Fire-Resistant Coatings: Advances in Flame-Retardant Technologies, Sustainable Approaches, and Industrial Implementation
Abstract
1. Introduction
2. Fire-Resistant Polymers
2.1. Fundamentals of Flame Retardancy
2.2. Classification of Flame Retardants
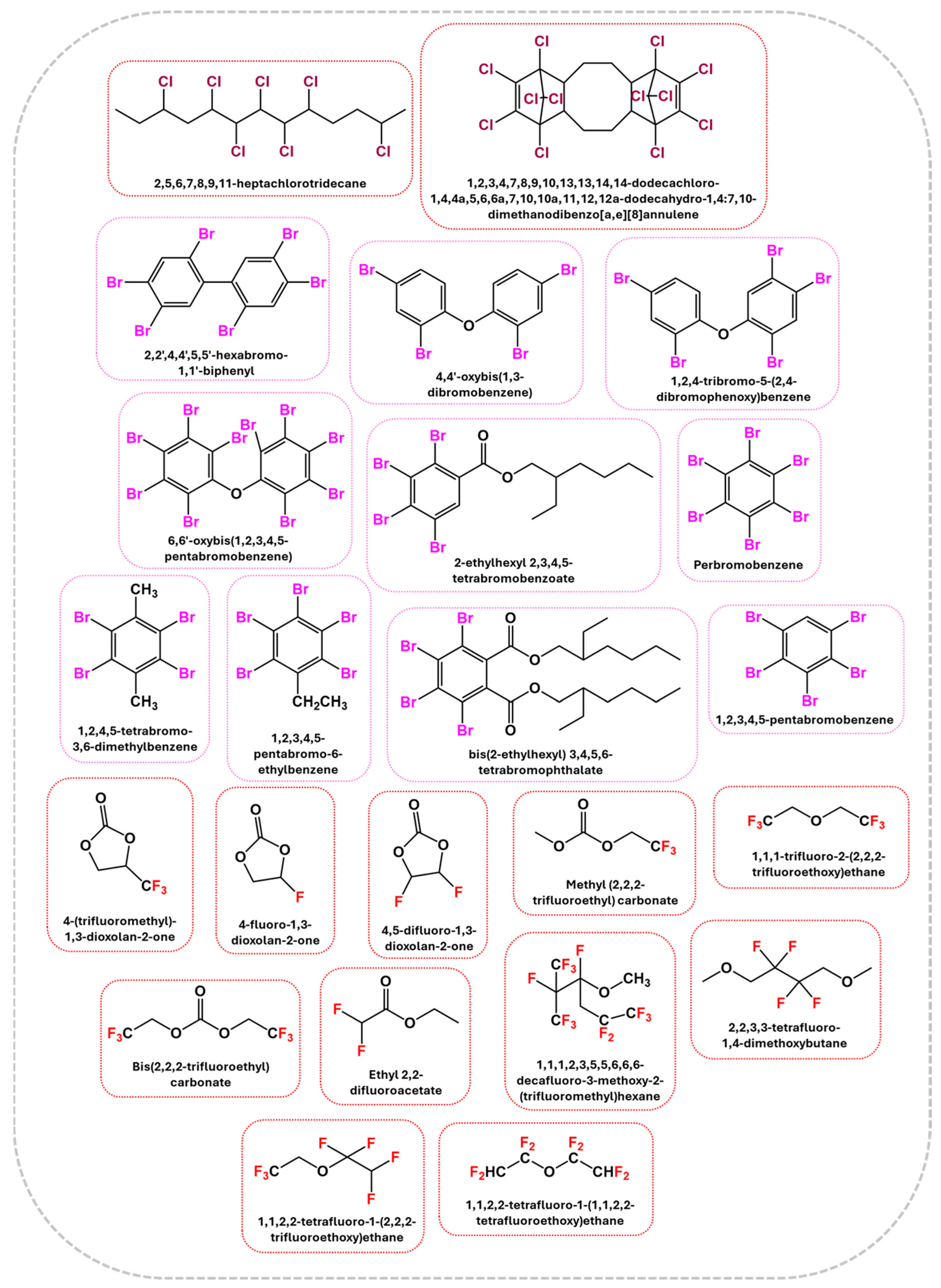
2.2.1. Halogenated Flame Retardants
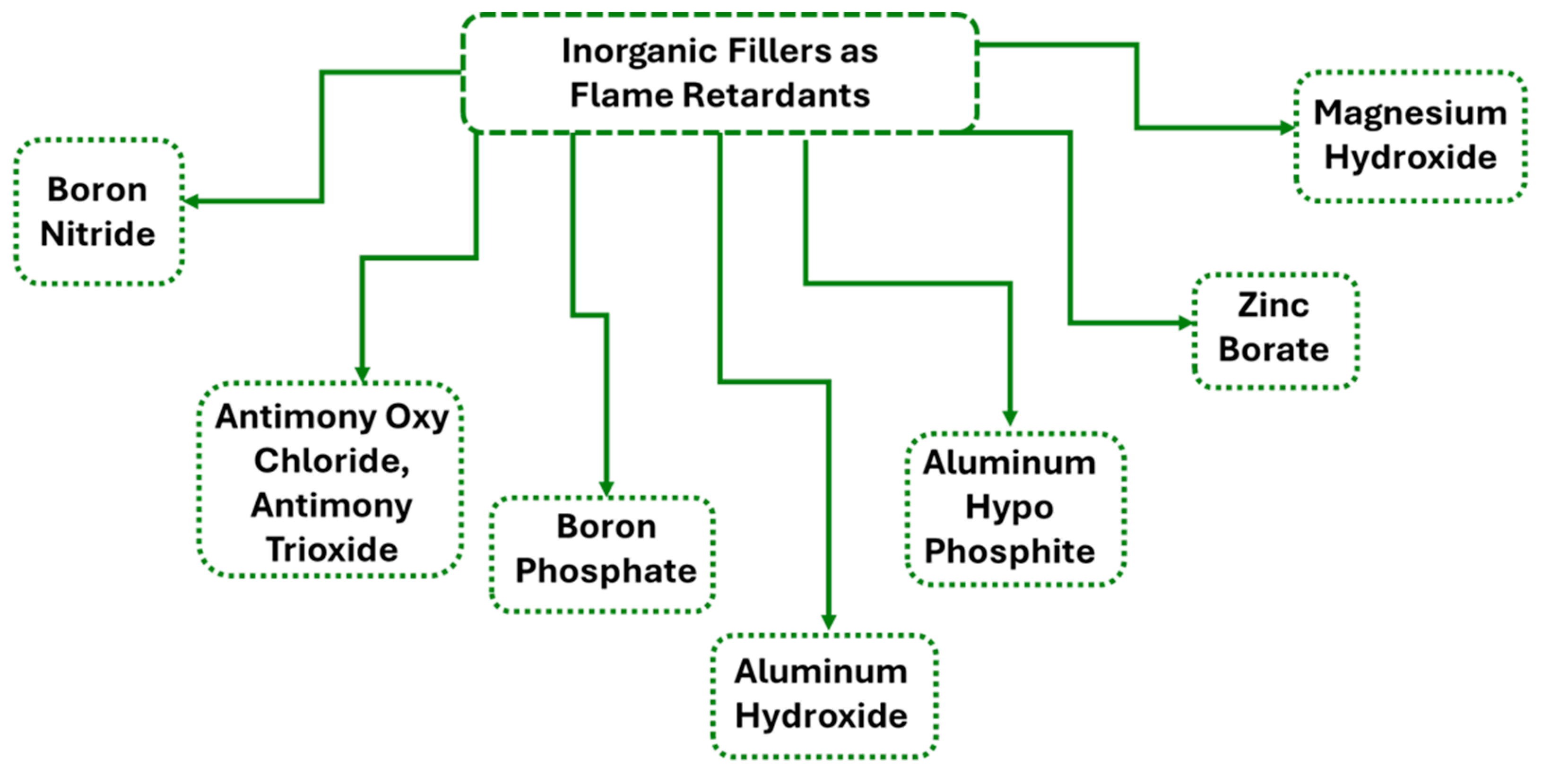
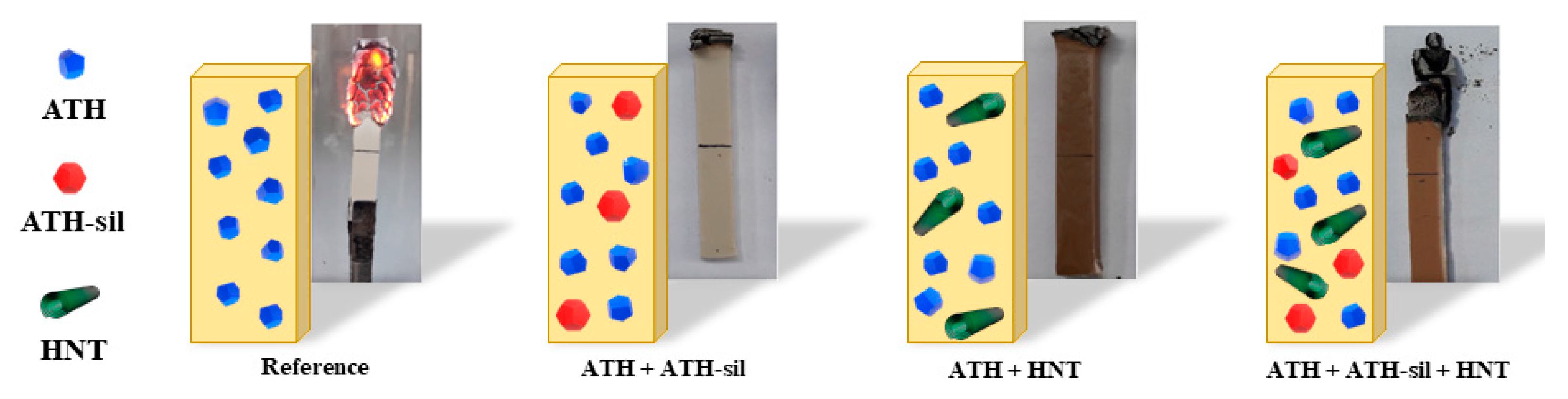
2.2.2. Inorganic Additives as Flame Retardants
2.2.3. Phosphorus-Based Systems
2.2.4. Nitrogen-Based Systems
2.2.5. Silicone-Based Flame Retardants
2.3. Hybrid and Synergistic Flame Retardants
3. Coating Matrix Systems and Compatibility
3.1. Epoxy-Based Coatings
3.2. Polyurethane and Polyurea Coatings
3.3. Acrylic- and Silicone-Based Coatings
3.4. Waterborne and Solvent-Based Systems
3.5. Nanocomposite for Flame Retardancy
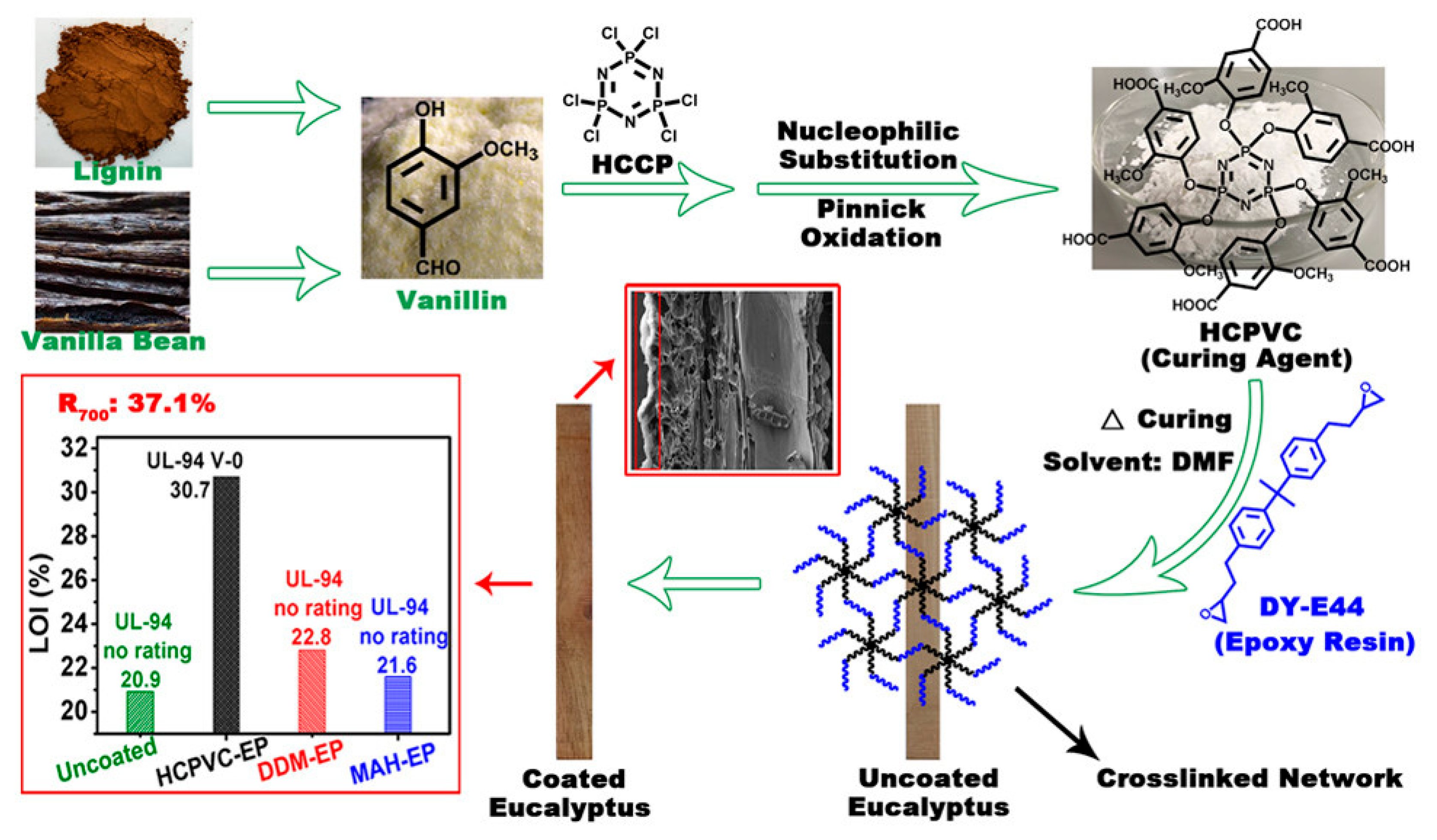
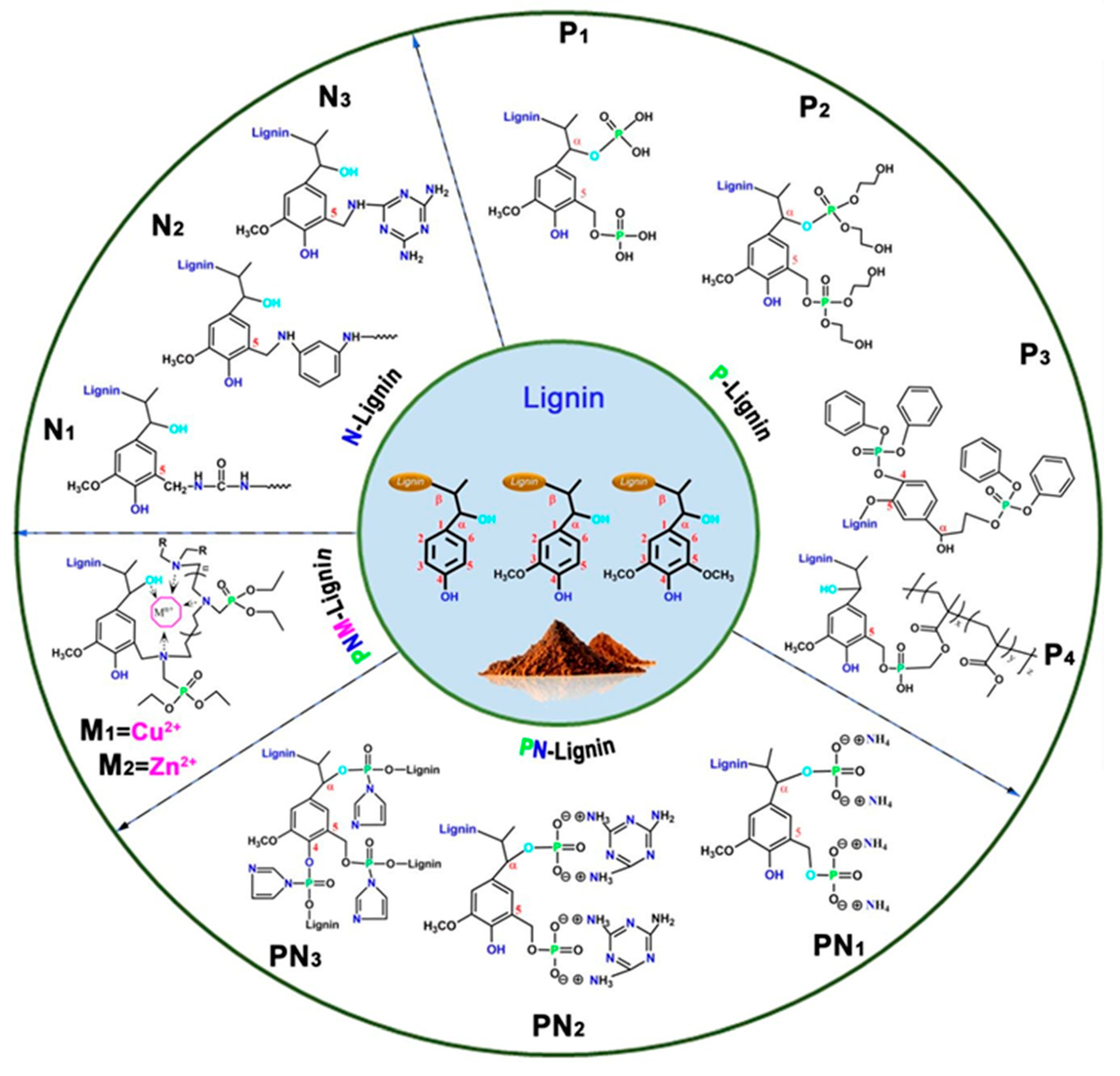
4. Bio-Based and Eco-Friendly Flame Retardants
5. Functional and Smart Flame-Retardant Coatings
5.1. Self-Healing Flame-Retardant Coatings
5.2. Coatings with Dual Functions
6. Processing and Application Techniques
6.1. Layer-by-Layer Assembly
6.2. Sol–Gel Coating Technique
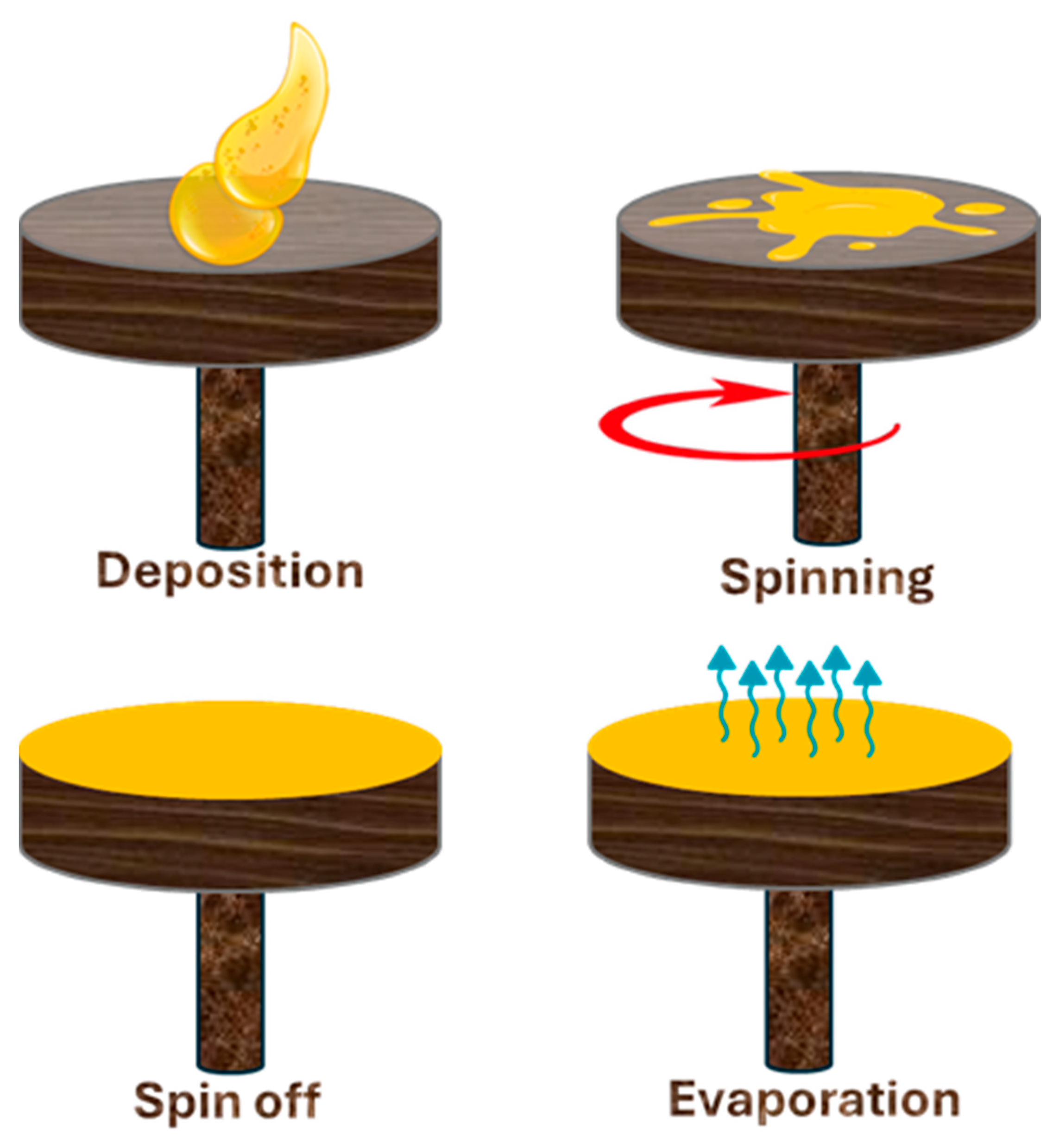
6.3. Spray, Dip, and Spin Coating Methods
7. Industrial and Real-World Applications
7.1. Flame-Retardant Coatings in Construction Materials
7.2. Automotive and Aerospace Coating Applications
7.3. Textile and Paper Coatings
7.4. Electronic and Cable Coatings
8. Challenges and Future Perspectives
8.1. Durability, Weatherability, and Aging of Fire-Resistant Coatings
8.2. Toxicity and Regulatory Issues
8.3. Toward Halogen-Free and Sustainable Alternatives
8.4. Research Gaps and Emerging Technologies
9. Conclusions
Funding
Conflicts of Interest
References
- Chaudhary, M.L.; Patel, R.; Chaudhari, S.; Gupta, R.K. Impact of Diverse Diols and Diisocyanates on Thermosetting Bio-Based Polyurethane Films. Next Mater. 2025, 8, 100609. [Google Scholar] [CrossRef]
- Chaudhary, M.L.; Patel, R.; Gupta, R.K. Beyond Isocyanates: Advances in Non-Isocyanate Polyurethane Chemistry and Applications. Polymer 2025, 332, 128553. [Google Scholar] [CrossRef]
- Patel, R.; Chaudhary, M.L.; Chaudhary, S.; Gupta, R.K. Effect of Distinct Molecular Structure of Diols on the Properties of Bio-Based Wood Adhesive. Int. J. Adhes. Adhes. 2025, 138, 103936. [Google Scholar] [CrossRef]
- Chaudhary, M.L.; Patel, R.; Gupta, R.K. Advances in Self-Healable and 3D Printable Biobased Elastomers. Polymer 2025, 319, 128020. [Google Scholar] [CrossRef]
- Patel, R.; Chaudhary, M.L.; Chaudhary, S.; Gupta, R.K. Comparative Analysis of Aliphatic and Aromatic Isocyanates on Soy-Based Polyurethane Films Modified with Schiff Base Diol. J. Polym. Environ. 2025, 33, 415–430. [Google Scholar] [CrossRef]
- Lazar, S.T.; Kolibaba, T.J.; Grunlan, J.C. Flame-Retardant Surface Treatments. Nat. Rev. Mater. 2020, 5, 259–275. [Google Scholar] [CrossRef]
- Morgan, A.B.; Gilman, J.W. An Overview of Flame Retardancy of Polymeric Materials: Application, Technology, and Future Directions. Fire Mater. 2013, 37, 259–279. [Google Scholar] [CrossRef]
- Wang, M.; Yin, G.-Z.; Yang, Y.; Fu, W.; Díaz Palencia, J.L.; Zhao, J.; Wang, N.; Jiang, Y.; Wang, D.-Y. Bio-Based Flame Retardants to Polymers: A Review. Adv. Ind. Eng. Polym. Res. 2023, 6, 132–155. [Google Scholar] [CrossRef]
- Bourbigot, S.; Duquesne, S. Fire Retardant Polymers: Recent Developments and Opportunities. J. Mater. Chem. 2007, 17, 2283–2300. [Google Scholar] [CrossRef]
- Qiu, X.; Li, Z.; Li, X.; Zhang, Z. Flame Retardant Coatings Prepared Using Layer by Layer Assembly: A Review. Chem. Eng. J. 2018, 334, 108–122. [Google Scholar] [CrossRef]
- Yew, M.C.; Ramli Sulong, N.H.; Yew, M.K.; Amalina, M.A.; Johan, M.R. Influences of Flame-Retardant Fillers on Fire Protection and Mechanical Properties of Intumescent Coatings. Prog. Org. Coat. 2015, 78, 59–66. [Google Scholar] [CrossRef]
- Chou, C.-S.; Lin, S.-H.; Wang, C.-I. Preparation and Characterization of the Intumescent Fire Retardant Coating with a New Flame Retardant. Adv. Powder Technol. 2009, 20, 169–176. [Google Scholar] [CrossRef]
- Luangtriratana, P.; Kandola, B.K.; Ebdon, J.R. UV-Polymerisable, Phosphorus-Containing, Flame-Retardant Surface Coatings for Glass Fibre-Reinforced Epoxy Composites. Prog. Org. Coat. 2015, 78, 73–82. [Google Scholar] [CrossRef]
- Özer, M.S.; Gaan, S. Recent Developments in Phosphorus Based Flame Retardant Coatings for Textiles: Synthesis, Applications and Performance. Prog. Org. Coat. 2022, 171, 107027. [Google Scholar] [CrossRef]
- Chen, X.; Hu, Y.; Jiao, C.; Song, L. Preparation and Thermal Properties of a Novel Flame-Retardant Coating. Polym. Degrad. Stab. 2007, 92, 1141–1150. [Google Scholar] [CrossRef]
- Yeoh, G.H.; De Cachinho Cordeiro, I.M.; Wang, W.; Wang, C.; Yuen, A.C.Y.; Chen, T.B.Y.; Vargas, J.B.; Mao, G.; Garbe, U.; Chua, H.T. Carbon-Based Flame Retardants for Polymers: A Bottom-up Review. Adv. Mater. 2024, 36, 2403835. [Google Scholar] [CrossRef]
- Gu, J.; Zhang, G.; Dong, S.; Zhang, Q.; Kong, J. Study on Preparation and Fire-Retardant Mechanism Analysis of Intumescent Flame-Retardant Coatings. Surf. Coat. Technol. 2007, 201, 7835–7841. [Google Scholar] [CrossRef]
- Wang, Z.; Han, E.; Ke, W. Influence of Nano-LDHs on Char Formation and Fire-Resistant Properties of Flame-Retardant Coating. Prog. Org. Coat. 2005, 53, 29–37. [Google Scholar] [CrossRef]
- Lu, H.; Song, L.; Hu, Y. A Review on Flame Retardant Technology in China. Part II: Flame Retardant Polymeric Nanocomposites and Coatings. Polym. Adv. Technol. 2011, 22, 379–394. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, Q.; Zhao, L.; Li, S.-N.; Wu, L.-B.; Jiang, J.-X.; Tang, L.-C. A Novel and Facile Strategy for Highly Flame Retardant Polymer Foam Composite Materials: Transforming Silicone Resin Coating into Silica Self-Extinguishing Layer. J. Hazard. Mater. 2017, 336, 222–231. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Pan, X.; Zhao, M.; Zhang, J. Rapid Preparation of Flame-Retardant Coatings Using Polyurethane Emulsion Mixed with Inorganic Fillers. Polymers 2023, 15, 754. [Google Scholar] [CrossRef] [PubMed]
- Venier, M.; Salamova, A.; Hites, R.A. Halogenated Flame Retardants in the Great Lakes Environment. Acc. Chem. Res. 2015, 48, 1853–1861. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Li, J.; Wang, Y.; Jiang, G. Distribution and Exposure Risk Assessment of Chlorinated Paraffins and Novel Brominated Flame Retardants in Toys. J. Hazard. Mater. 2023, 447, 130789. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Song, A.; Liu, H.; Li, Y.; Liu, M.; Sheng, G.; Peng, P.; Ying, G. Occurrence of Dechlorane Series Flame Retardants in Sediments from the Pearl River Delta, South China. Environ. Pollut. 2021, 279, 116902. [Google Scholar] [CrossRef]
- Mu, X.; Ding, H.; Wu, Y.; Hu, H.; Yu, B. Nonflammable Liquid Electrolytes for Safe Lithium Batteries. Small Struct. 2023, 4, 2300179. [Google Scholar] [CrossRef]
- Lu, S.; Feng, Y.; Zhang, P.; Hong, W.; Chen, Y.; Fan, H.; Yu, D.; Chen, X. Preparation of Flame-Retardant Polyurethane and Its Applications in the Leather Industry. Polymers 2021, 13, 1730. [Google Scholar] [CrossRef]
- Silva, N.G.S.; Zanini, N.C.; de Souza, A.G.; Barbosa, R.F.S.; Rosa, D.S.; Mulinari, D.R. Halogen-Based Flame Retardants in Polyurethanes. In Materials and Chemistry of Flame-Retardant Polyurethanes Volume 1: A Fundamental Approach; ACS Symposium Series; American Chemical Society: Washington, WA, USA, 2021; Volume 1399, pp. 141–171. ISBN 9780841298026. [Google Scholar]
- Dukarski, W.; Rykowska, I.; Krzyżanowski, P.; Gonsior, M. Flame Retardant Additives Used for Polyurea-Based Elastomers—A Review. Fire 2024, 7, 50. [Google Scholar] [CrossRef]
- Scionti, G.; Piperopoulos, E.; Atria, M.; Calabrese, L.; Proverbio, E. Effect of Magnesium Hydroxide and Aluminum Hydroxide as Thermal Barriers on the Flame-Retardant Behavior of Acrylic-Based Coating. Coatings 2023, 13, 1517. [Google Scholar] [CrossRef]
- Yin, S.; Ren, X.; Lian, P.; Zhu, Y.; Mei, Y. Synergistic Effects of Black Phosphorus/Boron Nitride Nanosheets on Enhancing the Flame-Retardant Properties of Waterborne Polyurethane and Its Flame-Retardant Mechanism. Polymers 2020, 12, 1487. [Google Scholar] [CrossRef]
- Soni, D.B.; Bhatt, G. A Review on Flame Retardants Used in Polyurethane Foam. ECS Trans. 2022, 107, 1153. [Google Scholar] [CrossRef]
- Xu, B.; Zhao, S.; Shan, H.; Qian, L.; Wang, J.; Xin, F. Effect of Two Boron Compounds on Smoke-Suppression and Flame-Retardant Properties for Rigid Polyurethane Foams. Polym. Int. 2022, 71, 1210–1219. [Google Scholar] [CrossRef]
- Liu, Y.; He, J.; Yang, R. Effects of Dimethyl Methylphosphonate, Aluminum Hydroxide, Ammonium Polyphosphate, and Expandable Graphite on the Flame Retardancy and Thermal Properties of Polyisocyanurate–Polyurethane Foams. Ind. Eng. Chem. Res. 2015, 54, 5876–5884. [Google Scholar] [CrossRef]
- Zhao, B.; Chen, L.; Long, J.-W.; Jian, R.-K.; Wang, Y.-Z. Synergistic Effect between Aluminum Hypophosphite and Alkyl-Substituted Phosphinate in Flame-Retarded Polyamide 6. Ind. Eng. Chem. Res. 2013, 52, 17162–17170. [Google Scholar] [CrossRef]
- Wang, X.; Pang, H.; Chen, W.; Lin, Y.; Zong, L.; Ning, G. Controllable Fabrication of Zinc Borate Hierarchical Nanostructure on Brucite Surface for Enhanced Mechanical Properties and Flame Retardant Behaviors. ACS Appl. Mater. Interfaces 2014, 6, 7223–7235. [Google Scholar] [CrossRef]
- Ai, L.; Chen, S.; Zeng, J.; Yang, L.; Liu, P. Synergistic Flame Retardant Effect of an Intumescent Flame Retardant Containing Boron and Magnesium Hydroxide. ACS Omega 2019, 4, 3314–3321. [Google Scholar] [CrossRef]
- Salmeia, K.A.; Gaan, S. An Overview of Some Recent Advances in DOPO-Derivatives: Chemistry and Flame Retardant Applications. Polym. Degrad. Stab. 2015, 113, 119–134. [Google Scholar] [CrossRef]
- Wendels, S.; Chavez, T.; Bonnet, M.; Salmeia, K.A.; Gaan, S. Recent Developments in Organophosphorus Flame Retardants Containing P-C Bond and Their Applications. Materials 2017, 10, 784. [Google Scholar] [CrossRef]
- Waaijers, S.L.; Bleyenberg, T.E.; Dits, A.; Schoorl, M.; Schütt, J.; Kools, S.A.E.; de Voogt, P.; Admiraal, W.; Parsons, J.R.; Kraak, M.H.S. Daphnid Life Cycle Responses to New Generation Flame Retardants. Environ. Sci. Technol. 2013, 47, 13798–13803. [Google Scholar] [CrossRef]
- Lu, S.; Chen, S.; Luo, L.; Yang, Y.; Wang, J.; Chen, Y.; Yang, Y.; Yuan, Z.; Chen, X. Molecules Featuring the Azaheterocycle Moiety toward the Application of Flame-Retardant Polymers. ACS Chem. Heal. Saf. 2023, 30, 343–361. [Google Scholar] [CrossRef]
- Shan, H.; Yan, L.; Xu, B.; Wang, D.; Wu, M. Polyphosphamide Containing Triazine and Melamine Cyanurate for Flame-Retardant PA6. ACS Appl. Polym. Mater. 2023, 5, 5322–5333. [Google Scholar] [CrossRef]
- Zhu, H.; Xu, S. Preparation of Flame-Retardant Rigid Polyurethane Foams by Combining Modified Melamine–Formaldehyde Resin and Phosphorus Flame Retardants. ACS Omega 2020, 5, 9658–9667. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wang, G.; Xu, J.; Liu, Y.; Chen, R.; Yan, H. Modification of Diatomite with Melamine Coated Zeolitic Imidazolate Framework-8 as an Effective Flame Retardant to Enhance Flame Retardancy and Smoke Suppression of Rigid Polyurethane Foam. J. Hazard. Mater. 2019, 379, 120819. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Zhang, W.; Xie, D.; Wang, Y.; Sun, X.; Zhou, R.; Jiang, J. A Flame Retardant Containing Dicyandiamide and Aluminum Hypophosphite for Polyethylene. Case Stud. Constr. Mater. 2023, 18, e01797. [Google Scholar] [CrossRef]
- Gilbertson, L.M.; Ng, C.A. Evaluating the Use of Alternatives Assessment To Compare Bulk Organic Chemical and Nanomaterial Alternatives to Brominated Flame Retardants. ACS Sustain. Chem. Eng. 2016, 4, 6019–6030. [Google Scholar] [CrossRef]
- Chiang, C.-L.; Ma, C.-C.M. Synthesis, Characterization and Thermal Properties of Novel Epoxy Containing Silicon and Phosphorus Nanocomposites by Sol–Gel Method. Eur. Polym. J. 2002, 38, 2219–2224. [Google Scholar] [CrossRef]
- Patel, R.; Patel, P.; Chaudhary, M.L.; Gupta, R.K. Fluorine-Free, Biobased Antismudge Polyurethane Coating with Enhanced Flame Retardancy. ACS Appl. Polym. Mater. 2024, 6, 7278–7287. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, W.; Yang, R. Flame Retardant Epoxy Composites with Epoxy-Containing Polyhedral Oligomeric Silsesquioxanes. Polym. Adv. Technol. 2020, 31, 2058–2074. [Google Scholar] [CrossRef]
- Kabir, I.I.; Fu, Y.; de Souza, N.; Nazir, M.T.; Baena, J.C.; Yuen, A.C.Y.; Yeoh, G.H. Improved Flame-Retardant Properties of Polydimethylsiloxane/Multi-Walled Carbon Nanotube Nanocomposites. J. Mater. Sci. 2021, 56, 2192–2211. [Google Scholar] [CrossRef]
- Tang, Q.; Yang, R.; He, J. Investigations of Thermoplastic Poly(Imide-Urethanes) Flame-Retarded by Hydroxyl-Terminated Poly(Dimethylsiloxane). Ind. Eng. Chem. Res. 2014, 53, 9714–9720. [Google Scholar] [CrossRef]
- Song, H.; Park, C.H.; Jeong, S.H.; Heo, J.H.; Lee, J.H. Synergistic Adenosine Triphosphate/Chitosan Bio-Coatings on Polyurethane Foam for Simultaneously Improved Flame Retardancy and Smoke Suppression. ACS Appl. Polym. Mater. 2023, 5, 4388–4399. [Google Scholar] [CrossRef]
- Paszkiewicz, S.; Irska, I.; Taraghi, I.; Piesowicz, E.; Sieminski, J.; Zawisza, K.; Pypeć, K.; Dobrzynska, R.; Terelak-Tymczyna, A.; Stateczny, K.; et al. Halloysite Nanotubes and Silane-Treated Alumina Trihydrate Hybrid Flame Retardant System for High-Performance Cable Insulation. Polymers 2021, 13, 2134. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Xue, Y.; Zuo, X.; Zhang, L.; Yang, Z.; Zhou, Y.; Marmorat, C.; He, S.; Rafailovich, M. Capitalizing on the Molybdenum Disulfide/Graphene Synergy to Produce Mechanical Enhanced Flame Retardant Ethylene-Vinyl Acetate Composites with Low Aluminum Hydroxide Loading. Polym. Degrad. Stab. 2017, 144, 155–166. [Google Scholar] [CrossRef]
- Vahabi, H.; Fouad, L.; Krzysztof, F.; Mohammad Reza, S.; Dubois, P. Flame-Retardant Polymer Materials Developed by Reactive Extrusion: Present Status and Future Perspectives. Polym. Rev. 2022, 62, 919–949. [Google Scholar] [CrossRef]
- Demir, H.; Balköse, D.; Ülkü, S. Influence of Surface Modification of Fillers and Polymer on Flammability and Tensile Behaviour of Polypropylene-Composites. Polym. Degrad. Stab. 2006, 91, 1079–1085. [Google Scholar] [CrossRef]
- Lee, J.; Park, J.H.; Shim, S.B.; Lee, J.E. Mechanical Properties of Polypropylene-Based Flame Retardant Composites by Surface Modification of Flame Retardants. Polymers 2022, 14, 3524. [Google Scholar] [CrossRef]
- Maurya, A.; Sinha, S.; Kumar, P.; Singh, V. A Review: Impact of Surface Treatment of Nanofillers for Improvement in Thermo Mechanical Properties of the Epoxy Based Nanocomposites. Mater. Today Proc. 2023, 78, 164–172. [Google Scholar] [CrossRef]
- Chen, M.-J.; Shao, Z.-B.; Wang, X.-L.; Chen, L.; Wang, Y.-Z. Halogen-Free Flame-Retardant Flexible Polyurethane Foam with a Novel Nitrogen–Phosphorus Flame Retardant. Ind. Eng. Chem. Res. 2012, 51, 9769–9776. [Google Scholar] [CrossRef]
- Neisius, M.; Liang, S.; Mispreuve, H.; Gaan, S. Phosphoramidate-Containing Flame-Retardant Flexible Polyurethane Foams. Ind. Eng. Chem. Res. 2013, 52, 9752–9762. [Google Scholar] [CrossRef]
- Price, E.J.; Covello, J.; Tuchler, A.; Wnek, G.E. Intumescent, Epoxy-Based Flame-Retardant Coatings Based on Poly(Acrylic Acid) Compositions. ACS Appl. Mater. Interfaces 2020, 12, 18997–19005. [Google Scholar] [CrossRef]
- Fahami, A.; Lee, J.; Lazar, S.; Grunlan, J.C. Mica-Based Multilayer Nanocoating as a Highly Effective Flame Retardant and Smoke Suppressant. ACS Appl. Mater. Interfaces 2020, 12, 19938–19943. [Google Scholar] [CrossRef]
- Yuan, L.; Yu, J.; Wang, Y.; Zhang, S.; Sun, Y.; Tan, C.; Liu, H.; Fan, Y.; Kang, D. Synergistic Effects of Sodium Polyacrylate-Loaded Ammonium Polyphosphate in Combination with ScYSZ to Enhance the Flame Retardancy and Thermal Insulation Properties of Epoxy-Polyamide Coatings. Prog. Org. Coat. 2025, 203, 109175. [Google Scholar] [CrossRef]
- Ou, M.; Lian, R.; Zhu, J.; Li, R.; Cui, J.; Guan, H.; Liu, L.; Jiao, C.; Chen, X. Aromatic P/N/Co-Containing Microsphere Flame Retardant for Enhancing Fire Safety and Mechanical Properties of Epoxy Coating with Lower Curing Temperature. Prog. Org. Coat. 2023, 183, 107728. [Google Scholar] [CrossRef]
- Gao, J.; Qi, L.; Wang, C.; Feng, Z.; Chen, L.; Li, S.; Hu, Y.; Xing, W. Construction of Multifunctional Coatings of Polyester Fabric for Flame Retardancy and Personal Thermal Management. Compos. Part A Appl. Sci. Manuf. 2025, 192, 108772. [Google Scholar] [CrossRef]
- Cai, J.; Zhang, X.; Wang, Z.; Xie, J.; Zhang, X. A Bio-Based Antibacterial Epoxy Resin Coating with Outstanding Flame Retardant and Mechanical Properties. Prog. Org. Coat. 2024, 190, 108369. [Google Scholar] [CrossRef]
- Qi, L.; Wang, B.; Zhang, W.; Yu, B.; Zhou, M.; Hu, Y.; Xing, W. Durable Flame Retardant and Dip-Resistant Coating of Polyester Fabrics by Plasma Surface Treatment and UV-Curing. Prog. Org. Coat. 2022, 172, 107066. [Google Scholar] [CrossRef]
- Wang, X.; Nabipour, H.; Kan, Y.-C.; Song, L.; Hu, Y. A Fully Bio-Based, Anti-Flammable and Non-Toxic Epoxy Thermosetting Network for Flame-Retardant Coating Applications. Prog. Org. Coat. 2022, 172, 107095. [Google Scholar] [CrossRef]
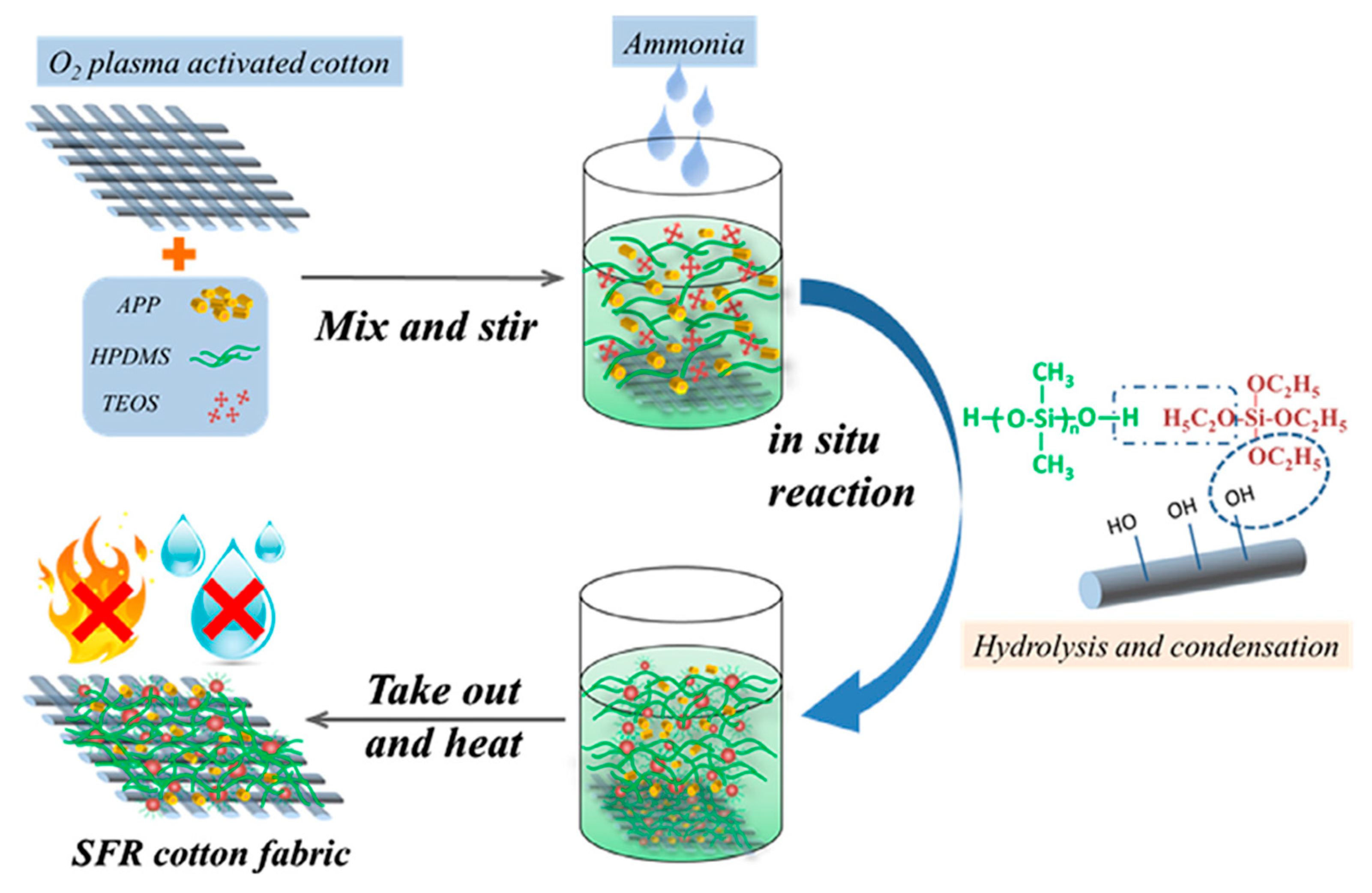
- Yang, Y.-T.; Huang, J.-L.; Wang, X.; Grunlan, J.; Song, L.; Hu, Y. Flame Retardant and Hydrophobic Cotton Using a Unique Phosphorus–Nitrogen–Silicon-Containing Coating. Cellulose 2022, 29, 8473–8488. [Google Scholar] [CrossRef]
- Wang, B.; Luo, C.-Y.; Zhu, P.; Liu, Y.; Xu, Y.-J. Facile Construction of H3PO3-Modified Chitosan/Montmorillonite Coatings for Highly Efficient Flame Retardation of Polyester–Cotton Fabrics. Prog. Org. Coat. 2023, 184, 107864. [Google Scholar] [CrossRef]
- Shi, J.; Song, X.; Wang, F.; Chen, J.; Wang, Y.; Wang, X.; Liu, Y. Phosphorus- and Nitrogen-Bridged Chitooligosaccharide as an Effective Flame-Retardant Coating of Fiber Cardboard. ACS Sustain. Chem. Eng. 2025, 13, 3413–3422. [Google Scholar] [CrossRef]
- Guo, S.; Mu, Y.; Li, Z.; Ma, M.; Zhou, W. Eco-Friendly Fabrication of Multifunctional and Double-Sided Heterochromatic Silk Fabric Using Tannin and Soil Humic Substances. ACS Sustain. Chem. Eng. 2024, 12, 8913–8922. [Google Scholar] [CrossRef]
- Wang, S.; Wang, S.; Shen, M.; Xu, X.; Liu, H.; Wang, D.; Wang, H.; Shang, S. Biobased Phosphorus Siloxane-Containing Polyurethane Foam with Flame-Retardant and Smoke-Suppressant Performances. ACS Sustain. Chem. Eng. 2021, 9, 8623–8634. [Google Scholar] [CrossRef]
- Cui, M.; Li, J.; Chen, X.; Hong, W.; Chen, Y.; Xiang, J.; Yan, J.; Fan, H. A Halogen-Free, Flame Retardant, Waterborne Polyurethane Coating Based on the Synergistic Effect of Phosphorus and Silicon. Prog. Org. Coat. 2021, 158, 106359. [Google Scholar] [CrossRef]
- Nizam, M.; Çakır Çanak, T.; Serhatlı, İ.E. Fabrication of Fluorine and Nitrogen-Based Flame Retardants Containing Rigid Polyurethane Foam with Improved Hydrophobicity and Flame Retardancy. ACS Omega 2025, 10, 17847–17858. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yao, M.; Zhang, H.; Zhang, Y.; Feng, J.; Fang, Z.; Song, P. Aqueous Self-Assembly of Bio-Based Flame Retardants for Fire-Retardant, Smoke-Suppressive, and Toughened Polylactic Acid. ACS Sustain. Chem. Eng. 2022, 10, 16313–16323. [Google Scholar] [CrossRef]
- Xiong, Z.; Zhang, Y.; Du, X.; Song, P.; Fang, Z. Green and Scalable Fabrication of Core–Shell Biobased Flame Retardants for Reducing Flammability of Polylactic Acid. ACS Sustain. Chem. Eng. 2019, 7, 8954–8963. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, X.; Peng, H.; Wang, F.; Liu, X.; Yang, Y.; Hao, J. Layer-by-Layer Assembly of Multifunctional Flame Retardant Based on Brucite, APTES, Alginate and Its Applications in EVA Resin. ACS Appl. Mater. Interfaces 2016, 8, 9925–9935. [Google Scholar] [CrossRef]
- Tan, Y.; Shao, Z.-B.; Chen, X.-F.; Long, J.-W.; Chen, L.; Wang, Y.-Z. Novel Multifunctional Organic–Inorganic Hybrid Curing Agent with High Flame-Retardant Efficiency for Epoxy Resin. ACS Appl. Mater. Interfaces 2015, 7, 17919–17928. [Google Scholar] [CrossRef]
- Pethsangave, D.A.; Khose, R.V.; Wadekar, P.H.; Some, S. Novel Approach toward the Synthesis of a Phosphorus-Functionalized Polymer-Based Graphene Composite as an Efficient Flame Retardant. ACS Sustain. Chem. Eng. 2019, 7, 11745–11753. [Google Scholar] [CrossRef]
- Köklükaya, O.; Carosio, F.; Grunlan, J.C.; Wågberg, L. Flame-Retardant Paper from Wood Fibers Functionalized via Layer-by-Layer Assembly. ACS Appl. Mater. Interfaces 2015, 7, 23750–23759. [Google Scholar] [CrossRef]
- Carosio, F.; Di Blasio, A.; Alongi, J.; Malucelli, G. Green DNA-Based Flame Retardant Coatings Assembled through Layer by Layer. Polymer 2013, 54, 5148–5153. [Google Scholar] [CrossRef]
- Zhao, W.; Zhao, H.-B.; Cheng, J.-B.; Li, W.; Zhang, J.; Wang, Y.-Z. A Green, Durable and Effective Flame-Retardant Coating for Expandable Polystyrene Foams. Chem. Eng. J. 2022, 440, 135807. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, W.; Liu, L.; Cheng, W.; Wang, W.; Liew, K.M.; Wang, B.; Hu, Y. Eco-Friendly Flame Retardant and Electromagnetic Interference Shielding Cotton Fabrics with Multi-Layered Coatings. Chem. Eng. J. 2019, 372, 1077–1090. [Google Scholar] [CrossRef]
- Yuan, H.; Xing, W.; Zhang, P.; Song, L.; Hu, Y. Functionalization of Cotton with UV-Cured Flame Retardant Coatings. Ind. Eng. Chem. Res. 2012, 51, 5394–5401. [Google Scholar] [CrossRef]
- Tao, Y.; Liu, C.; Li, P.; Wang, B.; Xu, Y.-J.; Jiang, Z.-M.; Liu, Y.; Zhu, P. A Flame-Retardant PET Fabric Coating: Flammability, Anti-Dripping Properties, and Flame-Retardant Mechanism. Prog. Org. Coat. 2021, 150, 105971. [Google Scholar] [CrossRef]
- Yan, L.; Xu, Z.; Deng, N. Effects of Polyethylene Glycol Borate on the Flame Retardancy and Smoke Suppression Properties of Transparent Fire-Retardant Coatings Applied on Wood Substrates. Prog. Org. Coat. 2019, 135, 123–134. [Google Scholar] [CrossRef]
- Yan, L.; Xu, Z.; Liu, D. Synthesis and Application of Novel Magnesium Phosphate Ester Flame Retardants for Transparent Intumescent Fire-Retardant Coatings Applied on Wood Substrates. Prog. Org. Coat. 2019, 129, 327–337. [Google Scholar] [CrossRef]
- Jin, X.; Wu, X.; Tang, W.; Tan, Z.; Wang, W.; Sun, S. Self-Assembled Coatings with Durable Flame Retardancy for EPS Foam. Chem. Eng. J. 2024, 494, 153285. [Google Scholar] [CrossRef]
- Wang, T.; Li, L.; Cao, Y.; Wang, Q.; Guo, C. Preparation and Flame Retardancy of Castor Oil Based UV-Cured Flame Retardant Coating Containing P/Si/S on Wood Surface. Ind. Crops Prod. 2019, 130, 562–570. [Google Scholar] [CrossRef]
- Guo, W.; Wang, X.; Huang, J.; Zhou, Y.; Cai, W.; Wang, J.; Song, L.; Hu, Y. Construction of Durable Flame-Retardant and Robust Superhydrophobic Coatings on Cotton Fabrics for Water-Oil Separation Application. Chem. Eng. J. 2020, 398, 125661. [Google Scholar] [CrossRef]
- Carosio, F.; Alongi, J.; Malucelli, G. Layer by Layer Ammonium Polyphosphate-Based Coatings for Flame Retardancy of Polyester–Cotton Blends. Carbohydr. Polym. 2012, 88, 1460–1469. [Google Scholar] [CrossRef]
- Chan, S.Y.; Si, L.; Lee, K.I.; Ng, P.F.; Chen, L.; Yu, B.; Hu, Y.; Yuen, R.K.K.; Xin, J.H.; Fei, B. A Novel Boron–Nitrogen Intumescent Flame Retardant Coating on Cotton with Improved Washing Durability. Cellulose 2018, 25, 843–857. [Google Scholar] [CrossRef]
- Fang, Y.; Sun, W.; Li, J.; Liu, H.; Liu, X. Eco-Friendly Flame Retardant and Dripping-Resistant of Polyester/Cotton Blend Fabrics through Layer-by-Layer Assembly Fully Bio-Based Chitosan/Phytic Acid Coating. Int. J. Biol. Macromol. 2021, 175, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Mohd Sabee, M.M.; Itam, Z.; Beddu, S.; Zahari, N.M.; Mohd Kamal, N.L.; Mohamad, D.; Zulkepli, N.A.; Shafiq, M.D.; Abdul Hamid, Z.A. Flame Retardant Coatings: Additives, Binders, and Fillers. Polymers 2022, 14, 2911. [Google Scholar] [CrossRef] [PubMed]
- Laoutid, F.; Jouyandeh, M.; Murariu, O.; Vahabi, H.; Saeb, M.R.; Brison, L.; Murariu, M.; Dubois, P. New Transparent Flame-Retardant (FR) Coatings Based on Epoxy-Aluminum Hypophosphite Nanocomposites. Coatings 2023, 13, 140. [Google Scholar] [CrossRef]
- Huang, Y.; Ma, T.; Wang, Q.; Guo, C. Synthesis of Biobased Flame-Retardant Carboxylic Acid Curing Agent and Application in Wood Surface Coating. ACS Sustain. Chem. Eng. 2019, 7, 14727–14738. [Google Scholar] [CrossRef]
- Cabo, M.J.; Manoj Narendra, P.; Lee, D.-W.; Yu, R.; Chanthavong, V.; Song, J.-I. Improving the Flame Retardancy and Mechanical Properties of Vinyl Ester Resins through Maleated Epoxidized Corn Oil/Epoxy Resin Additives for Sustainable Thermoset Composites. ACS Polym. Au J. 2025, 5, 45–58. [Google Scholar] [CrossRef]
- Ding, Y.; Su, Y.; Huang, J.; Wang, T.; Li, M.-Y.; Li, W. Flame Retardancy Behaviors of Flexible Polyurethane Foam Based on Reactive Dihydroxy P–N-Containing Flame Retardants. ACS Omega 2021, 6, 16410–16418. [Google Scholar] [CrossRef]
- Chatterjee, A.; Sen, S.; Bhardwaj, S.; Maji, P.K. Polysilazane-Cross-Linked Acrylic Coatings for Wood: A Versatile Solution for Weather Resistance, Stain Repellence, and Fire Safety. ACS Appl. Eng. Mater. 2025, 3, 502–512. [Google Scholar] [CrossRef]
- Lai, M.; Wang, Y.; Li, F.; Zhao, J. Synthesis and Characterization of Sodium Lignosulfonate-Based Phosphorus-Containing Intermediates and Its Composite Si–P–C Silicone-Acrylic Emulsion Coating for Flame-Retardant Plywood. Langmuir 2024, 40, 12573–12593. [Google Scholar] [CrossRef]
- Wang, Y.; Ran, Y.; Shao, Y.; Zhu, J.; Du, C.; Yang, F.; Bao, Q.; Shan, Y.; Zhang, W. A Novel Intumescent MCA-Modified Sodium Silicate/Acrylic Flame-Retardant Coating to Improve the Flame Retardancy of Wood. Molecules 2024, 29, 3021. [Google Scholar] [CrossRef]
- Yin, Y.; Li, W.; Feng, M.; Hu, X.; Niu, J.; Yao, J. Fabrication of Flame-Retardant and Anti-Dripping Waterborne Polyurethane Containing Phosphorus/Silicon via a Green and Feasible Strategy. ACS Sustain. Chem. Eng. 2025, 13, 3522–3533. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, D.; Liu, W.; Huang, J.; Huang, J.; Yang, D.; Qiu, X. Strong and Tough Lignin-Containing Waterborne Polyurethane Nanocomposites with Multiple Hydrogen Bonds as Photothermal Power Generation Coatings. ACS Sustain. Chem. Eng. 2023, 11, 17142–17156. [Google Scholar] [CrossRef]
- Guidugli, L.F.; Cheatham, R.; Calhoun, J.; Reza, M.T. Further Understanding of Phytic Acid–Based Deep Eutectic Solvents as Flame Retardants: COSMO Simulation, Physical Characterization, and Vertical Flame Testing. Ind. Eng. Chem. Res. 2024, 63, 14811–14820. [Google Scholar] [CrossRef]
- Murtaza, H.; Zhao, J.; Tabish, M.; Wang, J.; Mubeen, M.; Zhang, J.; Zhang, S.; Fan, B. Protective and Flame-Retardant Bifunctional Epoxy-Based Nanocomposite Coating by Intercomponent Synergy between Modified CaAl-LDH and RGO. ACS Appl. Mater. Interfaces 2024, 16, 13114–13131. [Google Scholar] [CrossRef]
- Panda, P.K.; Tasi, T.-P.; Wu, M.-W.; Dash, P.; Hsieh, C.-T.; Yang, P.-C.; Chang, J.-K. Development of Carbon Composite Coatings for Fire Retardancy and Electromagnetic Interference Shielding. Prog. Org. Coat. 2024, 194, 108628. [Google Scholar] [CrossRef]
- Panda, P.K.; Fu, H.-Y.; Tsai, T.-P.; Chu, C.-Y.; Dash, P.; Hsieh, C.-T. Development of Hexagonal Boron Nitride and Zinc Oxide Nanocomposite for Fire Retardant and Anti-Electromagnetic Construction Coatings. J. Indian Chem. Soc. 2025, 102, 101647. [Google Scholar] [CrossRef]
- Lee, I.; Jang, J.; Choi, D.; Park, Y.T.; Cho, C. Layer-by-Layer Assembly of TiO2 Nanoparticle/Poly(Acrylic Acid)/Montmorillonite Trilayer Composite Films as Flame-Retardant Coatings. ACS Appl. Nano Mater. 2024, 7, 26843–26853. [Google Scholar] [CrossRef]
- Wang, X.; Kalali, E.N.; Wan, J.-T.; Wang, D.-Y. Carbon-Family Materials for Flame Retardant Polymeric Materials. Prog. Polym. Sci. 2017, 69, 22–46. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Research on Nanomaterials. Available online: https://www.epa.gov/chemical-research/research-nanomaterials (accessed on 25 June 2025).
- Kulkarni, S.; Xia, Z.; Yu, S.; Kiratitanavit, W.; Morgan, A.B.; Kumar, J.; Mosurkal, R.; Nagarajan, R. Bio-Based Flame-Retardant Coatings Based on the Synergistic Combination of Tannic Acid and Phytic Acid for Nylon–Cotton Blends. ACS Appl. Mater. Interfaces 2021, 13, 61620–61628. [Google Scholar] [CrossRef]
- Kim, Y.-O.; Cho, J.; Yeo, H.; Lee, B.W.; Moon, B.J.; Ha, Y.-M.; Jo, Y.R.; Jung, Y.C. Flame Retardant Epoxy Derived from Tannic Acid as Biobased Hardener. ACS Sustain. Chem. Eng. 2019, 7, 3858–3865. [Google Scholar] [CrossRef]
- Deniz, A.; Zaytoun, N.; Hetjens, L.; Pich, A. Polyphosphazene–Tannic Acid Colloids as Building Blocks for Bio-Based Flame-Retardant Coatings. ACS Appl. Polym. Mater. 2020, 2, 5345–5351. [Google Scholar] [CrossRef]
- Weldemhret, T.G.; Lee, D.-W.; Prabhakar, M.N.; Park, Y.T.; Song, J. Il Polyurethane Foams Coated with Phosphorus-Doped Mesoporous Carbon for Flame-Retardant Triboelectric Nanogenerators. ACS Appl. Nano Mater. 2022, 5, 12464–12476. [Google Scholar] [CrossRef]
- Zhao, X.; Liang, Z.; Huang, Y.; Hai, Y.; Zhong, X.; Xiao, S.; Jiang, S. Influence of Phytic Acid on Flame Retardancy and Adhesion Performance Enhancement of Poly (Vinyl Alcohol) Hydrogel Coating to Wood Substrate. Prog. Org. Coat. 2021, 161, 106453. [Google Scholar] [CrossRef]
- Yang, H.; Yu, B.; Xu, X.; Bourbigot, S.; Wang, H.; Song, P. Lignin-Derived Bio-Based Flame Retardants toward High-Performance Sustainable Polymeric Materials. Green Chem. 2020, 22, 2129–2161. [Google Scholar] [CrossRef]
- Zhou, Q.; Chen, J.; Zhou, T.; Shao, J. In Situ Polymerization of Polyaniline on Cotton Fabrics with Phytic Acid as a Novel Efficient Dopant for Flame Retardancy and Conductivity Switching. New J. Chem. 2020, 44, 3504–3513. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, H.; Hu, X.; Liu, Y.; Zhang, S.; Xie, C. Self-Assembled Bio-Derived Microporous Nanosheet from Phytic Acid as Efficient Intumescent Flame Retardant for Polylactide. Polym. Degrad. Stab. 2021, 191, 109664. [Google Scholar] [CrossRef]
- Wong, E.H.H.; Fan, K.W.; Lei, L.; Wang, C.; Baena, J.C.; Okoye, H.; Fam, W.; Zhou, D.; Oliver, S.; Khalid, A.; et al. Fire-Resistant Flexible Polyurethane Foams via Nature-Inspired Chitosan-Expandable Graphite Coatings. ACS Appl. Polym. Mater. 2021, 3, 4079–4087. [Google Scholar] [CrossRef]
- Aaddouz, M.; Laoutid, F.; Mariage, J.; Yada, B.; Toncheva, A.; Lazko, J.; Azzaoui, K.; Sabbahi, R.; Mejdoubi, E.; Saeb, M.R.; et al. Mechanochemistry for the Synthesis of a Sustainable Phosphorus/Potassium Tannic Acid Flame-Retardant Additive and Its Application in Polypropylene. ACS Sustain. Chem. Eng. 2025, 13, 1450–1459. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Y.; Li, T.; Zhang, S.; Ma, P.; Shi, D.; Chen, M.; Dong, W. A Bio-Based Flame-Retardant Starch Based On Phytic Acid. ACS Sustain. Chem. Eng. 2020, 8, 10265–10274. [Google Scholar] [CrossRef]
- Wang, J.; Huo, S.; Wang, J.; Yang, S.; Chen, K.; Li, C.; Fang, D.; Fang, Z.; Song, P.; Wang, H. Green and Facile Synthesis of Bio-Based, Flame-Retardant, Latent Imidazole Curing Agent for Single-Component Epoxy Resin. ACS Appl. Polym. Mater. 2022, 4, 3564–3574. [Google Scholar] [CrossRef]
- Guo, Y.; Zuo, C.; Liu, Y.; Chen, X.; Ren, Y.; Liu, X. Construction of a Fully Bio-Based Intumescent Flame Retardant for Improving the Flame Retardancy of Polyacrylonitrile. Polym. Degrad. Stab. 2023, 214, 110385. [Google Scholar] [CrossRef]
- Ye, G.; Huo, S.; Wang, C.; Shi, Q.; Liu, Z.; Wang, H. One-Step and Green Synthesis of a Bio-Based High-Efficiency Flame Retardant for Poly (Lactic Acid). Polym. Degrad. Stab. 2021, 192, 109696. [Google Scholar] [CrossRef]
- Leng, Y.-M.; Zhao, X.; Fu, T.; Wang, X.-L.; Wang, Y.-Z. Bio-Based Flame-Retardant and Smoke-Suppressing Wood Plastic Composites Enabled by Phytic Acid Tyramine Salt. ACS Sustain. Chem. Eng. 2022, 10, 5055–5066. [Google Scholar] [CrossRef]
- Xu, S.; Han, Y.; Zhou, C.; Li, J.; Shen, L.; Lin, H. A Biobased Flame Retardant towards Improvement of Flame Retardancy and Mechanical Property of Ethylene Vinyl Acetate. Chin. Chem. Lett. 2023, 34, 107202. [Google Scholar] [CrossRef]
- Liu, W.; Shi, R.; Ge, X.; Huang, H.; Chen, X.; Mu, M. A Bio-Based Flame Retardant Coating Used for Polyamide 66 Fabric. Prog. Org. Coat. 2021, 156, 106271. [Google Scholar] [CrossRef]
- Luo, Y.; Lan, Y.; Cai, J.; Jiang, Q.; Wang, X.; Zhang, H.; Hou, L.; Xiao, L. Multi-Functional Bio-Based DOPO Derivative for Enhanced Flame Retardancy and Mechanical Strength in Epoxy Resin. Eur. Polym. J. 2025, 222, 113605. [Google Scholar] [CrossRef]
- Wang, F.; Wu, N.; Wang, M.; Deng, S.; Jia, H. Synthesis of Phenylphosphorylated Microcrystal Cellulose Biobased Flame Retardants and Its Flame-Retardant Modification on PLA Biomaterials. Polym. Degrad. Stab. 2024, 227, 110843. [Google Scholar] [CrossRef]
- Li, S.; Wang, X.; Xu, M.; Liu, L.; Wang, W.; Gao, S.; Li, B. Effect of a Biomass Based Waterborne Fire Retardant Coating on the Flame Retardancy for Wood. Polym. Adv. Technol. 2021, 32, 4805–4814. [Google Scholar] [CrossRef]
- Li, S.; Zhao, F.; Wang, X.; Liu, Z.; Guo, J.; Li, Y.; Tan, S.; Xin, Z.; Zhao, S.; Li, L. A Green Flame Retardant Coating Based on One-Step Aqueous Complexation of Phytic Acid and Urea for Fabrication of Lightweight and High Toughness Flame Retardant EPS Insulation Board. Polym. Degrad. Stab. 2024, 219, 110597. [Google Scholar] [CrossRef]
- Liu, J.; Qiu, S.; Qi, P.; Sun, J.; Li, H.; Gu, X.; Zhang, S. Constructing a Fully Biobased Coating to Improve the Flame Retardancy, Antibacterial Properties, and UV Resistance of Polyamide 6 Fabrics. ACS Appl. Eng. Mater. 2023, 1, 268–277. [Google Scholar] [CrossRef]
- Kianfar, M.; Ipakchi, H.; Mohajer, S.; Rasoulifard, M.H.; Seyed Dorraji, M.S.; Louaguef, D.B.; Azat, S.; Saeb, M.R.; Vahabi, H. Flame-Retardant Self-Healing Polymers: A Review. J. Polym. Sci. 2024, 5, 111–114. [Google Scholar] [CrossRef]
- Yang, H.; Yang, Y.; Li, Y.; Hope, J.; Choo, W. Extrinsic Conditions for the Occurrence and Characterizations of Self-Healing Polyurea Coatings for Improved Medical Device Reliability: A Mini Review. ACS Omega 2023, 8, 26650–26662. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Tan, M.; Shin, J.; Zhang, C.; Yang, S.; Song, N.; Zhang, W.; Jiao, Y.; Xie, J.; Geng, Z.; et al. Ultrarobust, Self-Healing Poly(Urethane-Urea) Elastomer with Superior Tensile Strength and Intrinsic Flame Retardancy Enabled by Coordination Cross-Linking. ACS Appl. Mater. Interfaces 2024, 16, 43979–43990. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.; Liu, X.; Wang, S.; Sun, J.; Li, H.; Wang, Z.; Gu, X.; Zhang, S. Self-Healing Polyelectrolyte Complex Coating for Flame Retardant Flexible Polyurethane Foam with Enhanced Mechanical Property. Compos. Part B Eng. 2021, 219, 108886. [Google Scholar] [CrossRef]
- Sun, F.-C.; Fu, J.-H.; Peng, Y.-X.; Jiao, X.-M.; Liu, H.; Du, F.-P.; Zhang, Y.-F. Dual-Functional Intumescent Fire-Retardant/Self-Healing Water-Based Plywood Coatings. Prog. Org. Coat. 2021, 154, 106187. [Google Scholar] [CrossRef]
- Li, P.; Liu, C.; Wang, B.; Tao, Y.; Xu, Y.-J.; Liu, Y.; Zhu, P. Eco-Friendly Coating Based on an Intumescent Flame-Retardant System for Viscose Fabrics with Multi-Function Properties: Flame Retardancy, Smoke Suppression, and Antibacterial Properties. Prog. Org. Coat. 2021, 159, 106400. [Google Scholar] [CrossRef]
- Sezer Hicyilmaz, A.; Altin, Y.; Bedeloglu, A. Polyimide-Coated Fabrics with Multifunctional Properties: Flame Retardant, UV Protective, and Water Proof. J. Appl. Polym. Sci. 2019, 136, 47616. [Google Scholar] [CrossRef]
- Shang, X.; Jin, Y.; Du, W.; Bai, L.; Zhou, R.; Zeng, W.; Lin, K. Flame-Retardant and Self-Healing Waterborne Polyurethane Based on Organic Selenium. ACS Appl. Mater. Interfaces 2023, 15, 16118–16131. [Google Scholar] [CrossRef]
- Zhang, T.; Xu, Y.; Dai, J.; Zhang, X. Epoxidized Soybean Oil-Based Waterborne Polyurethane Coated Polyethylene Terephthalate Fabrics with High Flame-Retardant, Self-Healing and Wash-Resistant Properties. Prog. Org. Coat. 2025, 205, 109315. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, Y.; Sun, P.; Hai, Y.; Jiang, S. A Self-Healing, Recyclable, and Degradable Fire-Retardant Gelatin-Based Biogel Coating for Green Buildings. Soft Matter 2021, 17, 5231–5239. [Google Scholar] [CrossRef]
- Du, W.; Jin, Y.; Lai, S.; Shi, L.; Shen, Y.; Yang, H. Multifunctional Light-Responsive Graphene-Based Polyurethane Composites with Shape Memory, Self-Healing, and Flame Retardancy Properties. Compos. Part A Appl. Sci. Manuf. 2020, 128, 105686. [Google Scholar] [CrossRef]
- Mahaninia, M.H.; Yan, N. Catalyst-Free Biodegradable Chitosan-Based Dual Dynamic Covalent Networks with Self-Healing and Flame-Retardant Properties. ACS Sustain. Chem. Eng. 2024, 12, 17117–17126. [Google Scholar] [CrossRef]
- Yang, S.; Wang, S.; Du, X.; Du, Z.; Cheng, X.; Wang, H. Mechanically Robust Self-Healing and Recyclable Flame-Retarded Polyurethane Elastomer Based on Thermoreversible Crosslinking Network and Multiple Hydrogen Bonds. Chem. Eng. J. 2020, 391, 123544. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, G.; Feng, Y.; Zhang, H.; Li, J.; Shi, X. Design of a Self-Healing and Flame-Retardant Cyclotriphosphazene-Based Epoxy Vitrimer. J. Mater. Sci. 2018, 53, 7030–7047. [Google Scholar] [CrossRef]
- Bai, S.; Zhang, K.; Zhang, Q.; Zhu, Y.; Wang, W.; Zhang, J.; Li, X.; Zhang, X.; Wang, R. Intrinsic Flame Retardancy and Flexible Solid–Solid Phase Change Materials with Self-Healing and Recyclability. ACS Appl. Mater. Interfaces 2023, 15, 48613–48622. [Google Scholar] [CrossRef]
- Li, W.; Xiao, L.; Wang, Y.; Huang, J.; Liu, Z.; Chen, J.; Nie, X. Thermal-Induced Self-Healing Bio-Based Vitrimers: Shape Memory, Recyclability, Degradation, and Intrinsic Flame Retardancy. Polym. Degrad. Stab. 2022, 202, 110039. [Google Scholar] [CrossRef]
- Abedin, R.; Feng, X.; Pojman, J.J.; Ibekwe, S.; Mensah, P.; Warner, I.; Li, G. A Thermoset Shape Memory Polymer-Based Syntactic Foam with Flame Retardancy and 3D Printability. ACS Appl. Polym. Mater. 2022, 4, 1183–1195. [Google Scholar] [CrossRef]
- Ding, X.-M.; Chen, L.; Xu, Y.-J.; Shi, X.-H.; Luo, X.; Song, X.; Wang, Y.-Z. Robust Epoxy Vitrimer with Simultaneous Ultrahigh Impact Property, Fire Safety, and Multipath Recyclability via Rigid-Flexible Imine Networks. ACS Sustain. Chem. Eng. 2023, 11, 14445–14456. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, Y.; Dong, H.; Xu, R.; Jiang, S. Flame-Retardant Shape Memory Polyurethane/MXene Paper and the Application for Early Fire Alarm Sensor. Compos. Part B Eng. 2021, 223, 109149. [Google Scholar] [CrossRef]
- Feng, X.; Li, G. High-Temperature Shape Memory Photopolymer with Intrinsic Flame Retardancy and Record-High Recovery Stress. Appl. Mater. Today 2021, 23, 101056. [Google Scholar] [CrossRef]
- Zhang, T.; Huo, S.; Ye, G.; Wang, C.; Zhang, Q.; Xue, Y.; Song, P.; Wang, H.; Liu, Z. Phosphorus/Boron-Containing, Flame-Retardant Polyurethane Elastomers with Great Mechanical, Shape-Memory, and Recycling Performances. Polym. Degrad. Stab. 2024, 230, 111047. [Google Scholar] [CrossRef]
- Du, X.; Jin, L.; Deng, S.; Zhou, M.; Du, Z.; Cheng, X.; Wang, H. Recyclable, Self-Healing, and Flame-Retardant Solid–Solid Phase Change Materials Based on Thermally Reversible Cross-Links for Sustainable Thermal Energy Storage. ACS Appl. Mater. Interfaces 2021, 13, 42991–43001. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.; Huo, S.; Wang, C.; Zhang, Q.; Wang, H.; Song, P.; Liu, Z. Strong yet Tough Catalyst-Free Transesterification Vitrimer with Excellent Fire-Retardancy, Durability, and Closed-Loop Recyclability. Small 2024, 20, 2404634. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Huo, S.; Ye, G.; Wang, C.; Zhang, Q.; Liu, Z. Tough, High-Strength, Flame-Retardant and Recyclable Polyurethane Elastomers Based on Dynamic Borate Acid Esters. React. Funct. Polym. 2024, 205, 106056. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, W.; MD Islam, Z.; Fu, Y.; Dong, Y. Polydopamine Modified Ammonium Polyphosphate Modified Shape Memory Water-Borne Epoxy Composites with Photo-Responsive Flame Retardant Property. J. Appl. Polym. Sci. 2021, 138, 49696. [Google Scholar] [CrossRef]
- Peng, J.; Xie, S.; Liu, T.; Wang, D.; Ou, R.; Guo, C.; Wang, Q.; Liu, Z. High-Performance Epoxy Vitrimer with Superior Self-Healing, Shape-Memory, Flame Retardancy, and Antibacterial Properties Based on Multifunctional Curing Agent. Compos. Part B Eng. 2022, 242, 110109. [Google Scholar] [CrossRef]
- Jin, L.; Hong, Y.; Hong, J.; Goh, M. Highly Flame-Retardant Biovitrimer Utilizing P–O–C Dynamic Bonds for Closed-Loop Recyclable Thermally Conductive Pads. ACS Appl. Polym. Mater. 2025, 7, 478–490. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Z.; Xu, X.; Qiu, Y.; Chang, G.; Yuan, K.; Xu, R.; Meng, L. Cyclotriphosphazene Based Epoxy Vitrimer with Excellent Recyclability and Flame Retardancy. Polym. Degrad. Stab. 2025, 238, 111346. [Google Scholar] [CrossRef]
- Skorb, E.V.; Andreeva, D.V. Layer-by-Layer Approaches for Formation of Smart Self-Healing Materials. Polym. Chem. 2013, 4, 4834–4845. [Google Scholar] [CrossRef]
- Carosio, F.; Alongi, J. Ultra-Fast Layer-by-Layer Approach for Depositing Flame Retardant Coatings on Flexible PU Foams within Seconds. ACS Appl. Mater. Interfaces 2016, 8, 6315–6319. [Google Scholar] [CrossRef]
- Yan, Y.; Dong, S.; Jiang, H.; Hou, B.; Wang, Z.; Jin, C. Efficient and Durable Flame-Retardant Coatings on Wood Fabricated by Chitosan, Graphene Oxide, and Ammonium Polyphosphate Ternary Complexes via a Layer-by-Layer Self-Assembly Approach. ACS Omega 2022, 7, 29369–29379. [Google Scholar] [CrossRef] [PubMed]
- Facco, L.; Parin, R.; Basso, M.; Martucci, A.; Colusso, E. Passive Ice Protection Systems for Unmanned Aerial Vehicles Applications: A Review. Small 2025, 21, 2412465. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Wang, C.; Fang, S.; Ji, P.; Wang, H.; Ji, C. Durable Flame-Retardant and Antidroplet Finishing of Polyester Fabrics with Flexible Polysiloxane and Phytic Acid through Layer-by-Layer Assembly and Sol–Gel Process. J. Appl. Polym. Sci. 2018, 135, 46414. [Google Scholar] [CrossRef]
- Lin, D.; Zeng, X.; Li, H.; Lai, X.; Wu, T. One-Pot Fabrication of Superhydrophobic and Flame-Retardant Coatings on Cotton Fabrics via Sol-Gel Reaction. J. Colloid Interface Sci. 2019, 533, 198–206. [Google Scholar] [CrossRef]
- Liang, F.; Xu, Y.; Chen, S.; Zhu, Y.; Huang, Y.; Fei, B.; Guo, W. Fabrication of Highly Efficient Flame-Retardant and Fluorine-Free Superhydrophobic Cotton Fabric by Constructing Multielement-Containing POSS@ZIF-67@PDMS Micro–Nano Hierarchical Coatings. ACS Appl. Mater. Interfaces 2022, 14, 56027–56045. [Google Scholar] [CrossRef]
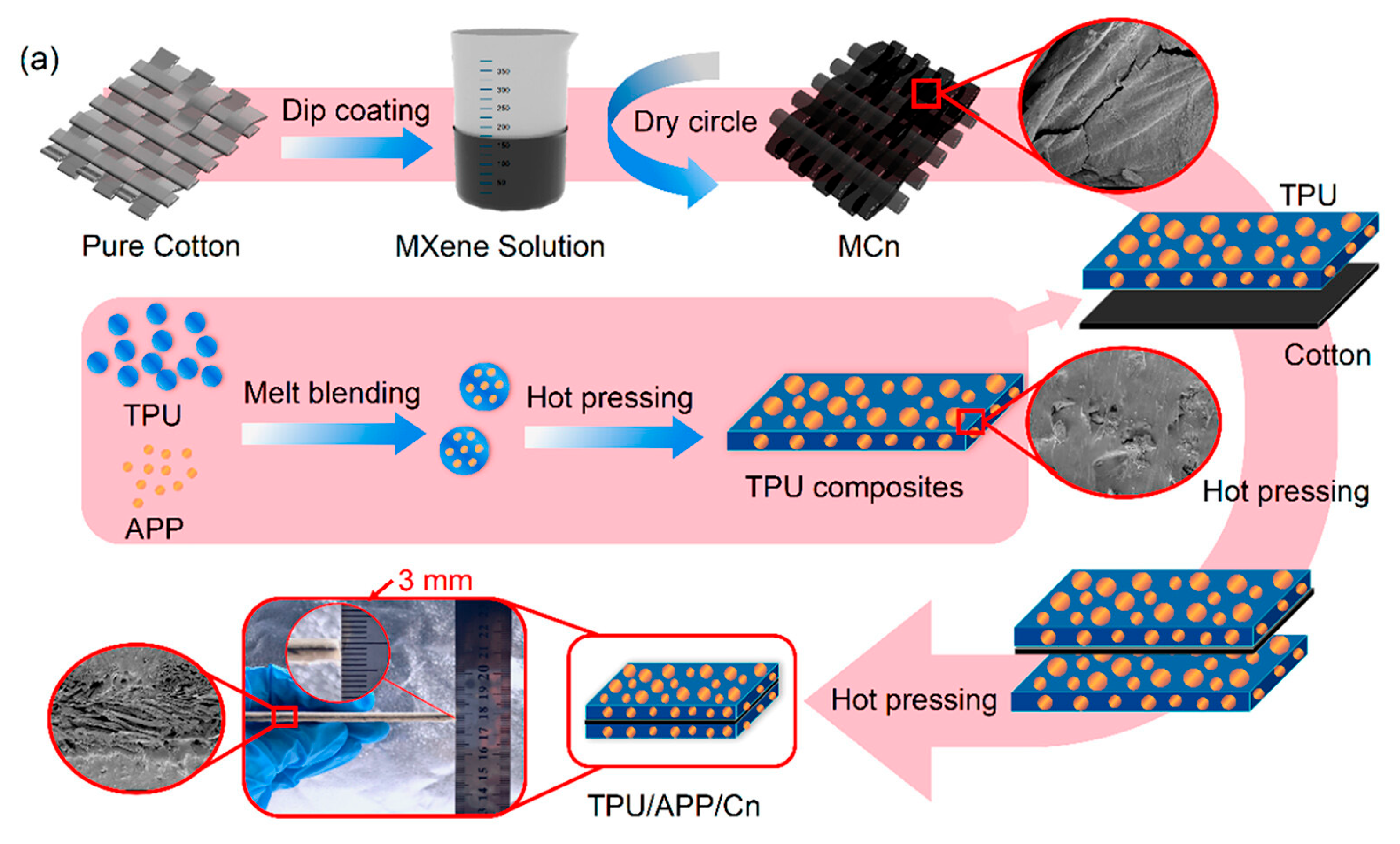
- Nie, C.; Shi, Y.; Jiang, S.; Wang, H.; Liu, M.; Huang, R.; Feng, Y.; Fu, L.; Yang, F. Constructing Fireproof MXene-Based Cotton Fabric/Thermoplastic Polyurethane Hierarchical Composites via Encapsulation Strategy. ACS Appl. Polym. Mater. 2023, 5, 7229–7239. [Google Scholar] [CrossRef]
- Liu, C.; Chen, K.; Wu, B.; Sun, W.; Ji, L.; Wu, Y. Preparation of a Novel Composite Coating of APP/MMT/APTES on Polyurethane with Improved Flame-Retarding Performance. ACS Omega 2024, 9, 48350–48360. [Google Scholar] [CrossRef]
- Yin, Y. Advances and Perspectives of Spin Coating Techniques. Appl. Comput. Eng. 2023, 7, 291–301. [Google Scholar] [CrossRef]
- Shen, J.; Liang, J.; Lin, X.; Lin, H.; Yu, J.; Wang, S. The Flame-Retardant Mechanisms and Preparation of Polymer Composites and Their Potential Application in Construction Engineering. Polymers 2022, 14, 82. [Google Scholar] [CrossRef]
- Vakhitova, L.; Kalafat, K.; Vakhitov, R.; Drizhd, V. Improving the Fire-Retardant Performance of Industrial Reactive Coatings for Steel Building Structures. Heliyon 2024, 10, e34729. [Google Scholar] [CrossRef]
- Zope, I.S.; Foo, S.; Seah, D.G.J.; Akunuri, A.T.; Dasari, A. Development and Evaluation of a Water-Based Flame Retardant Spray Coating for Cotton Fabrics. ACS Appl. Mater. Interfaces 2017, 9, 40782–40791. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.-L.; Ma, Z.-Y.; Yao, H.-X.; Qin, B.; Gao, Y.-C.; Xia, Z.-J.; Huang, Z.-H.; Yin, Y.-C.; Tu, H.; Ye, H.; et al. Economical Architected Foamy Aerogel Coating for Energy Conservation and Flame Resistance. ACS Mater. Lett. 2022, 4, 1453–1461. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, A.-N.; He, S.-M.; Zheng, G.-Q.; Zeng, F.-R.; Wang, Y.-Z.; Liu, B.-W.; Zhao, H.-B. Biomimetic Nanoporous Transparent Universal Fire-Resistant Coatings. ACS Appl. Mater. Interfaces 2024, 16, 19519–19528. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Wu, Y.; Xu, H.; Wang, B.; She, Y.; Chen, H.; Liu, Y.; Wang, S.; Xu, X. Enhancing Rigid Polyurethane Foam Properties with Lignin-Based Core–Shell Intumescent Flame Retardants. ACS Sustain. Chem. Eng. 2024, 12, 18126–18135. [Google Scholar] [CrossRef]
- Shie, C.Y.; Chen, C.-C.; Chen, H.F.; Lin, Y.H.; Liu, C.H.; Fuh, Y.-K.; Li, T. Flexible and Self-Powered Thermal Sensor Based on Graphene-Modified Intumescent Flame-Retardant Coating with Hybridized Nanogenerators. ACS Appl. Nano Mater. 2023, 6, 2429–2437. [Google Scholar] [CrossRef]
- Kolibaba, T.J.; Iverson, E.T.; Legendre, H.; Higgins, C.I.; Buck, Z.N.; Weeks, T.S.; Grunlan, J.C.; Killgore, J.P. Synergistic Fire Resistance of Nanobrick Wall Coated 3D Printed Photopolymer Lattices. ACS Appl. Mater. Interfaces 2023, 15, 16046–16054. [Google Scholar] [CrossRef]







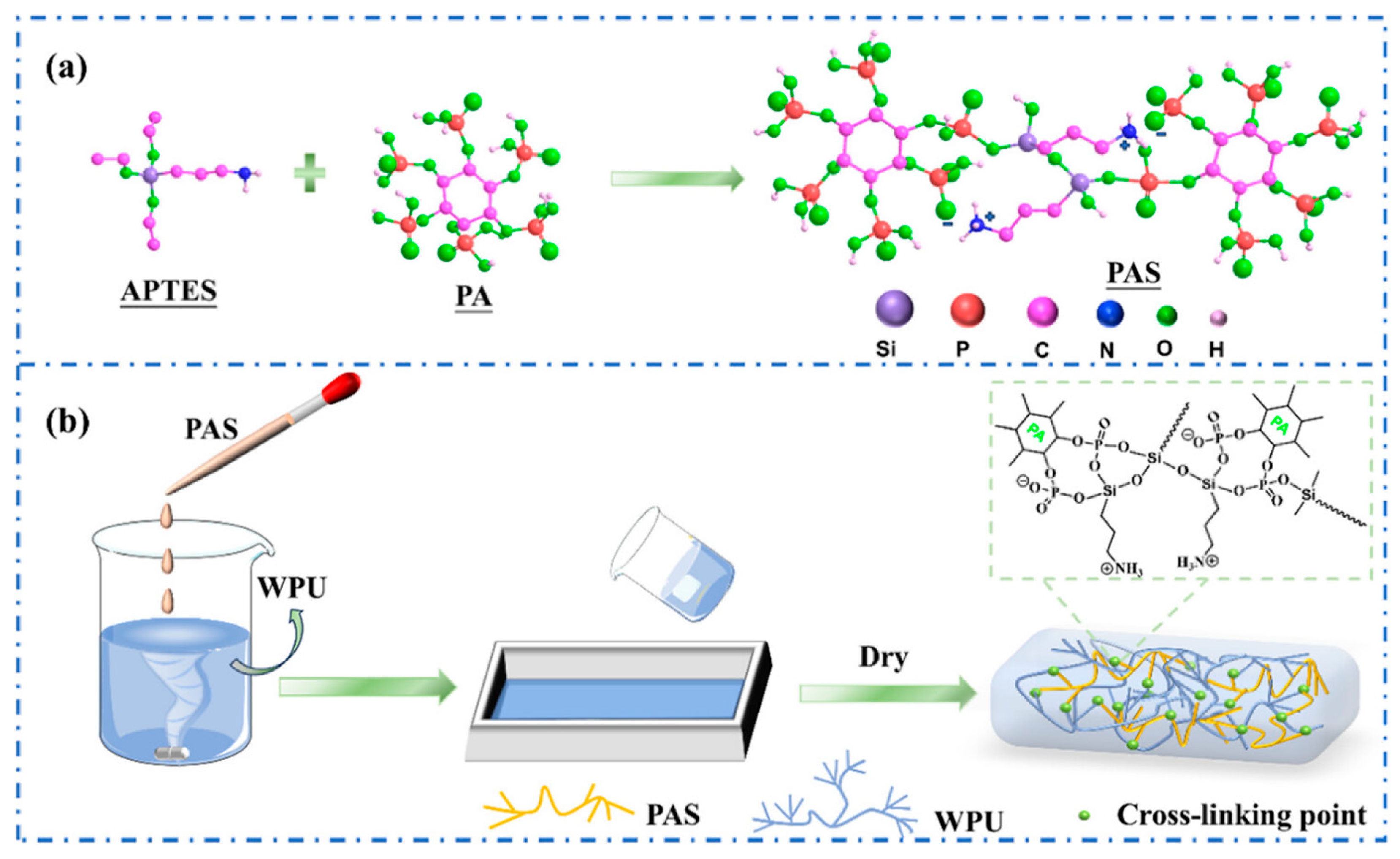
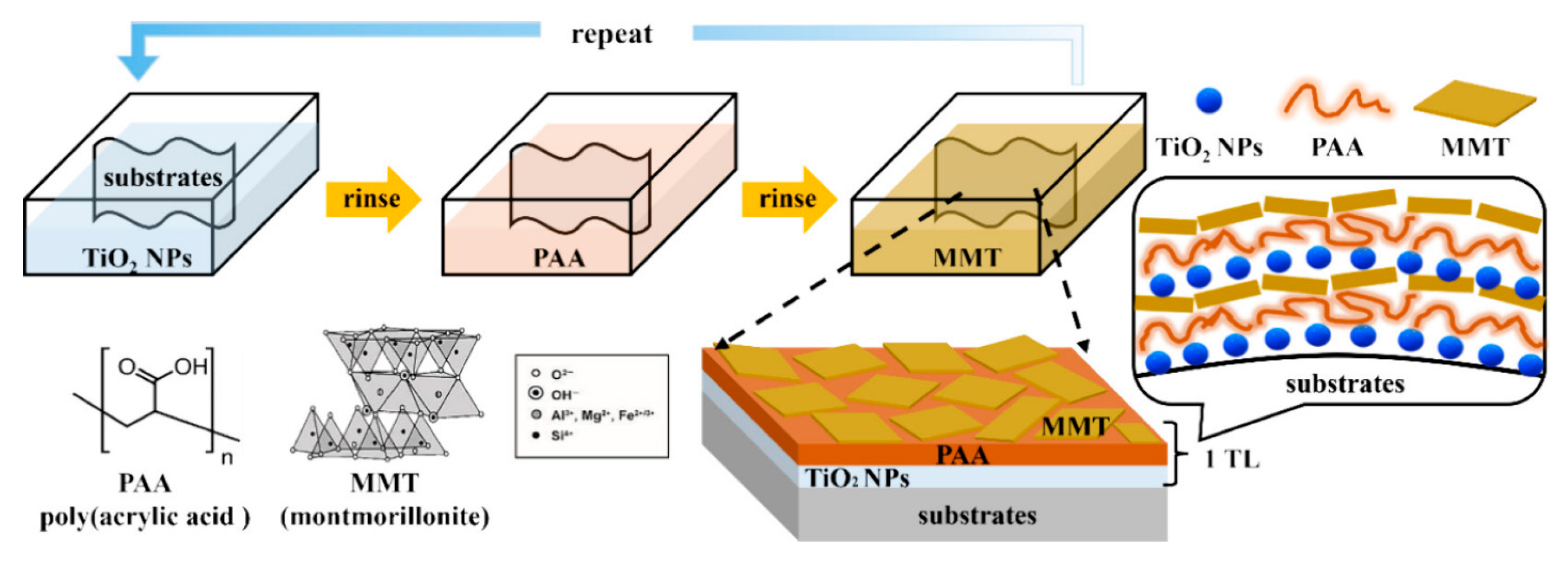
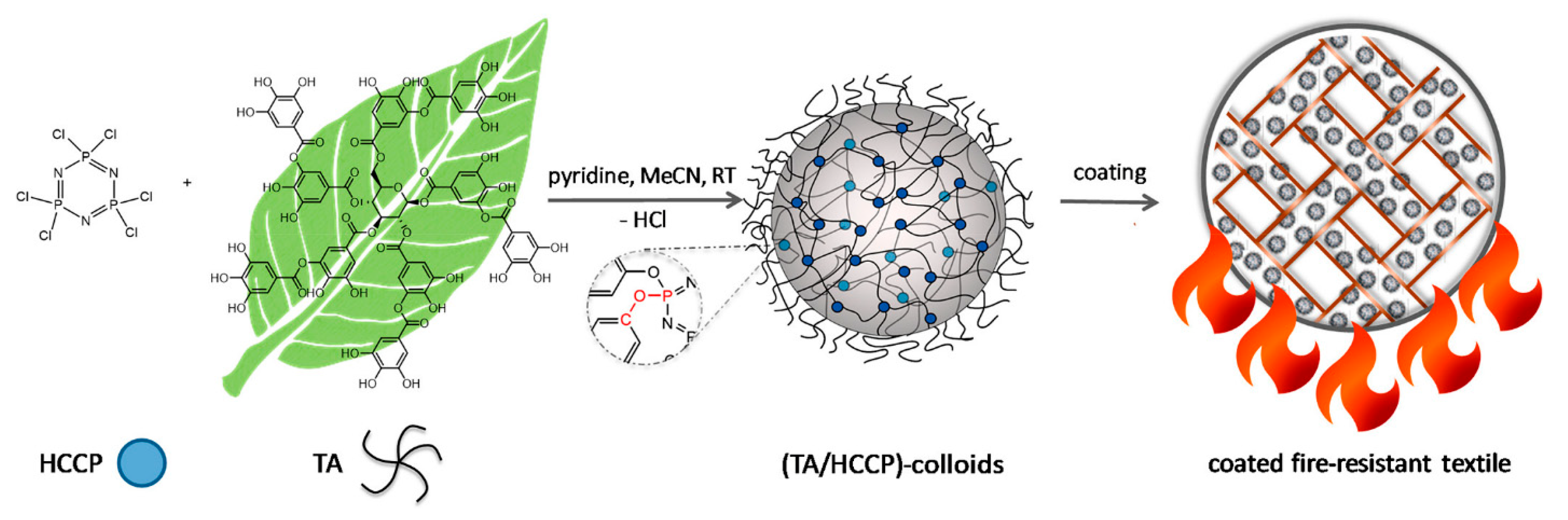
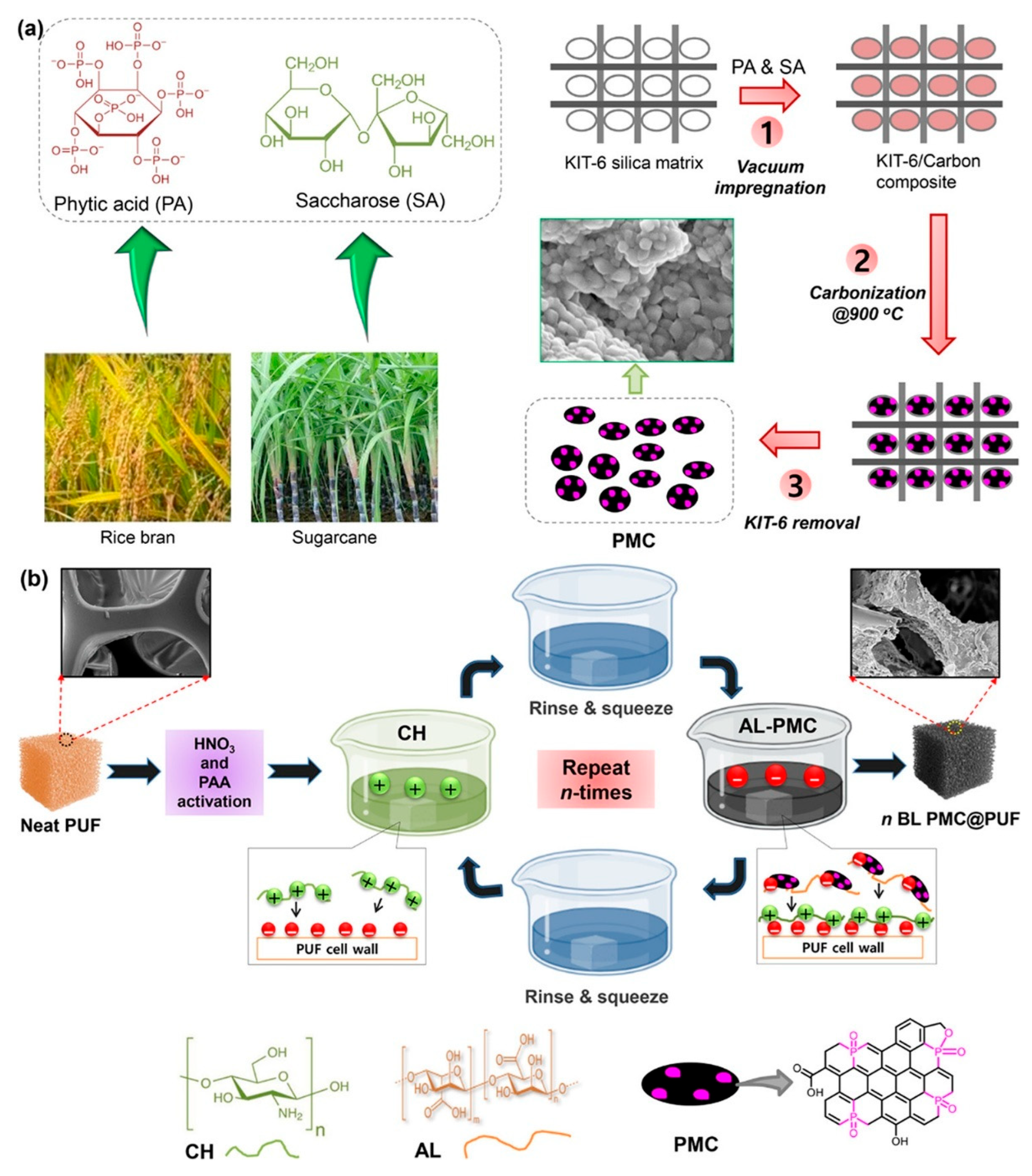



| Sr. No | Material | Flame Retardant | Mechanical Property | Thermal Property | Flame Retardancy (LOI %) | PHRR (kW/m2) | Char Yield (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | Polyether polyols, toluene diisocyanate | 2-carboxyethyl(phenyl)phosphinic acid (CEPP) and MA | Tensile—0.11 MPa | TGA—383 °C | 25.6 | 240 | 4.3 | [58] |
| 2 | Dimethyl/diphenyl phosphoramidates | Tris-(1-chloro-2-propyl) phosphate | - | TGA—407 °C | 23.9 | - | - | [59] |
| 3 | Epon 828 | Poly(acrylic acid), APP, MA | - | TGA—270 °C | - | 229 | - | [60] |
| 4 | Silicon wafers, cationic CH-stabilized mica | CS and poly(acrylic acid) (PAA) | - | TGA—600 °C | - | 305 | - | [61] |
| 5 | Epoxy–polyamide (EP) | APP, sodium polyacrylate | - | - | 31.3 | - | - | [62] |
| 6 | Epoxy resin | P/N/Co-containing microsphere FR (FNP-Co) | - | - | 30.2 | - | - | [63] |
| 7 | Polyester fiber | Polydimethylsiloxane (PDMS) | - | - | 22.5 | - | - | [64] |
| 8 | Vanillin, 4,4′-diaminodiphenyl ether | 4,4′-diaminodiphenylmethane (DDM) | - | Tg—188.9 °C | 35.2 | - | - | [65] |
| 9 | Polyester fabrics | 6-((((2-(acryloyloxy) ethoxy) (oxo)(phenyl)phosphonio) oxy) methyl) dibenzo[c,e] [1,2]oxaphosphinine 6-oxide and tri(acryloyloxyethyl) phosphate | - | - | 24 | - | - | [66] |
| 10 | Luteolin-derived epoxy resin, | 5, 5′-methylenedifurfurylamine | Tensile—66.3 MPa | Tg—216 °C | 38 | - | - | [67] |
| 11 | Tris(hydroxymethyl)phosphine oxide (THPO), 3,5-diamino-1,2,4-triazole (guanazole) | PDMS | - | - | 32 | 48.2 | 13.5 | [68] |
| 12 | Polyelectrolyte complexes | Phosphorus acid, CS | - | - | 64.3 | - | - | [69] |
| 13 | - | Chitooligosaccharide | - | TGA—372 °C | 28 | 380.35 | - | [70] |
| 14 | Silk fabric | Tannin, humic acid, fulic acid (FA), and green alum | - | - | 28.5 | - | - | [71] |
| 15 | Polyether polyol (ZS-4110), PDMS | DOPO with fumaropimaric acid-based siloxane | Flexural—1.08 MPa | TGA—332.8 °C | 26.1 | 170 | - | [72] |
| 16 | Polycarbonate diol, isophorone diisocyanate (IPDI) | 4-DOPO-((3-hydroxypropyl) imino) methyl) | Tensile—16.6 MPa | TGA—423 °C | 28.6 | 159 | 12.6 | [73] |
| 17 | Polyether polyol, methylene diphenyl diisocyanate | Fluorine- and nitrogen-based synthesized FRs | - | TGA—335 °C | 19.7 | 109.8 | - | [74] |
| 18 | Polylactic acid (PLA) (3001D) | APP and CS and carboxylated silicone oil (Si-COOH/Si)-based FRs | Tensile—53 MPa | TGA—381 °C | 34 | 387 | 3.1 | [75] |
| 19 | PLA (4032D) | APP- and CS-based FRs | Tensile—45.3 MPa | TGA—382 °C | 30.5 | 252 | 14 | [76] |
| 20 | Poly(ethylene–vinyl acetate) | Brucite/3-aminopropyltriethoxysilane (APTES)/nickel alginate/APTES (B/A/Nia/A)-based FRs | - | TGA—414 °C | 32.3 | 355. | - | [77] |
| 21 | Biphenol A (E-44) | APP- and diethylenetriamine-based FRs | - | TGA—329 °C | 30 | 310 | 66.5 | [78] |
| 22 | Graphene oxides | Polymer-functionalized graphene composites | Tensile—40.1 MPa | TGA—600 °C | 47.6 | - | - | [79] |
| 23 | Anionic poly(vinylphosphonic acid) | Cationic CS | - | TGA—476 °C | - | 76 | 1 | [80] |
| 24 | Cotton fabrics | Deoxyribonucleic acid and CS | - | - | 24 | 57 | 13 | [81] |
| 25 | Acrylic resin | Al(OH)3- and Mg(OH)2-based FRs | - | TGA—750 °C | 34 | - | - | [11] |
| 26 | Expandable polystyrene | Polysiloxane-based FRs | - | TGA—422 °C | 36 | 138.2 | - | [82] |
| 27 | Cotton fabrics | PA- and polyethylenimine-based FRs | Tensile—28.94 MPa | TGA—290 °C | 37 | 176 | - | [83] |
| 28 | Cotton fabrics | Phosphorus monomer | - | TGA—220 °C | 27 | 77.4 | - | [84] |
| 29 | Polyethylene terephthalate fabrics | GP-108- and PA-based FRs | Tensile—575 MPa | TGA—429 °C | 27 | 139 | - | [85] |
| 30 | Polyethylene glycol borate | Phosphoric acid, n-butyl alcohol, and PER | - | TGA—613 °C | - | 116.8 | - | [86] |
| 31 | Mg(OH)2 and fcyclic phosphate ester | Magnesium phosphate ester | - | TGA—361 °C | - | 162 | 26 | [87] |
| 32 | Branched polyethyleneimine (BPEI)-kaolinite (kaol)-BPEI-diethylene triamine penta (methylene phosphonic acid)) | Expandable polystyrene | - | - | 37.7 | 220.9 | 32.3 | [88] |
| 33 | Castor oil- and 3-mercaptopropionic acid-based | [(6-oxido-6H-dibenz [c,e] [1,2] oxaphosphorin-6-yl) methyl] butanedioic acid- and allyl glycidyl ether-based FRs | - | TGA—343 °C | 27.52 | 147 | 14 | [89] |
| 34 | Cotton fabrics | PDMS | - | TGA—496 °C | 29 | 81 | 15 | [90] |
| 35 | Polyester–cotton fabrics | APP | - | TGA—523 °C | - | 128 | 5.9 | [91] |
| 36 | Branched polyethylenimine | Phenylboronic acid | - | TGA—298 °C | 29.6 | 129 | - | [92] |
| 37 | Polyester–cotton blend fabrics | CS/PA | - | TGA—443 °C | 29.2 | 195.88 | 22.84 | [93] |
| Sr. No | Flame Retardant | Structure | Flame Retardancy (LOI%) | PHRR (kW/m2) | Char Yield (%) | Ref. |
|---|---|---|---|---|---|---|
| 1 | PA |  | 32 | - | - | [117] |
| 2 | Hexakis (4-aminophenoxy) cyclotriphosphazene-PA with microporous nanosheet | 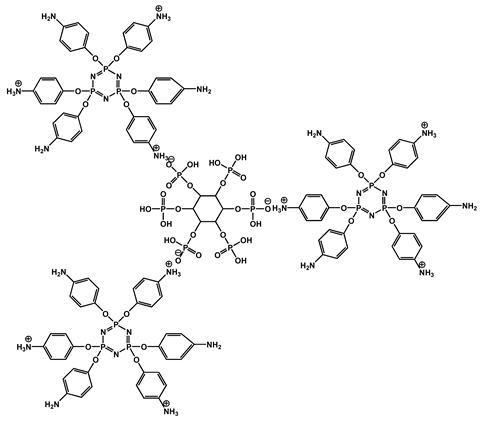 | 27.3 | 422.1 | 12 | [118] |
| 3 | CS |  | 31 | 66 | 87 | [119] |
| 4 | TA | 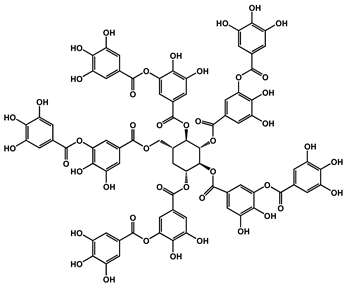 | - | 730 | - | [120] |
| 5 | Choline phytate | 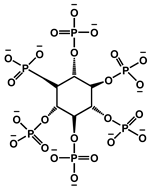 | 43.7 | 152.34 | - | [121] |
| 6 | Imidazole phytate | 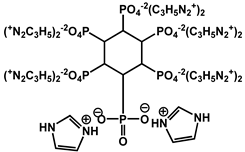 | 34.7 | 344 | - | [122] |
| 7 | Lignin, casein, and PA | 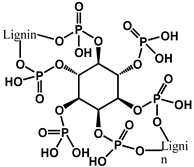 | 32.5 | 71.4% | - | [123] |
| 8 | PA and furfurylamine | 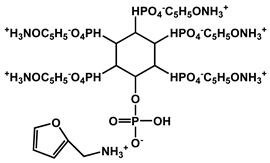 | 28.5 | 332.7 | - | [124] |
| 9 | PA–tyramine salt | 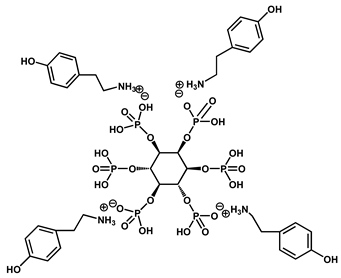 | 25 | 310 | 43.4 | [125] |
| 10 | PA and Mg(OH)2 |  | 30.8 | - | - | [126] |
| 11 | PA | 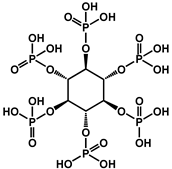 | 32.2 | 90.69 | - | [127] |
| 12 | A novel bio-based FR |  | 33.6 | 696.3 | 17 | [128] |
| 13 | Phenylphosphorylaminated microcrystal cellulose | 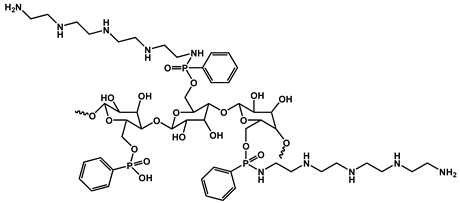 | 27 | 563.2 | - | [129] |
| 14 | CS/melamine formaldehyde-resin-coated APP/MMT | 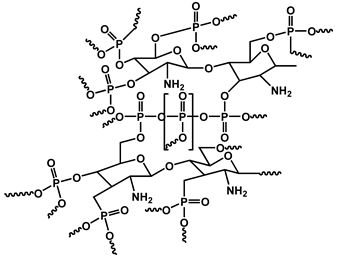 | 32.0 | 277.5 | 35.3 | [130] |
| 15 | Phosphorus–nitrogen flame retardant |  | 42 | - | - | [131] |
| 16 | PA |  | 32.3 | 90.69 | - | [127] |
| 17 | TA- and PA-based FRs | 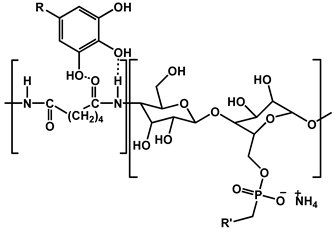 | - | 167 | 26.6 | [111] |
| 18 | PA 6-taurine (TN)/TA | 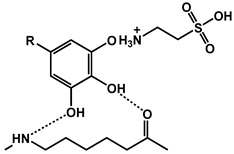 | 26.8 | 402 | - | [132] |
| No | Smart Material | FR Name | Mechanical Property | Thermal Property | Flame Retardancy (LOI %) | PHRR (kW/m2) | Char Yield (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | Polytetra methylene ether glycol, dimethylolpropionic acid (DMPA), IPDI | Di(1-hydroxyethylene) diselenide (DiSe) | Tensile—15.6 MPa elongation 852% | Tmax—365.9 °C Tg—66.8 °C | 28.1 | 519.22 | 0 | [140] |
| 2 | PVA | Sodium fluorosilicate (SFS), APP, sodium silicate | - | Tmax—362 °C | - | - | - | [137] |
| 3 | Epoxidized soybean oil, IPDI, DMPA, dimethylglyoxime | DOPO | Tensile—1082 N, elongation—24.1% | Tmax—443.7 °C | 22.5 | - | - | [141] |
| 4 | Gelatin, CS, glycerol | Bio-gel itself acts as an FR | Tensile—1.3 MPa | TGA—glycerol and CS degraded between 170 and 300 °C and gelatin degraded between 250 and 330 °C | - | 252.2 | - | [142] |
| 5 | dPTD (includes (DiSe) and dPTB (1,4-butanediol) | DOPO, isocyanatopropyltriethoxysilane, polyethylenimine | (dPTB-mfGO2) tensile—17.8 MPa elongation—1249.3%, (dPTD-mfGO2) tensile—15.6 MPa elongation—1128% | Tg (dPTB-mfGO2)—34.5 °C Tg (dPTD-mfGO2)—32.1 °C | 24.9 | - | - | [143] |
| 6 | CS citric acid | Vanillin-based phosphorus-containing epoxy | Tensile—41.2 ± 1.1 MPa elongation—78.8% | Tg—111 °C | 41.2 | 16 | - | [144] |
| 7 | Polypropylene glycol, IPDI | Tri(2-furyl) phosphoramide | Tensile—27.89 MPa elongation—827% | Tmax—383.5 °C | 28.5 | 572.5 | - | [145] |
| 8 | 4,4-dithiodianiline, p-hydroxybenzaldehyde benzyl mercaptan, 3-chloroperoxybenzoic acid | HCCP | Impact—18.2 MPa tensile-28.70 MPa | Tg—129 °C Tmax—335 °C | 30.5 | - | - | [146] |
| 9 | Polyethylene glycol, triethanolamine, IPDI | TBBPA | Tensile—6.37 MPa, elongation—21% | Tmax—320–350 °C | 23.6 | 937.94 | 7.96 | [147] |
| 10 | Pentaerythritol tetra(3-mercaptopropionate | 4-aminophenyl disulfide | Tensile—1.97 MPa, elongation—85.66% | Tg—22.62 °C Tmax—296.8 °C | 28.36 | 569.25 | 0.12 | [148] |
| 11 | Tris [2-(acryloyloxy)ethyl] isocyanurate | K20 HGM (hollow glass microspheres) | Compressive strength—81.8 ± 7.5 MPa | Tg—250 °C | - | - | - | [149] |
| 12 | Vanillin 1,3-Bis(3-aminopropyl) tetramethyldisiloxane, DDM | - | Tensile— 54.5 ± 1.1 MPa, elongation—13.2 ± 0.9% | Tg—72 °C, Tmax—334 °C | - | 586 | 33.7 | [150] |
| 13 | Shape memory thermoplastic PU | MXene (Ti3AlC2) | - | Tg—26.8 °C, Tmax—383 °C | - | 341.1 W/g | - | [151] |
| 14 | Tris [2-(acryloyloxy)ethyl] isocyanurate | Diphenyl(2,4,6 trimethylbenzoyl)phosphine oxide | Tensile—48.7 MPa elongation—6% compressive strength—370.7 MPa | Tg—280 °C, Tmax—454.4 °C | - | - | - | [152] |
| 15 | Poly(tetrahydrofuran) (PTMEG) | Phosphorus-containing diol (DPDF) | Tensile—41.8 MPa elongation—657.3% toughness—106.9 kJ/m3 | Tg—57.9 °C, Tmax—415 °C | 24.8 | 1191.3 | - | [153] |
| 16 | Furfuryl alcohol, polyethylene glycol, poly(propylene glycol) | Tri-maleimide end-capped cyclotriphospha zene | Tensile—19.8 MPa | Tmax—401.6 °C | 23.9 | 362.8 W/g | 8.74 | [154] |
| 17 | DGEBA | (Aminooxy)diphenylphosphine oxide | Tensile—86.2 MPa elongation—11.6% toughness—6.8 MJ/m3 | Tg—118 °C, Tmax—390 °C | 35.2 | 433.4 | 6.5 | [155] |
| 18 | PTMEG, IPDI | DOPO | Tensile—54.5 MPa elongation—891.3% toughness—207.8 kJ/m3 | Tg—61 °C, Tmax—398 °C | - | 744.3 W/g | - | [156] |
| 19 | Waterborne epoxy | Polydopamine-modified APP (PDA@APP) | - | Tmax—340.9 °C | 32.6 | 278.8 | 38.7 | [157] |
| 20 | Vanillin, DGEBA | Hexachlorocyclotriphosphazene | - | Tg—82 °C, Tmax—253.3 °C | 28.6 | 351.8 | 38.5 | [158] |
| 21 | TA, PVA | Phosphoric acid | Tensile—5.4 MPa elongation—253% hardness (shore D)—38 | Tmax—216 °C | 75.7 | - | - | [159] |
| 22 | DGEBA, eugenol | HCCP | Tensile—77.64 MPa elongation—4.4% impact strength—30.46 kJ/m2 | Tg—99.85 °C, Tmax—392.2 °C | 26.2 | 346 | 16.4 | [160] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, R.; Chaudhary, M.L.; Patel, Y.N.; Chaudhari, K.; Gupta, R.K. Fire-Resistant Coatings: Advances in Flame-Retardant Technologies, Sustainable Approaches, and Industrial Implementation. Polymers 2025, 17, 1814. https://doi.org/10.3390/polym17131814
Patel R, Chaudhary ML, Patel YN, Chaudhari K, Gupta RK. Fire-Resistant Coatings: Advances in Flame-Retardant Technologies, Sustainable Approaches, and Industrial Implementation. Polymers. 2025; 17(13):1814. https://doi.org/10.3390/polym17131814
Chicago/Turabian StylePatel, Rutu, Mayankkumar L. Chaudhary, Yashkumar N. Patel, Kinal Chaudhari, and Ram K. Gupta. 2025. "Fire-Resistant Coatings: Advances in Flame-Retardant Technologies, Sustainable Approaches, and Industrial Implementation" Polymers 17, no. 13: 1814. https://doi.org/10.3390/polym17131814
APA StylePatel, R., Chaudhary, M. L., Patel, Y. N., Chaudhari, K., & Gupta, R. K. (2025). Fire-Resistant Coatings: Advances in Flame-Retardant Technologies, Sustainable Approaches, and Industrial Implementation. Polymers, 17(13), 1814. https://doi.org/10.3390/polym17131814









