3.2. Rheological Analysis
Viscosity measurements were carried out using a 20 mL concentric cylinder system in which temperature was controlled at 25 °C by a Peltier device.
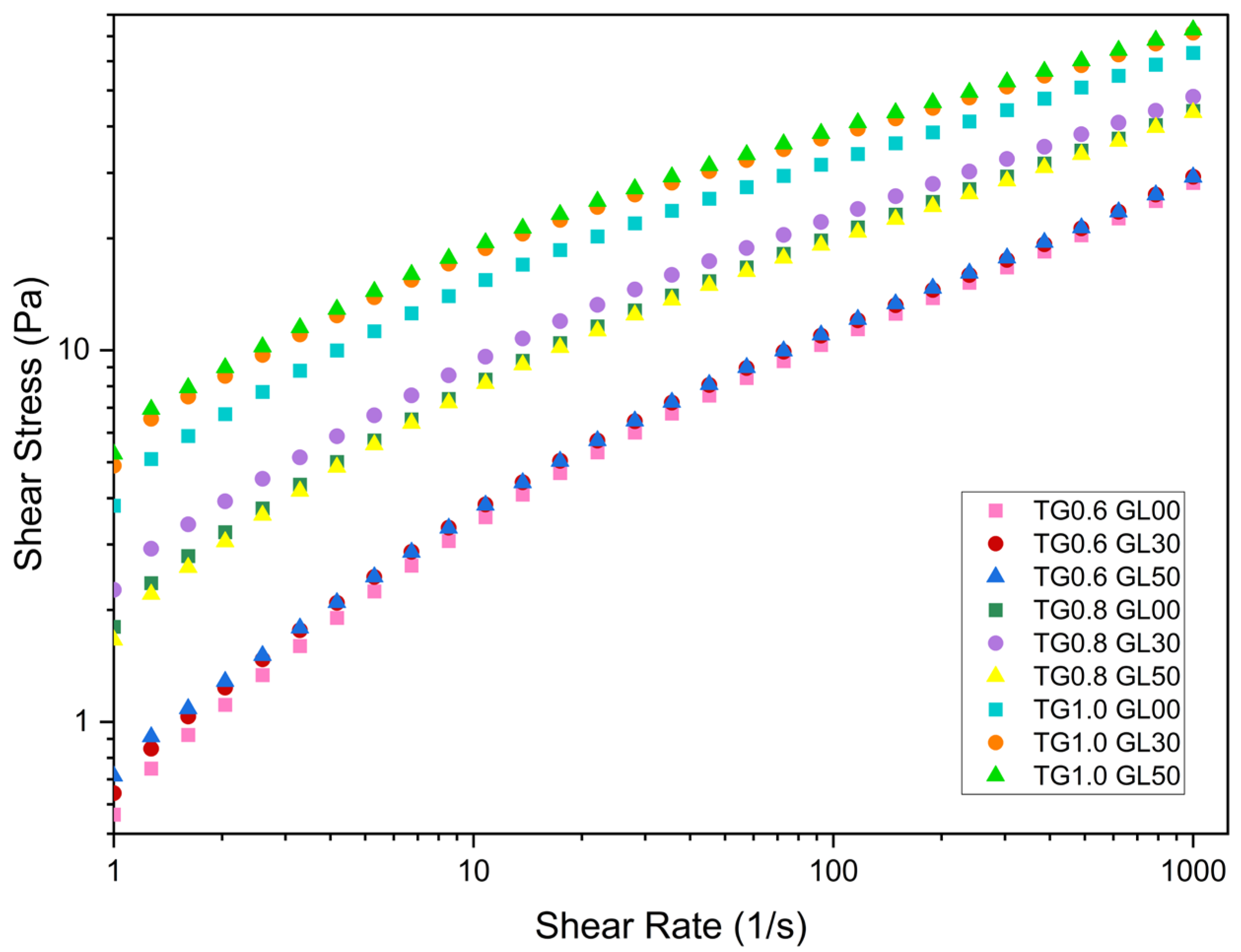
Rheological tests showed that viscosity increases with TG concentration. For a shear rate of less than 10 s−1, the viscosity of the 0.6% TG solutions is approximately 480 mPa.s, while the 0.8% TG solutions reach a viscosity of 700 mPa.s. Likewise, the solutions with a concentration of 1.0% TG presented viscosities of 1500 mPa.s, significantly higher than those obtained for the other TG concentrations.
As the shear rate increases, the viscosity decreases to 30 mPa.s for 0.6% TG, 45 mPa.s for 0.8% TG, and 70 mPa.s for 1.0% TG. This is because higher concentrations of TG generate a stiffer molecular chain network via hydrogen bridges [
10]. Moreover, the viscosity reduction with increasing shear rate is typical of pseudoplastic solutions [
1], where macromolecular chains become disentangled and aligned in the direction of flow, decreasing the interactions between them [
16]. This behavior was corroborated with the calculated parameters of the Herschel–Bulkley model (
Table 1).
GL possessing three hydroxyl groups (OH-) facilitates the formation of hydrogen bridges with the side chains of TG, which strengthens the network entanglement and increases the viscosity of the film-forming solutions [
17,
18]. However, as observed in
Figure 1, varying GL content between 30% and 50% has a smaller impact on the flow curves than modifying TG concentration.
From
Figure 1, it can be seen for all cases, the yield stress limit is affected by the concentration of GL and TG.
In
Table 1, we can see that when OP was added to the mixtures, the flow coefficient (k) subtly decreased, while the flow behavior index (n) slightly increased, maintaining a shear-thinning behavior, with TG as the dominant component of the solution. Similarly, OP was observed to increase the values of the flow behavior index (n), indicating that its incorporation enhances the viscous behavior of the solution. However, since it does not exceed the threshold of 1, the fluid remains pseudoplastic.
On the other hand, the data indicate that increasing the concentration of TG (from 0.8% to 1.0%
w/
v) led to a decrease in the 12.7% value from 0.487 (Composition 1) to 0.428 (Composition 7), which is consistent with the previous findings reported by Wu et al. [
1] and Santos et al. [
19], who studied the rheology of TG at different concentrations. According to these authors, the pseudoplastic nature of TG solutions becomes more pronounced as their concentration increases. This suggests that, although OP contributes to a slight increase, TG remains the predominant factor in determining the rheological behavior, reinforcing its pseudoplastic tendency at higher concentrations.
Before the frequency sweeps, amplitude sweeps were performed from the lowest TG concentration (0.6%) to the highest (1.0%), selecting the shear deformation value within the linear region of 5%. Frequency sweeps were then carried out with a shear deformation of 5% over a frequency range of 1 to 100 rad/s. In the first stage, TG concentrations of 0.6%, 0.8%, and 1.0% were evaluated with different GL concentrations. In the second stage, mixtures of 0.8% and 1.0% TG, 30% and 50% GL, and varying OP concentrations were tested.
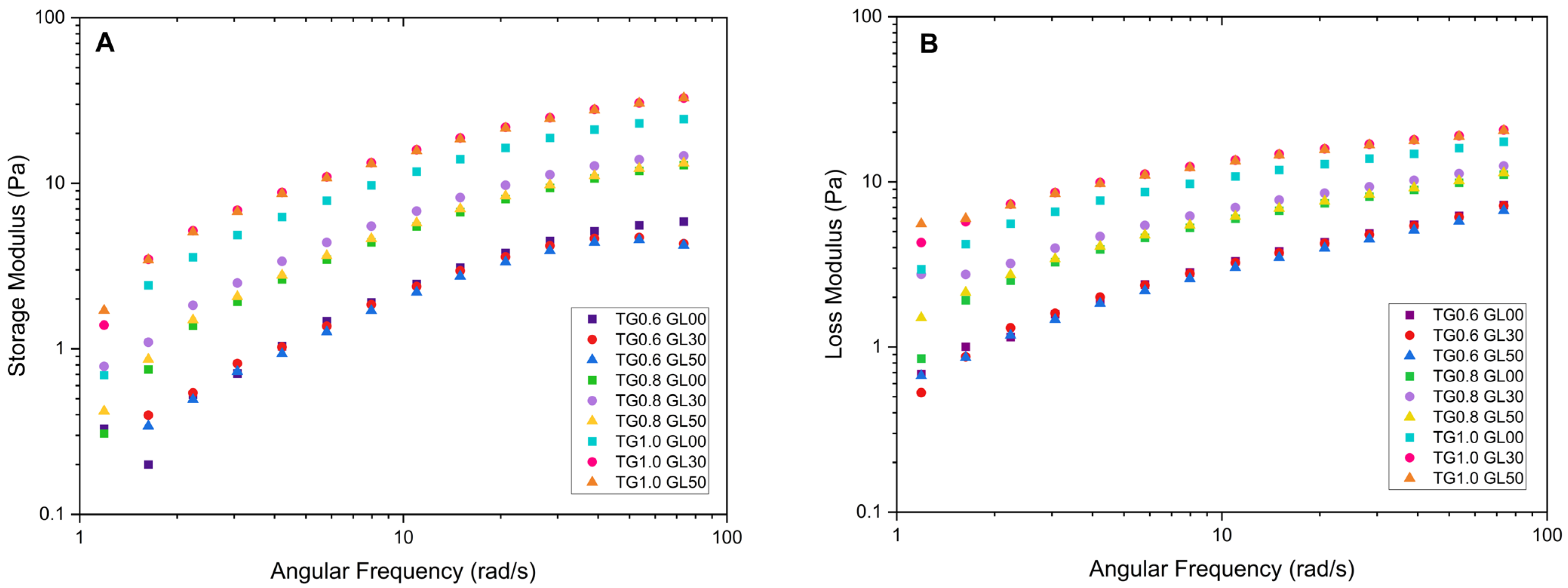
The storage modulus for the solutions with 1.0% TG shows a steep initial curve, followed by a decrease toward values close to zero. The highest peak of 62.10 Pa was recorded for the solution with 1% TG and 30% GL. Furthermore, it was observed that the storage modulus increases with angular frequency, which is attributed to the rearrangement of molecular chains due to the increase in frequency [
17], as evidenced in
Figure 2.
The loss modulus also increases with increasing angular frequency; however, it does not exceed the values reached by the storage modulus. The maximum value recorded was 20.454 Pa for the solution with 1.0% TG and 50% GL. In addition, the solutions with a higher concentration of TG exhibit moderate growth compared to the 0.6% solutions, which show a significant linear increment. This is due to an enlargement in the energy released, which causes the viscous component of the solution to predominate over the elastic component.
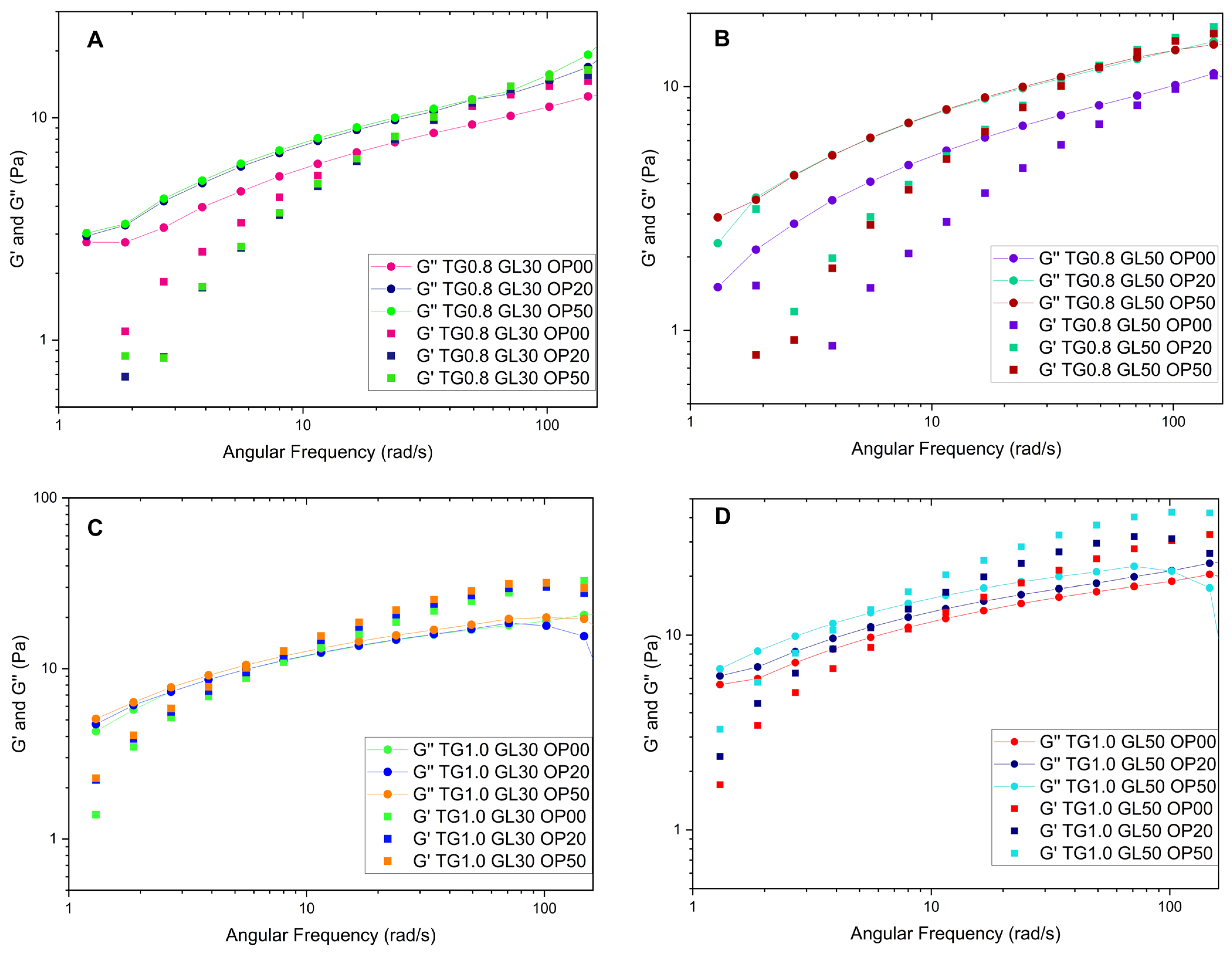
Figure 3 shows the behavior of some solutions. When OP is added to the solutions containing 0.8% TG, they behave as a liquid until a crossover point appears at 3.8 rad/s, after which they acquire a gelatinous character. The solutions with 1.0% TG observe a less-liquid behavior, since they comprise more TG and have higher values of the storage modulus at high frequencies, due to the increased crosslinking, forming a more cohesive network that can store more energy.
The addition of orange peel does not significantly affect the rheological behavior in terms of loss and storage modulus, with TG being the dominant component. At low frequencies, the loss modulus is higher, whereas at higher frequencies, the storage modulus prevails. This behavior has been observed in previous studies [
1,
10], where TG solutions exhibit an entangled solution behavior at higher frequencies.
A correlation of the frequency sweeps was performed using Maxwell’s equations, finding that the four-element model (
n = 4) is adequate to accurately describe the behavior of the solutions. The values of λ
i and G
i are reported in
Appendix A (
Table A1).
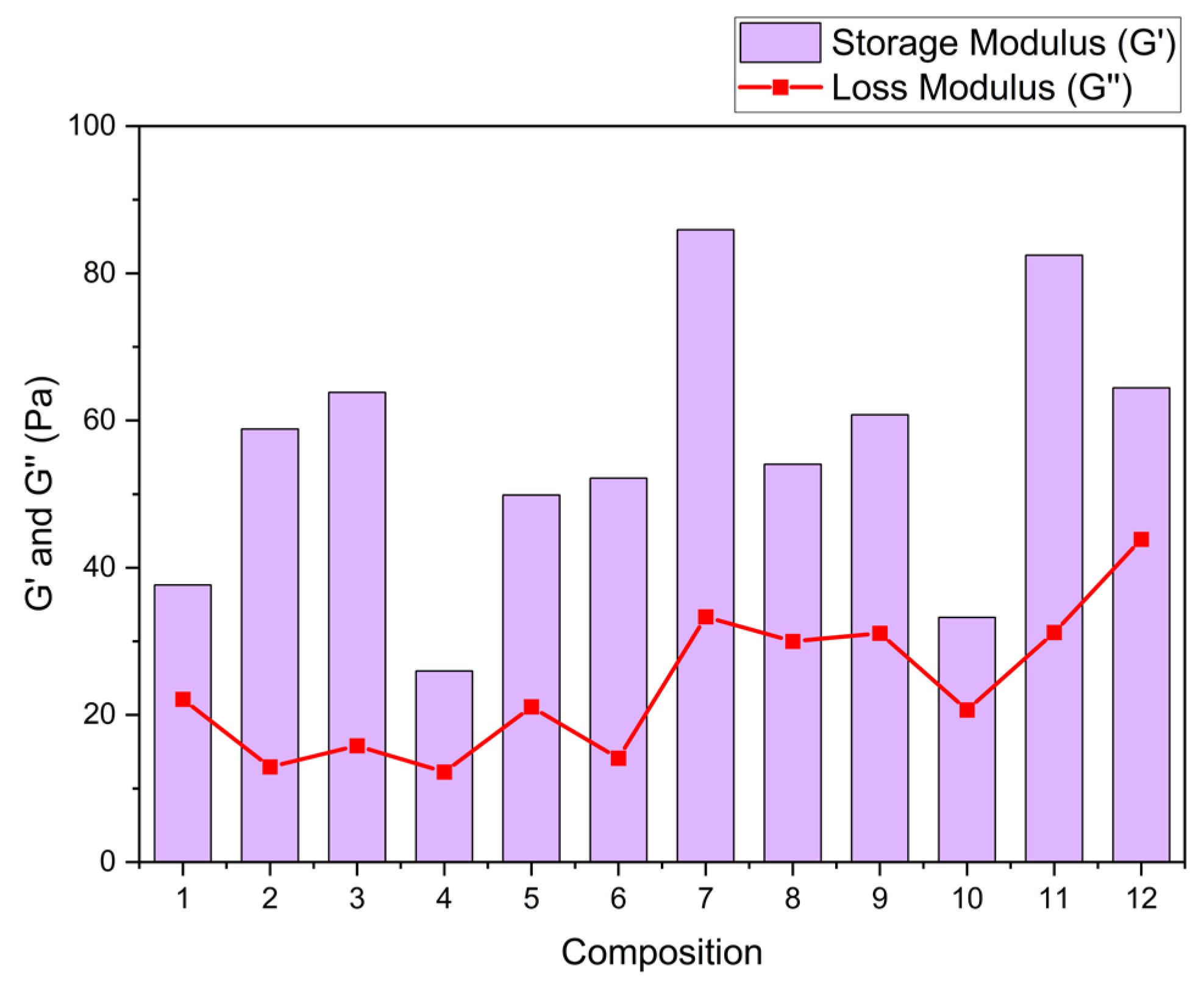
Table 2 shows the storage and loss modulus adjustments using Maxwell’s equations. Solutions with 0.8 TG in their composition present a better interaction between TG, GL, and OP, increasing the storage modulus with increasing OP concentration. Solutions that are composed of 1.0% TG and high OP content show a smaller storage modulus by about 35%. This effect can be attributed, on the one hand, to the saturation of the polymer matrix due to the formation of intermolecular bonds between OP cellulose, TG, and GL [
20,
21]. On the other hand, it could also be related to the intrinsic nature of OP as a filler material [
4], since an excess of its incorporation interferes with the cohesion of the polymeric network, affecting its structural integrity.
Figure 4 shows that the storage modulus G′, which reflects the stored elastic energy and elasticity of the matrix [
6], increases markedly in the compositions with higher contents of TG (1.0%). This increment suggests a more organized and cohesive polymeric network, attributed to hydrogen-bonding interactions between the hydroxyl groups of the TG, the OP compounds (cellulose and pectin), and the GL. On the other hand, the loss modulus G″, related to the energy dissipated as heat and the viscosity of the material [
10], shows a slight increment in the formulations with higher GL content (50%), indicating a higher flow capacity and higher plasticity.
It is important to emphasize that in this study, all rheological measurements were conducted at a controlled temperature of 25 ± 2 °C. Although the effect of temperature on the rheological properties was not investigated, this factor could have a significant influence on polymeric systems such as those evaluated.
3.4. Tensile Strength (TS)
The initial tensile strength of the films was 13.93 ± 0.44 MPa for the 0.8% TG concentration and 19.41 ± 0.67 MPa for 1.0% TG, reaching a maximum of 27.93 MPa with 50% OP in the TG 0.8 G 30 formulation. It was evidenced that the combination of TG and OP strengthened the polymeric matrix, improving the mechanical resistance by up to 50.21%. The increase in GL reduced the tensile strength due to the decrease in the intermolecular forces in the polymeric chains [
36,
37]. Tensile strength presented values between 10 and 20 MPa, being proportional to OP concentration. Previous studies containing nanocellulose, polyvinyl alcohol, and glutaraldehyde showed similar results [
21,
38]. Typically, food films report tensile strength values between 15 and 25 MPa, ensuring durability and flexibility, which is consistent with the formulations of TG between 0.8% and 1.0% with 50% OP.
3.4.1. Elongation (%)
The tests reported low elongation percentages: 2.86% for Composition 9 and 6.27% for Composition 8, both with 1.0% TG, suggesting a more compact polymeric matrix. In contrast, compositions with 0.8% TG and 50% GL showed higher elongations, 31.07% (Composition 5) and 40.68% (Composition 4), indicating that OP concentration reduces elongation due to the structural nature of cellulose [
22]. GL acted as a plasticizer, increasing flexibility by 78.86%. A higher GL concentration increases elongation by weakening intermolecular forces and favoring the formation of hydrogen bonds, which improves flexibility but decreases tensile strength [
39,
40].
Analyzing
Figure 7A,B,D, the important effect of OP on the tensile strength as well as elongation of the films can be observed. That effect is diminished in the compositions presented in
Figure 7C. The impact of TG on the mechanical properties can be appreciated by comparing
Figure 7B,D. The combination of high OP and TG concentrations produces an important increase in tensile strength and at the same time a reduction in the elongation of the films. The results suggest that important interactions take place between the film components.
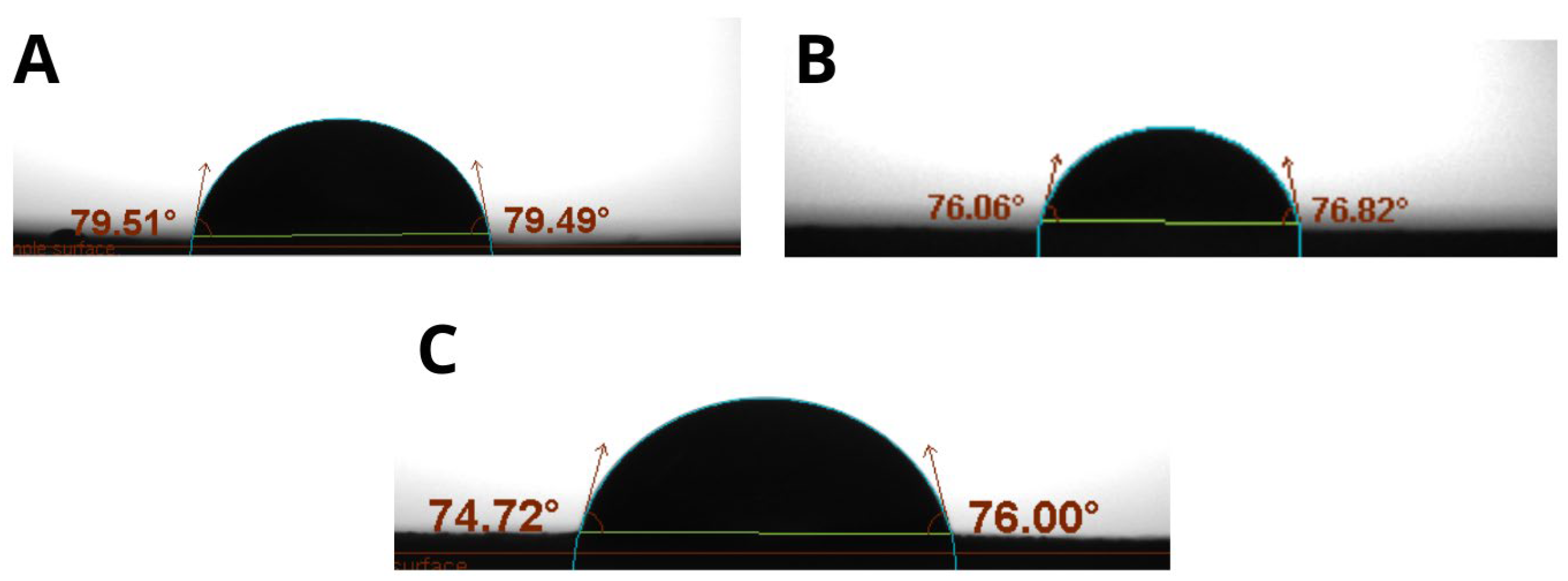
Additionally, film thickness exhibited a direct influence on mechanical properties. Formulations with a higher solid content, such as those containing 1.0% TG and 50% OP, produced thicker films associated with higher TS (20.57 MPa) and YM values (402.34 MPa), suggesting a denser and more rigid polymer matrix. In contrast, thinner films exhibited greater elongation, reflecting a more flexible structure. These findings indicate that thickness, governed by the composition of the film-forming solution, plays a modulatory role in the mechanical behavior of films.
3.4.2. Young’s Modulus (YM)
Figure 7 shows that films with 1.0% TG have slightly higher Young’s modulus values compared to those containing 0.8%. The highest values were 400 MPa and 402 MPa for Compositions 3 and 9 (See
Appendix C,
Table A3), which also have the highest concentrations of OP. This increase in the Young’s modulus is due to the increased stiffness in the film, caused by the molecular interactions between OP and TG, which strengthen the polymeric matrix [
4,
41].
Food coatings showed a Young’s modulus between 10 and 500 MPa, with greater flexibility in those formulations with more plasticizer. Films with 0.8% TG are more flexible, which is suitable for conforming to irregular surfaces, while those containing 1.0% TG have a higher Young’s modulus, suggesting a higher stiffness, preferable for foods requiring a stronger barrier.
The YM exhibited a broad range from 27 to 402 MPa, indicating the significant influence of each component and their interactions. The important role of OP in increasing the YM can be observed (See
Appendix C,
Table A3), showing a direct proportionality to OP concentration. For instance, in formulations with 0.8% TG and 30% G, the YM increased from 79.54 MPa in the absence of OP to 400.41 MPa with 50% OP. A similar trend was observed in formulations with 1.0% TG, where the YM reached its highest value of 402.34 MPa.
TG also plays a key role in the YM. Comparing samples 1 and 7, which do not contain OP and have the same glycerol content (30%), the YM increased from 79.54 MPa to 186.99 MPa when TG increased from 0.8% to 1.0%. Furthermore, glycerol’s plasticizing effect is evident when comparing samples with 30% and 50% GL, as a higher GL content leads to a decrease in the YM, highlighting its impact on the mechanical rigidity of the films.
3.5. Mechanical-Dynamic Analysis
For the mechanical-dynamic analysis of the solutions and films, it is noted that the storage modulus was obtained by applying a tangential force to the surface of the solution, while the Young’s modulus was determined by applying a normal force to the film. Both moduli are linked to the elasticity and energy stored in the material. Film-forming solutions and biofilms, composed of polymeric chains, exhibit both viscous and elastic behavior, which we previously defined as viscoelastic behavior.
The relationship between the storage modulus (G′) and Young’s modulus (YM) is a key factor in linking the rheological properties of the film-forming solutions to the mechanical properties of the corresponding films. These properties, although measured in different contexts, are connected because they describe the response of the material to deformation, either in the fluid (rheology) or solid (mechanical) state.
G′ is a parameter that describes how the solution components interact to form a cohesive network. During the film-formation process, which involves evaporation of the solvent, these interactions determine the final structure of the polymer network, which directly influences the YM.
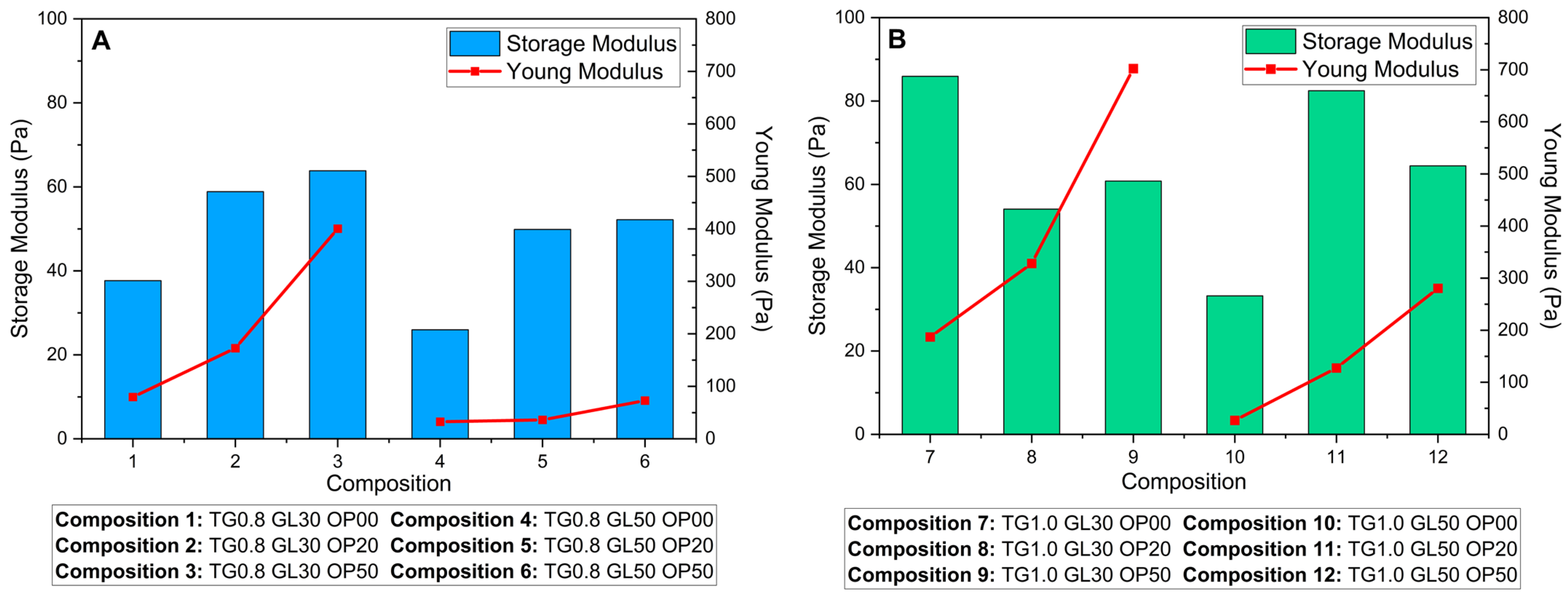
Figure 8 compares the G′ of the liquid phase with the YM of the solid films, revealing differences based on TG concentration. In solutions with 0.8% TG (
Figure 8A), a direct relationship between the G′ and YM is observed, where higher G′ values correspond to stiffer films. However, in solutions with 1.0% TG (
Figure 8B), the relationship becomes more complex, suggesting a stronger synergistic effect among the components. This deviation could be attributed to more intense molecular interactions at higher TG concentrations, which under certain mixing conditions, lead to an increase in viscosity and a transition from a dilute to a concentrated regime [
42,
43]. In this context, the interactions between the components become more intricate, resulting in variations in the structural rigidity of the films.
The solutions exhibit pseudoplastic behavior, characterized by a decrease in viscosity under increasing shear stresses. This behavior suggests that the matrix possesses some elasticity, which favors the formation of more uniform and flexible films due to a homogeneous distribution of polymers. A high G′ in solutions with a higher TG concentration indicates a higher flow resistance and stronger viscoelastic behavior. This attribute predicts a higher stiffness in the final film, aligning with the increase in related mechanical properties, as we can see in
Figure 8.
Data analysis was performed using IBM SPSS Statistics version 21. SPSS software was used to analyze the correlation between the two modules. First, normality tests were performed to determine whether parametric or nonparametric statistics should be applied, concluding that the data follow a normal distribution. Subsequently, a Pearson’s correlation index was calculated, which showed a significant relationship between the G′ and YM. This relationship is direct, with a positive Pearson’s coefficient of 0.840, indicating a high correlation between the two moduli.
Through a logarithmic multiple regression model, the relationship between the Young’s modulus (YM) and several predictor variables, including the storage modulus (G′) and the proportions of Tara gum (TG), glycerol (GL), and orange peel (OP), was evaluated. Calculations were performed with the logarithmic values of the YM and G′, obtaining the following equation:
In this equation, the coefficient associated with log(G′) is equal to 0.669, indicating that a 1% increase in the storage modulus generates an approximate 0.669% increase in log(YM), assuming all other variables are held constant. The coefficient of 0.467 associated with TG indicates that an increase in its concentration will cause a significant rise in the YM (
p = 0.004). This can mean that TG, being a polysaccharide with hydroxyl groups, facilitates the interaction with other polymer chains like pectin and cellulose, which improves the mechanical strength of films [
18,
34]. Its positive effect on the mechanical properties of films suggests that increasing TG enhances its interaction with other matrix components through hydrogen bonding, a behavior that has also been observed in other studies on gums [
42,
44]. On the other hand, the proportions of GL and OP have a less significant impact. For each unit increase in the GL ratio, the Young’s modulus decreases slightly (−0.024), reflecting a softening effect of the plasticizer on the interactions between the polymer chains. In contrast, the proportion of OP has a positive effect (0.002), which strengthens the structural cohesion of the films.
The model as a whole presents an R2 = 0.928, indicating that 92.8% of the variation in log(YM) can be explained by the included predictor variables. In terms of synergy, the storage modulus exhibits a strong relationship with the Young’s modulus, while the specific interactions between the components (TG, OP, and GL) play a decisive role in the final material properties. In particular, an increase in TG concentration enhances these interactions, generating a synergistic effect that influences the structural and mechanical properties of the films.
Due to its strong explanatory power, this model could be applied in the design and development of other biopolymeric film systems, facilitating the engineering of materials with optimized mechanical properties. In this context, the present study not only demonstrates the interdependence between rheology and mechanics but also validates the feasibility of mathematically modeling these interactions, making a notable contribution to biopolymer materials science.