Research Progress on Vegetable Oil-Based UV-Curing Resins
Abstract
1. Introduction
2. Research Background of Vegetable Oil-Based UV-Curable Materials
3. Research on Vegetable Oil-Based Ultraviolet Curing Materials
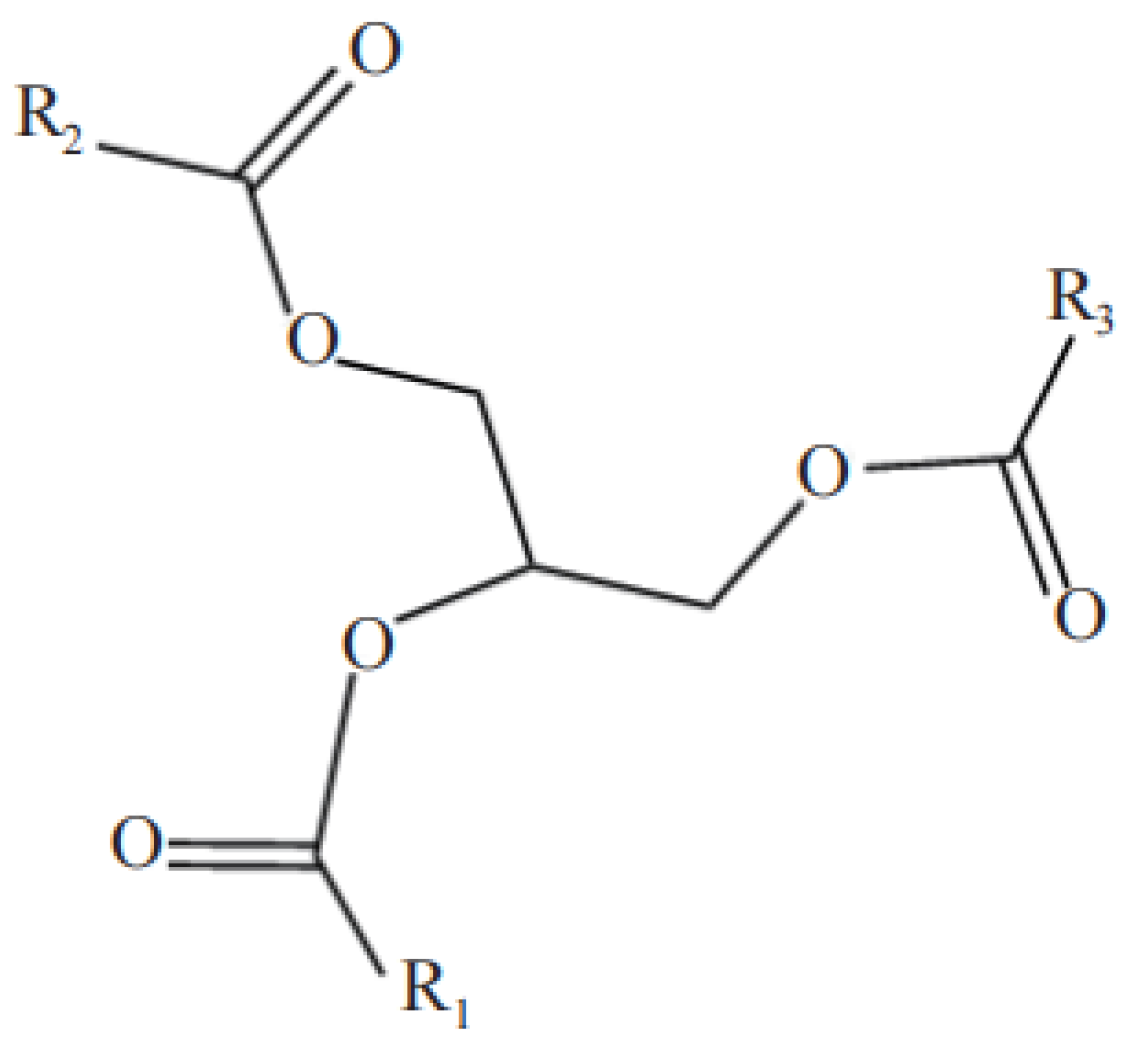
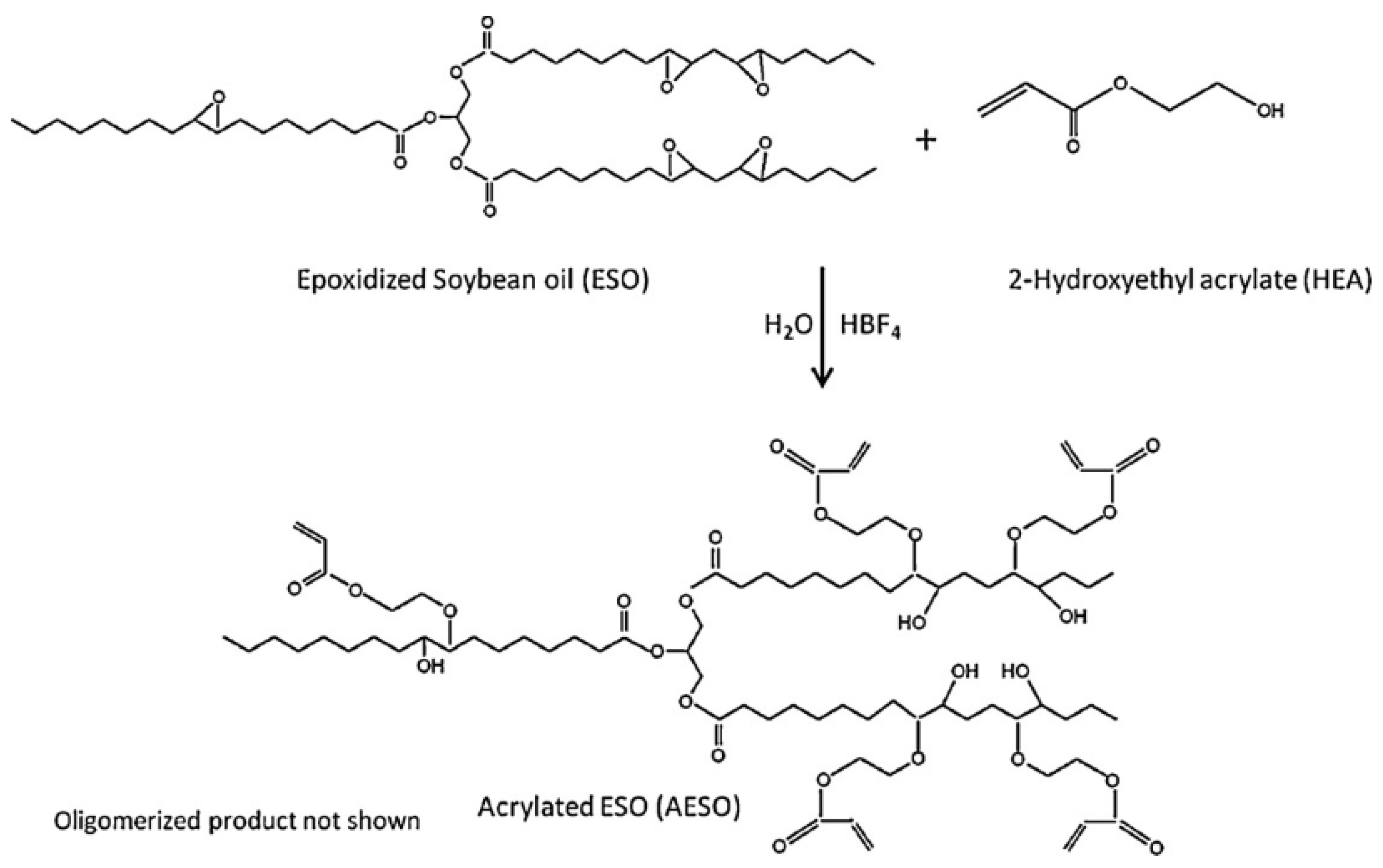
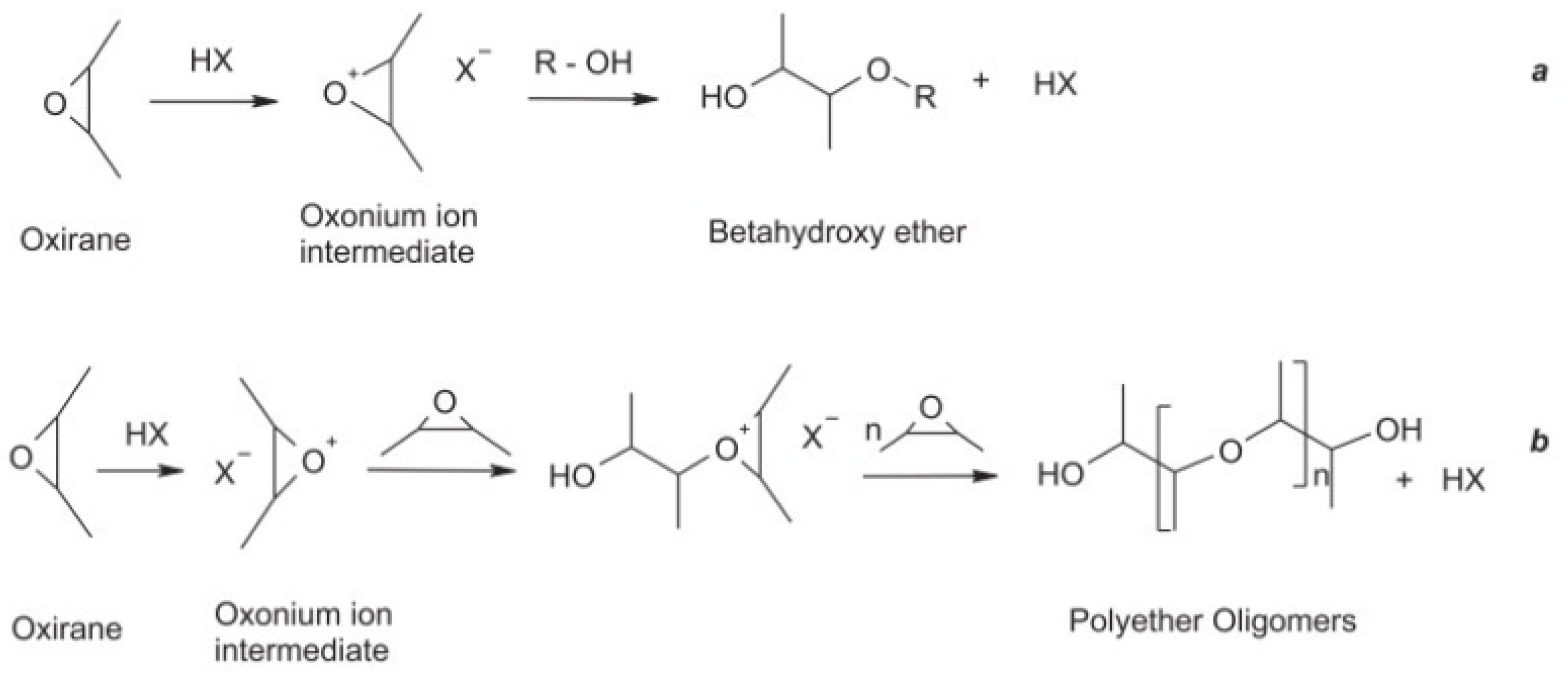
3.1. Soybean Oil
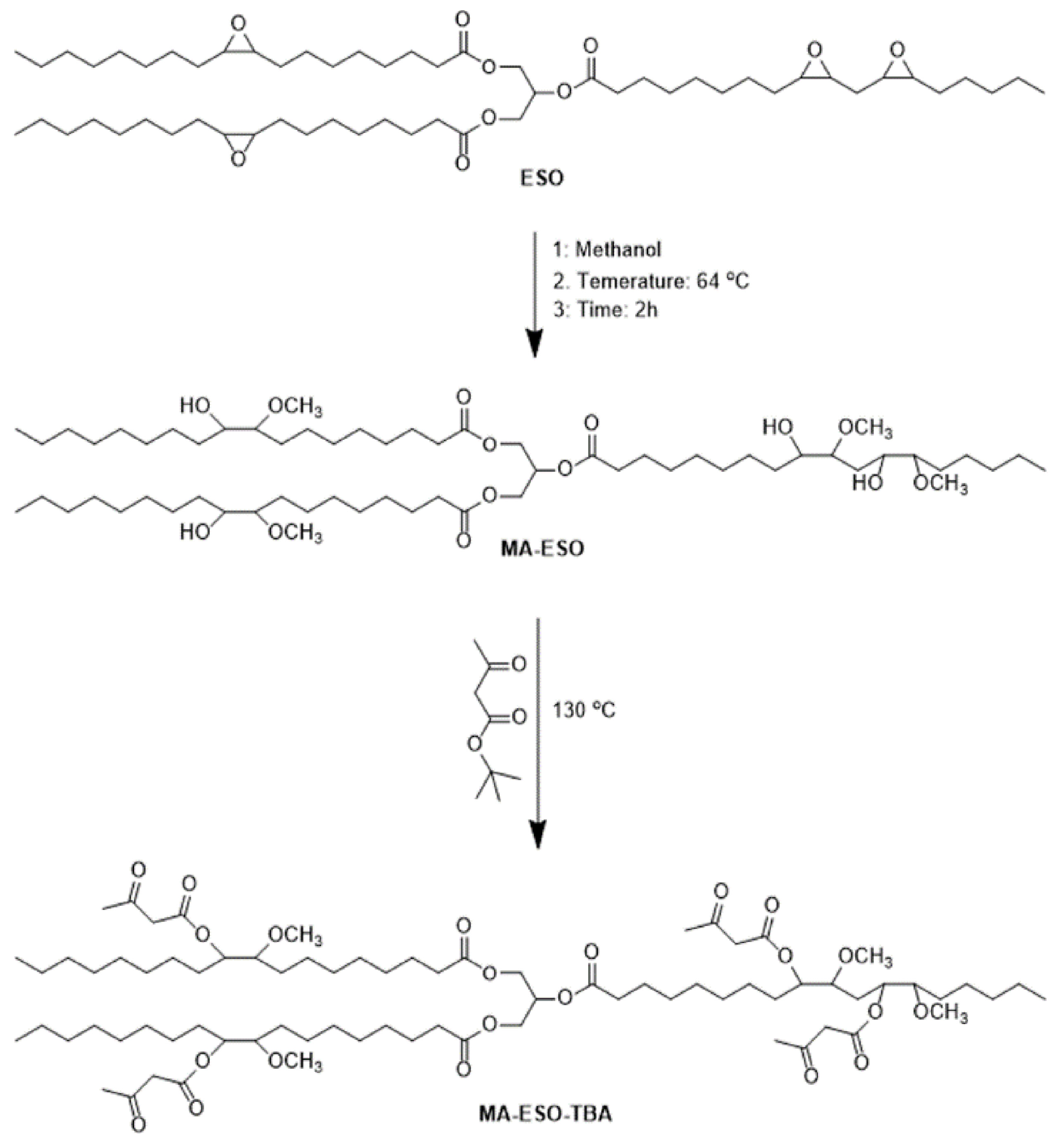
3.1.1. Epoxidation Ring-Opening Modification
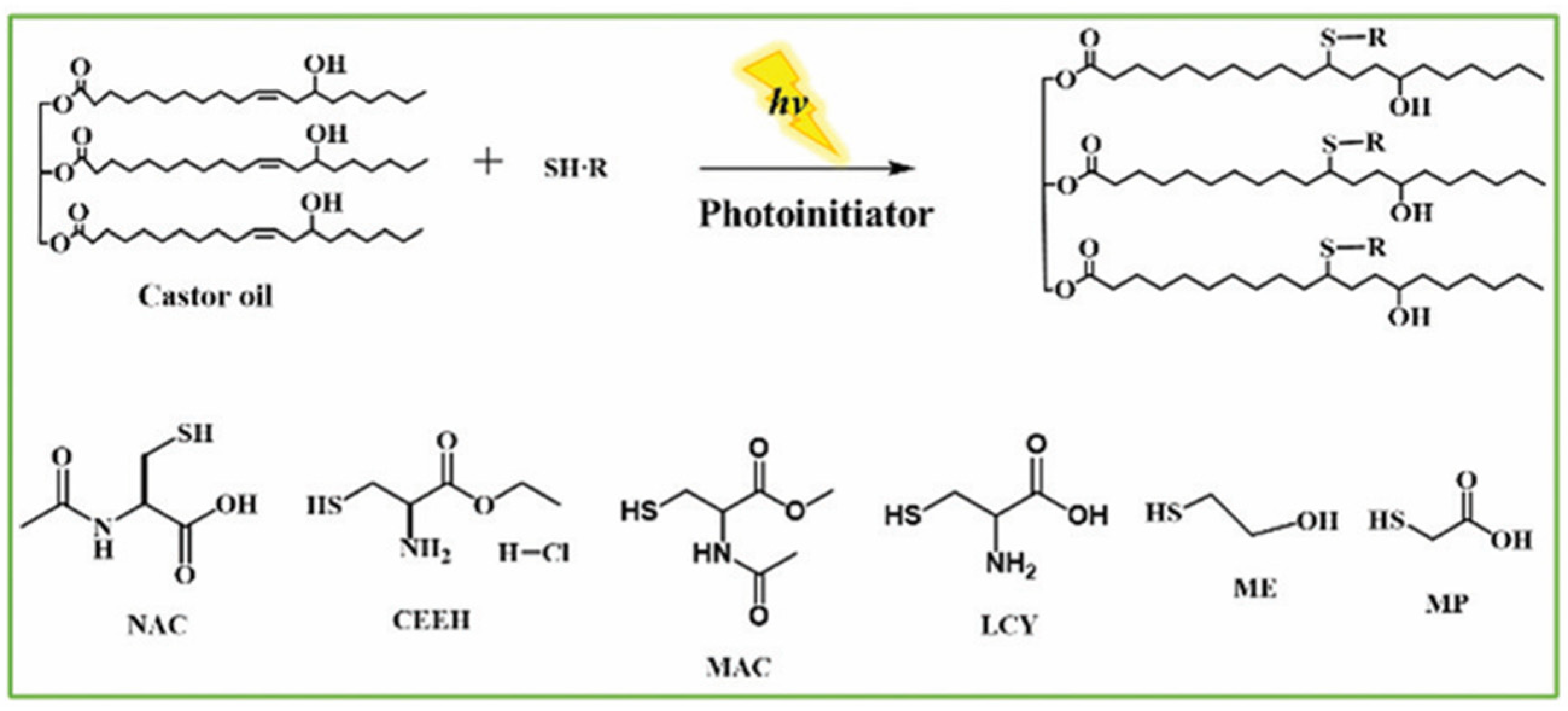
3.1.2. Mercapto-Enes Modification
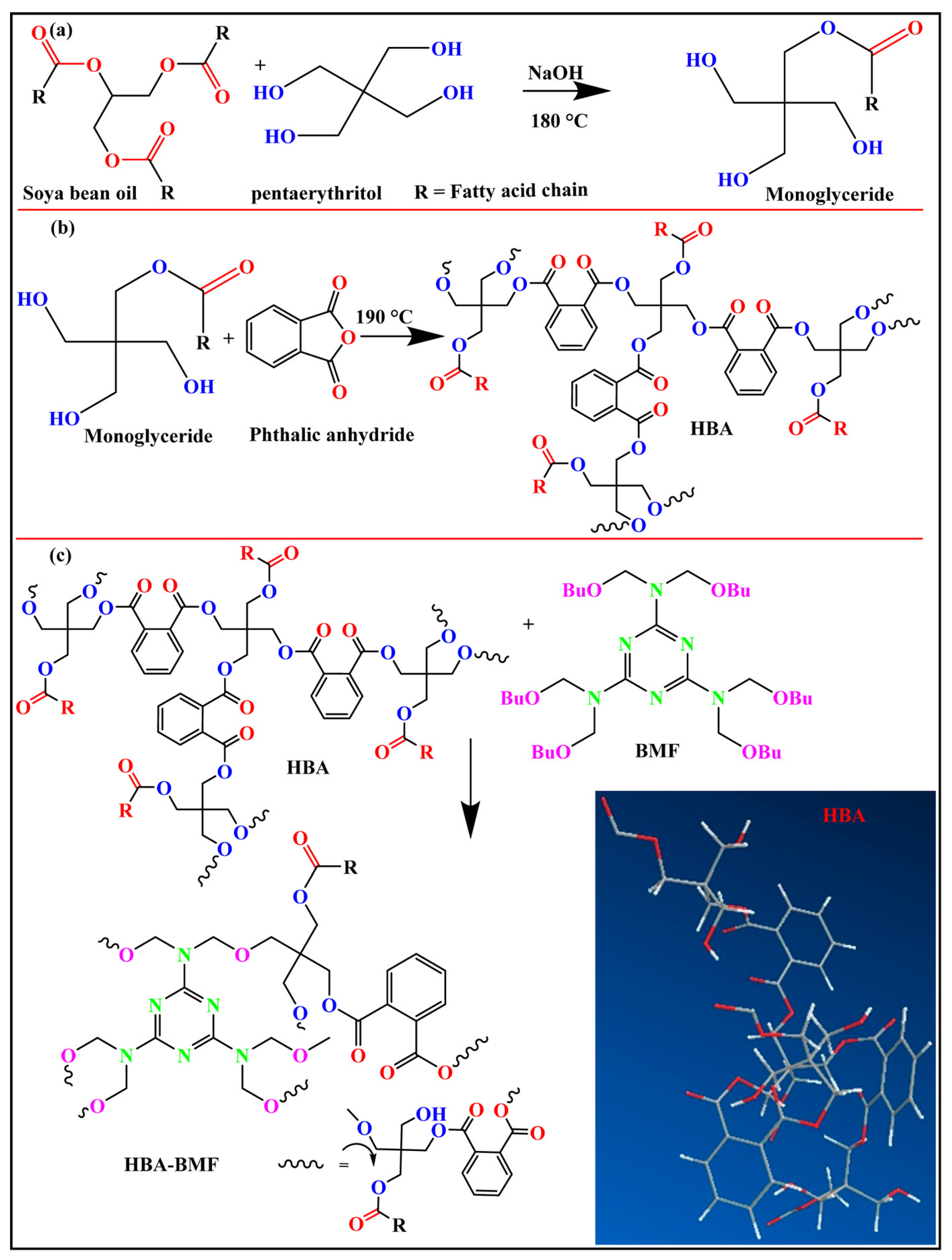
3.1.3. Alcohol Hydrolysis Modification
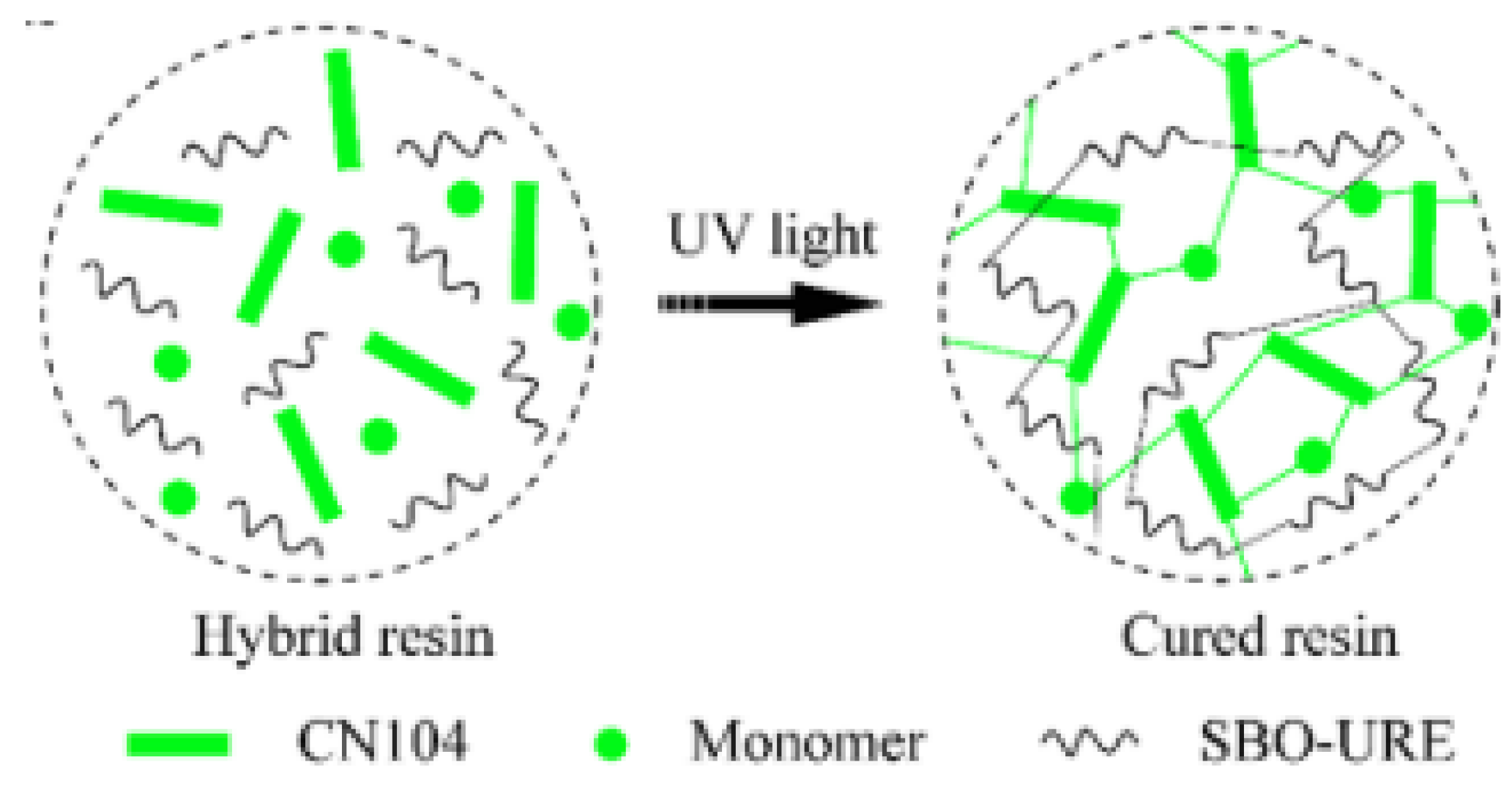
3.2. Castor Oil
3.2.1. Mercapto-Ene Modification
3.2.2. Polyurethane Acrylic Modification
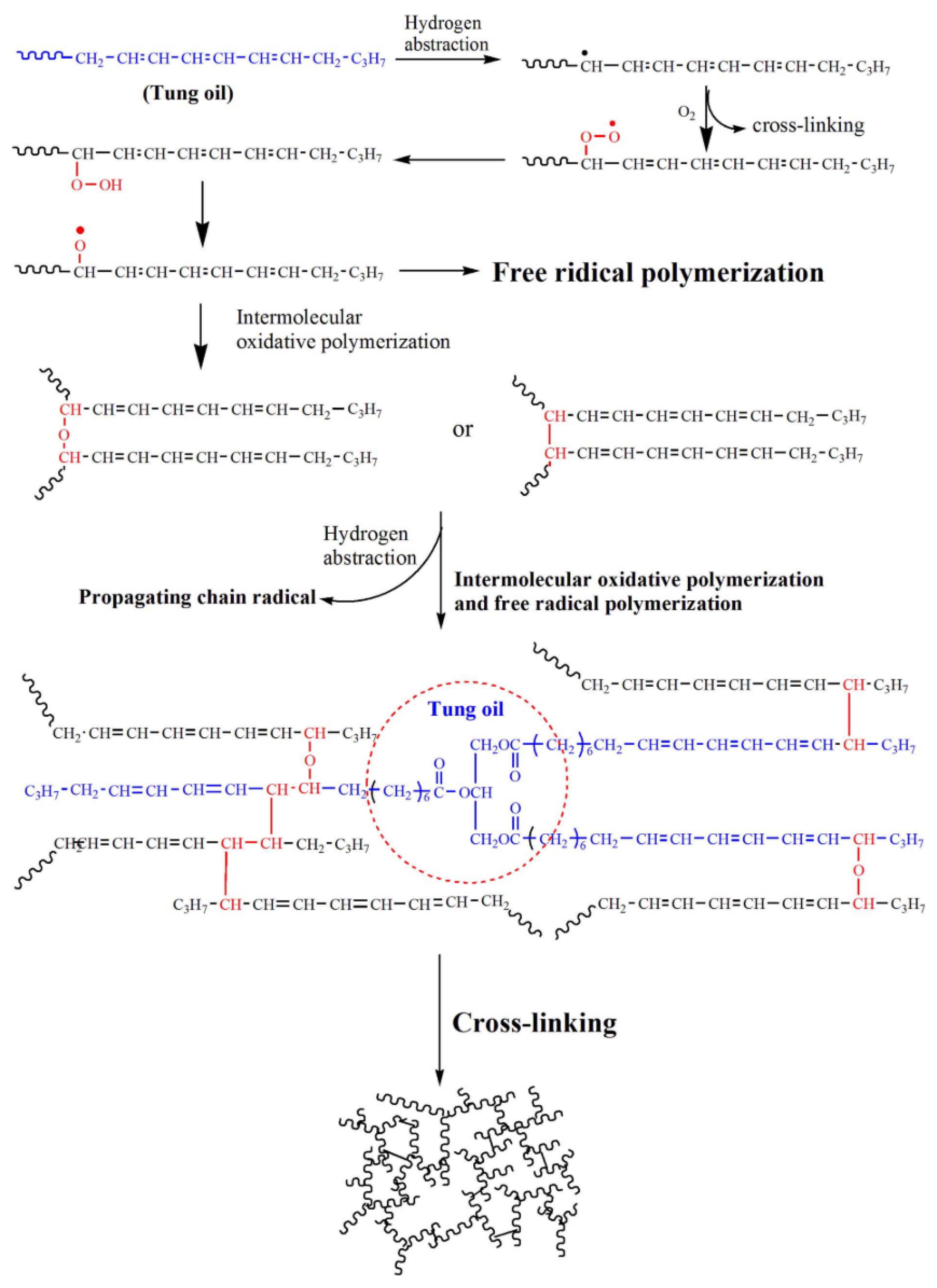
3.3. Other Vegetable Oils
4. Application
4.1. Coatings
4.2. Adhesive
4.3. Three-Dimensional Printing
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, Q.; Hu, Y.; Zhang, J.S.; Lu, J.Y.; Liu, C.G.; Lei, W. Research progress on vegetable oil-based light-curing resins. Polym. Mater. Sci. Eng. 2020, 36, 188–195. [Google Scholar]
- Wang, S.; Dai, J.; Teng, N.; Hu, J.; Zhao, W.; Liu, X. Synthesis of mechanically robust and self-healing UV-curable materials from renewable feedstock, ACS Sustain. Chem. Eng. 2020, 8, 16842–16852. [Google Scholar]
- Wu, Q.; Hu, Y.; Tang, J.; Zhang, J.; Wang, C.; Shang, Q.; Feng, G.; Liu, C.; Zhou, Y.; Lei, W. High-performance soybean-oil-based epoxy acrylate resins: “green” synthesis and application in UV-curable coatings, ACS Sustain. Chem. Eng. 2018, 6, 8340–8349. [Google Scholar] [CrossRef]
- Yang, Z.H.; Li, P.S.; Chu, Z.Z.; Yuan, T.; Hu, Y. Research progress of vegetable oil-based UV curable materials. J. South China Agric. Univ. 2022, 43, 1–12. [Google Scholar]
- Liu, K.; Madbouly, S.A.; Kessler, M.R. Biorenewable thermosetting copolymer based on soybean oil and eugenol. Eur. Polym. J. 2015, 69, 16–28. [Google Scholar] [CrossRef]
- Salih, A.M.; Ahmad, M.B.; Ibrahim, N.A.; Dahlan, K.Z.H.M.; Tajau, R.; Mahmood, M.H.; Yunus, W.M.; Wan, Z. Synthesis of radiation curable palm oil–based epoxy acrylate: NMR and FTIR spectroscopic investigations. Molecules 2015, 20, 14191–14211. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Qian, Y.; Sutton, C.A.; Scala, J.J.; Webster, D.C.; Sibi, M.P. Bio-based furanic di (meth) acrylates as reactive diluents for UV curable coatings: Synthesis and coating evaluation. ACS Sustain. Chem. Eng. 2021, 9, 15537–15544. [Google Scholar] [CrossRef]
- Chen, Z.; Chisholm, B.J.; Patani, R.; Wu, J.F.; Fernando, S.; Jogodzinski, K.; Webster, D.C. Soy-based UV-curable thiol–ene coatings. J. Coat. Technol. Res. 2010, 7, 603–613. [Google Scholar] [CrossRef]
- Hu, Y.; Shang, Q.; Tang, J.; Wang, C.; Zhang, F.; Jia, F.; Feng, G. Use of cardanol-based acrylate as reactive diluent in UV-curable castor oil-based polyurethane acrylate resins. Ind. Crop. Prod. 2018, 117, 295–302. [Google Scholar] [CrossRef]
- Yan, R.; Yang, D.; Zhang, N.; Zhao, Q.; Liu, B.; Xiang, W.; Sun, Z.; Xu, R.; Zhang, M.; Hu, W. Performance of UV curable lignin based epoxy acrylate coatings. Prog. Org. Coat. 2018, 116, 83–89. [Google Scholar] [CrossRef]
- Liu, R.; Zheng, J.; Guo, R.; Luo, J.; Yuan, Y.; Liu, X. Synthesis of new biobased antibacterial methacrylates derived from tannic acid and their application in UV-cured coatings. Ind. Eng. Chem. Res. 2014, 53, 10835–10840. [Google Scholar] [CrossRef]
- Mauro, C.D.; Tran, T.N.; Mija, A. One-pot terpolymerization synthesis of high carbon biocontent recyclable epoxy thermosets and their composites with flax woven fibers. ACS Sustain. Chem. Eng. 2021, 9, 8526–8538. [Google Scholar] [CrossRef]
- Arvin, Z.Y.; Rahimi, A.; Webster, D.C. High performance bio-based thermosets from dimethacrylated epoxidized sucrose soyate (DMESS). Eur. Polym. 2018, 99, 202–211. [Google Scholar]
- Mauro, C.D.; Genua, A.; Rymarczyk, M.; Dobbels, C.; Malburet, S.; Graillot, A.; Mija, A. Chemical and mechanical reprocessed resins and bio-composites based on five epoxidized vegetable oils thermosets reinforced with flax fibers or PLA woven. Compos. Sci. Technol. 2021, 205, 108678. [Google Scholar] [CrossRef]
- Bansal, K.; Webster, D.; Quadir, M. Self-assembled nanostructures from amphiphilic sucrose-soyates for solubilizing hydrophobic guest molecules. Langmuir 2022, 38, 2066–2075. [Google Scholar] [CrossRef]
- Monono, E.M.; Webster, D.C.; Wiesenborn, D.P. Pilot scale (10 kg) production and characterization of epoxidized sucrose soyate. Ind. Crop. Prod. 2015, 74, 987–997. [Google Scholar] [CrossRef]
- Alcock, T.D.; Salt, D.E.; Wilson, P.; Ramsden, S.J. More sustainable vegetable oil: Balancing productivity with carbon storage opportunities. Sci. Total Environ. 2022, 829, 154539. [Google Scholar] [CrossRef]
- Yuan, H.; Guo, H.X.; Huang, Z.S.; Zhang, P.; Wang, Z.; Jin, C.D. Synthesis of multifunctional polyurethane wood coating from epoxidized soybean oil and CO2-based polyol. Colloids Surf. A Physicochem. Eng. Asp. 2025, 709, 136162. [Google Scholar] [CrossRef]
- Shan, B.L.; Cao, X.J.; Zhang, X.Q.; Yang, X.Y.; Tang, B.M. Sustainable synthesis of polyurethane using epoxidized soybean oil for enhanced performance of modified asphalt. Case Stud. Constr. Mater. 2025, 22, e04795. [Google Scholar] [CrossRef]
- Jiang, J.P.; Yang, W.Q.; Lee, S.H.; Lum, W.C.; Du, G.B.; Ren, Y.H.; Zhou, X.J.; Zhang, J. Reactive bio-based flame retardant derived from chitosan, phytic acid, and epoxidized soybean oil for high-performance biodegradable foam. Int. J. Biol. Macromol. 2025, 307, 142137. [Google Scholar] [CrossRef]
- Xu, T.L.; Ju, X.R.; Tang, H.; Xiang, W.L. Preparation and performance evaluation of bio-based wood-plastic composites from ricinoleic acid ring-openning epoxidized soybean oil. React. Funct. Polym. 2024, 203, 106015. [Google Scholar] [CrossRef]
- Varganici, C.D.; Rosu, L.; Rosu, D.; Mustata, F.; Rusu, T. Sustainable wood coatings made of epoxidized vegetable oils for ultraviolet protection. Environ. Chem. Lett. 2021, 19, 307–328. [Google Scholar] [CrossRef]
- Lim, S.J.; Kim, D.S. Effect of functionality and content of epoxidized soybean oil on the physical properties of a modified diglycidyl ether of bisphenol A resin system. J. Appl. Polym. Sci. 2021, 138, e50411. [Google Scholar] [CrossRef]
- Rengasamy, S.; Mannari, V. Development of soy-based UV-curable acrylate oligomers and study of their film properties. Prog. Org. Coat. 2013, 76, 78–85. [Google Scholar] [CrossRef]
- Cui, Y.Y.; Yang, J.L.; Lei, D.H.; Su, J.H. 3D Printing of a Dual-Curing Resin with Cationic Curable Vegetable Oil. Ind. Eng. Chem. Res. 2020, 59, 11381–11388. [Google Scholar] [CrossRef]
- Jurinovs, M.; Barkane, A.; Platnieks, O.; Beluns, S.; Grase, L.; Dieden, R.; Staropoli, M.; Schmidt, D.F.; Gaidukovs, S. Vat Photopolymerization of Nanocellulose-Reinforced Vegetable Oil-Based Resins: Synergy in Morphology and Functionalization. ACS Appl. Polym. Mater. 2023, 5, 3104–3118. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Li, P.; Sun, S.D. Biopolyol synthesis via ring-opening of epoxidized soybean oil with glycerol utilizing a novel sulfonic-functionalized ionic liquid as the catalyst at low temperature. Ind. Crops Prod. 2024, 222, 119546. [Google Scholar] [CrossRef]
- Hoyle, C.E.; Bowman, C.N. Thiol-ene click chemistry. Angew. Chem. 2010, 49, 1540–1573. [Google Scholar] [CrossRef]
- Fertier, L.; Koleilat, H.; Stemmelen, M.; Giani, O.; Joly-Duhamel, C.; Lapinte, V.; Robin, J.-J. The use of renewable feedstock in UV-curable materials–a new age for polymers and green chemistry. Prog. Polym. Sci. 2013, 38, 932–962. [Google Scholar] [CrossRef]
- Lligadas, G.; Ronda, J.C.; Galià, M.; Cádiz, V. Monomers and polymers from plant oils via click chemistry reactions. J. Polym. Sci. A Polym. Chem. 2013, 51, 2111–2124. [Google Scholar] [CrossRef]
- Desroches, M.; Caillol, S.; Lapinte, V.; Auvergne, R.; Boutevin, B. Synthesis of Biobased Polyols by Thiol−Ene Coupling from Vegetable Oils. Macromolecules 2011, 44, 2489–2500. [Google Scholar] [CrossRef]
- He, M.H.; Jiang, S.; Xu, R.X.; Yang, J.; Zeng, Z.; Chen, G. Facile functionalization of soybean oil by thiol-ene photo-click reaction for the synthesis of polyfunctional acrylate. Prog. Org. Coat. 2014, 77, 868–871. [Google Scholar] [CrossRef]
- Alagi, P.; Ye, J.C.; Seog, J.; Hong, S.C. Efficient and quantitative chemical transformation of vegetable oils to polyols through a thiol-ene reaction for thermoplastic polyurethanes. Ind. Crops Prod. 2016, 87, 78–88. [Google Scholar] [CrossRef]
- Feng, Y.; Liang, H.; Yang, Z.; Yuan, T.; Luo, Y.; Li, P.; Yang, Z.; Zhang, C. A Solvent-Free and Scalable Method To Prepare Soybean-Oil-Based Polyols by Thiol–Ene Photo-Click Reaction and Biobased Polyurethanes Therefrom. ACS Sustain. Chem. Eng. 2017, 5, 7365–7373. [Google Scholar] [CrossRef]
- Denis, M.; Paré, A.; Borgne, D.L.; Caillol, S.; Negrell, C. HydroxyUrethane Modifiers (HUM): An environmentally-friendly route to improve chemical resistance of alkyd coatings. Prog. Org. Coat. 2023, 183, 107734. [Google Scholar] [CrossRef]
- Rahman, O.U.; Bhat, O.I.; Yu, H.B.; Ahmad, S. Hyperbranched Soya Alkyd Nanocomposite: A Sustainable Feedstock-Based Anticorrosive Nanocomposite Coatings. ACS Sustain. Chem. Eng. 2017, 5, 9725–9734. [Google Scholar] [CrossRef]
- Irfan, M.; Bhat, S.I.; Ahmad, S. Reduced Graphene Oxide Reinforced Waterborne Soy Alkyd Nanocomposites: Formulation, Characterization, and Corrosion Inhibition Analysis. ACS Sustain. Chem. Eng. 2018, 6, 14820–14830. [Google Scholar] [CrossRef]
- Chukwuebuka, A.S.; Samuel, E.O.; Asadu, C.O.; Onoghwarite, O.E.; Celestine, O.; Ezema, C.A. Optimal analysis of the effects of process conditions on the yield of alkyd resins from castor and soybean seed oils using response surface methodology. Chem. Eng. J. Adv. 2022, 9, 100217. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, H.; Zeng, W.; Zhou, Q. High Biobased Carbon Content Polyurethane Dispersions Synthesized from Fatty Acid-Based Isocyanate. Ind. Eng. Chem. Res. 2019, 58, 5195–5201. [Google Scholar] [CrossRef]
- Jian, X.Y.; He, Y.; Li, Y.D.; Wang, M.; Zeng, J.B. Curing of epoxidized soybean oil with crystalline oligomeric poly(butylene succinate) towards high performance and sustainable epoxy resins. Chem. Eng. J. 2017, 326, 875–885. [Google Scholar] [CrossRef]
- Li, M.; Xia, J.; Mao, W.; Yang, X.; Xu, L.; Huang, K.; Li, S. Preparation and Properties of Castor Oil-Based Dual Cross-Linked Polymer Networks with Polyurethane and Polyoxazolidinone Structures. ACS Sustain. Chem. Eng. 2017, 5, 6883–6893. [Google Scholar] [CrossRef]
- Xu, D.D.; Cao, Z.Y.; Wang, T.; Zhong, J.; Zhao, J.Z.; Gao, F.; Luo, X.; Fang, Z.; Cao, J.; Xu, S.; et al. An ambient-cured coating film obtained via a Knoevenagel and Michael addition reactions based on modified acetoacetylated castor oil prepared by a thiol-ene coupling reaction. Prog. Org. Coat. 2019, 135, 510–516. [Google Scholar] [CrossRef]
- Wang, X.; Liang, H.; Jiang, J.; Wang, Q.; Luo, Y.; Feng, P.; Zhang, C. A cysteine derivative-enabled ultrafast thiol–ene reaction for scalable synthesis of a fully bio-based internal emulsifier for high-toughness waterborne polyurethanes. Green Chem. 2020, 22, 5722–5729. [Google Scholar] [CrossRef]
- Liang, B. Preparation and Performance of Vegetable Oil-Based UV-Curing Coatings. Ph.D. Thesis, South China Agricultural University, Guangzhou, China, 2020; pp. 50–52. [Google Scholar]
- Su, Y.; Ma, S.Q.; Wang, B.; Xu, X.; Feng, H.; Hu, K.; Zhang, W.; Zhou, S.; Weng, G.; Zhu, J. High-performance castor oil-based polyurethane thermosets: Facile synthesis and properties. React. Funct. Polym. 2023, 183, 105496. [Google Scholar] [CrossRef]
- Paramarta, A.; Pan, X.; Webster, D.C. Synthesis and photopolymerization of highly functional acrylated biobased resins. Polym. Prep. 2011, 52, 552–553. [Google Scholar]
- Sitaramam, B.S.; Chatterjee, P.C.; Sivasamban, M.A. Use of castorbased products in formulating UV-curable coatings. Paintindia 1986, 36, 17–18. [Google Scholar]
- Patel, K.I.; Parmar, R.J.; Parmar, J.S. Novel binder system for ultraviolet curable coatings based on tobacco seed (Nicotiana rustica) oil derivatives as a renewable resource. J. Appl. Polym. Sci. 2008, 107, 71–81. [Google Scholar] [CrossRef]
- Hu, Y. Preparation and Properties of UV-Curable Resin Based on Castor Oil and Cardanol. Ph.D. Thesis, Beijing Forestry University, Beijing, China, 2019; pp. 23–25. [Google Scholar]
- Li, P.S.; Chu, Z.Z.; Chen, Y.W.; Yuan, T.; Yang, Z. One-pot and solvent-free synthesis of castor oil-based polyurethane acrylate oligomers for UV-curable coatings applications. Prog. Org. Coat. 2021, 159, 106398. [Google Scholar] [CrossRef]
- Cheng, F.; Fan, Y.X.; He, N.; Song, Y.; Shen, J.; Gong, Z.; Tong, X.; Yang, X. Castor oil based high transparent UV cured silicone modified polyurethane acrylate coatings with outstanding tensile strength and good chemical resistance. Prog. Org. Coat. 2022, 163, 106624. [Google Scholar] [CrossRef]
- Zhu, G.Q.; Zhang, J.S.; Huang, J.; Qiu, Y.; Liu, M.; Yu, J.; Liu, C.; Shang, Q.; Hu, Y.; Hu, L.; et al. Recyclable and reprintable biobased photopolymers for digital light processing 3D printing. Chem. Eng. J. 2023, 452, 139401. [Google Scholar] [CrossRef]
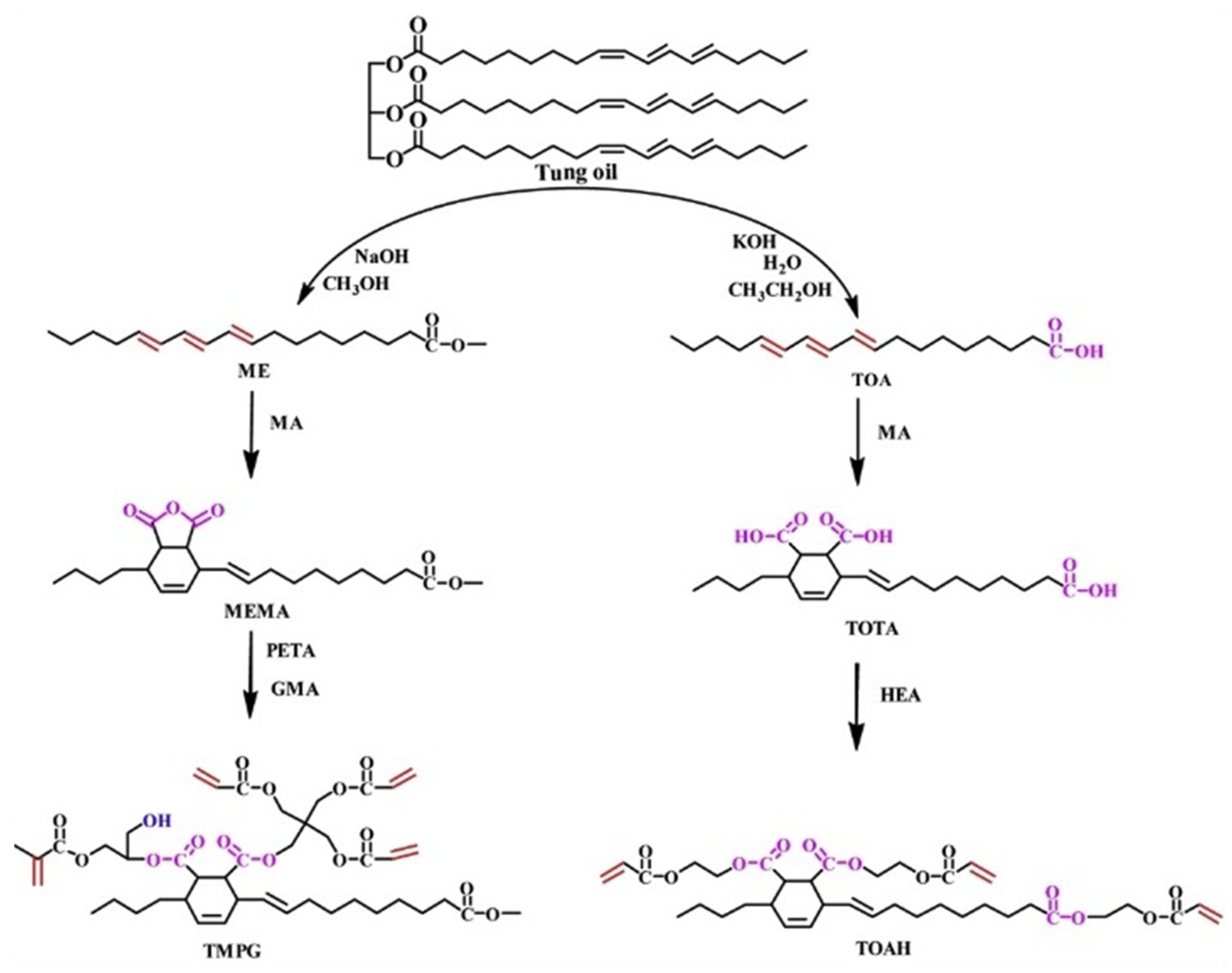
- Huang, J.J.; Yuan, T.; Ye, X.Y.; Man, L.M.; Zhou, C.; Hu, Y.; Zhang, C.Q.; Yang, Z.H. Study on the UV curing behavior of tung oil: Mechanism, curing activity and film-forming property. Ind. Crops Prod. 2018, 112, 61–69. [Google Scholar] [CrossRef]
- Liang, B.; Kuang, S.; Huang, J.; Man, L.; Yang, Z.; Yuan, T. Synthesis and characterization of novel renewable tung oil-based UV curable active monomers and bio-based copolymers. Prog. Org. Coat. 2019, 129, 116–124. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Khandelwal, V.; Manik, G. Synthesis and characterization of low viscous and highly acrylated epoxidized methyl ester based green adhesives derived from linseed oil. Int. J. Adhes. Adhes. 2019, 89, 174–177. [Google Scholar] [CrossRef]
- Samper, M.D.; Ferri, J.M.; Carbonell-Verdu, A.; Balart, R.; Fenollar, O. Properties of biobased epoxy resins from epoxidized linseed oil (ELO) crosslinked with a mixture of cyclic anhydride and maleinized linseed oil. eXPRESS Polym. Lett. 2019, 13, 407–418. [Google Scholar] [CrossRef]
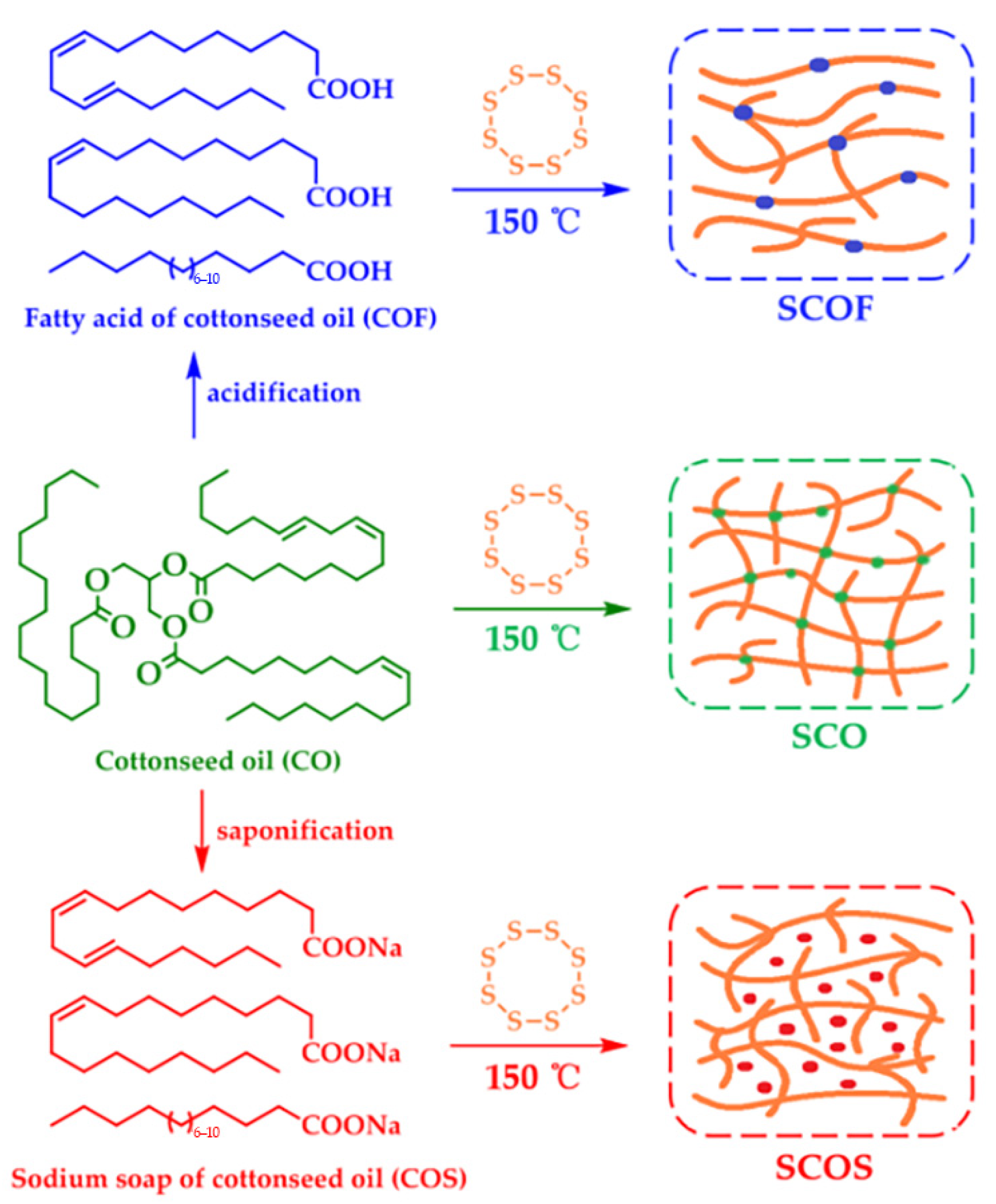
- Chen, Y.R.; Liu, Y.X.; Chen, Y.D.; Zhang, Y.G.; Zan, X.J. Design and Preparation of Polysulfide Flexible Polymers Based on Cottonseed Oil and Its Derivatives. Polymer 2020, 12, 1858. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.P.; Zhang, S.T.; Chen, Y.W.; Yuan, T.; Yang, Z.H. One-step synthesis of novel renewable multi-functional linseed oil-based acrylate prepolymers and its application in UV-curable coatings. Prog. Org. Coat. 2020, 148, 105820. [Google Scholar] [CrossRef]
- Hubmann, M.; Gunten, K.V.; Alessi, D.S.; Curtis, J.M. Epoxidized linseed lipids as a durable and fast-curing alternative to drying oils. Prog. Org. Coat. 2021, 159, 106406. [Google Scholar] [CrossRef]
- Hegde, M.B.; Mohana, K.N.S.; Rajitha, K.; Ambale Murthy Madhusudhana. Reduced graphene oxide-epoxidized linseed oil nanocomposite: A highly efficient bio-based anti-corrosion coating material for mild steel. Prog. Org. Coat. 2021, 159, 106399. [Google Scholar] [CrossRef]
- Zhao, Z.C.; Wu, H.; Liu, X.D.; Kang, D.S.; Xiao, Z.H.; Lin, Q.Q.; Zhang, A.H. Synthesis and characterization of tung oil-based UV curable for three-dimensional printing resins. RSC Adv. 2022, 12, 22119–22130. [Google Scholar] [CrossRef]
- Vonsul, M.I.; Webste, D.C. Investigation of cottonseed oil as renewable source for the development of highly functional UV-curable materials. Prog. Org. Coat. 2023, 185, 107883. [Google Scholar] [CrossRef]
- Liu, X.X.; Hu, Y.; Hu, L.H.; Zhang, M.; Jia, P.Y.; Zhou, Y.H. Recent progress in bio-based light-curable resins used for 3D printing: From synthetic strategies to structural properties and resin applications. React. Funct. Polym. 2025, 215, 106359. [Google Scholar] [CrossRef]
- Alves, L.R.; Carriello, G.M.; Pegoraro, G.M.; Filho, J.F. A utilização de óleos vegetais como fonte de polióis para a síntese de poliuretano: Uma revisão. Discip. Sci.|Nat. E Tecnológicas 2021, 22, 99–118. [Google Scholar] [CrossRef]
- Macalino, A.D.; Salen, V.A.; Reyes, L.Q. Castor oil based polyurethanes: Synthesis and characterization. IOP Conf. Ser. Mater. Sci. Eng. 2017, 229, 012016. [Google Scholar] [CrossRef]
- Mohanty, S.; Borah, K.; Kashyap, S.S.; Sarmah, S.; Bera, M.K.; Basak, P.; Narayan, R. Development of hydrophobic polyurethane film from structurally modified castor oil and its anticorrosive performance. Polym. Adv. Technol. 2023, 34, 351–362. [Google Scholar] [CrossRef]
- Yin, J.; Xiong, Y.H.; Zhou, X.H.; Yang, Z.H.; Yuan, T. An efficient halogen-free reactive flame-retardant active diluent for soy-castor oil-based fire safety UV-curable coatings. Prog. Org. Coat. 2022, 163, 106683. [Google Scholar] [CrossRef]
- Zhou, Y.M.; Feng, L.X.; Qu, J.Q. Preparation of high-performance epoxy soybean oil-based UV-curable oligomers and coatings. J. Coat. Technol. Res. 2023, 20, 1923–1933. [Google Scholar] [CrossRef]
- Tuo, Y.; Luo, X.B.; Xiong, Y.H.; Xu, C.A.; Yuan, T. A Novel Polyfunctional Polyurethane Acrylate Derived from Castor Oil-Based Polyols for Waterborne UV-Curable Coating Application. Polymers 2024, 16, 949. [Google Scholar] [CrossRef]
- Peng, J.; Chen, B.; Tang, H.; Luo, X.H.; Zhou, C.L.; Liu, Y.L. Vinyl-terminated photoactive citric acid derivatives for fabricating hard yet flexible soybean oil-based UV-curable coatings. Ind. Crops Prod. 2025, 228, 120966. [Google Scholar] [CrossRef]
- Peng, J.; Zhou, C.L.; Chen, B.; Zhang, H.J.; Pan, X.Y.; Xiong, W.T.; Luo, X.H.; Liu, Y.L. Rational design of waterborne biobased epoxy methacrylates using citric acid and epoxy soybean oil for UV-curable coatings. Ind. Crops Prod. 2024, 209, 117958. [Google Scholar] [CrossRef]
- Sánchez, M.C.; Tenorio-Alfonso, A.; Franco, J.M. Synthesis and mechanical properties of bio-sourced polyurethane adhesives obtained from castor oil and MDI-modified cellulose acetate: Influence of cellulose acetate modification. Int. J. Adhes. Adhes. 2019, 95, 102404. [Google Scholar]
- Chattopadhyay, D.K.; Raju, K.V.S.N. Structural engineering of polyurethane coatings for high performance applications. Prog. Polym. Sci. 2007, 32, 352–418. [Google Scholar] [CrossRef]
- Luo, Z.; Shi, Y.; Zhao, D.; He, M. Synthesis of epoxidatied castor oil and its effect on the properties of waterborne polyurethane. Procedia Eng. 2011, 18, 37–42. [Google Scholar] [CrossRef]
- Yuan, T.; Yang, Z. A renewable and multifunctional eco-friendly coating from novel tung oil-based cationic waterborne polyurethane dispersions. J. Clean. Prod. 2019, 241, 118341. [Google Scholar]
- Tavares, L.B.; Boas, C.V.; Schleder, G.R.; Nacas, A.M.; Rosa, D.S.; Santos, D.J. Bio-Based Polyurethane Prepared from Kraft Lignin and Modified Castor Oil. Express Polym. Lett. 2016, 10, 927–940. [Google Scholar] [CrossRef]
- Sardon, H.; Mecerreyes, D.; Basterretxea, A.; Avérous, L.; Jehanno, C. From Lab to Market: Current Strategies for the Production of Biobased Polyols. ACS Sustain. Chem. Eng. 2021, 9, 10664–10677. [Google Scholar] [CrossRef]
- Alhanish, A.; Abu Ghalia, M. Developments of Biobased Plasticizers for Compostable Polymers in the Green Packaging Applications: A Review. Biotechnol. Prog. 2021, 37, e3210. [Google Scholar] [CrossRef]
- Kaikade, D.S.; Sabnis, A.S. Recent Advances in Polyurethane Coatings and Adhesives Derived from Vegetable Oil-Based Polyols. J. Polym. Environ. 2023, 31, 4583–4605. [Google Scholar] [CrossRef]
- Li, C.H.; Xiao, H.; Wang, X.F.; Zhao, T. Development of green waterborne UV-curable vegetable oil-based urethane acrylate pigment prints adhesive: Preparation and application. J. Clean. Prod. 2018, 180, 272–279. [Google Scholar] [CrossRef]
- Yan, Y.Y.; Wu, J.; Wang, Y.; Fang, X.Z.; Wang, Z.K.; Yang, G.; Hua, Z. Strong and UV-Responsive Plant Oil-Based Ethanol Aqueous Adhesives Fabricated Via Surfactant-free RAFT-Mediated Emulsion Polymerization. ACS Sustain. Chem. Eng. 2021, 9, 13695–13702. [Google Scholar] [CrossRef]
- Li, C.; Chen, Y.Z.; Zeng, Y.; Wu, Y.C.; Liu, W.D.; Qiu, R.H. Strong and recyclable soybean oil-based epoxy adhesives based on dynamic borate. Eur. Polym. J. 2022, 162, 110923. [Google Scholar] [CrossRef]
- Calignano, F.; Manfredi, D.; Ambrosio, E.P.; Biamino, S.; Lombardi, M.; Atzeni, E.; Salmi, A.; Minetola, P.; Iuliano, L.; Fino, P. Overview on additive manufacturing technologies. Proc. IEEE 2017, 105, 593–612. [Google Scholar] [CrossRef]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3d printing and customized additive manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Qin, Q.; Wang, J. A review of stereolithography: Processes and systems. Processes 2020, 8, 1138. [Google Scholar] [CrossRef]
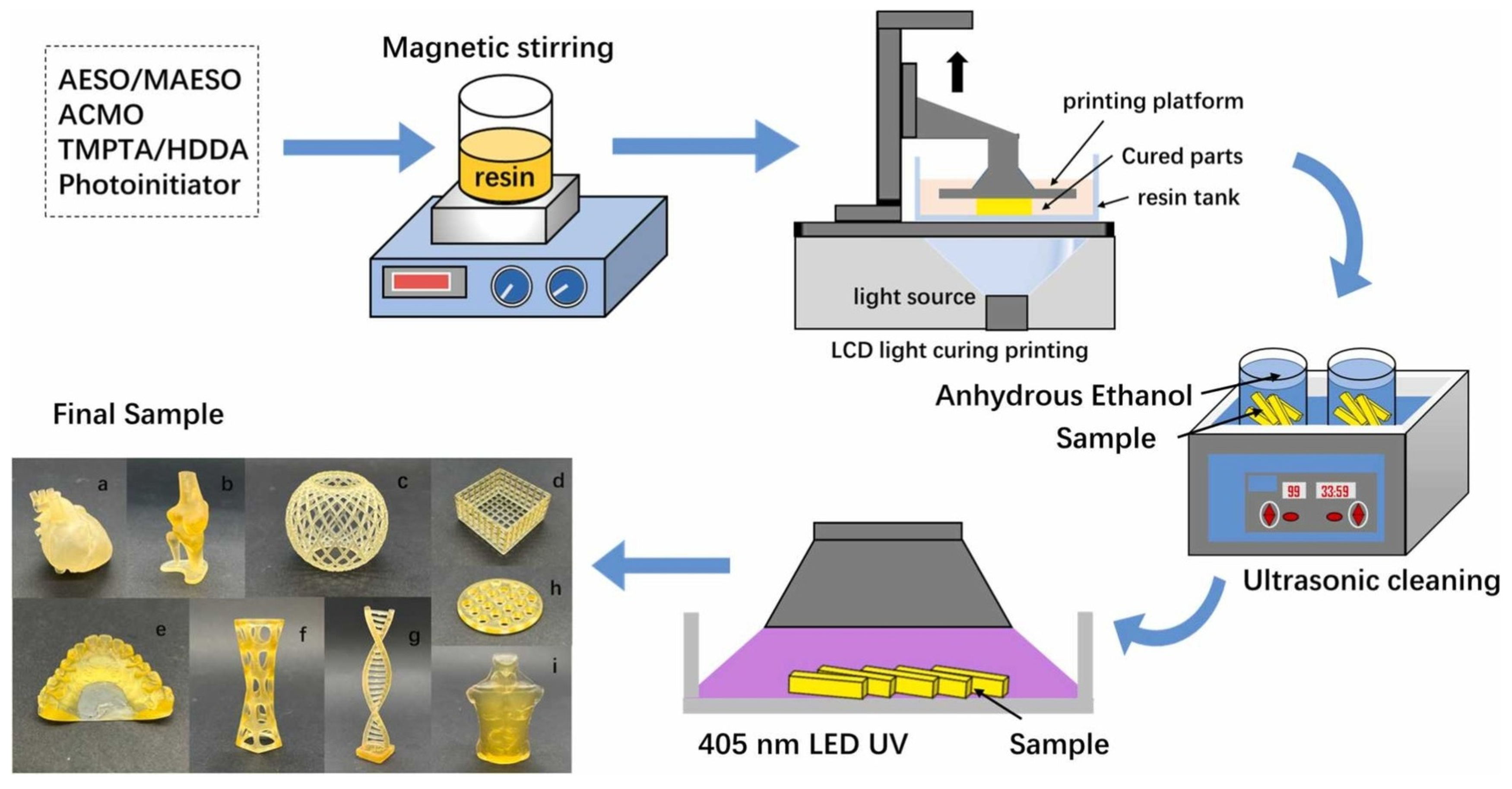
- Barkane, A.; Platnieks, O.; Jurinovs, M.; Kasetaite, S.; Ostrauskaite, J.; Gaidukovs, S.; Habibi, Y. UV-Light Curing of 3D Printing Inks from Vegetable Oils for Stereolithography. Polymers 2021, 13, 1195. [Google Scholar] [CrossRef]
- Lebedevaite, M.; Gineika, A.; Talacka, V.; Baltakys, K.; Ostrauskaite, J. Development and optical 3D printing of acrylated epoxidized soybean oil-based composites with functionalized calcium silicate hydrate filler derived from aluminium fluoride production waste. Compos. Part A Appl. Sci. Manuf. 2022, 157, 106929. [Google Scholar] [CrossRef]
- Briede, S.; Jurinovs, M.; Nechausov, S.; Platnieks, O.; Gaidukovs, S. State-of-the-art UV-assisted 3D printing via a rapid syringe-extrusion approach for photoactive vegetable oil acrylates produced in one-step synthesis. Mol. Syst. Des. Eng. 2022, 7, 1434–1448. [Google Scholar] [CrossRef]
- Chen, S.Y.; Zhang, Q.D.; Yang, Z.J.; Bian, Y.S.; Chen, G.G. Fabrication and characterization of light-curing soybean oil-basedepoxy resin applied for LCD additive manufacturing. Ind. Crops Prod. 2023, 202, 117037. [Google Scholar] [CrossRef]
- Bhanushali, H.; Mestry, S.; Mhaske, S.T. Castor oil-based UV-curable polyurethane acrylate resins for digital light processing (DLP) 3D printing technology. J. Appl. Polym. Sci. 2023, 140, 18. [Google Scholar] [CrossRef]












| The Specific Research Direction: | Selection and Modification |
|---|---|
| Diversification of raw materials | study the influence of structural properties (unsaturation, hydroxyl value, epoxy value) of different vegetable oils (soybean oil, flaxseed oil, castor oil, tung oil, etc.) on the properties of the final resin [3,8,14]. |
| Efficient Modification Technology | Epoxidation: Epoxy groups are introduced to improve reactivity. Acrylication/Methacrylate: Introduction of double bonds ((meth)acrylate groups required for light-curing) [2,6,16]. Optimize reaction conditions: (temperature, catalyst, polymerization inhibitor) to improve conversion and reduce side reactions. Hydroxylation: The hydroxyl group of the vegetable oil itself or the introduction of more hydroxyl groups is used to synthesize polyurethane acrylates [4,7]. Exchange/alcohololysis: Changing the fatty acid chain length or introducing polyols. Development of highly functional monomers: design and synthesis of vegetable oil derivatives with higher double bond functionality to increase curing speed and crosslinking density, and improve hardness, heat resistance and chemical resistance. |
| MNA/MLO [wt/wt] | Flexural Strength [MPa] | Flexural Modulus [MPa] | Shore D |
|---|---|---|---|
| 50/0 | 60.8 (5.8) | 1772.0 (132.0) | 82.2 (2.5) |
| 45/5 | 54.9 (5.4) | 1460.0 (73.5) | 81.9 (1.3) |
| 40/10 | 47.2 (3.1) | 1027.0 (46.5) | 78.2 (1.9) |
| 35/15 | 31.9 (4.8) | 718.2 (45.2) | 76.1 (1.5) |
| 30/20 | 23.8 (0.5) | 485.8 (33.0) | 71.4 (1.8) |
| 25/25 | 14.5 (0.6) | 272.3 (20.9) | 64.1 (1.5) |
| Type of Vegetable Oil | Structural Features | Modification Method | Superior Performance | Limitations | Fields of Application |
|---|---|---|---|---|---|
| Soybean oil, such as [24,27,34,40,67,68,80]. | 1. Unconjugated double bonds 2. Iodine value 120–140 (Semi-dry oil) | Epoxidation, acrylication, maleoylation, copolymerization with citric acid | High flexibility, low viscosity, bio-based content > 50% | Low functionality and poor chemical resistance | Wood coatings, water-based coatings, 3D printing, adhesives |
| Castor oil, such as [5,9,41,42,45,74]. | 1. Contains hydroxyl (ricinoleic acid) 2. Iodine value 80–90 (Non-drying oil) | Hydroxyl group is directly acrylated, and PU prepolymer is synthesized with isocyanate | High reactivity, fast curing speed (≤30 s) and high biodegradation rate | The distribution of hydroxyl groups is uneven, and the reaction conditions are difficult to control | Biodegradable adhesives, coatings |
| Tung oil, such as [53,54,61,75]. | 1. Conjugated trienes 2. Iodine value 160–175 (Dry oil) | Diels–Alder reaction, thiolene click reactions, acrylication | High functionality, high hardness, adhesion grade 0 | The color is dark, the synthesis steps are complex | High hardness coatings, electronic packaging inks |
| Linseed oil, such as [55,56,58,59,60]. | 1. Highly unsaturated unconjugated double bond (linolenic acid) 2. Iodine value 170–190 (Dry oil) | Epoxidation, acrylication, compound with nano-SiO2 | High cross-linking density, good thermal stability (>180 °C) | High viscosity, need to add diluent | Industrial coatings, packaging inks |
| Cottonseed oil, such as [57,62]. | 1. Unconjugated double bonds 2. The composition of fatty acids varies greatly | Epoxidation, acrylication | Flexibility is superior to petroleum-based materials and is suitable for flexible substrates | Research is not yet complete | Coating of flexible printed electronic and paper products |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Hu, Z.; Lei, W. Research Progress on Vegetable Oil-Based UV-Curing Resins. Polymers 2025, 17, 1890. https://doi.org/10.3390/polym17141890
Wang W, Hu Z, Lei W. Research Progress on Vegetable Oil-Based UV-Curing Resins. Polymers. 2025; 17(14):1890. https://doi.org/10.3390/polym17141890
Chicago/Turabian StyleWang, Wei, Zhengru Hu, and Wen Lei. 2025. "Research Progress on Vegetable Oil-Based UV-Curing Resins" Polymers 17, no. 14: 1890. https://doi.org/10.3390/polym17141890
APA StyleWang, W., Hu, Z., & Lei, W. (2025). Research Progress on Vegetable Oil-Based UV-Curing Resins. Polymers, 17(14), 1890. https://doi.org/10.3390/polym17141890






