Structure–Property Relationship in Isotactic Polypropylene Under Contrasting Processing Conditions
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Characterization Techniques
3. Results and Discussion
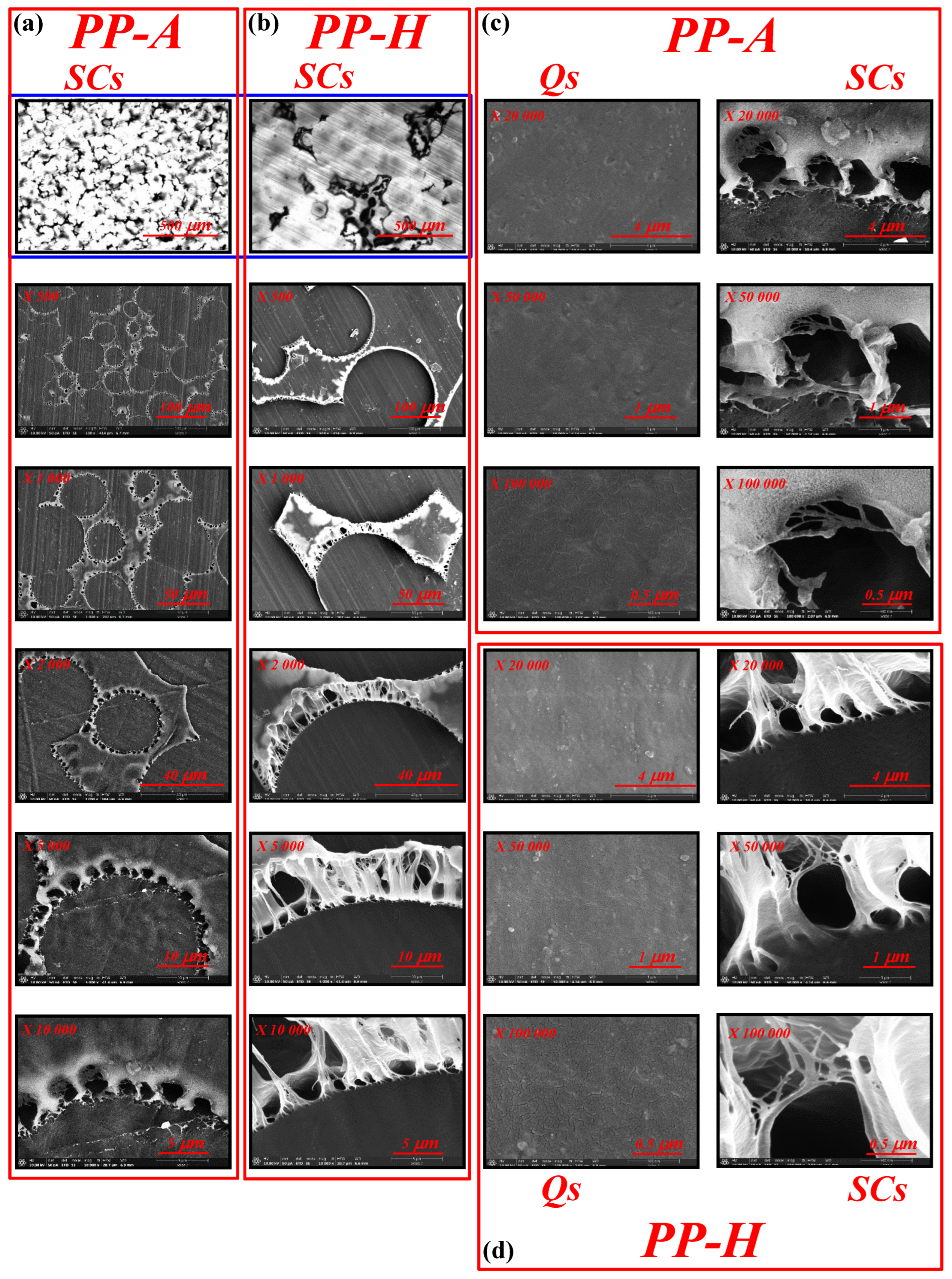
3.1. Microstructure Investigation
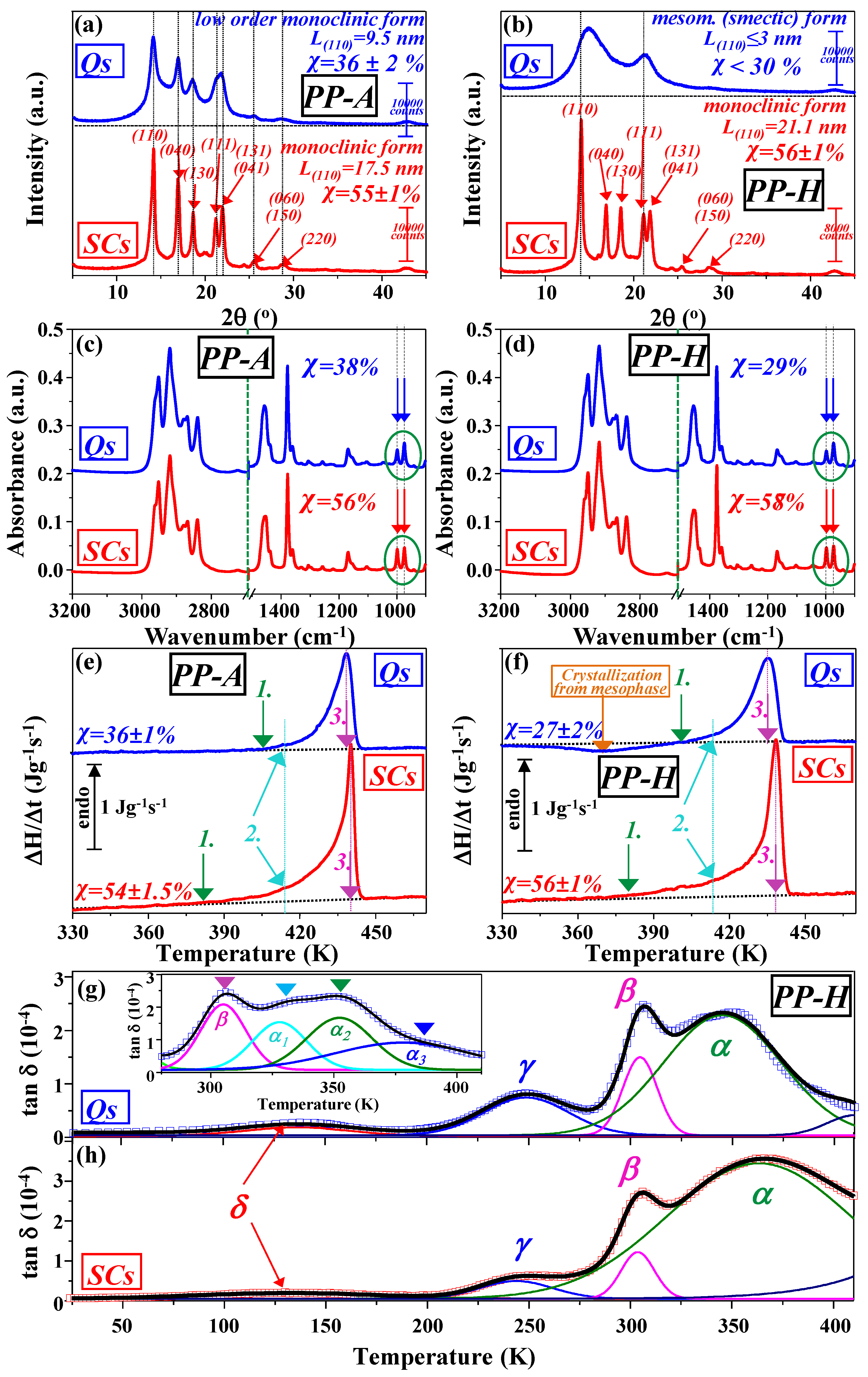
3.2. WAXD Study
3.3. ATR-FTIR Spectroscopy
3.4. Calorimetric Study
3.5. DDS Study
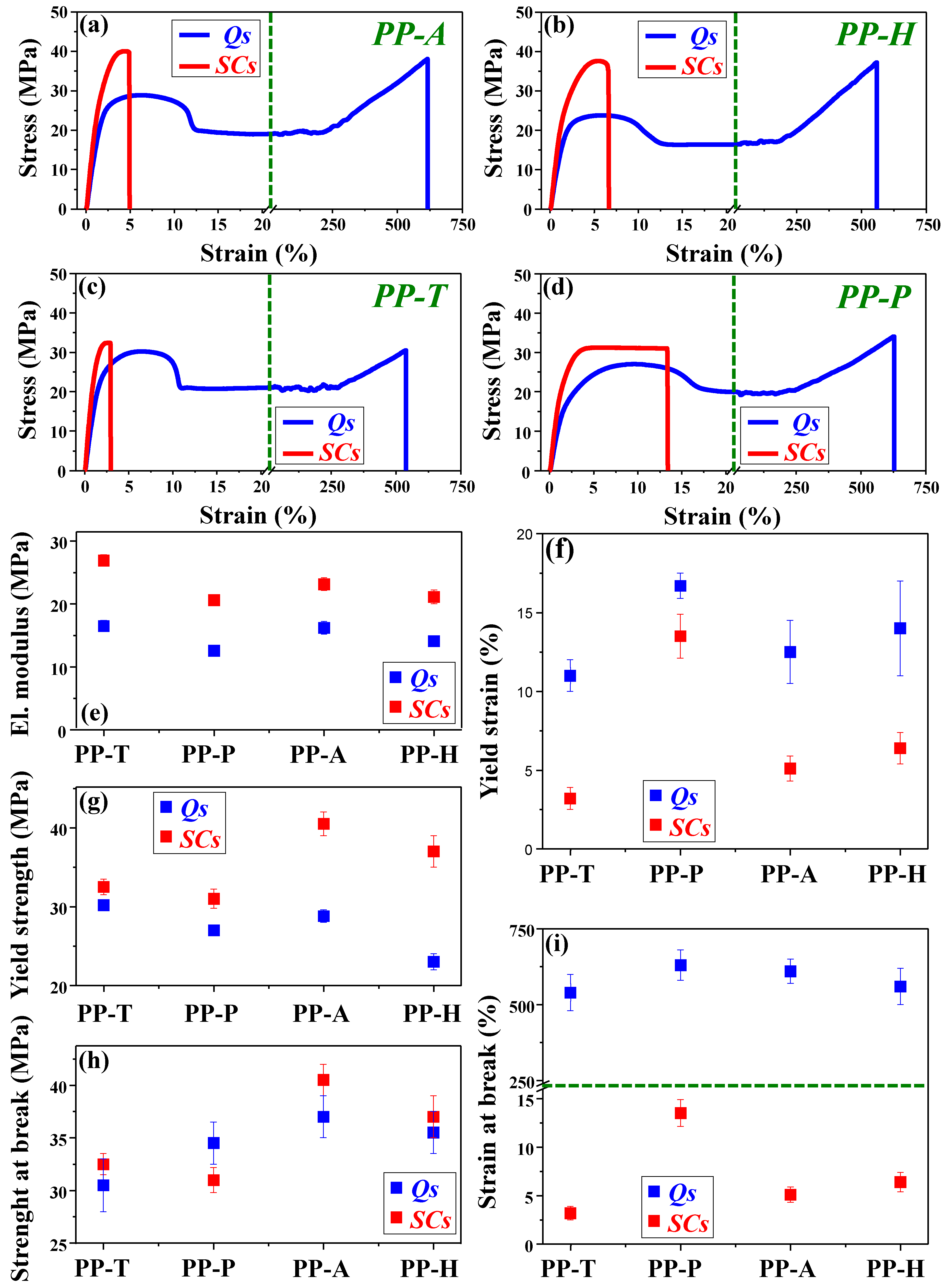
3.6. Mechanical Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Mileva, D.; Tranchida, D.; Gahleitner, M. Designing polymer crystallinity: An industrial perspective. Polym. Cryst. 2018, 1, e10009. [Google Scholar] [CrossRef]
- Amer, I.; van Reenen, A.; Mokrani, T. Molecular weight and tacticity effect on morphological and mechanical properties of Ziegler–Natta catalyzed isotactic polypropylenes. Polímeros 2015, 25, 556–563. [Google Scholar] [CrossRef]
- Moore, E.P. Polypropylene Handbook: Polymerization, Characterization, Properties, Processing, Applications; Hanser Publishers: Cincinnati, OH, USA, 1996. [Google Scholar]
- Ozzetti, R.A.; De Oliveira Filho, A.P.; Schuchardt, U.; Mandelli, D. Determination of tacticity in polypropylene by FTIR with multivariate calibration. J. Appl. Polym. Sci. 2002, 85, 734–745. [Google Scholar] [CrossRef]
- Arranz-Andrés, J.; Peña, B.; Benavente, R.; Pérez, E.; Cerrada, M.L. Influence of isotacticity and molecular weight on the properties of metallocenic isotactic polypropylene. Eur. Polym. J. 2007, 43, 2357–2370. [Google Scholar] [CrossRef]
- Cheng, S.Z.D.; Janimak, J.J.; Zhang, A.; Hsieh, E.T. Isotacticity effect on crystallization and melting in polypropylene fractions: 1. Crystalline structures and thermodynamic property changes. Polymer 1991, 32, 648–655. [Google Scholar] [CrossRef]
- Paukkeri, R.; Lehtinen, A. Thermal behaviour of polypropylene fractions: 2. The multiple melting peaks. Polymer 1993, 34, 4083–4088. [Google Scholar] [CrossRef]
- Paukkeri, R.; Lehtinen, A. Thermal behaviour of polypropylene fractions: 1. Influence of tacticity and molecular weight on crystallization and melting behaviour. Polymer 1993, 34, 4075–4082. [Google Scholar] [CrossRef]
- Fukuda, Y.; Kida, T.; Yamaguchi, M. Mechanical properties of isotactic polypropylene with nodular or spherulite morphologies. Polym. Eng. Sci. 2023, 63, 4043–4050. [Google Scholar] [CrossRef]
- Polypropylene Handbook; Pasquini, N., Ed.; Carl Hanser Verlag: Munich, Germany, 2005. [Google Scholar]
- Yamada, K.; Matsumoto, S.; Tagashira, K.; Hikosaka, M. Isotacticity dependence of spherulitic morphology of isotactic polypropylene. Polymer 1998, 39, 5327–5333. [Google Scholar] [CrossRef]
- Tripathi, D. Practical Guide to Polypropylene; Rapra Publishing: Shrewsbury, UK, 2001. [Google Scholar]
- Addeo, A. Polypropylene Handbook; Hanser Gardner: Cincinnati, OH, USA, 2005. [Google Scholar]
- Ariff, Z.; Ariffin, A.; Jikan, S.; Abdul Rahim, N. Rheological Behaviour of Polypropylene Through Extrusion and Capillary Rheometry. In Polypropylene; Dogan, F., Ed.; InTech: Houston, TX, USA, 2012; pp. 29–48. [Google Scholar]
- Maddah, H. Polypropylene as a Promising Plastic: A Review. Am. J. Polym. Sci. 2016, 6, 1–11. [Google Scholar] [CrossRef]
- Suljovrujic, E.; Milicevic, D.; Stolic, A.; Dudic, D.; Vasalic, D.; Dzunuzovic, E.; Stamboliev, G. Thermal, mechanical, and dielectric properties of radiation sterilized mesomorphic PP: Comparison between gamma and electron beam irradiation modalities. Polym. Degrad. Stab. 2024, 229, 110940. [Google Scholar] [CrossRef]
- Natta, G. Une nouvelle classe de polymeres d’α-olefines ayant une régularité de structure exceptionnelle. J. Polym. Sci. 1955, 16, 143–154. [Google Scholar] [CrossRef]
- Bogoeva-Gaceva, G. Advances in polypropylene based materials. Contrib. Sect. Nat. Math. Biotech. Sci. 2014, 35, 121–138. [Google Scholar] [CrossRef]
- Brückner, S.; Meille, S.V.; Petraccone, V.; Pirozzi, B. Polymorphism in isotactic polypropylene. Prog. Polym. Sci. 1991, 16, 361–404. [Google Scholar] [CrossRef]
- Seguela, R.; Staniek, E.; Escaig, B.; Fillon, B. Plastic deformation of polypropylene in relation to crystalline structure. J. Appl. Polym. Sci. 1999, 71, 1873–1885. [Google Scholar] [CrossRef]
- Shang, Y.; Zhao, J.; Li, J.; Wu, Z.; Jiang, S. Investigations in annealing effects on structure and properties of β-isotactic polypropylene with X-ray synchrotron experiments. Colloid Polym. Sci. 2014, 292, 3205–3221. [Google Scholar] [CrossRef]
- Arvidson, S.A.; Khan, S.A.; Gorga, R.E. Mesomorphic−α-Monoclinic Phase Transition in Isotactic Polypropylene: A Study of Processing Effects on Structure and Mechanical Properties. Macromolecules 2010, 43, 2916–2924. [Google Scholar] [CrossRef]
- Sharaf, M.A.; Kloczkowski, A. Evolution of the Deformation- and Flow-Induced Crystallization and Characterization of the Microstructure of a Single Spherulite, Lamella, and Chain of Isotactic Polypropylene. Macromol. Chem. Phys. 2024, 225, 2300203. [Google Scholar] [CrossRef]
- Suljovrujic, E. Radiation Modification of the Physical Properties of Polyolefins; University of Belgrade: Belgrade, Serbia, 2000. [Google Scholar]
- van der Meer, D.W. Structure-Property Relationships in Isotactic Polypropylene. Ph.D. Thesis, Twente University, Enschede, The Netherlands, 2003. [Google Scholar]
- Scoti, M.; De Stefano, F.; Di Girolamo, R.; Malafronte, A.; Talarico, G.; De Rosa, C. Crystallization Behavior and Properties of Propylene/4-Methyl-1-pentene Copolymers from a Metallocene Catalyst. Macromolecules 2023, 56, 1446–1460. [Google Scholar] [CrossRef]
- De Rosa, C.; Auriemma, F.; Circelli, T.; Waymouth, R.M. Crystallization of the α and γ Forms of Isotactic Polypropylene as a Tool To Test the Degree of Segregation of Defects in the Polymer Chains. Macromolecules 2002, 35, 3622–3629. [Google Scholar] [CrossRef]
- Suljovrujic, E.; Trifunovic, S.; Milicevic, D. The influence of gamma radiation on the dielectric relaxation behaviour of isotactic polypropylene. The α relaxation. Polym. Degrad. Stab. 2010, 95, 164–171. [Google Scholar] [CrossRef]
- Vittoria, V.; Perullo, A. Effect of quenching temperature on the structure of isotactic polypropylene films. J. Macromol. Sci. Part B 1986, 25, 267–281. [Google Scholar] [CrossRef]
- Auriemma, F.; De Rosa, C.; Corradini, P. Solid Mesophases in Semicrystalline Polymers: Structural Analysis by DiffractionTechniques. In Interphases and Mesophases in Polymer Crystallization II; Allegra, G., Ed.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 1–74. [Google Scholar]
- Mileva, D.; Androsch, R.; Radusch, H.-J. Effect of structure on light transmission in isotactic polypropylene and random propylene-1-butene copolymers. Polym. Bull. 2009, 62, 561–571. [Google Scholar] [CrossRef]
- Stupp, S.I.; Supan, T.J.; Belton, D.J. Ice-water quenching technique for polypropylene. Orthot. Prosthet. 1979, 33, 16–21. [Google Scholar]
- Kim, Y.C.; Ahn, W.; Kim, C.Y. A study on multiple melting of isotactic polypropylene. Polym. Eng. Sci. 1997, 37, 1003–1011. [Google Scholar] [CrossRef]
- Karger-Kocsis, J.; Bárány, T. Polypropylene Handbook Morphology, Blends and Composites: Morphology, Blends and Composites; Springer Nature: Cham, Switzerland, 2019. [Google Scholar]
- Rungswang, W.; Jarumaneeroj, C.; Patthamasang, S.; Phiriyawirut, P.; Jirasukho, P.; Soontaranon, S.; Rugmai, S.; Hsiao, B.S. Influences of tacticity and molecular weight on crystallization kinetic and crystal morphology under isothermal crystallization: Evidence of tapering in lamellar width. Polymer 2019, 172, 41–51. [Google Scholar] [CrossRef]
- Bassett, D.C.; Olley, R.H. On the lamellar morphology of isotactic polypropylene spherulites. Polymer 1984, 25, 935–943. [Google Scholar] [CrossRef]
- Ryan, A.J.; Stanford, J.L.; Bras, W.; Nye, T.M.W. A synchrotron X-ray study of melting and recrystallization in isotactic polypropylene. Polymer 1997, 38, 759–768. [Google Scholar] [CrossRef]
- Chan, C.-M.; Li, L. Direct Observation of the Growth of Lamellae and Spherulites by AFM. In Intrinsic Molecular Mobility and Toughness of Polymers II; Kausch, H.-H., Ed.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 1–41. [Google Scholar]
- Cong, Y.; Hong, Z.; Zhou, W.; Chen, W.; Su, F.; Li, H.; Li, X.; Yang, K.; Yu, X.; Qi, Z.; et al. Conformational Ordering on the Growth Front of Isotactic Polypropylene Spherulite. Macromolecules 2012, 45, 8674–8680. [Google Scholar] [CrossRef]
- Jiang, C.; Miao, C.; Zhou, J.; Yuan, M. Insights into damage mechanisms and advances in numerical simulation of spherulitic polymers. Polymer 2025, 318, 128001. [Google Scholar] [CrossRef]
- Michaeli, W.; Gutberlet, D.; Glißmann, M. Characterisation of the spherulite structure of polypropylene using light-microscope methods. Polym. Test. 2001, 20, 459–467. [Google Scholar] [CrossRef]
- Park, J.; Eom, K.; Kwon, O.; Woo, S. Chemical Etching Technique for the Investigation of Melt-crystallized Isotactic Polypropylene Spherulite and Lamellar Morphology by Scanning Electron Microscopy. Microsc. Microanal. 2001, 7, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-G.; Zhang, L.-Q.; Chen, C.; Cui, J.; Zeng, X.-b.; Liu, L.; Liu, F.; Ungar, G. 3D Morphology of Different Crystal Forms in β-Nucleated and Fiber-Sheared Polypropylene: α-Teardrops, α-Teeth, and β-Fans. Macromolecules 2023, 56, 5502–5511. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.-J.; Liu, J.-G.; Yan, S.-K.; Dong, J.-Y.; Li, L.; Chan, C.-M.; Schultz, J.M. Atomic force microscopy study of the lamellar growth of isotactic polypropylene. Polymer 2005, 46, 4077–4087. [Google Scholar] [CrossRef]
- Zhou, Z.; Ma, L.; Zhen, W.; Sun, X.; Ren, Z.; Li, H.; Yan, S. An abnormal melting behavior of isotactic polypropylene spherulites grown at low temperatures. Polymer 2017, 111, 183–191. [Google Scholar] [CrossRef]
- Raimo, M.; Silvestre, C. Topographic Analysis of Isotactic Polypropylene Spherulites by Atomic Force Microscopy. J. Scanning Probe Microsc. 2009, 4, 45–47. [Google Scholar] [CrossRef]
- Schönherr, H.; Snétivy, D.; Vansco, G.J. A nanoscopic view at the spherulitic morphology of isotactic polypropylene by atomic force microscopy. Polym. Bull. 1993, 30, 567–574. [Google Scholar] [CrossRef]
- Bassett, D.C. Principles of Polymer Morphology; Cambridge: New York, NY, USA, 1981. [Google Scholar]
- Bassett, D.C.; Keller, A.; Mitsuhashi, S. New features in polymer crystal growth from concentrated solutions. J. Polym. Sci. Part A Gen. Pap. 1963, 1, 763–788. [Google Scholar] [CrossRef]
- Bassett, D.C.; Vaughan, A.S. On the lamellar morphology of melt-crystallized isotactic polystyrene. Polymer 1985, 26, 717–725. [Google Scholar] [CrossRef]
- Imai, M.; Kaji, K. Polymer crystallization from the metastable melt: The formation mechanism of spherulites. Polymer 2006, 47, 5544–5554. [Google Scholar] [CrossRef]
- Norton, D.R.; Keller, A. The spherulitic and lamellar morphology of melt-crystallized isotactic polypropylene. Polymer 1985, 26, 704–716. [Google Scholar] [CrossRef]
- Padden, F.J., Jr.; Keith, H.D. Spherulitic Crystallization in Polypropylene. J. Appl. Phys. 1959, 30, 1479–1484. [Google Scholar] [CrossRef]
- Stachurski, Z.H.; Macnicol, J. The geometry of spherulite boundaries. Polymer 1998, 39, 5717–5724. [Google Scholar] [CrossRef]
- Androsch, R.; Di Lorenzo, M.L.; Schick, C.; Wunderlich, B. Mesophases in polyethylene, polypropylene, and poly(1-butene). Polymer 2010, 51, 4639–4662. [Google Scholar] [CrossRef]
- Kida, T.; Yamaguchi, M. Role of Rigid–Amorphous chains on mechanical properties of polypropylene solid using DSC, WAXD, SAXS, and Raman spectroscopy. Polymer 2022, 249, 124834. [Google Scholar] [CrossRef]
- Schawe, J.E.K. Mobile amorphous, rigid amorphous and crystalline fractions in isotactic polypropylene during fast cooling. J. Therm. Anal. Calorim. 2017, 127, 931–937. [Google Scholar] [CrossRef]
- Zia, Q.; Mileva, D.; Androsch, R. Rigid Amorphous Fraction in Isotactic Polypropylene. Macromolecules 2008, 41, 8095–8102. [Google Scholar] [CrossRef]
- Di Lorenzo, M.L.; Righetti, M.C. Crystallization-induced formation of rigid amorphous fraction. Polym. Cryst. 2018, 1, e10023. [Google Scholar] [CrossRef]
- Zuo, F.; Keum, J.K.; Chen, X.; Hsiao, B.S.; Chen, H.; Lai, S.-Y.; Wevers, R.; Li, J. The role of interlamellar chain entanglement in deformation-induced structure changes during uniaxial stretching of isotactic polypropylene. Polymer 2007, 48, 6867–6880. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, Q.; Zhao, J.; Li, L. Molecular and thermodynamics descriptions of flow-induced crystallization in semi-crystalline polymers. J. Appl. Phys. 2020, 127, 241101. [Google Scholar] [CrossRef]
- Sigalas, N.I.; Van Kraaij, S.A.T.; Lyulin, A.V. Effect of Temperature on Flow-Induced Crystallization of Isotactic Polypropylene: A Molecular-Dynamics Study. Macromolecules 2023, 56, 8417–8427. [Google Scholar] [CrossRef]
- Hine, P.; Broome, V.; Ward, I. The incorporation of carbon nanofibres to enhance the properties of self reinforced, single polymer composites. Polymer 2005, 46, 10936–10944. [Google Scholar] [CrossRef]
- Laura, D.M.; Keskkula, H.; Barlow, J.W.; Paul, D.R. Effect of rubber particle size and rubber type on the mechanical properties of glass fiber reinforced, rubber-toughened nylon 6. Polymer 2003, 44, 3347–3361. [Google Scholar] [CrossRef]
- Haggenmueller, R.; Guthy, C.; Lukes, J.R.; Fischer, J.E.; Winey, K.I. Single Wall Carbon Nanotube/Polyethylene Nanocomposites: Thermal and Electrical Conductivity. Macromolecules 2007, 40, 2417–2421. [Google Scholar] [CrossRef]
- Tangirala, R.; Baer, E.; Hiltner, A.; Weder, C. Photopatternable reflective films produced by nanolayer extrusion. Adv. Funct. Mater. 2004, 14, 595–604. [Google Scholar] [CrossRef]
- Dudić, D.; Kostoski, D.; Djoković, V.; Dramićanin, M.D. Formation and behaviour of low-temperature melting peak of quenched and annealed isotactic polypropylene. Polym. Int. 2002, 51, 111–116. [Google Scholar] [CrossRef]
- Brucato, V.; Piccarolo, S.; La Carrubba, V. An experimental methodology to study polymer crystallization under processing conditions. The influence of high cooling rates. Chem. Eng. Sci. 2002, 57, 4129–4143. [Google Scholar] [CrossRef]
- De Rosa, C.; Auriemma, F.; Tarallo, O.; Malafronte, A.; Di Girolamo, R.; Esposito, S.; Piemontesi, F.; Liguori, D.; Morini, G. The “Nodular” α Form of Isotactic Polypropylene: Stiff and Strong Polypropylene with High Deformability. Macromolecules 2017, 50, 5434–5446. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhao, Y.; Zhang, C.; Yang, J.; Xu, Y.; Wang, D. In-situ investigation on the structural evolution of mesomorphic isotactic polypropylene in a continuous heating process. Polymer 2016, 105, 133–143. [Google Scholar] [CrossRef]
- Mollova, A.; Androsch, R.; Mileva, D.; Gahleitner, M.; Funari, S.S. Crystallization of isotactic polypropylene containing beta-phase nucleating agent at rapid cooling. Eur. Polym. J. 2013, 49, 1057–1065. [Google Scholar] [CrossRef]
- Fu, X.; Jia, W.; Li, X.; Wang, Y.; Wang, Z.; Liu, C.; Shen, C.; Shao, C. Phase transitions of the rapid-compression-induced mesomorphic isotactic polypropylene under high-pressure annealing. J. Polym. Sci. Part B Polym. Phys. 2019, 57, 651–661. [Google Scholar] [CrossRef]
- Hendra, P.J.; Vile, J.; Willis, H.A.; Zichy, V.; Cudby, M.E.A. The effect of cooling rate upon the morphology of quenched melts of isotactic polypropylenes. Polymer 1984, 25, 785–790. [Google Scholar] [CrossRef]
- Konishi, T.; Nishida, K.; Kanaya, T.; Kaji, K. Effect of Isotacticity on Formation of Mesomorphic Phase of Isotactic Polypropylene. Macromolecules 2005, 38, 8749–8754. [Google Scholar] [CrossRef]
- Ferrero, A.; Ferracini, E.; Mazzavillani, A.; Malta, V. A New X-Ray Study of the Quenched Isotactic Polypropylene Transition by Annealing. J. Macromol. Sci. Part B 2000, 39, 109–129. [Google Scholar] [CrossRef]
- Di Sacco, F.; Saidi, S.; Hermida-Merino, D.; Portale, G. Revisiting the Mechanism of the Meso-to-α Transition of Isotactic Polypropylene and Ethylene–Propylene Random Copolymers. Macromolecules 2021, 54, 9681–9691. [Google Scholar] [CrossRef]
- Lei, C.; Huang, W.; Xu, R.; Xu, Y. The correlation between the lower temperature melting plateau endotherm and the stretching-induced pore formation in annealed polypropylene films. J. Plast. Film. Sheeting 2012, 28, 151–164. [Google Scholar] [CrossRef]
- Nitta, K.-h.; Odaka, K. Influence of structural organization on tensile properties in mesomorphic isotactic polypropylene. Polymer 2009, 50, 4080–4088. [Google Scholar] [CrossRef]
- Zia, Q.; Radusch, H.-J.; Androsch, R. Deformation behavior of isotactic polypropylene crystallized via a mesophase. Polym. Bull. 2009, 63, 755–771. [Google Scholar] [CrossRef]
- Androsch, R. In Situ Atomic Force Microscopy of the Mesomorphic−Monoclinic Phase Transition in Isotactic Polypropylene. Macromolecules 2008, 41, 533–535. [Google Scholar] [CrossRef]
- Miller, R.L. On the existence of near-range order in isotactic polypropylenes. Polymer 1960, 1, 135–143. [Google Scholar] [CrossRef]
- Martorana, A.; Piccarolo, S.; Sapoundjieva, D. SAXS/WAXS study of the annealing process in quenched samples of isotactic poly(propylene). Macromol. Chem. Phys. 1999, 200, 531–540. [Google Scholar] [CrossRef]
- Hanna, L.A.; Hendra, P.J.; Maddams, W.; Willis, H.A.; Zichy, V.; Cudby, M.E.A. Vibrational spectroscopic study of structural changes in isotactic polypropylene below the melting point. Polymer 1988, 29, 1843–1847. [Google Scholar] [CrossRef]
- Konishi, T.; Nishida, K.; Kanaya, T. Crystallization of Isotactic Polypropylene from Prequenched Mesomorphic Phase. Macromolecules 2006, 39, 8035–8040. [Google Scholar] [CrossRef]
- Zia, Q.; Androsch, R.; Radusch, H.-J.; Piccarolo, S. Morphology, reorganization and stability of mesomorphic nanocrystals in isotactic polypropylene. Polymer 2006, 47, 8163–8172. [Google Scholar] [CrossRef]
- Gomez, M.A.; Tanaka, H.; Tonelli, A.E. High-resolution solid-state 13C nuclear magnetic resonance study of isotactic polypropylene polymorphs. Polymer 1987, 28, 2227–2232. [Google Scholar] [CrossRef]
- Suljovrujic, E.; Stojanovic, Z.; Dudic, D.; Milicevic, D. Radiation, thermo-oxidative and storage induced changes in microstructure, crystallinity and dielectric properties of (un)oriented isotactic polypropylene. Polym. Degrad. Stab. 2021, 188, 109564. [Google Scholar] [CrossRef]
- Lanyi, F.J.; Wenzke, N.; Kaschta, J.; Schubert, D.W. On the Determination of the Enthalpy of Fusion of α-Crystalline Isotactic Polypropylene Using Differential Scanning Calorimetry, X-Ray Diffraction, and Fourier-Transform Infrared Spectroscopy: An Old Story Revisited. Adv. Eng. Mater. 2020, 22, 1900796. [Google Scholar] [CrossRef]
- Lanyi, F.J.; Wenzke, N.; Kaschta, J.; Schubert, D.W. A method to reveal bulk and surface crystallinity of Polypropylene by FTIR spectroscopy—Suitable for fibers and nonwovens. Polym. Test. 2018, 71, 49–55. [Google Scholar] [CrossRef]
- Tarani, E.; Arvanitidis, I.; Christofilos, D.; Bikiaris, D.N.; Chrissafis, K.; Vourlias, G. Calculation of the degree of crystallinity of HDPE/GNPs nanocomposites by using various experimental techniques: A comparative study. J. Mater. Sci. 2023, 58, 1621–1639. [Google Scholar] [CrossRef]
- Reddy, K.R.; Tashiro, K.; Sakurai, T.; Yamaguchi, N.; Sasaki, S.; Masunaga, H.; Takata, M. Isothermal Crystallization Behavior of Isotactic Polypropylene H/D Blends as Viewed from Time-Resolved FTIR and Synchrotron SAXS/WAXD Measurements. Macromolecules 2009, 42, 4191–4199. [Google Scholar] [CrossRef]
- Stojanović, Z.; Kačarević-Popović, Z.; Galović, S.; Miličević, D.; Suljovrujić, E. Crystallinity changes and melting behavior of the uniaxially oriented iPP exposed to high doses of gamma radiation. Polym. Degrad. Stab. 2005, 87, 279–286. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhou, Q.; Ren, Z.; Sun, X.; Li, H.; Li, H.; Yan, S. The αβ-iPP growth transformation of commercial-grade iPP during non-isothermal crystallization. CrystEngComm 2015, 17, 9221–9227. [Google Scholar] [CrossRef]
- Caldas, V.; Brown, G.R.; Nohr, R.S.; MacDonald, J.G.; Raboin, L.E. The structure of the mesomorphic phase of quenched isotactic polypropylene. Polymer 1994, 35, 899–907. [Google Scholar] [CrossRef]
- Cohen, Y.; Saraf, R. A direct correlation function for mesomorphic polymers and its application to the ‘smectic’ phase of isotactic polpropylene. Polymer 2001, 42, 5865–5870. [Google Scholar] [CrossRef]
- An, H.; Li, X.; Geng, Y.; Wang, Y.; Wang, X.; Li, L.; Li, Z.; Yang, C. Shear-Induced Conformational Ordering, Relaxation, and Crystallization of Isotactic Polypropylene. J. Phys. Chem. B 2008, 112, 12256–12262. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Wang, G.; Cong, Y.; Bai, L.; Li, L.; Yang, C. Shear-Induced Nucleation and Growth of Long Helices in Supercooled Isotactic Polypropylene. Macromolecules 2009, 42, 4751–4757. [Google Scholar] [CrossRef]
- Qian, C.; Zhao, Y.; Wang, Z.; Liu, L.; Wang, D. Probing the difference of crystalline modifications and structural disorder of isotactic polypropylene via high-resolution FTIR spectroscopy. Polymer 2021, 224, 123722. [Google Scholar] [CrossRef]
- Zhu, X.; Yan, D.; Fang, Y. In Situ FTIR Spectroscopic Study of the Conformational Change of Isotactic Polypropylene during the Crystallization Process. J. Phys. Chem. B 2001, 105, 12461–12463. [Google Scholar] [CrossRef]
- Luongo, J.P. Infrared study of polypropylene. J. Appl. Polym. Sci. 1960, 3, 302–309. [Google Scholar] [CrossRef]
- Kissin, Y.V. Isospecific Polymerization of Olefins with Heterogeneous Ziegler-Natta Catalysts; Springer: Berlin/Heidelberg, Germany, 1985. [Google Scholar]
- Cong, Y.; Hong, Z.; Qi, Z.; Zhou, W.; Li, H.; Liu, H.; Chen, W.; Wang, X.; Li, L. Conformational Ordering in Growing Spherulites of Isotactic Polypropylene. Macromolecules 2010, 43, 9859–9864. [Google Scholar] [CrossRef]
- Burfield, D.R.; Loi, P.S.T. The use of infrared spectroscopy for determination of polypropylene stereoregularity. J. Appl. Polym. Sci. 1988, 36, 279–293. [Google Scholar] [CrossRef]
- Huy, T.A.; Adhikari, R.; Lüpke, T.; Henning, S.; Michler, G.H. Molecular deformation mechanisms of isotactic polypropylene in α- and β-crystal forms by FTIR spectroscopy. J. Polym. Sci. Part B Polym. Phys. 2004, 42, 4478–4488. [Google Scholar] [CrossRef]
- Kilic, A.; Jones, K.; Shim, E.; Pourdeyhimi, B. Surface crystallinity of meltspun isotactic polypropylene filaments. Macromol. Res. 2016, 24, 25–30. [Google Scholar] [CrossRef]
- Heinen, W. Infrared determination of the crystallinity of polypropylene. J. Polym. Sci. 1959, 38, 545–547. [Google Scholar] [CrossRef]
- Li, L.; Liu, T.; Zhao, L.; Yuan, W.-k. Effect of compressed CO2 on the melting behavior and βα-recrystallization of β-form in isotactic polypropylene. J. Supercrit. Fluids 2011, 60, 137–143. [Google Scholar] [CrossRef]
- Quynn, R.G.; Riley, J.L.; Young, D.A.; Noether, H.D. Density, crystallinity, and heptane insolubility in isotactic polypropylene. J. Appl. Polym. Sci. 1959, 2, 166–173. [Google Scholar] [CrossRef]
- Lamberti, G.; Brucato, V. Real-time orientation and crystallinity measurements during the isotactic polypropylene film-casting process. J. Polym. Sci. Part B Polym. Phys. 2003, 41, 998–1008. [Google Scholar] [CrossRef]
- Tadokoro, H.; Kobayashi, M.; Ukita, M.; Yasufuku, K.; Murahashi, S.; Torii, T. Normal Vibrations of the Polymer Molecules of Helical Conformation. V. Isotactic Polypropylene and Its Deuteroderivatives. J. Chem. Phys. 1965, 42, 1432–1449. [Google Scholar] [CrossRef]
- Wu, H.Y.; Li, X.X.; Xiang, F.M.; Huang, T.; Shi, Y.Y.; Wang, Y. Microstructure evolution of isotactic polypropylene during annealing: Effect of poly(ethylene oxide). Chin. J. Polym. Sci. 2012, 30, 199–208. [Google Scholar] [CrossRef]
- Milicevic, D.; Micic, M.; Stamboliev, G.; Leskovac, A.; Mitric, M.; Suljovrujic, E. Microstructure and crystallinity of polyolefins oriented via solid-state stretching at an elevated temperature. Fibers Polym. 2012, 13, 466–470. [Google Scholar] [CrossRef]
- Milicevic, D.; Trifunovic, S.; Galovic, S.; Suljovrujic, E. Thermal and crystallization behaviour of gamma irradiated PLLA. Radiat. Phys. Chem. 2007, 76, 1376–1380. [Google Scholar] [CrossRef]
- Brandrup, J.; Immergut, E.H.; Grulke, E.A. (Eds.) Polymer Handbook; Wiley-Interscience: New York, NY, USA, 1999. [Google Scholar]
- Wunderlich, B. Thermal Analysis; Academic Press, Inc.: Cambridge, MA, USA, 1990. [Google Scholar]
- Chang, B.; Schneider, K.; Vogel, R.; Heinrich, G. Influence of Annealing on Mechanical αc-Relaxation of Isotactic Polypropylene: A Study from the Intermediate Phase Perspective. Macromol. Mater. Eng. 2017, 302, 1700291. [Google Scholar] [CrossRef]
- Rodriguez-Arnold, J.; Zhang, A.; Cheng, S.Z.D.; Lovinger, A.J.; Hsieh, E.T.; Chu, P.; Johnson, T.W.; Honnell, K.G.; Geerts, R.G.; Palackal, S.J.; et al. Crystallization, melting and morphology of syndiotactic polypropylene fractions: 1. Thermodynamic properties, overall crystallization and melting. Polymer 1994, 35, 1884–1895. [Google Scholar] [CrossRef]
- Lee, M.; Kim, C.-H.; Koo, C.-S.; Kim, B.-R.; Lee, Y. The variation of structure and physical properties of XLPE during thermal aging process. Polymer 2003, 27, 249–254. [Google Scholar]
- Haftka, S.; Könnecke, K. Physical properties of syndiotactic polypropylene. J. Macromol. Sci. Part B 1991, 30, 319–334. [Google Scholar] [CrossRef]
- Farrow, G. Crystallinity, ‘crystallite size’ and melting point of polypropylene. Polymer 1963, 4, 191–197. [Google Scholar] [CrossRef]
- Teodorescu, G.M.; Vuluga, Z.; Ion, R.M.; Fistoș, T.; Ioniță, A.; Slămnoiu-Teodorescu, S.; Paceagiu, J.; Nicolae, C.A.; Gabor, A.R.; Ghiurea, M. The Effect of Thermoplastic Elastomer and Fly Ash on the Properties of Polypropylene Composites with Long Glass Fibers. Polymers 2024, 16, 1238. [Google Scholar] [CrossRef]
- Weeks, J.J. Melting Temperature and Change of Lamellar Thickness with Time for Bulk Polyethylene. J. Res. Natl. Bur. Stand. Sect. A Phys. Chem. 1963, 67a, 441–451. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, Z.; Fu, L.; Lu, Y.; Men, Y. Lamellar Thickness and Stretching Temperature Dependency of Cavitation in Semicrystalline Polymers. PLoS ONE 2014, 9, e97234. [Google Scholar] [CrossRef]
- Huang, S.; Li, H.; Jiang, S. Crystal structure and unique lamellar thickening for poly(l-lactide) induced by high pressure. Polymer 2019, 175, 81–86. [Google Scholar] [CrossRef]
- Ronkay, F.; Molnár, B.; Nagy, D.; Szarka, G.; Iván, B.; Kristály, F.; Mertinger, V.; Bocz, K. Melting temperature versus crystallinity: New way for identification and analysis of multiple endotherms of poly(ethylene terephthalate). J. Polym. Res. 2020, 27, 372. [Google Scholar] [CrossRef]
- Tencé-Girault, S.; Lebreton, S.; Bunau, O.; Dang, P.; Bargain, F. Simultaneous SAXS-WAXS Experiments on Semi-Crystalline Polymers: Example of PA11 and Its Brill Transition. Crystals 2019, 9, 271. [Google Scholar] [CrossRef]
- Guleria, D.; Ge, S.; Cardon, L.; Vervoort, S.; den Doelder, J. Impact of resin density and short-chain branching distribution on structural evolution and enhancement of tensile modulus of MDO-PE films. Polym. Test. 2024, 139, 108560. [Google Scholar] [CrossRef]
- Banford, H.M.; Fouracre, R.A.; Faucitano, A.; Buttafava, A.; Martinotti, F. The influence of chemical structure on the dielectric behavior of polypropylene. IEEE Trans. Dielectr. Electr. Insul. 1996, 3, 594–598. [Google Scholar] [CrossRef]
- Brandrup, J.; Immergut, E.H. Polymer Handbook; Wiley: New York, NY, USA, 1975. [Google Scholar]
- Castejón, M.L.; Tiemblo, P.; Gómez-Elvira, J.M. Photo-oxidation of thick isotactic polypropylene films. II. Evolution of the low temperature relaxations and of the melting endotherm along the kinetic stages. Polym. Degrad. Stab. 2001, 71, 99–111. [Google Scholar] [CrossRef]
- Hara, T. Dielectric Property of Some Polymers in Low Temperature Region. Jpn. J. Appl. Phys. 1967, 6, 147–150. [Google Scholar] [CrossRef]
- Hoyos, M.; Tiemblo, P.; Gómez-Elvira, J.M. The role of microstructure, molar mass and morphology on local relaxations in isotactic polypropylene. The α relaxation. Polymer 2007, 48, 183–194. [Google Scholar] [CrossRef]
- Fouracre, R.A.; MacGregor, S.J.; Judd, M.; Banford, H.M. Condition monitoring of irradiated polymeric cables. Radiat. Phys. Chem. 1999, 54, 209–211. [Google Scholar] [CrossRef]
- Gitsas, A.; Floudas, G. Pressure Dependence of the Glass Transition in Atactic and Isotactic Polypropylene. Macromolecules 2008, 41, 9423–9429. [Google Scholar] [CrossRef]
- Jourdan, C.; Cavaille, J.Y.; Perez, J. Mechanical relaxations in polypropylene: A new experimental and theoretical approach. J. Polym. Sci. Part B Polym. Phys. 1989, 27, 2361–2384. [Google Scholar] [CrossRef]
- McCrum, N.G. Density-independent relaxations in polypropylene. J. Polym. Sci. Part B Polym. Lett. 1964, 2, 495–498. [Google Scholar] [CrossRef]
- Olivares, N.; Tiemblo, P.; Gomez-Elvira, J.M. Physicochemical processes along the early stages of the thermal degradation of isotactic polypropylene I. Evolution of the γ relaxation under oxidative conditions. Polym. Degrad. Stab. 1999, 65, 297–302. [Google Scholar] [CrossRef]
- Perepechko, I.I. Svoistva Polimerov Pri Nizkih Temperaturah; Khimiya: Moscow, Russia, 1977. [Google Scholar]
- Pluta, M.; Kryszewski, M. Studies of alpha-relaxation process in spherulitic and non-spherulitic samples of isotactic polypropylene with different molecular ordering. Acta Polym. 1987, 38, 42–52. [Google Scholar] [CrossRef]
- Read, B.E. Mechanical relaxation in isotactic polypropylene. Polymer 1990, 30, 1439–1445. [Google Scholar] [CrossRef]
- Quijada-Garrido, I.; Barrales-Rienda, J.M.; Pereña, J.M.; Frutos, G. Dynamic mechanical and dielectric behavior of erucamide (13-Cis-Docosenamide), isotactic poly(propylene), and their blends. J. Polym. Sci. Part B Polym. Phys. 1997, 35, 1473–1482. [Google Scholar] [CrossRef]
- Sakai, A.; Tanaka, K.; Fujii, Y.; Nagamura, T.; Kajiyama, T. Structure and thermal molecular motion at surface of semi-crystalline isotactic polypropylene films. Polymer 2005, 46, 429–437. [Google Scholar] [CrossRef]
- Starkweather, H.W.; Avakian, P.; Matheson, R.R.; Fontanella, J.J.; Wintersgill, M.C. Ultralow temperature dielectric relaxations in polyolefins. Macromolecules 1992, 25, 6871–6875. [Google Scholar] [CrossRef]
- Suljovrujic, E. Gel production, oxidative degradation and dielectric properties of isotactic polypropylene irradiated under various atmospheres. Polym. Degrad. Stab. 2009, 94, 521–526. [Google Scholar] [CrossRef]
- Tiemblo, P.; Gomez-Elvira, J.M.; García Beltrán, S.; Matisova-Rychla, L.; Rychly, J. Melting and α relaxation effects on the kinetics of polypropylene thermooxidation in the range 80–170 °C. Macromolecules 2002, 35, 5922–5926. [Google Scholar] [CrossRef]
- Umemura, T.; Suzuki, T.; Kashiwazaki, T. Impurity Effect of the Dielectric Properties of Isotactic Polypropylene. IEEE Trans. Electr. Insul. 1982, EI-17, 300–305. [Google Scholar]
- Dintilhac, N.; Lewandowski, S.; Planes, M.; Lectez, A.S.; Dantras, E. Tuning dielectric response of polyethylene by low gamma dose: Molecular mobility study improvement by dipolar probes implementation. J. Non·Cryst. Solids 2023, 621, 122606. [Google Scholar] [CrossRef]
- Suljovrujic, E. Some aspects of structural electrophysics of irradiated polyethylenes. Polymer 2005, 46, 6353–6359. [Google Scholar] [CrossRef]
- Suljovrujic, E. Complete relaxation map of polypropylene: Radiation-induced modification as dielectric probe. Polym. Bull. 2012, 68, 2033–2047. [Google Scholar] [CrossRef]
- Suljovrujic, E. Dielectric studies of molecular β-relaxation in low density polyethylene: The influence of drawing and ionizing radiation. Polymer 2002, 43, 5969–5978. [Google Scholar] [CrossRef]
- Wang, Y.; Bao, Z.; Ding, S.; Jia, J.; Dai, Z.; Li, Y.; Shen, S.; Chu, S.; Yin, Y.; Li, X. γ-Ray Irradiation Significantly Enhances Capacitive Energy Storage Performance of Polymer Dielectric Films. Adv. Mater. 2024, 36, e2308597. [Google Scholar] [CrossRef]
- Hedvig, P. Dielectric Spectroscopy of Polymers; Academia Kiado: Budapest, Hungary, 1977. [Google Scholar]
- Suljovrujic, E.; Kostoski, D.; Kacarevic-Popovic, Z.; Dojcilovic, J. Effect of gamma irradiation on the dielectric relaxation of uniaxially oriented low density polyethylene. Polym. Int. 1999, 48, 1193–1196. [Google Scholar] [CrossRef]
- Zhuravlev, S.P.; Zhuravleva, N.M.; Polonskij, Y.A. Deformation characteristics of polypropylene film and thermal stability of capacitor insulation made on the base of polypropylene film. Elektrotekhnika 2002, 11, 36–40. [Google Scholar]
- Fournie, R. All film power capacitors. Endurance tests and degradation mechanisms. Bulletin de la Direction des etudes et recherches. Bull. Dir. Etudes Rech. Ser. B 1990, 1, 1–31. [Google Scholar]
- Montanari, G.C.; Fabiani, D.; Palmieri, F.; Kaempfer, D.; Thomann, R.; Mulhaupt, R. Modification of electrical properties and performance of EVA and PP insulation through nanostructure by organophilic silicates. IEEE Trans. Dielectr. Electr. Insul. 2004, 11, 754–762. [Google Scholar] [CrossRef]
- Jia, C.; Das, P.; Kim, I.; Yoon, Y.-J.; Tay, C.Y.; Lee, J.-M. Applications, treatments, and reuse of plastics from electrical and electronic equipment. J. Ind. Eng. Chem. 2022, 110, 84–99. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, Y.; Wang, X.; Liu, H.; Cheng, L.; Liu, W.; Li, S.; Guo, J.; Xu, Y. Failure mechanism of metallized film capacitors under DC field superimposed AC harmonic: From equipment to material. High Volt. 2024, 9, 1081–1089. [Google Scholar] [CrossRef]
- Zhang, Y.-T.; Hou, S.; Li, D.-L.; Cao, Y.-J.; Zhan, Y.-P.; Jia, L.; Fu, M.-l.; Huang, H.-D. Hierarchical Structural Evolution, Electrical and Mechanical Performance of Polypropylene Containing Intrinsic Elastomers under Stretching and Annealing for Cable Insulation Applications. Ind. Eng. Chem. Res. 2024, 63, 11982–11991. [Google Scholar] [CrossRef]
- Zhang, C.; Dai, X.-Y.; Xing, Z.-L.; Guo, S.-W.; Li, F.; Chen, X.; Zhou, J.-J.; Li, L. Investigation on the Structure and Performance of Polypropylene Sheets and Bi-axially Oriented Polypropylene Films for Capacitors. Chin. J. Polym. Sci. 2022, 40, 1688–1696. [Google Scholar] [CrossRef]
- Suljovrujic, E.; Kostoski, D.; Dojcilovic, J. Charge trapping in gamma irradiated low-density polyethylene. Polym. Degrad. Stab. 2001, 74, 167–170. [Google Scholar] [CrossRef]
- Suljovrujic, E.; Micic, M.; Milicevic, D. Structural Changes and Dielectric Relaxation Behavior of Uniaxially Oriented High Density Polyethylene. J. Eng. Fibers Fabr. 2013, 8, 155892501300800316. [Google Scholar] [CrossRef]
- Kostoski, D.; Galovic, S.; Suljovrujic, E. Charge trapping and dielectric relaxations of gamma irradiated radiolytically oxidized highly oriented LDPE. Radiat. Phys. Chem. 2004, 69, 245–248. [Google Scholar] [CrossRef]
- Bohning, M.; Goering, H.; Fritz, A.; Brzezinka, K.-W.; Turky, G.; Schönhals, A.; Schartel, B. Dielectric study of molecular mobility in poly(propylene-graft-maleic anhydride)/clay nanocomposites. Macromolecules 2005, 38, 2764–2774. [Google Scholar] [CrossRef]
- Beuguel, Q.; Mija, A.; Vergnes, B.; Peuvrel-Disdier, E. Structural, thermal, rheological and mechanical properties of polypropylene/graphene nanoplatelets composites: Effect of particle size and melt mixing conditions. Polym. Eng. Sci. 2018, 58, 1937–1944. [Google Scholar] [CrossRef]
- Banford, H.M.; Fouracre, R.; Faucitano, A.; Buttafava, A.; Martinotti, F. The influence of γ-irradiation and chemical structure on the dielectric properties of PP. Radiat. Phys. Chem. 1996, 48, 129–130. [Google Scholar] [CrossRef]
- Milicevic, D.; Micic, M.; Suljovrujic, E. Radiation-induced modification of dielectric relaxation spectra of polyolefins: Polyethylenes vs. polypropylene. Polym. Bull. 2014, 71, 2317–2334. [Google Scholar] [CrossRef]
- Qian, S.; Igarashi, T.; Nitta, K.-h. Thermal degradation behavior of polypropylene in the melt state: Molecular weight distribution changes and chain scission mechanism. Polym. Bull. 2011, 67, 1661–1670. [Google Scholar] [CrossRef]
- Nitta, K.h.; Yamaguchi, N. Influence of Morphological Factors on Tensile Properties in the Pre-yield Region of Isotactic Polypropylenes. Polym. J. 2006, 38, 122–131. [Google Scholar] [CrossRef]
- Nitta, K.-H. Tensile Properties in β-Modified Isotactic Polypropylene. In Polypropylene—Polymerization and Characterization of Mechanical and Thermal Properties; Wang, W., Zeng, Y., Eds.; IntechOpen: Rijeka, Croatia, 2018. [Google Scholar]
- Kida, T.; Fukuda, Y.; Yamaguchi, M.; Otsuki, Y.; Kimura, T.; Mizukawa, T.; Murakami, T.; Hato, K.; Okawa, T. Morphological transformation of extruded isotactic polypropylene film from the Mesophase to α-form crystals. React. Funct. Polym. 2023, 191, 105682. [Google Scholar] [CrossRef]
- Li, J.; Zhu, Z.; Li, T.; Peng, X.; Jiang, S.; Turng, L.-S. Quantification of the Young’s modulus for polypropylene: Influence of initial crystallinity and service temperature. J. Appl. Polym. Sci. 2020, 137, 48581. [Google Scholar] [CrossRef]
- Makarewicz, C.; Safandowska, M.; Idczak, R.; Rozanski, A. Plastic Deformation of Polypropylene Studied by Positron Annihilation Lifetime Spectroscopy. Macromolecules 2022, 55, 10062–10076. [Google Scholar] [CrossRef]
- Peterson, J.M. Thermal Initiation of Screw Dislocations in Polymer Crystal Platelets. J. Appl. Phys. 1966, 37, 4047–4050. [Google Scholar] [CrossRef]
- Peterson, J.M. Peierls Stress for Screw Dislocations in Polyethylene. J. Appl. Phys. 1968, 39, 4920–4928. [Google Scholar] [CrossRef]
- Peterlin, A. Molecular model of drawing polyethylene and polypropylene. J. Mater. Sci. 1971, 6, 490–508. [Google Scholar] [CrossRef]
- Morosoff, N.; Peterlin, A. Plastic deformation of polypropylene. IV. Wide-angle x-ray scattering in the neck region. J. Polym. Sci. Part A-2: Polym. Phys. 1972, 10, 1237–1254. [Google Scholar] [CrossRef]
- Suljovrujic, E. The influence of molecular orientation on the crosslinking/oxidative behaviour of iPP exposed to gamma radiation. Eur. Polym. J. 2009, 45, 2068–2078. [Google Scholar] [CrossRef]
- Milicevic, D.; Trifunovic, S.; Popovic, M.; Vukasinovic-Milic, T.; Suljovrujic, E. The influence of orientation on the radiation-induced crosslinking/oxidative behavior of different PEs. Nucl. Instrum. Methods Phys. Res. Sect. B 2007, 260, 603–612. [Google Scholar] [CrossRef]
- Butler, M.F.; Donald, A.M.; Bras, W.; Mant, G.R.; Derbyshire, G.E.; Ryan, A.J. A Real-Time Simultaneous Small- and Wide-Angle X-ray Scattering Study of In-Situ Deformation of Isotropic Polyethylene. Macromolecules 1995, 28, 6383–6393. [Google Scholar] [CrossRef]
- Furuta, M.; Kojima, K. Morphological study of deformation process for linear polyethylene. J. Macromol. Sci. Part B 1986, 25, 349–364. [Google Scholar] [CrossRef]
- Liu, T.-M.; Juska, T.D.; Harrison, I.R. Plastic deformation of polypropylene. Polymer 1986, 27, 247–249. [Google Scholar] [CrossRef]
- Aboulfaraj, M.; G’Sell, C.; Ulrich, B.; Dahoun, A. In situ observation of the plastic deformation of polypropylene spherulites under uniaxial tension and simple shear in the scanning electron microscope. Polymer 1995, 36, 731–742. [Google Scholar] [CrossRef]
- G’sell, C.; Favier, V.; Hiver, J.M.; Dahoun, A.; Philippe, M.J.; Canova, G.R. Microstructure transformation and stress-strain behavior of isotactic polypropylene under large plastic deformation. Polym. Eng. Sci. 1997, 37, 1702–1711. [Google Scholar] [CrossRef]
- Ariyama, T.; Mori, Y.; Kaneko, K. Tensile properties and stress relaxation of polypropylene at elevated temperatures. Polym. Eng. Sci. 1997, 37, 81–90. [Google Scholar] [CrossRef]
- Chodák, I. High modulus polyethylene fibres: Preparation, properties and modification by crosslinking. Prog. Polym. Sci. 1998, 23, 1409–1442. [Google Scholar] [CrossRef]
- Séguéla, R. Dislocation approach to the plastic deformation of semicrystalline polymers: Kinetic aspects for polyethylene and polypropylene. J. Polym. Sci. Part B Polym. Phys. 2002, 40, 593–601. [Google Scholar] [CrossRef]
- Na, B.; Lv, R. Effect of cavitation on the plastic deformation and failure of isotactic polypropylene. J. Appl. Polym. Sci. 2007, 105, 3274–3279. [Google Scholar] [CrossRef]
- Pawlak, A.; Rozanski, A.; Galeski, A. Thermovision studies of plastic deformation and cavitation in polypropylene. Mech. Mater. 2013, 67, 104–118. [Google Scholar] [CrossRef]
- Chen, W.; Li, X.-y.; Liu, Y.-p.; Li, J.; Zhou, W.-m.; Chen, L.; Li, L.-b. The spatial correlation between crystalline and amorphous orientations of isotactic polypropylene during plastic deformation: An in situ observation with FTIR imaging. Chin. J. Polym. Sci. 2015, 33, 613–620. [Google Scholar] [CrossRef]
- Kim, M.; Park, T.Y.; Hong, S. Experimental determination of the plastic deformation and fracture behavior of polypropylene composites under various strain rates. Polym. Test. 2021, 93, 107010. [Google Scholar] [CrossRef]
- Liparoti, S.; Sorrentino, A.; Speranza, V. Morphology-Mechanical Performance Relationship at the Micrometrical Level within Molded Polypropylene Obtained with Non-Symmetric Mold Temperature Conditioning. Polymers 2021, 13, 462. [Google Scholar] [CrossRef] [PubMed]
- Shirinbayan, M.; Nouira, S.; Imaddahen, M.-A.; Fitoussi, J. Microstructure-sensitive investigation on the plastic deformation and damage initiation of fiber-reinforced polypropylene composite. Compos. Part B Eng. 2024, 286, 111790. [Google Scholar] [CrossRef]
- An, Y.; Wang, S.; Li, R.; Shi, D.; Gao, Y.; Song, L. Effect of different nucleating agent on crystallization kinetics and morphology of polypropylene. e-Polymers 2019, 19, 32–39. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suljovrujic, E.; Milicevic, D.; Djordjevic, K.; Rogic Miladinovic, Z.; Stamboliev, G.; Galovic, S. Structure–Property Relationship in Isotactic Polypropylene Under Contrasting Processing Conditions. Polymers 2025, 17, 1889. https://doi.org/10.3390/polym17141889
Suljovrujic E, Milicevic D, Djordjevic K, Rogic Miladinovic Z, Stamboliev G, Galovic S. Structure–Property Relationship in Isotactic Polypropylene Under Contrasting Processing Conditions. Polymers. 2025; 17(14):1889. https://doi.org/10.3390/polym17141889
Chicago/Turabian StyleSuljovrujic, Edin, Dejan Milicevic, Katarina Djordjevic, Zorana Rogic Miladinovic, Georgi Stamboliev, and Slobodanka Galovic. 2025. "Structure–Property Relationship in Isotactic Polypropylene Under Contrasting Processing Conditions" Polymers 17, no. 14: 1889. https://doi.org/10.3390/polym17141889
APA StyleSuljovrujic, E., Milicevic, D., Djordjevic, K., Rogic Miladinovic, Z., Stamboliev, G., & Galovic, S. (2025). Structure–Property Relationship in Isotactic Polypropylene Under Contrasting Processing Conditions. Polymers, 17(14), 1889. https://doi.org/10.3390/polym17141889







