Multifunctional Graphite Nanosheet–Hydrophilic Epoxy Anticorrosion Coatings via Size Confinement of Exfoliated Graphite
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Synthesis
2.2. Characterization
2.3. Electrochemical Measurement
2.4. Wear Resistance Measurement
3. Results and Discussion
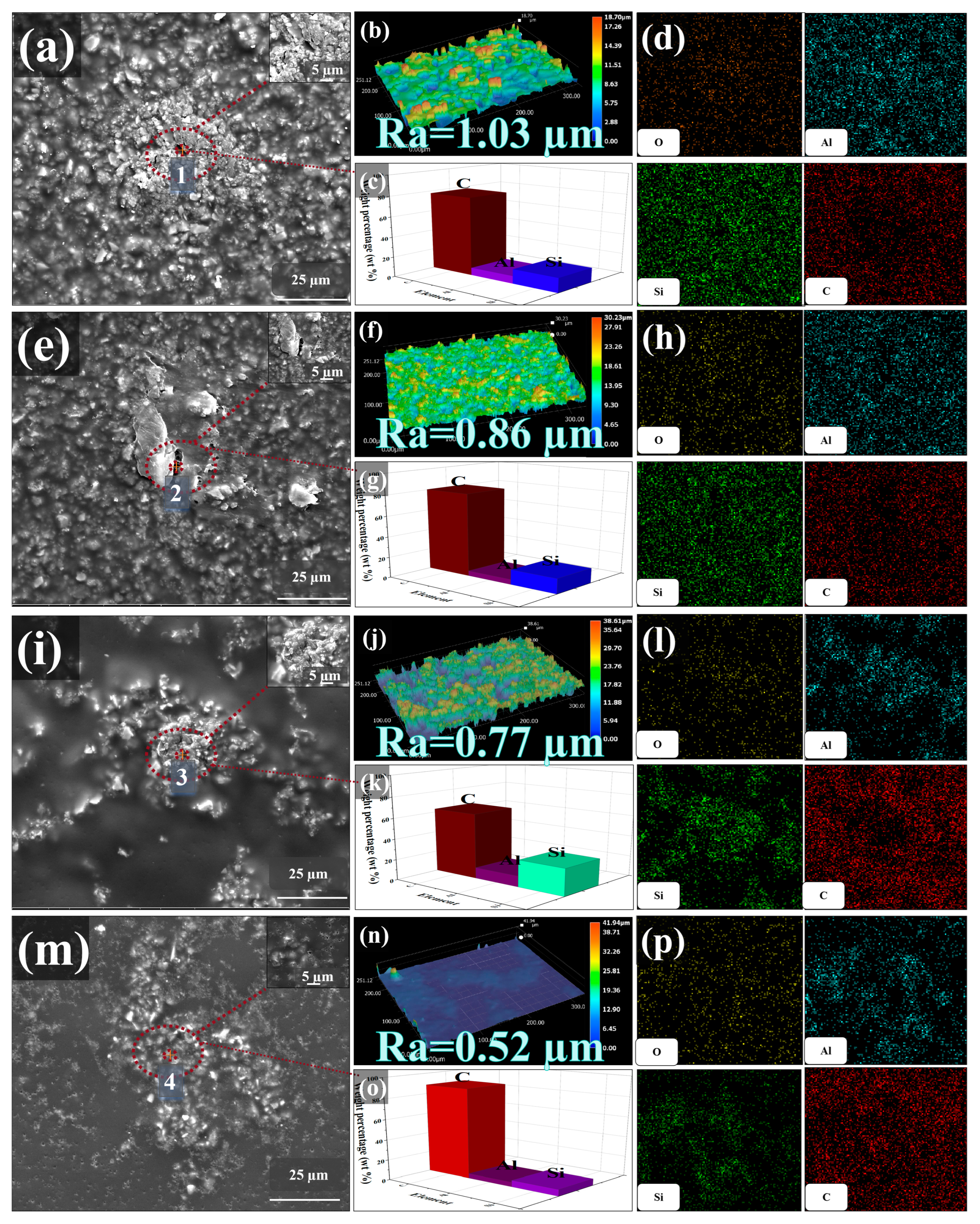
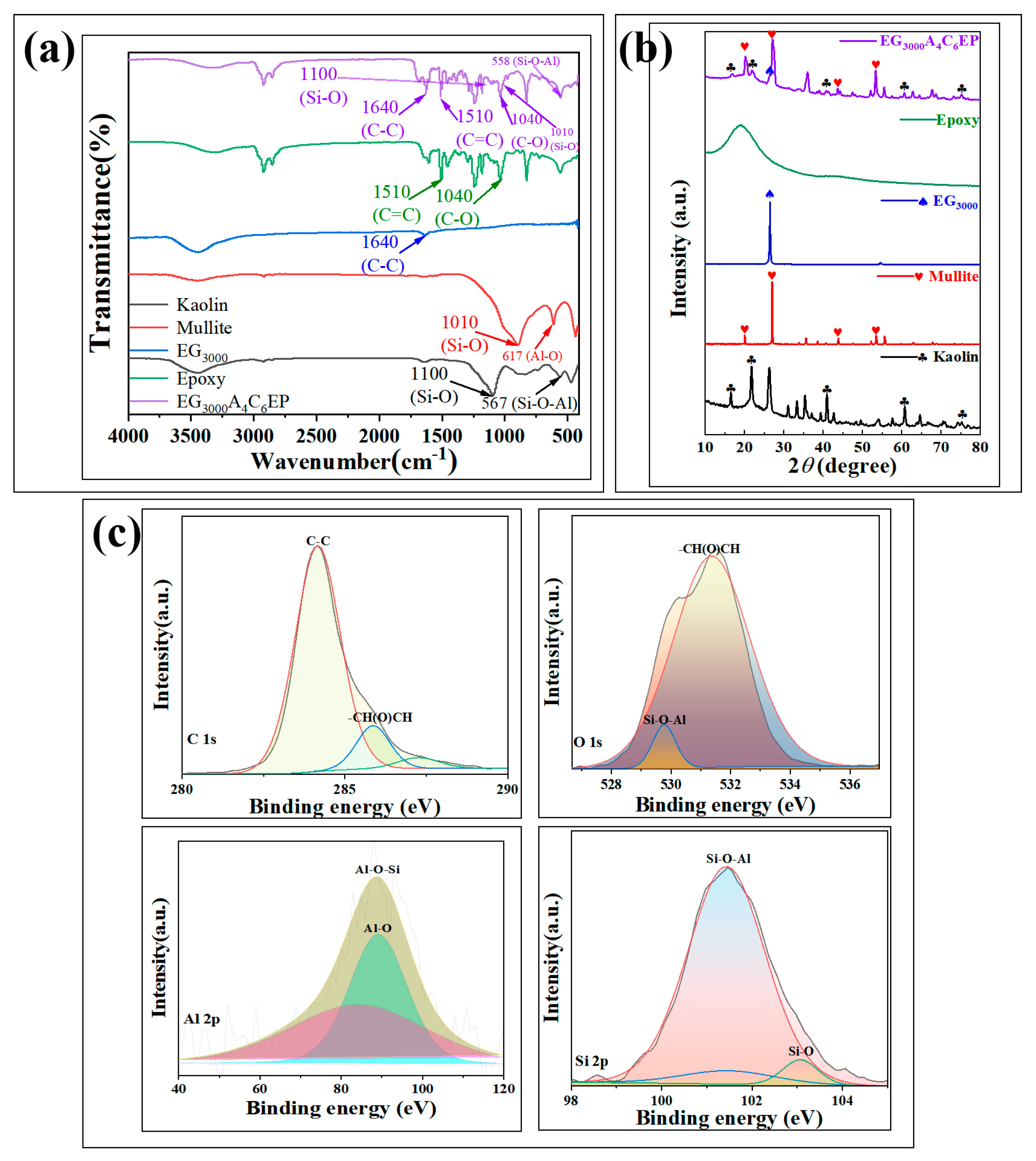
3.1. Structure and Morphology
3.1.1. Morphology Characterization of Samples and Coatings
3.1.2. Structural Characterization of Samples and Coatings
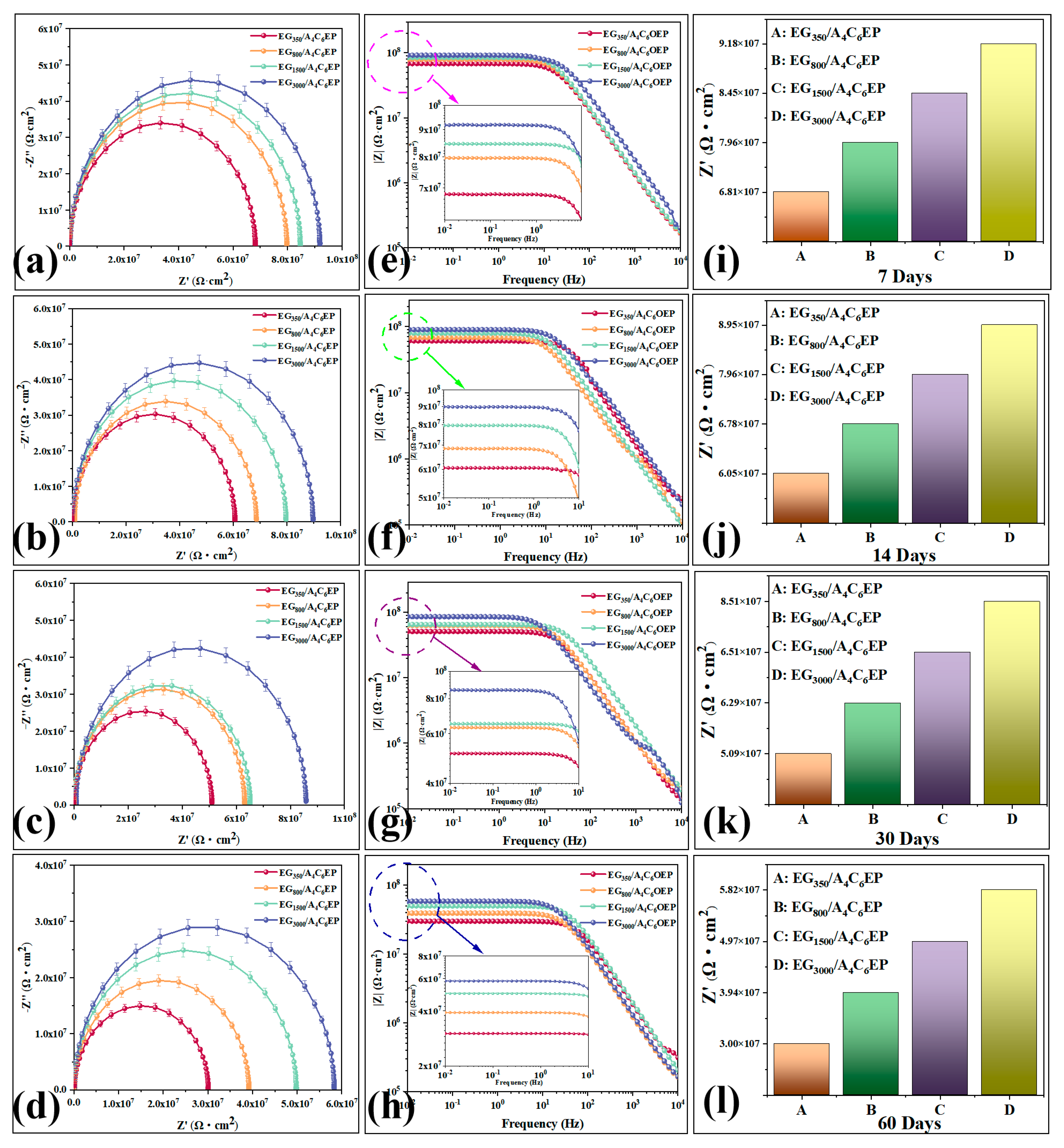
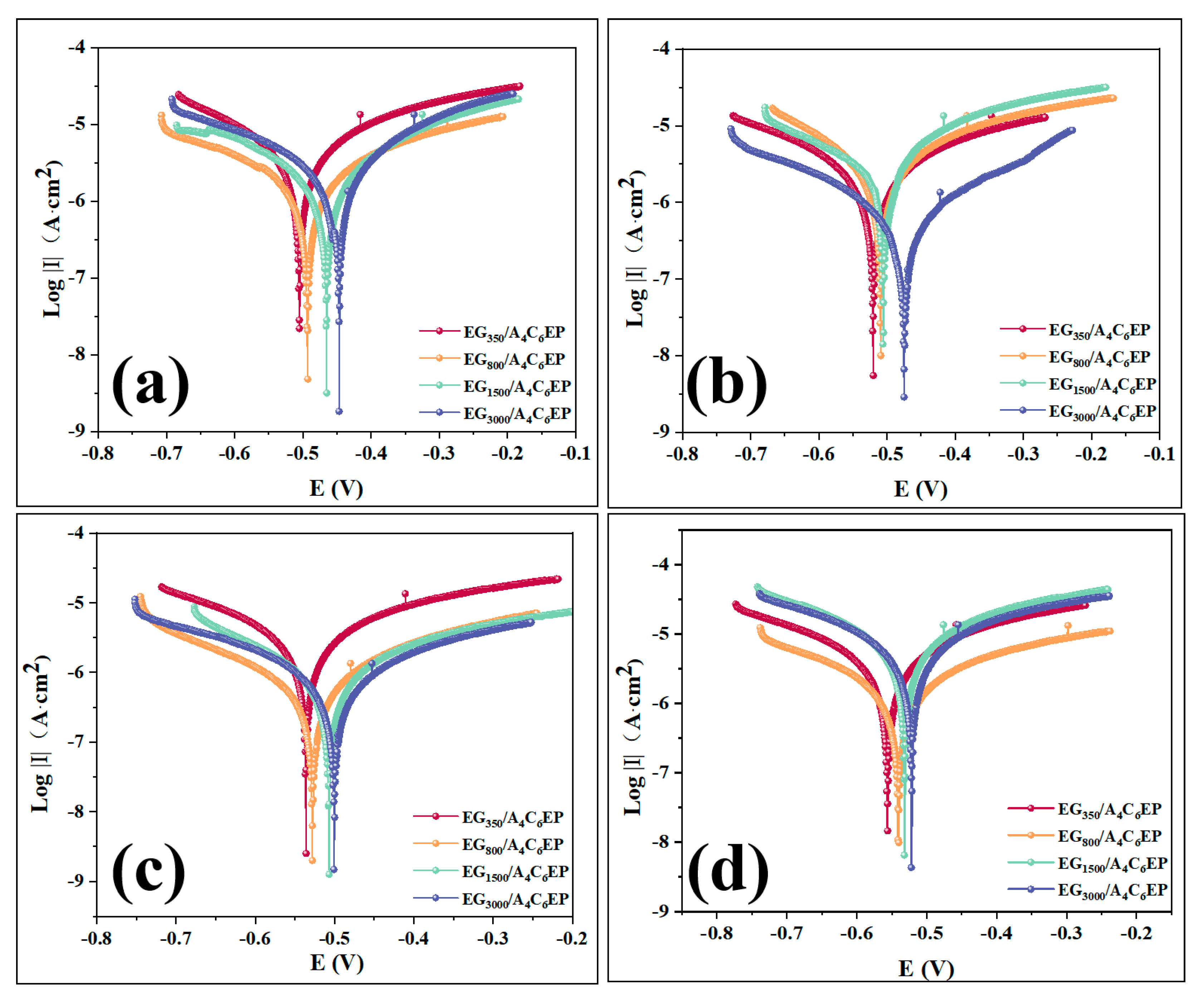
3.2. Corrosion Resistance Test
3.2.1. Electrochemical Measurement Result
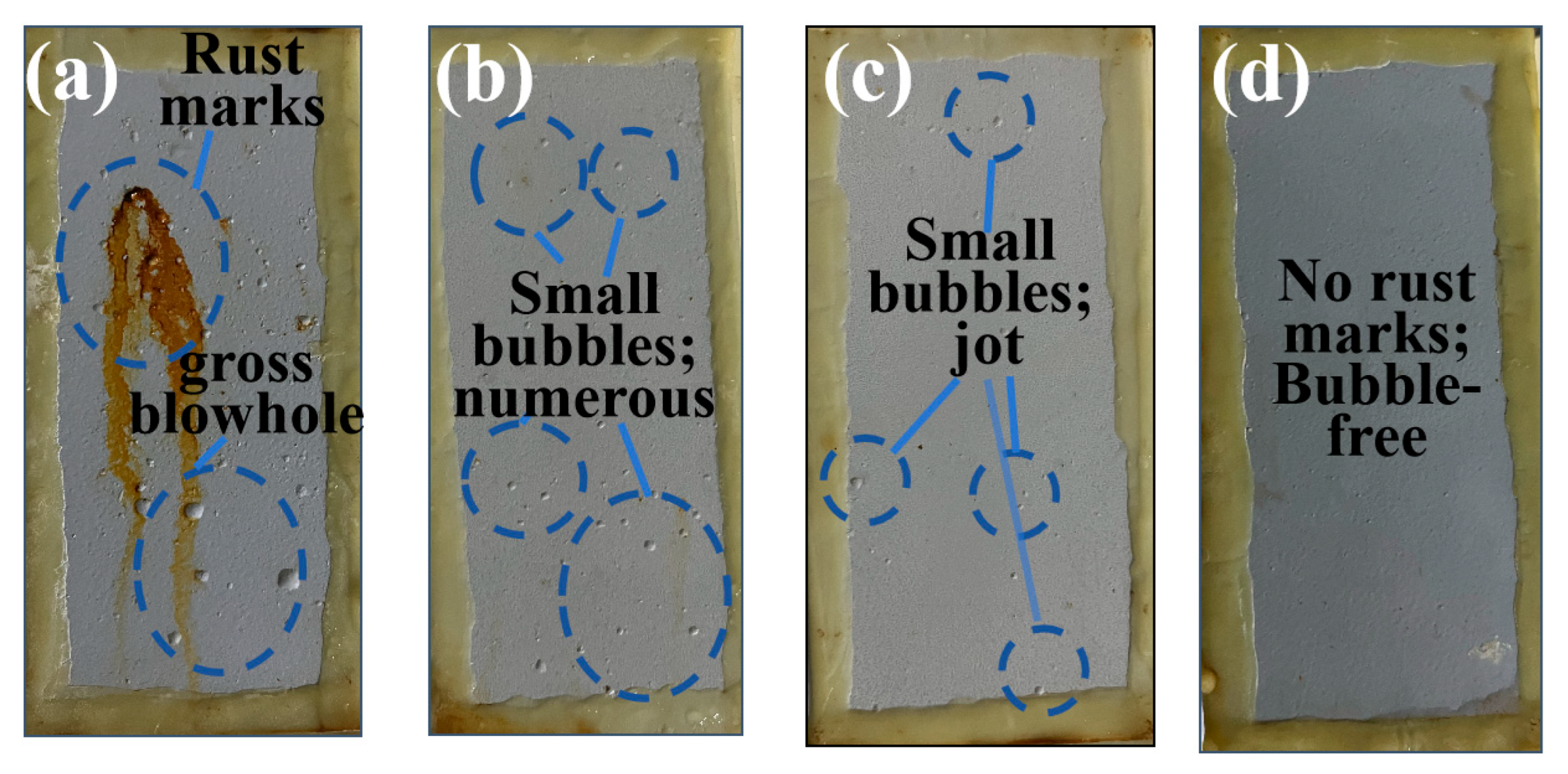
3.2.2. Salt Spray Test
3.3. Mechanical Wear Test
3.4. Coating Corrosion Resistance Mechanism Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lv, K.; Wan, D.; Pan, R.; Suo, W.; Zhu, Y. Curvature of NCNTs induced selectivity of CO2 electroreduction into CO. Carbon Neutraliz. 2022, 1, 189–197. [Google Scholar] [CrossRef]
- Resen, A.M.; Jasim, A.N.; Qasim, H.S.; Hanoon, M.M.; Al-Kaabi, M.H.H.; Al-Amiery, A.A.; Al-Azzawi, W.K. A combined experimental and theoretical study of a novel corrosion inhibitor derived from thiophen. Carbon Neutraliz. 2023, 2, 661–677. [Google Scholar] [CrossRef]
- Aljibori, H.S.; Alamiery, A.; Gaaz, T.S.; Al-Azzawi, W.K. Exploring corrosion protection for mild steel in HCl solution: An experimental and theoretical analysis of an antipyrine derivative as an anticorrosion agent. Carbon Neutraliz. 2024, 3, 74–93. [Google Scholar] [CrossRef]
- Foorginezhad, S.; Mohseni-Dargah, M.; Firoozirad, K.; Aryai, V.; Razmjou, A.; Abbassi, R.; Garaniya, V.; Beheshti, A.; Asadnia, M. Recent advances in sensing and assessment of corrosion in sewage pipelines. Process. Saf. Environ. Prot. 2021, 147, 192–213. [Google Scholar] [CrossRef]
- Gu, Y.; Chi, X.; Dong, T.; Zhang, Y.; Li, Z.; Wang, Z. Urea-based construction of hydrogen bonding networks for poly (biphenyl alkylene) s anion exchange membrane for fuel cells. Carbon Neutraliz. 2024, 3, 1092–1100. [Google Scholar] [CrossRef]
- Zhang, B.; Sun, Y.; Xu, H.; He, X. Hydrogen storage mechanism of metal–organic framework materials based on metal centers and organic ligands. Carbon Neutraliz. 2023, 2, 632–645. [Google Scholar] [CrossRef]
- Liu, T.; Zhao, Y.; Deng, Y.; Ge, H. Preparation of fully epoxy resin microcapsules and their application in self-healing epoxy anti-corrosion coatings. Prog. Org. Coat. 2024, 188, 108247. [Google Scholar] [CrossRef]
- Xiang, M.; Fan, W.; Lin, W.; Zhou, S.; Li, F.; Yang, Z.; Dong, S. Triple kill: Fabrication of composites coming from waste face masks, polystyrene microplastics, graphene, and their electromagnetic interference shielding behaviors. Carbon Neutraliz. 2023, 2, 616–628. [Google Scholar] [CrossRef]
- Emmerick, A.A.; Barbosa, M.N.; Silva, N.T.; Batalha, J.A.; Bott, I.S. Porous Density Influence on Anti-Corrosive Epoxy Resin-Based Coat Reinforced with Reduced Graphene Oxide (rGO). Mater. Res. 2024, 27, e20240020. [Google Scholar] [CrossRef]
- Chopra, I.; Ola, S.K.; Priyanka, C.; Dhayal, V.; Shekhawat, D.S. Recent advances in epoxy coatings for corrosion protection of steel: Experimental and modelling approach-A review. Mater. Today Proc. 2022, 62, 1658–1663. [Google Scholar] [CrossRef]
- Zaghloul, M.M.Y.; Zaghloul, M.M.Y.; Fuseini, M. Recent progress in Epoxy Nanocomposites: Corrosion, structural, flame retardancy and applications—A comprehensive review. Polym. Adv. Technol. 2023, 34, 3438–3472. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, Y.; Sun, Y.; Zhao, W.; Wang, L. 2D nanomaterials reinforced organic coatings for marine corrosion protection: State of the art, challenges, and future prospectives. Adv. Mater. 2024, 36, 2312460. [Google Scholar] [CrossRef] [PubMed]
- Syamkumar, K.; Babu, N.; Govindarajan, S.; Arya, S.B. Hot corrosion behaviour of mullite thermal barrier coatings for marine diesel engines. Ceram. Int. 2024, 50, 2808–2818. [Google Scholar]
- Luo, Y.; Wang, X.; Liu, Z.; Yu, C.; Deng, C.; Ding, J. Enhanced oxidation resistance and thermal shock resistance of low-carbon Al2O3–C refractories with nano-BN: A synergistic of mullitization behavior. J. Alloys Compd. 2024, 975, 172937. [Google Scholar] [CrossRef]
- Wang, W.; Cheng, C.; Qiao, J.; Han, L.; Li, Y.; Miao, Y. Fabrication and mechanical properties of multi-principal cation (Mg, Ti, Cr, Zr, Al, Si) mullite ceramic. Ceram. Int. 2024, 50, 25738–25748. [Google Scholar] [CrossRef]
- Romero, M.; Padilla, I.; Contreras, M.; López-Delgado, A. Mullite-based ceramics from mining waste: A review. Minerals 2021, 11, 332. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, B.; Zhu, N.; Huang, Y.; Wang, F.; Li, R.; Zhao, Y.; Jiang, Q.; Wu, X.; Zhang, Q. Dual-band electrochromic materials for energy-saving smart windows. Carbon Neutraliz. 2023, 2, 4–27. [Google Scholar] [CrossRef]
- Li, X.; Li, S.; Wen, Q.; Wan, Y.; Yang, L.; Kong, Y.; Liu, Y.; Tian, S.; Ma, C. Multilayered mullite ceramics with anisotropic properties. J. Eur. Ceram. Soc. 2023, 43, 5606–5615. [Google Scholar] [CrossRef]
- Pei, X.; Mu, Y.; Dong, X.; Ding, C.; Xu, L.; Cui, M.; Meng, C.; Zhang, Y. Ion-change promoting Co nanoparticles@ N-doped carbon framework on Co2SiO4/rGO support forming “double-triple-biscuit” structure boosts oxygen evolution reaction. Carbon Neutraliz. 2023, 2, 115–126. [Google Scholar] [CrossRef]
- Miao, J.; Fan, T. Flexible and stretchable transparent conductive graphene-based electrodes for emerging wearable electronics. Carbon 2023, 202, 495–527. [Google Scholar] [CrossRef]
- Yan, M.; Fang, Q.; Ding, R.; Li, Y.; Guo, J.; Xie, J.; Zhang, Y.; He, Y.; Yan, Z.; Chen, Z.; et al. Unveiling Na-ion storage mechanism and interface property of layered perovskite Bi2TiO4F2@ rGO anode in ether-based electrolyte. Carbon Neutraliz. 2024, 3, 818–831. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, L.; Sun, W.; Li, S.; Zhu, T.; Liu, W.; Liu, G. Superhydrophobic epoxy coating modified by fluorographene used for anti-corrosion and self-cleaning. Appl. Surf. Sci. 2017, 401, 146–155. [Google Scholar] [CrossRef]
- Lv, K.; Bao, Y.; Ma, H.; Liu, X.; Zhu, Y.; Wan, D. Size effect of graphite nanosheet-induced anti-corrosion of hydrophobic epoxy coatings. Coatings 2024, 14, 769. [Google Scholar] [CrossRef]
- Ma, H.; Zhang, X.; Liu, X.; Wan, D.; Lv, K. Controllable size of graphite nanosheets and functionalized SiO2 microspheres with excellent anti-corrosive/wear function for superhydrophobic coating. Mater. Today Commun. 2024, 40, 110026. [Google Scholar] [CrossRef]
- Wang, G.; Song, D.; Qiao, Y.; Cheng, J.; Liu, H.; Jiang, J.; Ma, A.; Ma, X. Developing super-hydrophobic and corrosion-resistant coating on magnesium-lithium alloy via one-step hydrothermal processing. J. Magnes. Alloys 2023, 11, 1422–1439. [Google Scholar] [CrossRef]
- Zhao, Y.; Ma, H.; Gao, Z.; Huang, Z.; Wu, Y.; Lv, K. Enhancement of the Corrosion and Wear Resistance of an Epoxy Coating Using a Combination of Mullite Powder and PVB. Coatings 2025, 15, 41. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, L.; Niu, Y.; Ma, H.; Lv, Y.; Lv, K. Shell-like ZnO–Graphene/Epoxy Coating with Outstanding Anticorrosion Performance and Weather Resistance. Coatings 2025, 15, 63. [Google Scholar] [CrossRef]
- Thakkar, H.K.; Joshi, K.K.; Pataniya, P.M.; Bhadu, G.; Siraj, S.; Sahatiya, P.; Sumesh, C.K. Photo-sensitive CuS/NiO heterostructure electrocatalysts for energy-saving hydrogen evolution reaction at all pH conditions. Int. J. Hydrogen Energy 2023, 48, 38266–38278. [Google Scholar] [CrossRef]
- Parmar, K.; Sumesh, C.; Pataniya, P.M.; Iyer, B. An electrically conducting adhesive paste based on adhesive exopolysaccharide of Priestia aryabhattai KAG-18. Int. J. Adhes. Adhes. 2025, 138, 103957. [Google Scholar] [CrossRef]
- Pataniya, P.M.; Sumesh, C.K. MoS2 nanosheets on Cu-foil for rapid electrocatalytic hydrogen evolution reaction. J. Electroanal. Chem. 2022, 912, 116270. [Google Scholar] [CrossRef]
- Lv, K.; Ma, H.; Liu, X.; Zhu, Y.; Wan, D. Exfoliated MoS2 with tunable size effect towards anticorrosion application. J. Alloys Compd. 2024, 1005, 176225. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, H.; Wan, D.; Liu, X.; Lv, K.; Wang, X. Fabrication of a Hydrophilic Al2O3/AlN EP Coating with Self-Cleaning and High-Efficiency Anti-Corrosion Properties. ChemistrySelect 2024, 9, e202402728. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, H.; Zhang, X.; Zhang, D.; Peng, Y.; Wan, D.; Peng, T.; Lv, K. Multifunctional Graphite Nanosheet–Hydrophilic Epoxy Anticorrosion Coatings via Size Confinement of Exfoliated Graphite. Polymers 2025, 17, 1803. https://doi.org/10.3390/polym17131803
Ma H, Zhang X, Zhang D, Peng Y, Wan D, Peng T, Lv K. Multifunctional Graphite Nanosheet–Hydrophilic Epoxy Anticorrosion Coatings via Size Confinement of Exfoliated Graphite. Polymers. 2025; 17(13):1803. https://doi.org/10.3390/polym17131803
Chicago/Turabian StyleMa, Huachao, Xuyang Zhang, Dongxing Zhang, Yizhan Peng, Detian Wan, Tai Peng, and Kuilin Lv. 2025. "Multifunctional Graphite Nanosheet–Hydrophilic Epoxy Anticorrosion Coatings via Size Confinement of Exfoliated Graphite" Polymers 17, no. 13: 1803. https://doi.org/10.3390/polym17131803
APA StyleMa, H., Zhang, X., Zhang, D., Peng, Y., Wan, D., Peng, T., & Lv, K. (2025). Multifunctional Graphite Nanosheet–Hydrophilic Epoxy Anticorrosion Coatings via Size Confinement of Exfoliated Graphite. Polymers, 17(13), 1803. https://doi.org/10.3390/polym17131803





