Polymethyl Methacrylate-like Photopolymer Resin with Titanium Metal Nanoparticles Is a Promising Material for Biomedical Applications
Abstract
1. Introduction
2. Materials and Methods
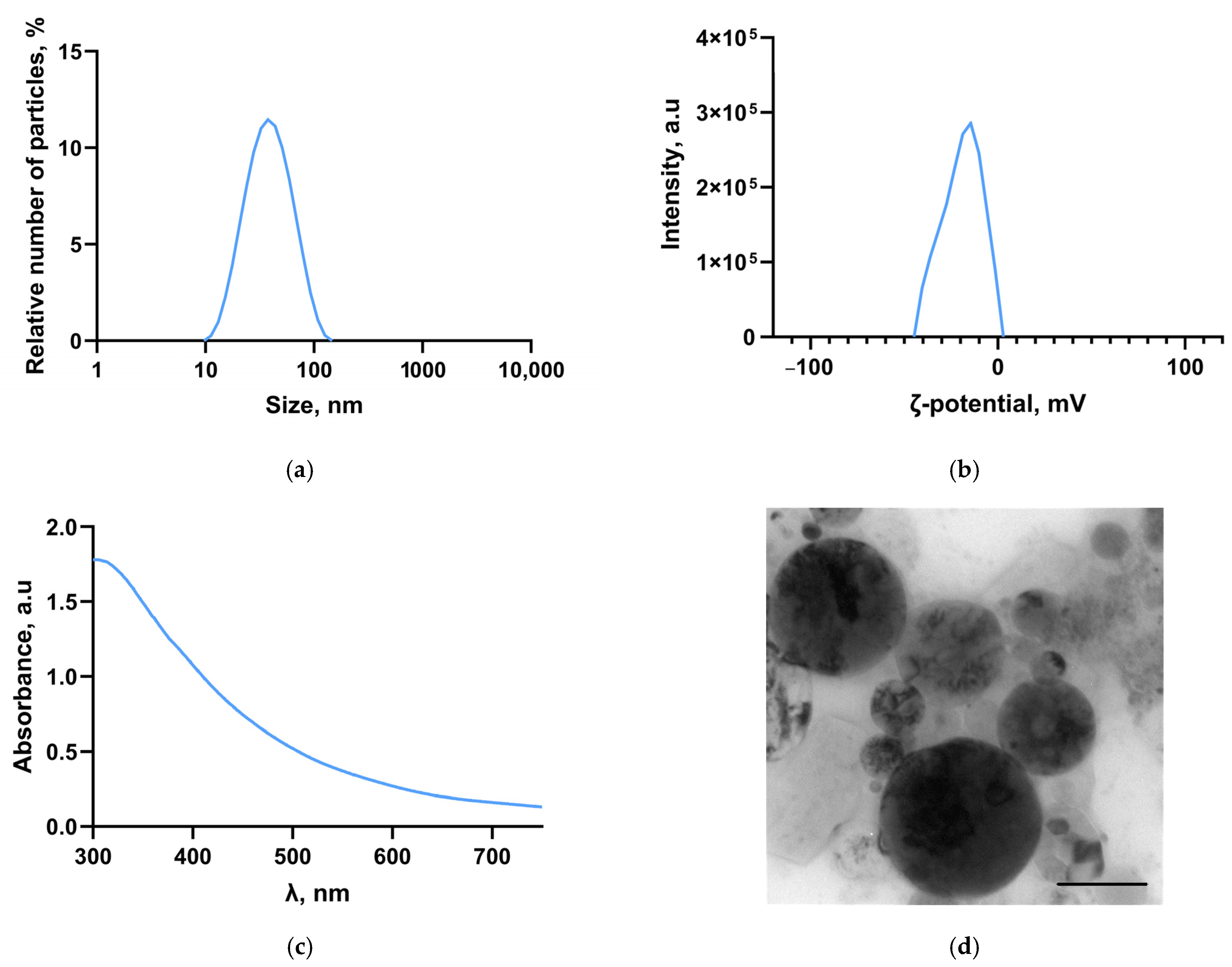
2.1. Synthesis and Characterization of Ti-NPs; Preparation of PMMA/Ti-NPs Composite Material
2.2. Additive Fabrication of PMMA/Ti-NPs Composite Samples
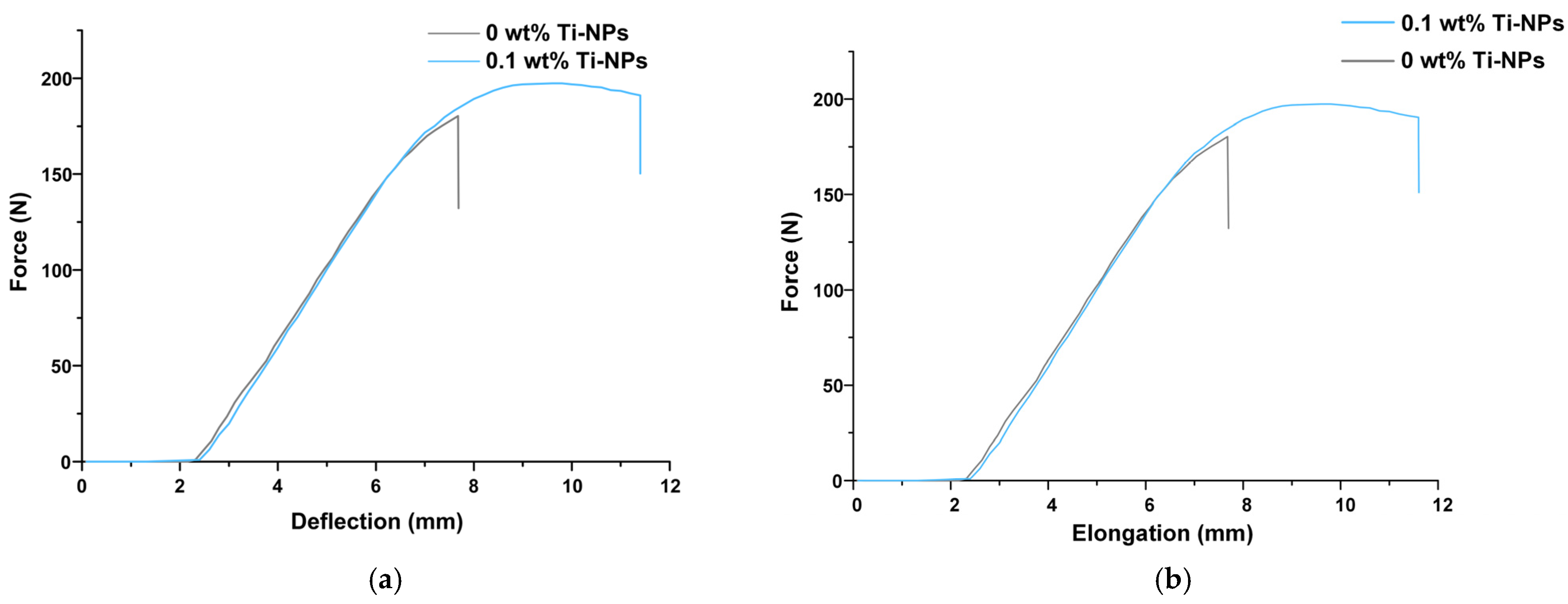
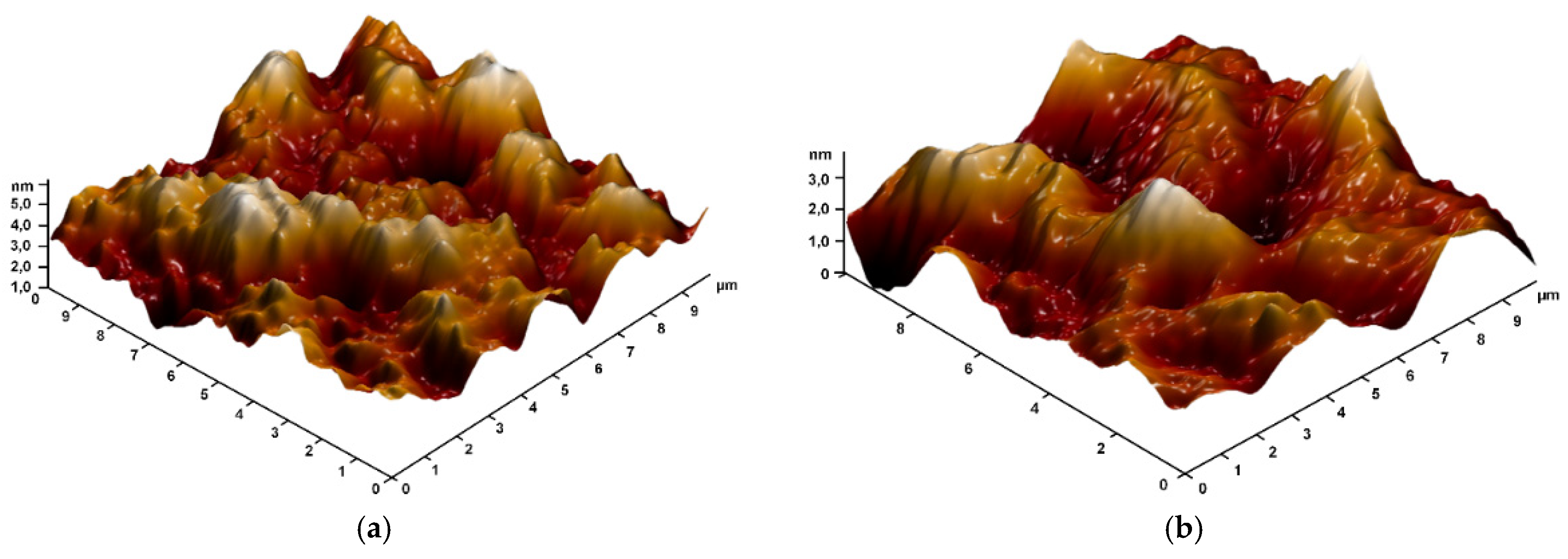
2.3. Characterization of PMMA/Ti-NPs Material Samples
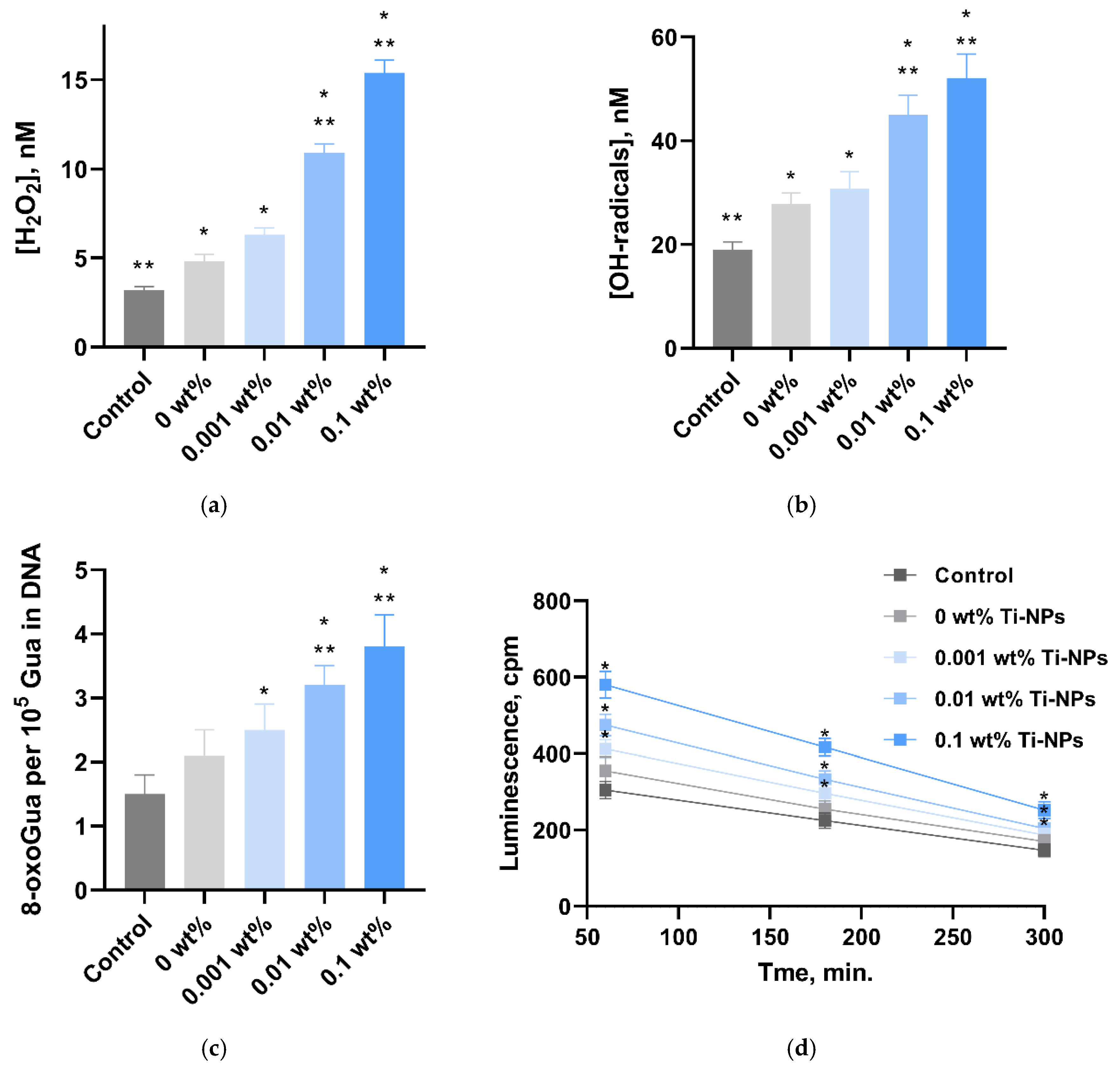
2.4. Quantitative Assessment of Generated ROS in Aqueous Solutions (H2O2 and •OH)
2.5. Quantitative Determination of 8-Oxoguanine and Long-Lived Reactive Proteins Species (LRPS)
2.6. E. coli Growth Curves
2.7. Evaluation of Antibacterial Activity by Flow Cytofluorometry
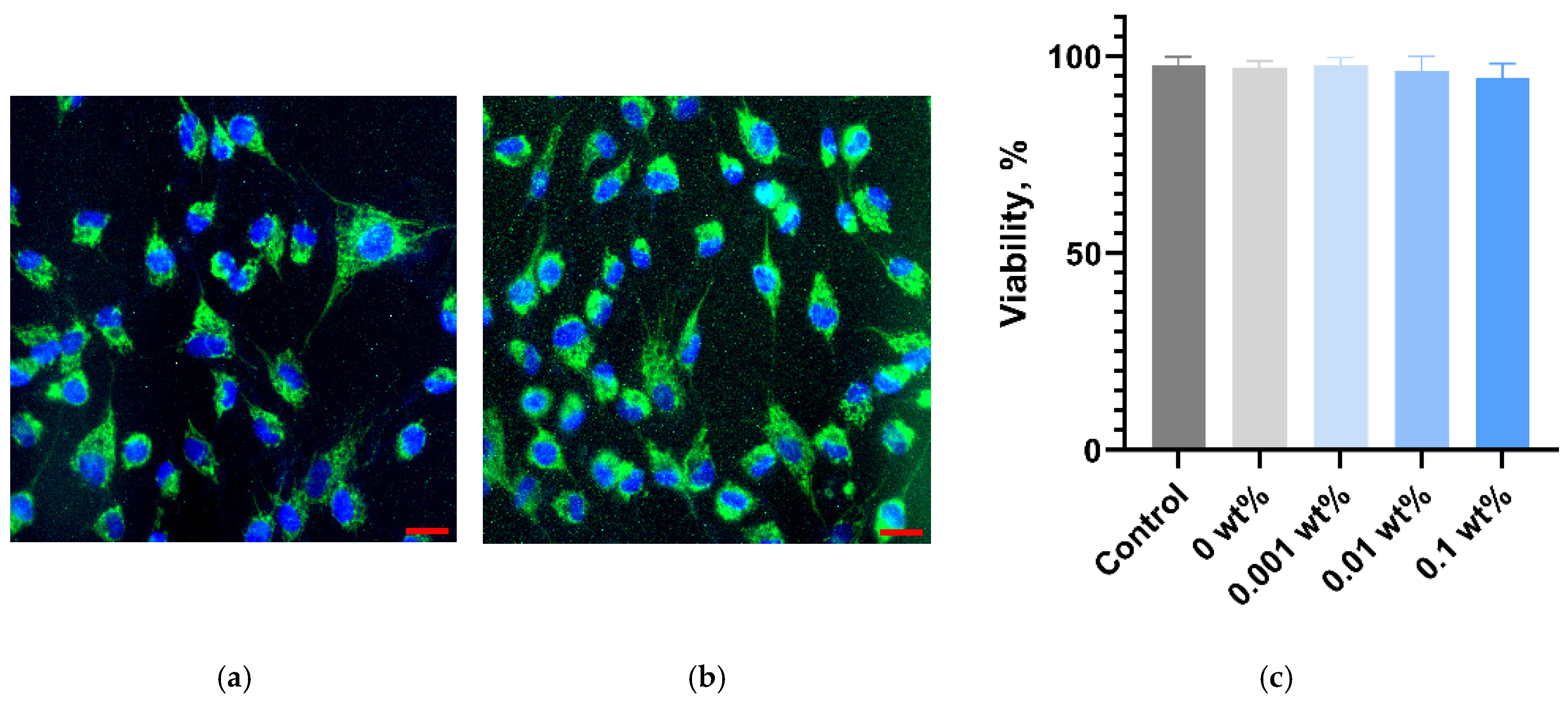
2.8. In Vitro Cytotoxicity Assessment
2.9. Statistical Data Analysis and Visualization
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PMMA | Polymethyl methacrylate |
| Ti-NPs | Titanium nanoparticles |
| H2O2 | Hydrogen peroxide |
| •OH | Hydroxyl radical |
| PI | Propidium iodide |
| MIM | Modulation interference microscopy |
| MSLA | Masked Stereolithography |
| ROS | Reactive oxygen species |
References
- Yüceer, Ö.M.; Kaynak Öztürk, E.; Çiçek, E.S.; Aktaş, N.; Bankoğlu Güngör, M. Three-Dimensional-Printed Photopolymer Resin Materials: A Narrative Review on Their Production Techniques and Applications in Dentistry. Polymers 2025, 17, 316. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Gao, W.; Liu, F.; Cui, J.; Feng, S.; Liang, C.; Guo, Y.; Wang, Z.; Mao, Z.; Zhang, B. Vat photopolymerization based digital light processing 3D printing hydrogels in biomedical fields: Key parameters and perspective. Addit. Manuf. 2024, 94, 104443. [Google Scholar] [CrossRef]
- AlAzzam, N.F.; Bajunaid, S.O.; Mitwalli, H.A.; Baras, B.H.; Weir, M.D.; Xu, H.H.K. The Effect of Incorporating Dimethylaminohexadecyl Methacrylate and/or 2-Methacryloyloxyethyl Phosphorylcholine on Flexural Strength and Surface Hardness of Heat Polymerized and 3D-Printed Denture Base Materials. Materials 2024, 17, 4625. [Google Scholar] [CrossRef]
- Shabalina, A.V.; Kozlov, V.A.; Popov, I.A.; Gudkov, S.V. A Review on Recently Developed Antibacterial Composites of Inorganic Nanoparticles and Non-Hydrogel Polymers for Biomedical Applications. Nanomaterials 2024, 14, 1753. [Google Scholar] [CrossRef]
- Mosley, A.P. Acrylic Plastics. In Brydson’s Plastics Materials; Elsevier: Amsterdam, The Netherlands, 2017; pp. 441–456. [Google Scholar]
- Hassan, M.; Asghar, M.; Din, S.U.; Zafar, M.S. Thermoset polymethacrylate-based materials for dental applications. In Materials for Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 273–308. [Google Scholar]
- Zafar, M.S. Prosthodontic Applications of Polymethyl Methacrylate (PMMA): An Update. Polymers 2020, 12, 2299. [Google Scholar] [CrossRef] [PubMed]
- Alam, F.; Elsherif, M.; Salih, A.E.; Butt, H. 3D printed polymer composite optical fiber for sensing applications. Addit. Manuf. 2022, 58, 102996. [Google Scholar] [CrossRef]
- Luo, Y.; Canning, J.; Zhang, J.; Peng, G.-D. Toward optical fibre fabrication using 3D printing technology. Opt. Fiber Technol. 2020, 58, 102299. [Google Scholar] [CrossRef]
- van der Elst, L.; Faccini de Lima, C.; Gokce Kurtoglu, M.; Koraganji, V.N.; Zheng, M.; Gumennik, A. 3D Printing in Fiber-Device Technology. Adv. Fiber Mater. 2021, 3, 59–75. [Google Scholar] [CrossRef]
- Chekkaramkodi, D.; Ahmed, I.; Al-Rub, R.K.A.; Schiffer, A.; Butt, H. 3D-printed multi-material optical fiber sensor for dual sensing applications. Adv. Compos. Hybrid Mater. 2025, 8, 107. [Google Scholar] [CrossRef]
- Zhao, J.; Xiao, C.; Feng, Y.; Xu, N. A Review: Polymethacrylate Fibers as Oil Absorbents. Polym. Rev. 2013, 53, 527–545. [Google Scholar] [CrossRef]
- Gavrilenko, N.A.; Saranchina, N.V.; Sukhanov, A.V.; Gavrilenko, M.A.; Zenkova, E.V. Colorimetric Polymethacrylate Sensor. Adv. Mater. Res. 2014, 880, 19–24. [Google Scholar] [CrossRef]
- Dimitrova, M.; Corsalini, M.; Kazakova, R.; Vlahova, A.; Chuchulska, B.; Barile, G.; Capodiferro, S.; Kazakov, S. Comparison between Conventional PMMA and 3D Printed Resins for Denture Bases: A Narrative Review. J. Compos. Sci. 2022, 6, 87. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Zhang, G.; Xu, B.; Zhao, Z.; Yin, T. Fabrication of Polymethyl Methacrylate (PMMA) Hydrophilic Surfaces Using Combined Offset-Tool-Servo Flycutting and Hot Embossing Methods. Polymers 2023, 15, 4532. [Google Scholar] [CrossRef] [PubMed]
- Abad-Coronel, C.; Vélez Chimbo, D.; Lupú, B.; Pacurucu, M.; Fárez, M.V.; Fajardo, J.I. Comparative Analysis of the Structural Weights of Fixed Prostheses of Zirconium Dioxide, Metal Ceramic, PMMA and 3DPP Printing Resin—Mechanical Implications. Dent. J. 2023, 11, 249. [Google Scholar] [CrossRef] [PubMed]
- Rudenko, Y.; Kozlov, V.; Burmistrov, D.; Fedyakova, N.; Bermeshev, M.; Chapala, P. Comprehensive investigation of phosphine oxide photoinitiators for vat photopolymerization. Prog. Addit. Manuf. 2025. [Google Scholar] [CrossRef]
- FDA. Class II Special Controls Guidance Document: Polymethylmethacrylate (PMMA) Bone Cement. In Guidance for Industry and FDA; FDA: Silver Spring, MD, USA, 2002. [Google Scholar]
- Class, I. Special Controls Guidance Document: Polymethylmethacrylate (PMMA) Bone Cement; Guidance for Industry and FDA. Center for Devices and Radiological Health. 2002; pp. 1–18. Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf19/K193059.pdf (accessed on 4 June 2025).
- Gold, M.H.; Sadick, N.S. Optimizing outcomes with polymethylmethacrylate fillers. J. Cosmet. Dermatol. 2018, 17, 298–304. [Google Scholar] [CrossRef]
- Kostić, M.; Igić, M.; Gligorijević, N.; Nikolić, V.; Stošić, N.; Nikolić, L. The Use of Acrylate Polymers in Dentistry. Polymers 2022, 14, 4511. [Google Scholar] [CrossRef]
- Worzakowska, M. UV Polymerization of Methacrylates—Preparation and Properties of Novel Copolymers. Polymers 2021, 13, 1659. [Google Scholar] [CrossRef]
- Soleymani Eil Bakhtiari, S.; Bakhsheshi-Rad, H.R.; Karbasi, S.; Tavakoli, M.; Razzaghi, M.; Ismail, A.F.; RamaKrishna, S.; Berto, F. Polymethyl Methacrylate-Based Bone Cements Containing Carbon Nanotubes and Graphene Oxide: An Overview of Physical, Mechanical, and Biological Properties. Polymers 2020, 12, 1469. [Google Scholar] [CrossRef]
- Mohsen, A.H.; Ali, N.A.; Hussein, S.I.; Mohammed, A.Y.A.; Alraih, A.M.; Abd-Elnaiem, A.M. Investigation of optical, mechanical, thermal, wettability, and antibacterial activity of polyvinyl alcohol mixed with clay nanoparticles. Inorg. Chem. Commun. 2024, 167, 112841. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Sarimov, R.M.; Astashev, M.E.; Pishchalnikov, R.Y.; Yanykin, D.V.; Simakin, A.V.; Shkirin, A.V.; Serov, D.A.; Konchekov, E.M.; Gusein-zade, N.; et al. Modern physical methods and technologies in agriculture. Phys.-Uspekhi 2024, 67, 194–210. [Google Scholar] [CrossRef]
- Kuang, T.; Guo, H.; Guo, W.; Liu, W.; Li, W.; Saeb, M.R.; Vatankhah-Varnosfaderani, M.; Sheiko, S.S. Boosting the Strength and Toughness of Polymer Blends via Ligand-Modulated MOFs. Adv. Sci. 2024, 11, e2407593. [Google Scholar] [CrossRef] [PubMed]
- Alhussain, H.; Alghamdi, A.M.; Elamin, N.Y.; Rajeh, A. Recent Progress in Enhanced Optical, Mechanical, Thermal Properties, and Antibacterial Activity of the Chitosan/Polyvinylalcohol/Co3O4 Nanocomposites for Optoelectronics and Biological Applications. J. Polym. Environ. 2024, 32, 3735–3748. [Google Scholar] [CrossRef]
- Halder, S.; Prasad, T.; Khan, N.I.; Goyat, M.S.; Ram Chauhan, S. Superior mechanical properties of poly vinyl alcohol-assisted ZnO nanoparticle reinforced epoxy composites. Mater. Chem. Phys. 2017, 192, 198–209. [Google Scholar] [CrossRef]
- Abbas, Z.A.; Aleabi, S.H. Studying some of mechanical properties (tensile, impact, hardness) and thermal conductivity of polymer blend reinforce by magnesium oxide. In Proceedings of the Xiamen-Custipen Workshop on the Equation of State of Dense Neutron-Rich Matter in the Era of Gravitational Wave Astronomy, Xiamen, China, 3–7 January 2019. [Google Scholar]
- Kaurani, P.; Hindocha, A.D.; Jayasinghe, R.M.; Pai, U.Y.; Batra, K.; Price, C. Effect of addition of titanium dioxide nanoparticles on the antimicrobial properties, surface roughness and surface hardness of polymethyl methacrylate: A Systematic Review. F1000Research 2023, 12, 577. [Google Scholar] [CrossRef]
- Siwińska-Ciesielczyk, K.; Andrzejczak, A.; Jesionowski, T.; Gierz, Ł.; Marcinkowska, A.; Robakowska, M. New Insights into the Application of Biocompatible (Un)Modified TiO2 and TiO2-ZrO2 Oxide Fillers in Light-Curing Materials. Materials 2024, 17, 2908. [Google Scholar] [CrossRef]
- Rudko, G.; Kovalchuk, A.; Fediv, V.; Chen, W.M.; Buyanova, I.A. Enhancement of polymer endurance to UV light by incorporation of semiconductor nanoparticles. Nanoscale Res. Lett. 2015, 10, 81. [Google Scholar] [CrossRef]
- Wang, B.; Duan, Y.; Zhang, J. Titanium dioxide nanoparticles-coated aramid fiber showing enhanced interfacial strength and UV resistance properties. Mater. Des. 2016, 103, 330–338. [Google Scholar] [CrossRef]
- Thakur, N.; Thakur, N.; Kumar, A.; Thakur, V.K.; Kalia, S.; Arya, V.; Kumar, A.; Kumar, S.; Kyzas, G.Z. A critical review on the recent trends of photocatalytic, antibacterial, antioxidant and nanohybrid applications of anatase and rutile TiO2 nanoparticles. Sci. Total Environ. 2024, 914, 169815. [Google Scholar] [CrossRef]
- El-Khawaga, A.M.; Elsayed, M.A.; Gobara, M.; Suliman, A.A.; Hashem, A.H.; Zaher, A.A.; Mohsen, M.; Salem, S.S. Green synthesized ZnO nanoparticles by Saccharomyces cerevisiae and their antibacterial activity and photocatalytic degradation. Biomass Convers. Biorefinery 2023, 15, 2673–2684. [Google Scholar] [CrossRef]
- Lekkala, V.D.V.V.; Muktinutalapati, A.V.; Lebaka, V.R.; Lomada, D.; Korivi, M.; Li, W.; Reddy, M.C. Green Synthesis and Characterization of Silver Nanoparticles from Tinospora cordifolia Leaf Extract: Evaluation of Their Antioxidant, Anti-Inflammatory, Antibacterial, and Antibiofilm Efficacies. Nanomaterials 2025, 15, 381. [Google Scholar] [CrossRef] [PubMed]
- Jahan, I.; Matpan Bekler, F.; Tunç, A.; Güven, K. The Effects of Silver Nanoparticles (AgNPs) on Thermophilic Bacteria: Antibacterial, Morphological, Physiological and Biochemical Investigations. Microorganisms 2024, 12, 402. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Hong, D.; Qiu, S.; Fang, Y.; Zhu, Y. Intrinsic antibacterial photopolymerization 3D-printed thermosets from citronellol and cinnamyl alcohol: Synthesis and properties. Polym. Testing. 2024, 140, 108582. [Google Scholar] [CrossRef]
- Burunkova, J.A.; Semykina, V.V.; Sitnikova, V.E.; Dolgintsev, D.M.; Zaripova, F.F.; Ponomareva, A.A.; Mizina, D.R.; Csick, A.; Kokenyesi, S.; Zhilenkov, A. Antibacterial UV-Curable Gel with Hydroxyapatite Nanoparticles for Regenerative Medicine in the Field of Orthopedics. J. Compos. Sci. 2025, 9, 65. [Google Scholar] [CrossRef]
- Preobrazhenskii, I.I.; Putlyaev, V.I. 3D Printing of Hydrogel-Based Biocompatible Materials. Russ. J. Appl. Chem. 2022, 95, 775–788. [Google Scholar] [CrossRef]
- Wang, Y.; Frascella, F.; Gaglio, C.; Pirri, C.; Wei, Q.; Roppolo, I. Vat Photopolymerization 3D Printing of Hydrogels Embedding Metal–Organic Frameworks for Photodynamic Antimicrobial Therapy. ACS Appl. Mater. Interfaces 2024, 16, 57778–57791. [Google Scholar] [CrossRef]
- Idumah, C.I. Novel trends in conductive polymeric nanocomposites, and bionanocomposites. Synth. Met. 2021, 273, 116674. [Google Scholar] [CrossRef]
- Meshalkin, V.P.; Kolmakov, A.G.; Nzioka, A.M.; Bannykh, I.O.; Sevostyanov, M.A.; Konushkin, S.V.; Kaplan, M.A.; Chistyakova, T.B. Structural metal alloys: Towards environmentally friendly materials. Russ. Chem. Rev. 2025, 94, RCR5165. [Google Scholar] [CrossRef]
- Schoenitz, M.; Chen, C.-M.; Dreizin, E.L. Oxidation of Aluminum Particles in the Presence of Water. J. Phys. Chem. B 2009, 113, 5136–5140. [Google Scholar] [CrossRef]
- Maldybayev, G.K.; Korabayev, A.S.; Shayakhmetova, R.A.; Khabiyev, A.T.; Baigenzhenov, O.S.; Sharipov, R.H.; Amirkhan, A.A. Separation of iron and titanium from titanium magnetite raw materials by low-temperature treatment and magnetic separation. Case Stud. Chem. Environ. Eng. 2024, 10, 100848. [Google Scholar] [CrossRef]
- Myers, J.R.; Bomberger, H.B.; Froes, F.H. Corrosion Behavior and Use of Titanium and Its Alloys. JOM 1984, 36, 50–60. [Google Scholar] [CrossRef]
- Shabatina, T.; Vernaya, O.; Shumilkin, A.; Semenov, A.; Melnikov, M. Nanoparticles of Bioactive Metals/Metal Oxides and Their Nanocomposites with Antibacterial Drugs for Biomedical Applications. Materials 2022, 15, 3602. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Kim, J.; Lee, C.; Lee, G.; Hyeon, J.; Jeong, S.-k.; Cho, N. Enhancing the Mechanical Strength of a Photocurable 3D Printing Material Using Potassium Titanate Additives for Craniofacial Applications. Biomimetics 2024, 9, 698. [Google Scholar] [CrossRef]
- Kainz, M.; Perak, S.; Stubauer, G.; Kopp, S.; Kauscheder, S.; Hemetzberger, J.; Martínez Cendrero, A.; Díaz Lantada, A.; Tupe, D.; Major, Z.; et al. Additive and Lithographic Manufacturing of Biomedical Scaffold Structures Using a Versatile Thiol-Ene Photocurable Resin. Polymers 2024, 16, 655. [Google Scholar] [CrossRef]
- Silva, L.F.O.; Hower, J.C.; Dotto, G.L.; Oliveira, M.L.S.; Pinto, D. Titanium nanoparticles in sedimented dust aggregates from urban children’s parks around coal ashes wastes. Fuel 2021, 285, 119162. [Google Scholar] [CrossRef]
- Avolio, R.; D’Albore, M.; Guarino, V.; Gentile, G.; Cocca, M.C.; Zeppetelli, S.; Errico, M.E.; Avella, M.; Ambrosio, L. Pure titanium particle loaded nanocomposites: Study on the polymer/filler interface and hMSC biocompatibility. J. Mater. Sci. Mater. Med. 2016, 27, 153. [Google Scholar] [CrossRef]
- Mutaf, T.; Caliskan, G.; Ozel, H.; Akagac, G.; Öncel, S.Ş.; Elibol, M. Green synthesis of titanium nanoparticles using a sustainable microalgal metabolite solution for potential biotechnological activities. Asia-Pac. J. Chem. Eng. 2023, 18, e2954. [Google Scholar] [CrossRef]
- Seydi, N.; Mahdavi, B.; Paydarfard, S.; Zangeneh, A.; Zangeneh, M.M.; Najafi, F.; Jalalvand, A.R.; Pirabbasi, E. Preparation, characterization, and assessment of cytotoxicity, antioxidant, antibacterial, antifungal, and cutaneous wound healing properties of titanium nanoparticles using aqueous extract of Ziziphora clinopodioides Lam leaves. Appl. Organomet. Chem. 2019, 33, e5009. [Google Scholar] [CrossRef]
- Dikovskaya, A.O.; Simakin, A.V.; Baimler, I.V.; Gudkov, S.V. The concentration limit of stability for individual gold nanoparticles in aqueous colloid during water evaporation. Chem. Phys. 2024, 586, 112399. [Google Scholar] [CrossRef]
- ISO 10993-1:2018; Biological Evaluation of Medical Devices. ISO: Geneva, Switzerland, 2018.
- ISO 179-1:2010; Plastics—Determination of Charpy Impact Properties—Part 1: Non-Instrumented Impact Test. ISO: Geneva, Switzerland, 2010.
- ISO 527-2:2012; Plastics–Determination of Tensile Properties. ISO: Geneva, Switzerland, 2012.
- ASTM D790; Standard Test Methods for Flexural Properties of Unreinforced and Reinforced Plastics and Electrical Insulating Materials. Annual Book of ASTM Standards. ASTM Intemational: West Conshohocken, PA, USA, 1997.
- ASTM D638-14; Standard Test Method for Tensile Properties of Plastics. ASTM International: West Conshohocken, PA, USA, 1998.
- Sevostyanov, M.A.; Kolmakov, A.G.; Sergiyenko, K.V.; Kaplan, M.A.; Baikin, A.S.; Gudkov, S.V. Mechanical, physical–chemical and biological properties of the new Ti–30Nb–13Ta–5Zr alloy. J. Mater. Sci. 2020, 55, 14516–14529. [Google Scholar] [CrossRef]
- Chernikov, A.V.; Gudkov, S.V.; Shtarkman, I.N.; Bruskov, V.I. Oxygen effect in heat-induced DNA damage. Biophysics 2007, 52, 185–190. [Google Scholar] [CrossRef]
- Sevostyanov, M.A.; Baikin, A.S.; Sergienko, K.V.; Shatova, L.A.; Kirsankin, A.A.; Baymler, I.V.; Shkirin, A.V.; Gudkov, S.V. Biodegradable stent coatings on the basis of PLGA polymers of different molecular mass, sustaining a steady release of the thrombolityc enzyme streptokinase. React. Funct. Polym. 2020, 150, 104550. [Google Scholar] [CrossRef]
- Astashev, M.E.; Sarimov, R.M.; Serov, D.A.; Matveeva, T.A.; Simakin, A.V.; Ignatenko, D.N.; Burmistrov, D.E.; Smirnova, V.V.; Kurilov, A.D.; Mashchenko, V.I.; et al. Antibacterial behavior of organosilicon composite with nano aluminum oxide without influencing animal cells. React. Funct. Polym. 2022, 170, 105143. [Google Scholar] [CrossRef]
- Burmistrov, D.E.; Simakin, A.V.; Smirnova, V.V.; Uvarov, O.V.; Ivashkin, P.I.; Kucherov, R.N.; Ivanov, V.E.; Bruskov, V.I.; Sevostyanov, M.A.; Baikin, A.S.; et al. Bacteriostatic and Cytotoxic Properties of Composite Material Based on ZnO Nanoparticles in PLGA Obtained by Low Temperature Method. Polymers 2021, 14, 49. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Li, R.; Serov, D.A.; Burmistrov, D.E.; Baimler, I.V.; Baryshev, A.S.; Simakin, A.V.; Uvarov, O.V.; Astashev, M.E.; Nefedova, N.B.; et al. Fluoroplast Doped by Ag2O Nanoparticles as New Repairing Non-Cytotoxic Antibacterial Coating for Meat Industry. Int. J. Mol. Sci. 2023, 24, 869. [Google Scholar] [CrossRef]
- Baimler, I.V.; Simakin, A.V.; Dorokhov, A.S.; Gudkov, S.V. Mini-review on laser-induced nanoparticle heating and melting. Front. Chem. 2024, 12, 1463612. [Google Scholar] [CrossRef]
- Ragab, H.M.; Diab, N.S.; Khaled, A.M.; Aboelnaga, S.M.; Qasem, A.; Farea, M.O.; Morsi, M.A. Tailoring optical and electrical properties of hybrid polymer nanodielectrics: Synthesis and characterization of CuO/TiO2 nanoparticle-embedded HPMC/NaAlg blend. Ceram. Int. 2025, 51, 17302–17310. [Google Scholar] [CrossRef]
- Căprărescu, S.; Tihan, G.T.; Zgârian, R.G.; Grumezescu, A.M.; Lazau, C.; Bandas, C.; Atanase, L.I.; Nicolae, C.-A. Synthesis and Characterization of Cellulose Acetate/Polyethylene Glycol/Poly(Styrene)-b-Poly(4-Vinylpyridine) Membrane Embedded with Hydrotermaly Activated TiO2 Nanoparticles for Waste-Waters Treatment by Membrane Processes. Polymers 2025, 17, 446. [Google Scholar] [CrossRef]
- Simakin, A.V.; Baymler, I.V.; Smirnova, V.V.; Astashev, M.E.; Voronov, V.V.; Dorokhov, A.S.; Gudkov, S.V. Comparison of the Intensities of Chemical and Physical Processes Occurring during Laser-Induced Breakdown of Colloidal Solutions of Tb Nanoparticles with Different Oxidation States. Dokl. Phys. 2023, 67, 465–471. [Google Scholar] [CrossRef]
- Trembecka-Wójciga, K.; Jankowska, M.; Lepcio, P.; Sevriugina, V.; Korčušková, M.; Czeppe, T.; Zubrzycka, P.; Ortyl, J. Optimization of Zirconium Oxide Nanoparticle-Enhanced Photocurable Resins for High-Resolution 3D Printing Ceramic Parts. Adv. Mater. Interfaces 2025, 12, 2400951. [Google Scholar] [CrossRef]
- Tkachev, D.; Dubkova, Y.; Zhukov, A.; Verkhoshanskiy, Y.; Vorozhtsov, A.; Zhukov, I. Photocurable High-Energy Polymer-Based Materials for 3D Printing. Polymers 2023, 15, 4252. [Google Scholar] [CrossRef] [PubMed]
- Gil, L.D.; Monteiro, S.N.; Colorado, H.A. Polymer Matrix Nanocomposites Fabricated with Copper Nanoparticles and Photopolymer Resin via Vat Photopolymerization Additive Manufacturing. Polymers 2024, 16, 2434. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.I.; Harrysson, O.L.A.; Chowdhury, M.A.; Hossain, N. Impact of graphene nanoparticles on DLP-printed parts’ mechanical behavior. Adv. Ind. Manuf. Eng. 2025, 10, 100153. [Google Scholar] [CrossRef]
- Yüksel, N.; Eren, O.; Şahin, İ. Exploration of tungsten carbide reinforcement in photopolymer nano-composites via DLP 3D printing. Ceram. Int. 2025; in press. [Google Scholar] [CrossRef]
- Papaléo, R.M.; Mota, E.G.; Subramani, K.; De Oliveira, E.M.N.; Mussatto, C.M.B. Evaluation of Mechanical Properties of Self-cured, Heat-cured, and Photosensitive 3D Printing Resins After the Addition of Silica Nanoparticles. J. Mater. Appl. 2024, 13, 1–10. [Google Scholar] [CrossRef]
- Maleki, H.; Asadi, P.; Moghanian, A.; Safaee, S.; Najjar, I.M.R.; Fathy, A. Hybrid PLA-based biocomposites produced by DLP 3D printer with alumina and silica nanoparticles. Mater. Today Commun. 2025, 42, 111469. [Google Scholar] [CrossRef]
- Makvandi, P.; Nikfarjam, N.; Sanjani, N.S.; Qazvini, N.T. Effect of silver nanoparticle on the properties of poly (methyl methacrylate) nanocomposite network made by in situ photoiniferter-mediated photopolymerization. Bull. Mater. Sci. 2015, 38, 1625–1631. [Google Scholar] [CrossRef]
- Check, C.; Chartoff, R.; Chang, S. Effects of nanoparticles on photopolymerization of acrylate monomers in forming nano-composites. Eur. Polym. J. 2015, 70, 166–172. [Google Scholar] [CrossRef]
- Bondarenko, A.V.; Islamov, S.R.; Ignatyev, K.V.; Mardashov, D.V. Laboratory studies of polymer compositions for well-kill under increased fracturing. Perm J. Pet. Min. Eng. 2020, 20, 37–48. [Google Scholar] [CrossRef]
- Belousov, A.; Lushpeev, V.; Sokolov, A.; Sultanbekov, R.; Tyan, Y.; Ovchinnikov, E.; Shvets, A.; Bushuev, V.; Islamov, S. Experimental Research of the Possibility of Applying the Hartmann–Sprenger Effect to Regulate the Pressure of Natural Gas in Non-Stationary Conditions. Processes 2025, 13, 1189. [Google Scholar] [CrossRef]
- Dejene, B.K. Advancing Natural Fiber-Reinforced Composites Through Incorporating ZnO Nanofillers in the Polymeric Matrix: A Review. J. Nat. Fibers 2024, 21, 2356015. [Google Scholar] [CrossRef]
- Bailly, M.; Kontopoulou, M.; El Mabrouk, K. Effect of polymer/filler interactions on the structure and rheological properties of ethylene-octene copolymer/nanosilica composites. Polymer 2010, 51, 5506–5515. [Google Scholar] [CrossRef]
- Hiremath, A.; Murthy, A.A.; Thipperudrappa, S.; K N., B.; Jones, I.P. Nanoparticles Filled Polymer Nanocomposites: A Technological Review. Cogent Eng. 2021, 8, 1991229. [Google Scholar] [CrossRef]
- Ahmad, K.H.; Mohamad, Z.; Khan, Z.I.; Habib, M. Tailoring UV Penetration Depth in Photopolymer Nanocomposites: Advancing SLA 3D Printing Performance with Nanofillers. Polymers 2025, 17, 97. [Google Scholar] [CrossRef] [PubMed]
- Abdollahpour, S.; Estedlal, T.; Chiniforush, N.; Rafeie, N.; Nikparto, N.; Abbasi, M.; Ranjbar Omrani, L.; Kukiattrakoon, B. Effect of Different Bleaching Methods on Monomer Release from Aged Microhybrid and Nanohybrid Resin Composites. Int. J. Dent. 2023, 2023, 2773879. [Google Scholar] [CrossRef]
- Schneider, T.R.; Hakami-Tafreshi, R.; Tomasino-Perez, A.; Tayebi, L.; Lobner, D. Effects of dental composite resin monomers on dental pulp cells. Dent. Mater. J. 2019, 38, 579–583. [Google Scholar] [CrossRef]
- Filimonova, E.A.; Lozovaya, A.V.; Prosyankin, E.E.; Mustafina, A.R.; Chapala, P. Thermal treatment influence on optical properties of 3D printed objects by vat photopolymerization. Prog. Addit. Manuf. 2024, 10, 2703–2713. [Google Scholar] [CrossRef]
- Asmussen, S.V.; Arenas, G.F.; Vallo, C.I. Enhanced degree of polymerization of methacrylate and epoxy resins by plasmonic heating of embedded silver nanoparticles. Prog. Org. Coat. 2015, 88, 220–227. [Google Scholar] [CrossRef]
- Toh-ae, P.; Lee-Nip, R.; Nakaramontri, Y. Releasing of zinc ions from modified zinc oxide surfaces for improvement chemical crosslinks and antibacterial properties of acrylonitrile butadiene rubber films. Express Polym. Lett. 2023, 17, 944–963. [Google Scholar] [CrossRef]
- Sharapov, M.G.; Gudkov, S.V.; Lankin, V.Z. Hydroperoxide-Reducing Enzymes in the Regulation of Free-Radical Processes. Biochemistry 2021, 86, 1256–1274. [Google Scholar] [CrossRef]
- Kruchinin, A.A.; Kamzeeva, P.N.; Zharkov, D.O.; Aralov, A.V.; Makarova, A.V. 8-Oxoadenine: A «New» Player of the Oxidative Stress in Mammals? Int. J. Mol. Sci. 2024, 25, 1342. [Google Scholar] [CrossRef]
- Mohammed, M.R.; Hadi, A.N.; Ali, S. Enhancing the Mechanical Behaviour and Antibacterial Activity of Bioepoxy Using Hybrid Nanoparticles for Dental Applications. Int. J. Biomater. 2022, 2022, 2124070. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi Anzabi, R.; Divband, B.; Tukmachi, M.S.; Vahedifar, S.; Esmaeilzadeh, M.; Yeganeh Sefidan, F.; Jahanbani, M.; Rafighi, A.; Lopes, M.B. Assessment of Physicochemical Properties, Cytotoxicity, Antimicrobial Activity, and Flexural Strength of Self-Cured Acrylic Resin with Silver Nanoparticles on Delaminated Clay for Removable Appliances. Int. J. Dent. 2025, 2025, 7939455. [Google Scholar] [CrossRef] [PubMed]
- Soniya Pauline, G.; Kaleemulla, S. Properties of Ti doped ZnO nanoparticles under solid state reaction method involving vacuum annealing. Phys. B Condens. Matter 2023, 649, 414409. [Google Scholar] [CrossRef]
- Zalega, M.; Bociong, K. Antibacterial Agents Used in Modifications of Dental Resin Composites: A Systematic Review. Appl. Sci. 2024, 14, 3710. [Google Scholar] [CrossRef]
- AlAzzam, N.F.; Bajunaid, S.O.; Baras, B.H.; Mitwalli, H.A.; Weir, M.D.; Xu, H.H. Microbial Adhesion and Cytotoxicity of Heat-Polymerized and 3D-Printed Denture Base Materials when Modified with Dimethylaminohexadecyl Methacrylate and/or 2-Methacryloyloxyethyl Phosphorylcholine as Antimicrobial and Protein-Repellent Materials. Polymers 2025, 17, 228. [Google Scholar] [CrossRef]
- Garza, E.G.; Wadajkar, A.; Ahn, C.; Zhu, Q.; Opperman, L.A.; Bellinger, L.L.; Nguyen, K.T.; Komabayashi, T. Cytotoxicity evaluation of methacrylate-based resins for clinical endodontics in vitro. J. Oral Sci. 2012, 54, 213–217. [Google Scholar] [CrossRef]
- Sulek, J.; Luczaj-Cepowicz, E.; Marczuk-Kolada, G.; Rosłan, M.; Holownia, A. Cytotoxicity of Methacrylate Dental Resins to Human Gingival Fibroblasts. J. Funct. Biomater. 2022, 13, 56. [Google Scholar] [CrossRef]
- Van Noort, R. Titanium: The implant material of today. J. Mater. Sci. 1987, 22, 3801–3811. [Google Scholar] [CrossRef]
- Kim, K.T.; Eo, M.Y.; Nguyen, T.T.H. General review of titanium toxicity. Int. J. Implant Dent. 2019, 5, 10. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burmistrov, D.E.; Serov, D.A.; Baimler, I.V.; Gritsaeva, A.V.; Chapala, P.; Simakin, A.V.; Astashev, M.E.; Karmanova, E.E.; Dubinin, M.V.; Nizameeva, G.R.; et al. Polymethyl Methacrylate-like Photopolymer Resin with Titanium Metal Nanoparticles Is a Promising Material for Biomedical Applications. Polymers 2025, 17, 1830. https://doi.org/10.3390/polym17131830
Burmistrov DE, Serov DA, Baimler IV, Gritsaeva AV, Chapala P, Simakin AV, Astashev ME, Karmanova EE, Dubinin MV, Nizameeva GR, et al. Polymethyl Methacrylate-like Photopolymer Resin with Titanium Metal Nanoparticles Is a Promising Material for Biomedical Applications. Polymers. 2025; 17(13):1830. https://doi.org/10.3390/polym17131830
Chicago/Turabian StyleBurmistrov, Dmitriy E., Dmitriy A. Serov, Ilya V. Baimler, Ann V. Gritsaeva, Pavel Chapala, Aleksandr V. Simakin, Maxim E. Astashev, Ekaterina E. Karmanova, Mikhail V. Dubinin, Guliya R. Nizameeva, and et al. 2025. "Polymethyl Methacrylate-like Photopolymer Resin with Titanium Metal Nanoparticles Is a Promising Material for Biomedical Applications" Polymers 17, no. 13: 1830. https://doi.org/10.3390/polym17131830
APA StyleBurmistrov, D. E., Serov, D. A., Baimler, I. V., Gritsaeva, A. V., Chapala, P., Simakin, A. V., Astashev, M. E., Karmanova, E. E., Dubinin, M. V., Nizameeva, G. R., Validov, S. Z., Yanbaev, F. M., Synyashin, O. G., & Gudkov, S. V. (2025). Polymethyl Methacrylate-like Photopolymer Resin with Titanium Metal Nanoparticles Is a Promising Material for Biomedical Applications. Polymers, 17(13), 1830. https://doi.org/10.3390/polym17131830












