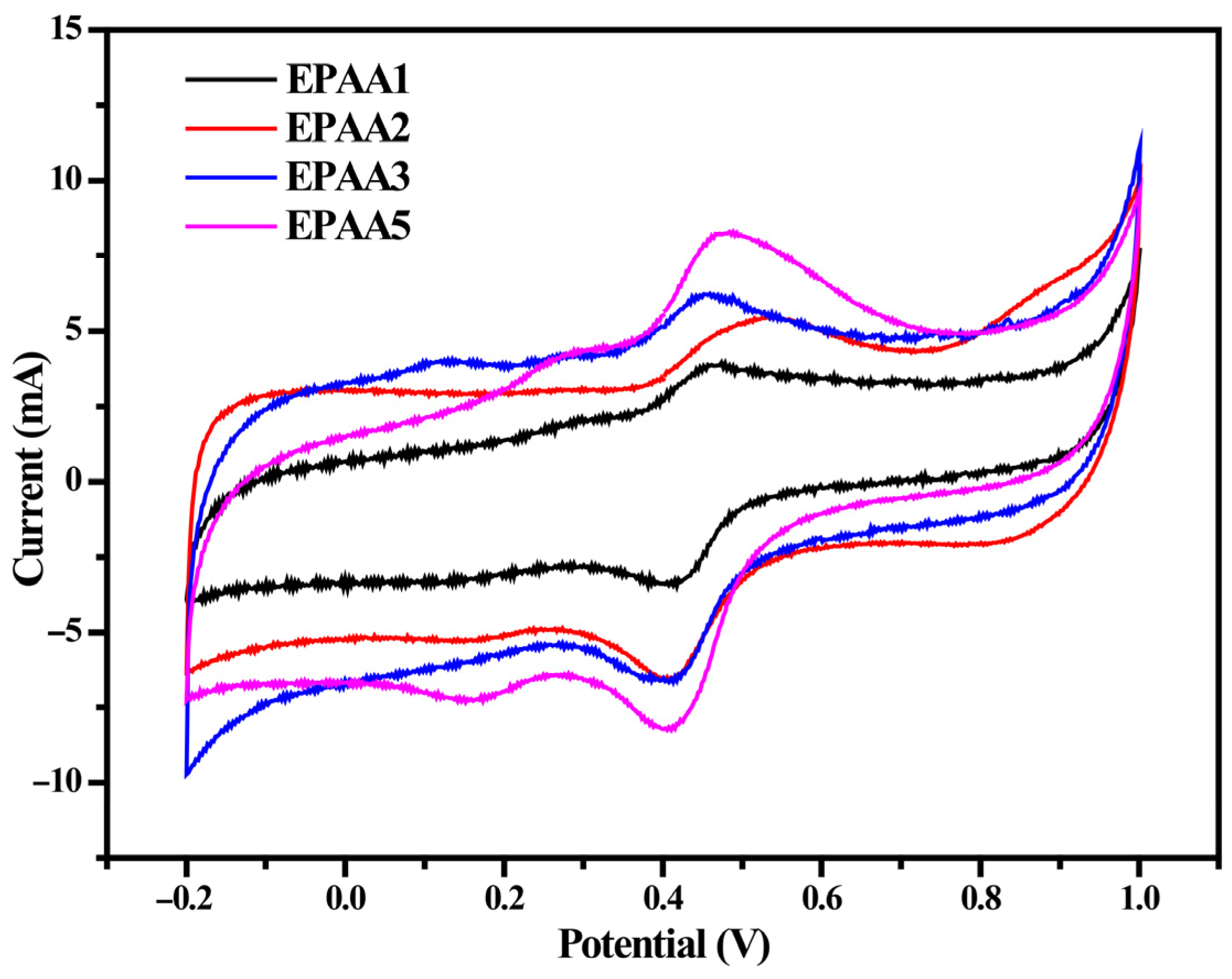
3.4. Electrochemical Redox Properties of EPAA
3.5. Conductivity Test of EPAA by Four-Probe
3.6. Morphology of EPAA Films
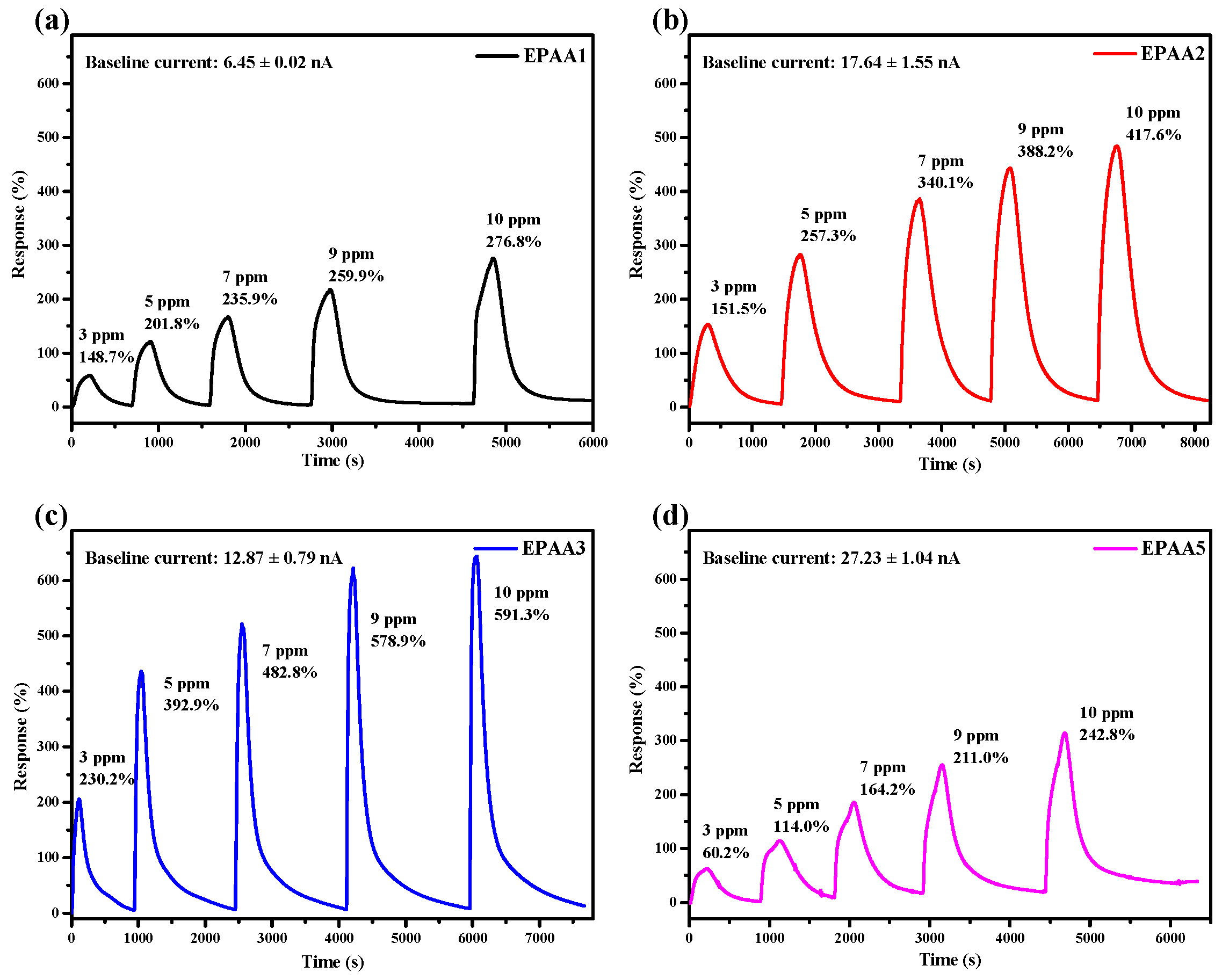
3.7. Gas Sensing Performance
3.7.1. Sensitivity and Calibration Curve
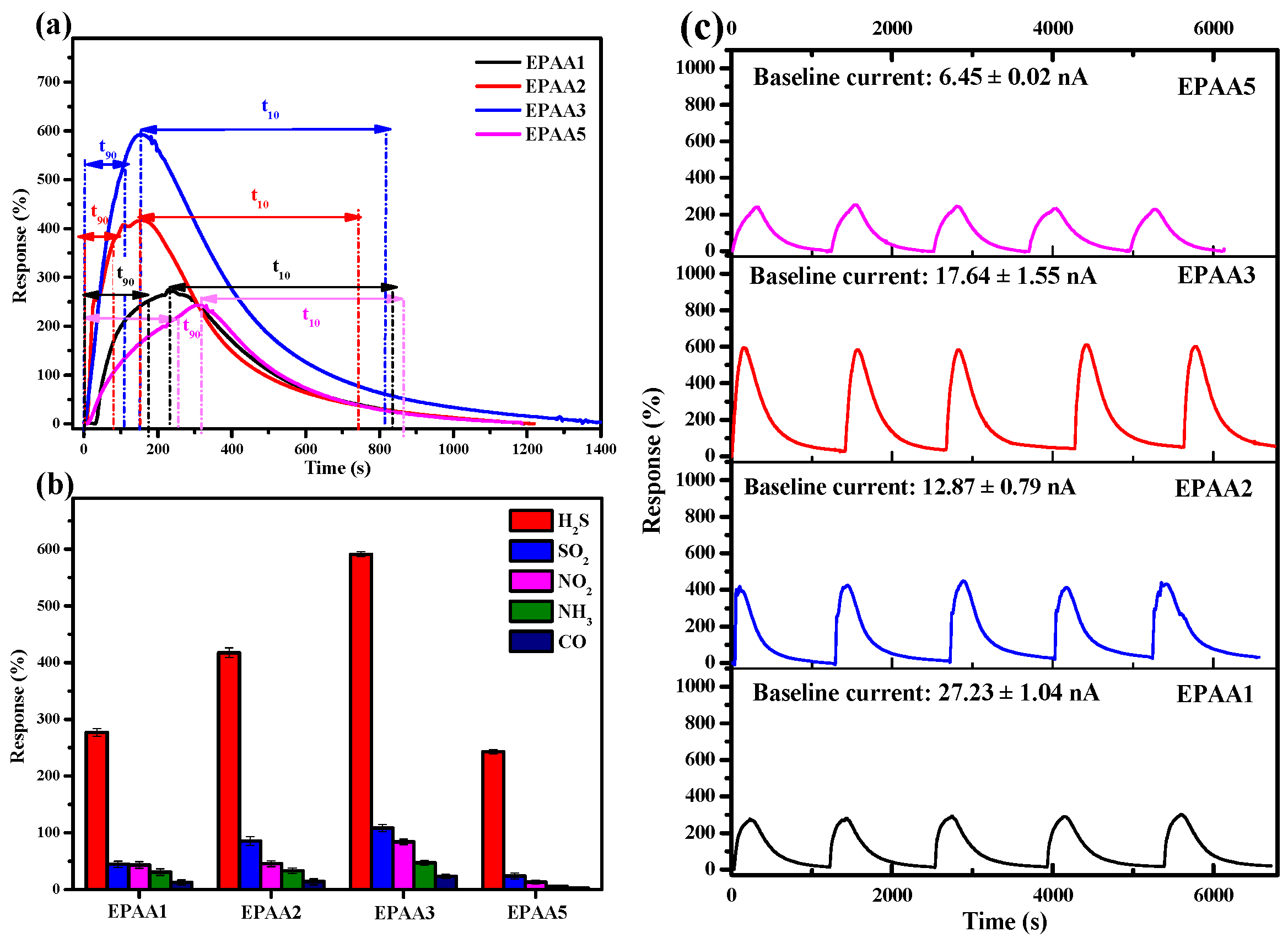
3.7.2. Response and Recovery Time, Selectivity, and Repeatability
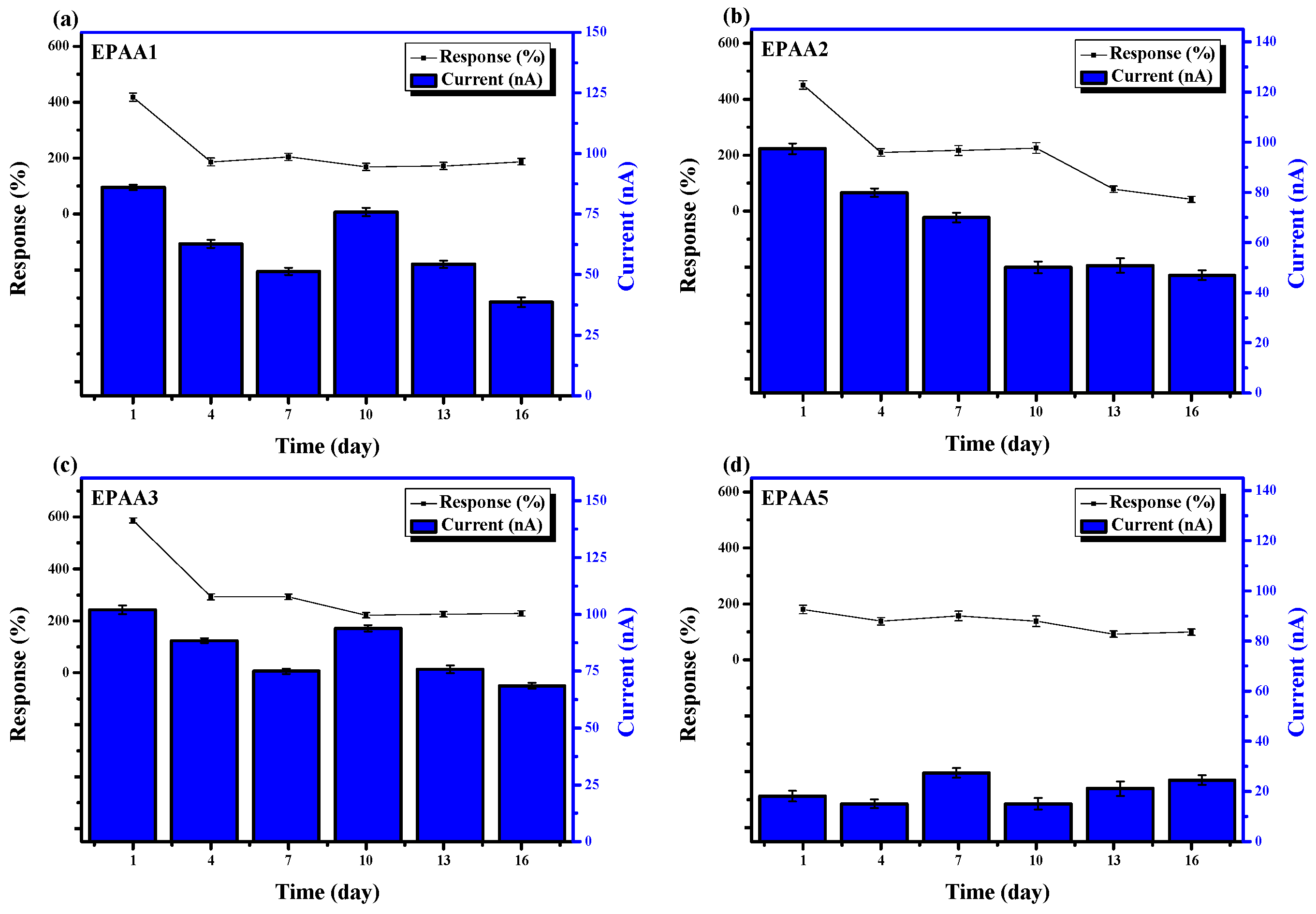
3.7.3. Stability and the Effect of Humidity
3.8. Gas Sensing Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Z.; Song, J.; Wang, T.; Liu, H.; Chen, J.; Sun, Y.; Zhang, P.; Cui, G. Ultrasensitive H2S sensor based on Cu2O/Graphene heterostructures at room temperature. Appl. Surf. Sci. 2025, 702, 163339. [Google Scholar] [CrossRef]
- Rath, R.J.; Talebian, S.; Giaretta, J.; Naficy, S.; Dehghani, F. Organic-Based Chemiresistive Sensors for Detection of Water-Soluble Gases: Strategies and Roadmap for Enhancing Sensing Performance. Adv. Funct. Mater. 2025, 35, 2417323. [Google Scholar] [CrossRef]
- Turlybekuly, A.; Shynybekov, Y.; Soltabayev, B.; Yergaliuly, G.; Mentbayeva, A. The Cross-Sensitivity of Chemiresistive Gas Sensors: Nature, Methods, and Peculiarities: A Systematic Review. ACS Sens. 2024, 9, 6358–6371. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, T.-J.; Peng, C.-H.; Chen, W.-S. A transparent ZnO nanowire MEMS gas sensor prepared by an ITO micro-heater. Sens. Actuators B Chem. 2020, 304, 127319. [Google Scholar] [CrossRef]
- Keshtkar, S.; Rashidi, A.; Kooti, M.; Askarieh, M.; Pourhashem, S.; Ghasemy, E.; Izadi, N. A novel highly sensitive and selective H2S gas sensor at low temperatures based on SnO2 quantum dots-C60 nanohybrid: Experimental and theory study. Talanta 2018, 188, 531–539. [Google Scholar] [CrossRef]
- Choi, S.-J.; Jang, B.-H.; Lee, S.-J.; Min, B.K.; Rothschild, A.; Kim, I.-D. Selective Detection of Acetone and Hydrogen Sulfide for the Diagnosis of Diabetes and Halitosis Using SnO2 Nanofibers Functionalized with Reduced Graphene Oxide Nanosheets. ACS Appl. Mater. Interfaces 2014, 6, 2588–2597. [Google Scholar] [CrossRef]
- Su, P.-G.; Peng, Y.-T. Fabrication of a room-temperature H2S gas sensor based on PPy/WO3 nanocomposite films by in-situ photopolymerization. Sens. Actuators B Chem. 2014, 193, 637–643. [Google Scholar] [CrossRef]
- Preethichandra, D.M.G.; Gholami, M.D.; Izake, E.L.; O’Mullane, A.P.; Sonar, P. Conducting Polymer Based Ammonia and Hydrogen Sulfide Chemical Sensors and Their Suitability for Detecting Food Spoilage. Adv. Mater. Technol. 2023, 8, 2200841. [Google Scholar] [CrossRef]
- Ragab, H.M.; Diab, N.S.; Aleid, G.M.; Aziz, R.A.; Obeidat, S.T.; Yusof, N.; Farea, M.A. Selective H2S sensor with CdS@PPy/rGO nanocomposite for sustainable air quality monitoring. Diam. Relat. Mater. 2025, 154, 112155. [Google Scholar] [CrossRef]
- Mousavi, S.; Kang, K.; Park, J.; Park, I. A room temperature hydrogen sulfide gas sensor based on electrospun polyaniline–polyethylene oxide nanofibers directly written on flexible substrates. RSC Adv. 2016, 6, 104131–104138. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, Z.; Zong, X. Flexible and highly sensitive H2S gas sensor based on in-situ polymerized SnO2/rGO/PANI ternary nanocomposite with application in halitosis diagnosis. Sens. Actuators B Chem. 2019, 289, 32–41. [Google Scholar] [CrossRef]
- Bulemo, P.M.; Kim, D.-H.; Shin, H.; Cho, H.-J.; Koo, W.-T.; Choi, S.-J.; Park, C.; Ahn, J.; Güntner, A.T.; Penner, R.M.; et al. Selectivity in Chemiresistive Gas Sensors: Strategies and Challenges. Chem. Rev. 2025, 125, 4111–4183. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hu, J.; Zhuang, X.; Zhang, P.; Wei, Y.; Wang, X.; Chen, X. Synthesis and Characterization of Novel Biodegradable and Electroactive Hydrogel Based on Aniline Oligomer and Gelatin. Macromol. Biosci. 2012, 12, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Moini, N.; Jahandideh, A.; Shahkarami, F.; Kabiri, K.; Piri, F. Linear and star-shaped π-conjugated oligoanilines: A review on molecular design in syntheses and properties. Polym. Chem. 2022, 13, 2714–2756. [Google Scholar] [CrossRef]
- Tropp, J.; Rivnay, J. Design of biodegradable and biocompatible conjugated polymers for bioelectronics. J. Mater. Chem. C 2021, 9, 13543–13556. [Google Scholar] [CrossRef]
- Yazgan, I.; Du, N.; Congdon, R.; Okello, V.; Sadik, O.A. Biofunctionalized poly (amic) acid membranes for absolute disinfection of drinking water. J. Membr. Sci. 2014, 472, 261–271. [Google Scholar] [CrossRef]
- Hooley, E.N.; Tilley, A.J.; White, J.M.; Ghiggino, K.P.; Bell, T.D.M. Energy transfer in PPV-based conjugated polymers: A defocused widefield fluorescence microscopy study. Phys. Chem. Chem. Phys. 2014, 16, 7108–7114. [Google Scholar] [CrossRef][Green Version]
- Husted, K.E.L.; Herzog-Arbeitman, A.; Kleinschmidt, D.; Zhang, W.; Sun, Z.; Fielitz, A.J.; Le, A.N.; Zhong, M.; Johnson, J.A. Pendant Group Modifications Provide Graft Copolymer Silicones with Exceptionally Broad Thermomechanical Properties. ACS Cent. Sci. 2023, 9, 36–47. [Google Scholar] [CrossRef]
- Lan, Y.-X.; Weng, C.W.; Ahmed, M.M.M.; Luo, K.-H.; Ji, W.-F.; Liu, W.-R.; Yeh, J.-M. Aniline pentamer-modified reduced graphene oxide/epoxy composites as anticorrosion coatings. Mater. Chem. Phys. 2021, 264, 124446. [Google Scholar] [CrossRef]
- Petrovski, A.; Paunović, P.; Avolio, R.; Errico, M.E.; Cocca, M.; Gentile, G.; Grozdanov, A.; Avella, M.; Barton, J.; Dimitrov, A. Synthesis and characterization of nanocomposites based on PANI and carbon nanostructures prepared by electropolymerization. Mater. Chem. Phys. 2017, 185, 83–90. [Google Scholar] [CrossRef]
- Abirami, S.; Kumar, E.; Vigneshwaran, B.; Vijayalakshmi, P. Fabrication of symmetric PANI/CeVO4 nanocomposites supercapacitor device: Electrochemical characterization and performance evaluation. Electrochim. Acta 2025, 532, 146512. [Google Scholar] [CrossRef]
- Ding, Y.; Zhuang, Q.; Guo, X.; Li, H.; Liang, C.; Du, B.; Zhao, C.; Shi, Y.; Meng, G.; Li, R.; et al. NiO-CuO hydrangeas-like composite derived from Ni/Cu bimetallic MOFs for sensitive detection of H2S at room temperature. Appl. Surf. Sci. 2023, 612, 155792. [Google Scholar] [CrossRef]
- Chen, B.-X.; Chen, L.-Y.; Zan, H.-W.; Meng, H.-F.; Hsieh, C.-A.; Yang, J.-B.; Chen, M.-H.; Cheng, Y.-H. Enhancement in operational current of PTB7 based ammonia gas sensor utilizing F4-TCNQ as P-type dopant. Sens. Actuators B Chem. 2022, 361, 131723. [Google Scholar] [CrossRef]
- Yao, P.-C.; Niu, J.-S.; Dai, G.-Y.; Jian, J.-J.; Hsu, W.-C.; Lin, K.-W.; Liu, W.-C. Ammonia sensing characteristics of an ITO-V2O5 based chemoresistive dual-type gas sensors (CDGS) decorated with platinum nanoparticles. Sens. Actuators B Chem. 2023, 392, 134071. [Google Scholar] [CrossRef]
- Kuo, C.-K.; Luo, K.-H.; Chu, P.-Y.; Wang, J.-C.; Tan, S.-W.; Liu, W.-C. Study of a highly sensitive ammonia gas sensor based on a sputtered Ga-doped SnO2 (GTO) thin film decorated with evaporated platinum nanoparticles. Sens. Actuators B Chem. 2024, 417, 136043. [Google Scholar] [CrossRef]
- Huang, S.; Liu, W.; Lin, H.; Wen, Z.; Hang, C.; An, R.; Li, Y.; Tian, Y. Hierarchical flower-like Cu-doped SnO2/Ag2S heterojunctions decorated with Ag for excellent sensing performance toward n-butanol at low operating temperatures. Sens. Actuators B Chem. 2025, 435, 137638. [Google Scholar] [CrossRef]
- Chan, Y.H.; Lock, S.S.M.; Wong, M.K.; Yiin, C.L.; Loy, A.C.M.; Cheah, K.W.; Chai, S.Y.W.; Li, C.; How, B.S.; Chin, B.L.F.; et al. A state-of-the-art review on capture and separation of hazardous hydrogen sulfide (H2S): Recent advances, challenges and outlook. Environ. Pollut. 2022, 314, 120219. [Google Scholar] [CrossRef]
- Muthumalai, K.; Manoharan, M.; Govindharaj, K.; Saravanan, P.; Haldorai, Y.; Sofer, Z.; Rajendra Kumar, R.T. Development of Dual-Selective Chemiresistive Sensor for NH3 and NOx at Room Temperature Using MoS2/MoO2 Heterostructures. ACS Appl. Nano Mater. 2024, 7, 14164–14173. [Google Scholar] [CrossRef]
- Wu, Z.; Li, Z.; Li, H.; Sun, M.; Han, S.; Cai, C.; Shen, W.; Fu, Y. Ultrafast Response/Recovery and High Selectivity of the H2S Gas Sensor Based on α-Fe2O3 Nano-Ellipsoids from One-Step Hydrothermal Synthesis. ACS Appl. Mater. Interfaces 2019, 11, 12761–12769. [Google Scholar] [CrossRef]
- Zhao, N.; Cheng, M.; Huang, S.; Liu, D.; Zhao, Q.; Bai, Y.; Zhang, X. Various Multicharged Anions of Ginsenosides in Negative Electrospray Ionization with QTOF High-Resolution Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2019, 30, 403–418. [Google Scholar] [CrossRef]
- Gendensuren, B.; Sugartseren, N.; Kim, M.; Oh, E.-S. Incorporation of aniline tetramer into alginate-grafted-polyacrylamide as polymeric binder for high-capacity silicon/graphite anodes. Chem. Eng. J. 2022, 433, 133553. [Google Scholar] [CrossRef]
- Shin, Y.S.; Chae, B.; Jung, Y.M.; Lee, S.W. Thermal imidization behaviors of 6FDA-ODA poly(amic acid) containing curing accelerators by in-situ FTIR spectroscopy. Vib. Spectrosc. 2020, 106, 103007. [Google Scholar] [CrossRef]
- Xu, F.; Jamal, R.; Ubul, A.; Shao, W.; Abdiryim, T. Characterization and electrochemical properties of poly(aniline-co-o-methoxyaniline)/multi-walled carbon nanotubes composites synthesized by solid-state method. Fibers Polym. 2013, 14, 8–15. [Google Scholar] [CrossRef]
- Ahmed, M.M.M.; Chi, Y.-T.; Hung, Y.-H.; Reyes, L.M.C.; Yeh, J.-M. UV-cured electroactive polyurethane acrylate coatings with superhydrophobic surface structure of biomimetic peacock feather for anticorrosion application. Prog. Org. Coat. 2022, 165, 106679. [Google Scholar] [CrossRef]
- Querner, C.; Reiss, P.; Zagorska, M.; Renault, O.; Payerne, R.; Genoud, F.; Rannou, P.; Pron, A. Grafting of oligoaniline on CdSe nanocrystals: Spectroscopic, electrochemical and spectroelectrochemical properties of the resulting organic/inorganic hybrid. J. Mater. Chem. 2005, 15, 554–563. [Google Scholar] [CrossRef]
- Aoki, K.J.; Chen, J.; Liu, Y.; Jia, B. Peak potential shift of fast cyclic voltammograms owing to capacitance of redox reactions. J. Electroanal. Chem. 2020, 856, 113609. [Google Scholar] [CrossRef]
- Bibi, A.; Rubio, Y.R.M.; Xian-Lun, L.; Sathishkumar, N.; Chen, C.-Y.; Santiago, K.S.; Chen, H.-T.; Lin, Y.-F.; Yeh, J.-M. Detection of hydrogen sulfide using polyaniline incorporated with graphene oxide aerogel. Synth. Met. 2021, 282, 116934. [Google Scholar] [CrossRef]
- Zhang, B.; Shang, F.; Shi, X.; Yao, R.; Wei, F.; Hou, X.; Li, W.; Zhang, J. Polyaniline/CuO Nanoparticle Composites for Use in Selective H2S Sensors. ACS Appl. Nano Mater. 2023, 6, 18413–18425. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, Y.; Wang, Y.; Li, Z.; Xiao, D.; Zhou, T.; Sun, M. Flexible Gas Sensor Based on PANI/WO3/CuO for Room-Temperature Detection of H2S. Sensors 2025, 25, 2640. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, J.; Zhou, R.; Liu, H.; Chang, Y.; Babichuk, I.S. A polymer gel-gated flexible transistor based on chitosan physically cross-linked films for hydrogen sulfide gas sensing. Sens. Actuators A Phys. 2025, 390, 116593. [Google Scholar] [CrossRef]
- Agbor, N.E.; Petty, M.C.; Monkman, A.P. Polyaniline thin films for gas sensing. Sens. Actuators B Chem. 1995, 28, 173–179. [Google Scholar] [CrossRef]







| Code | Dianhydride | Diamine * | |
|---|---|---|---|
| BPDA Content | ODA Content | PEDA Content | |
| EPAA1 | 1.47 g | 0.99 | 0.03 |
| EPAA2 | 0.98 | 0.05 | |
| EPAA3 | 0.97 | 0.08 | |
| EPAA5 | 0.96 | 0.13 | |
| Code | Conductivity (S/cm) | |||
|---|---|---|---|---|
| Intrinsic | Doped | Dedoped | Redoped | |
| EPAA1 | 2.19 × 10−6 | 1.69 × 10−4 | 1.16 × 10−6 | 9.59 × 10−5 |
| EPAA2 | 4.62 × 10−6 | 1.97 × 10−4 | 1.32 × 10−6 | 9.62 × 10−5 |
| EPAA3 | 7.53 × 10−6 | 2.51 × 10−4 | 1.45 × 10−6 | 9.56 × 10−5 |
| EPAA5 | 2.01 × 10−6 | 1.82 × 10−4 | 1.02 × 10−6 | 9.46 × 10−5 |
| Sample Code | Response | Gas Concentration (ppm) | Response/Recovery Time (s) | Ref. |
|---|---|---|---|---|
| PANI–PEO | 25% | 10 | 120/250 | [10] |
| SnO2/rGO/PANI | 91.11% | 10 | 80/88 | [11] |
| PANI/CuO | 188% | 25 | 306/296 | [38] |
| PANI/WO3/CuO | 90.1% | 10 | 353/4958 | [39] |
| CS/1,3-propanediol conductive polymer gel | 32% | 10 | 23/17 | [40] |
| EPAA3 | 591.3% | 10 | 108/1128 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, K.-H.; Chen, Y.-T.; Wu, H.-Y.; Ni, Z.-K.; Yeh, J.-M. Electroactive Poly(amic acid) Films Grafted with Pendant Aniline Tetramer for Hydrogen Sulfide Gas Sensing Applications. Polymers 2025, 17, 1915. https://doi.org/10.3390/polym17141915
Luo K-H, Chen Y-T, Wu H-Y, Ni Z-K, Yeh J-M. Electroactive Poly(amic acid) Films Grafted with Pendant Aniline Tetramer for Hydrogen Sulfide Gas Sensing Applications. Polymers. 2025; 17(14):1915. https://doi.org/10.3390/polym17141915
Chicago/Turabian StyleLuo, Kun-Hao, Yun-Ting Chen, Hsuan-Yu Wu, Zong-Kai Ni, and Jui-Ming Yeh. 2025. "Electroactive Poly(amic acid) Films Grafted with Pendant Aniline Tetramer for Hydrogen Sulfide Gas Sensing Applications" Polymers 17, no. 14: 1915. https://doi.org/10.3390/polym17141915
APA StyleLuo, K.-H., Chen, Y.-T., Wu, H.-Y., Ni, Z.-K., & Yeh, J.-M. (2025). Electroactive Poly(amic acid) Films Grafted with Pendant Aniline Tetramer for Hydrogen Sulfide Gas Sensing Applications. Polymers, 17(14), 1915. https://doi.org/10.3390/polym17141915









