Design of Biomass Adsorbents Based on Bacterial Cellulose and E. crassipes for the Removal of Cr (VI)
Abstract
1. Introduction
2. Materials and Methods
2.1. Creations of Biomass Adsorbents
2.2. Model Evaluation
2.3. Measurement of the Pore Volume of Biomass
3. Results
3.1. Measurement of Biomass Pore Volume
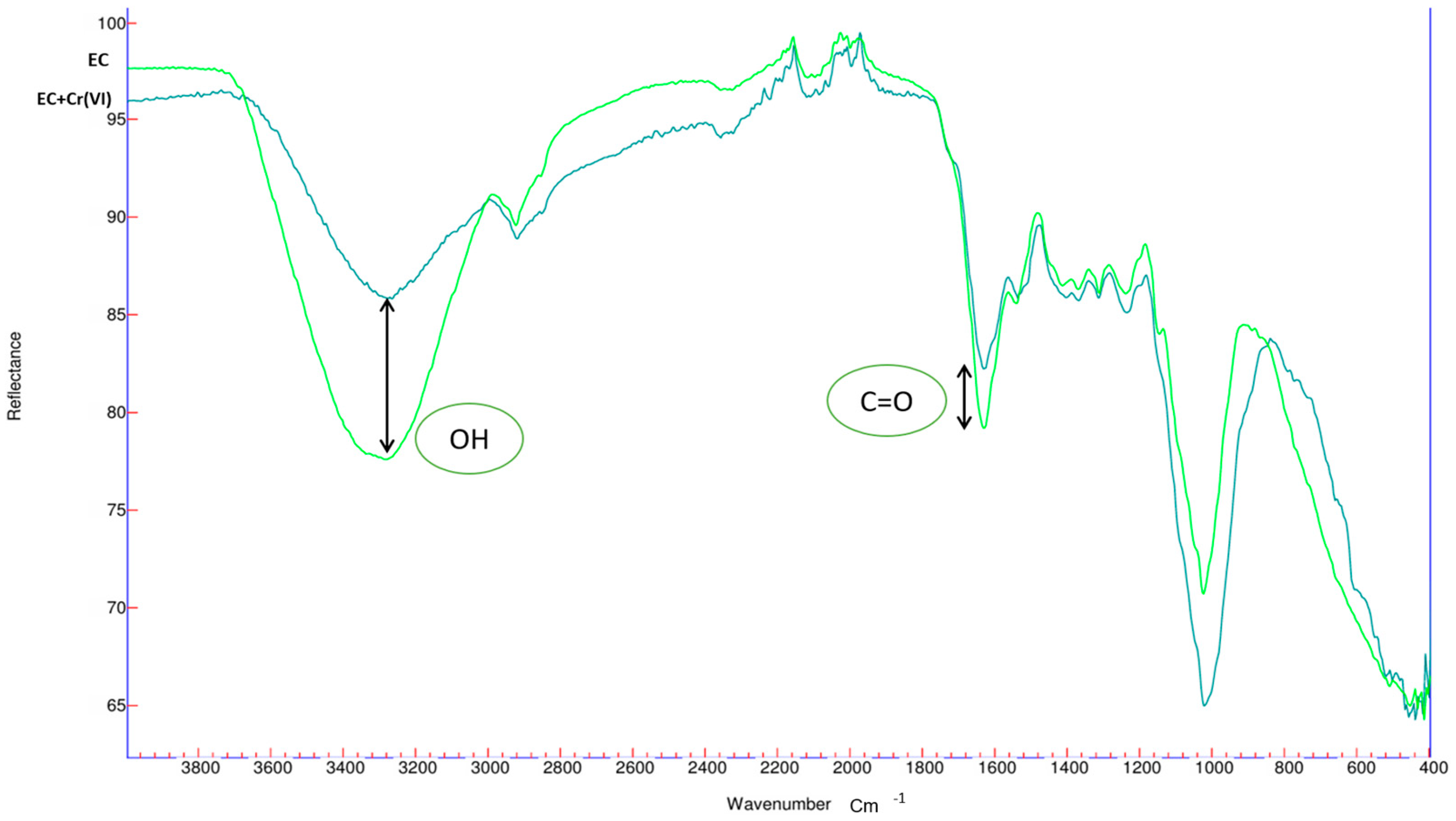
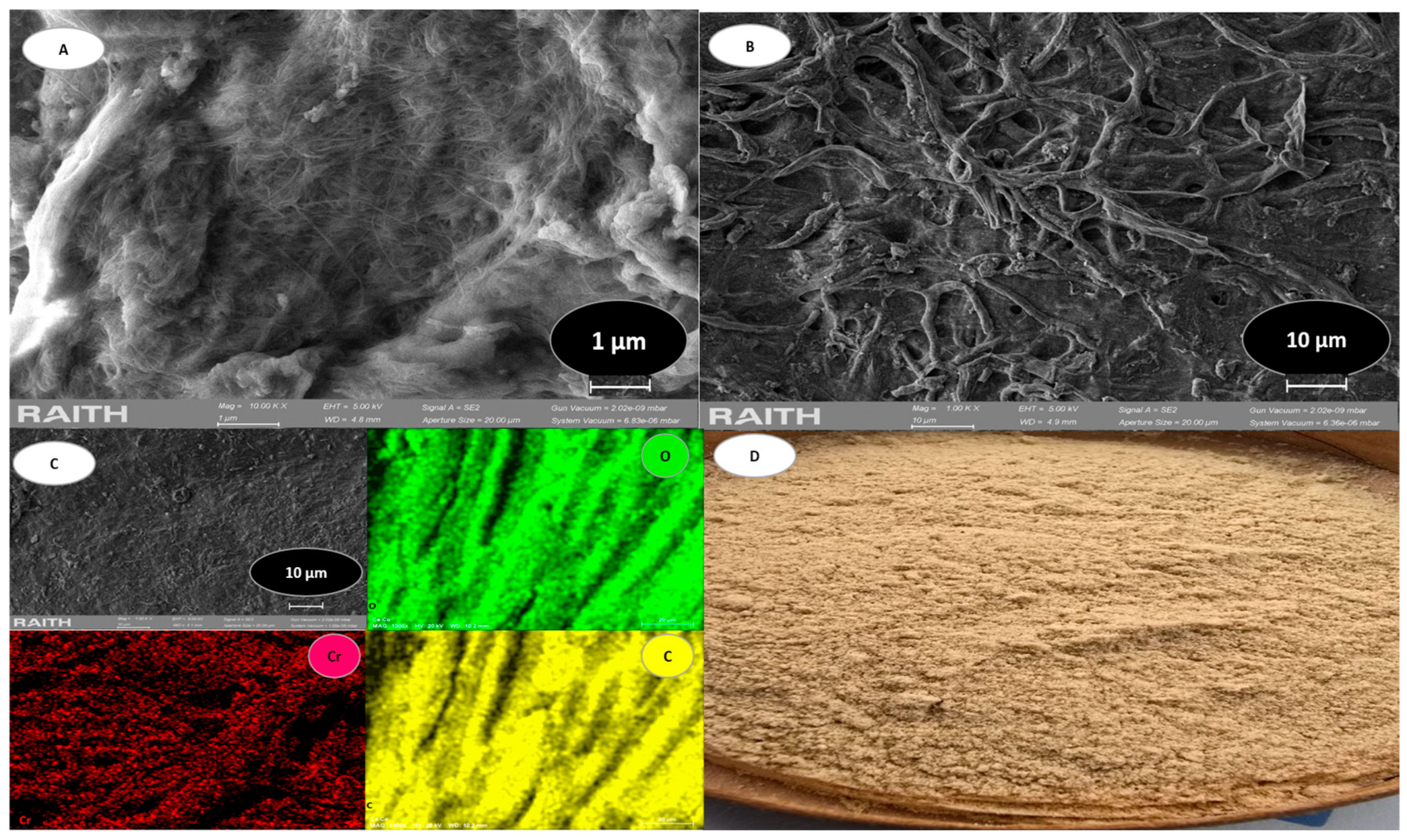
3.2. Material Characterization
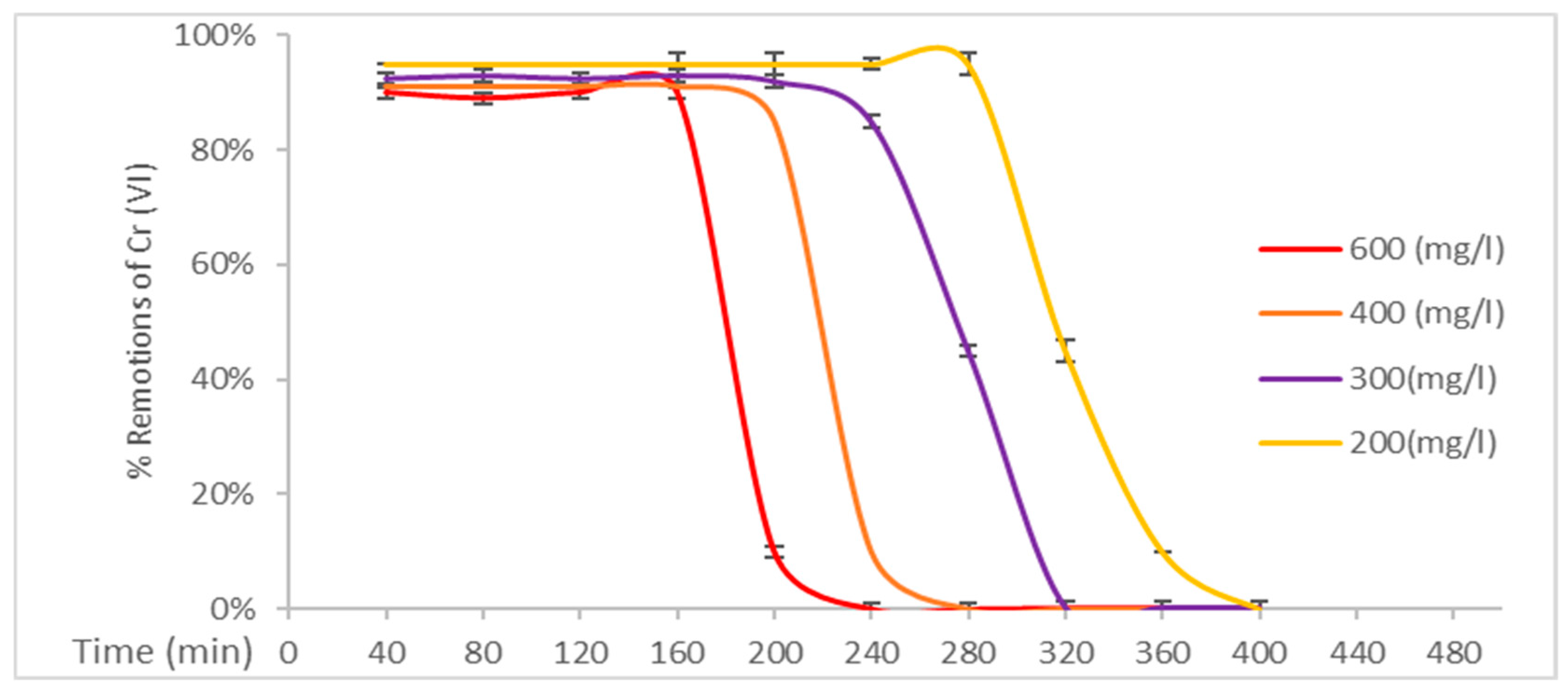
3.3. Results of Remotion’s of Cr (VI)
3.4. Adsorption Capacities
3.5. Isotherms
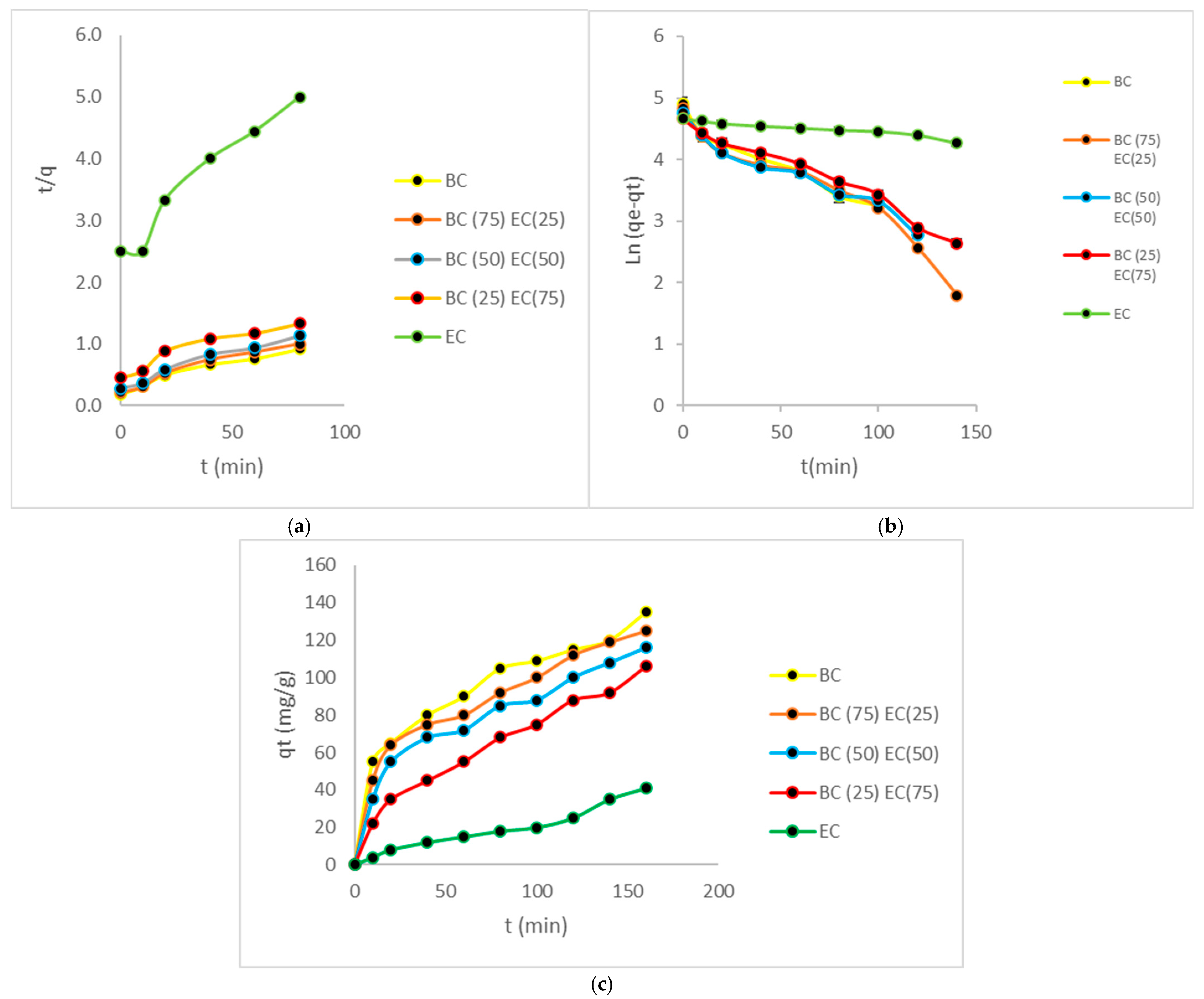
3.6. Kinetic Studies
3.7. Process of Elutions
3.8. Treatment System Costs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- de Paiva, G.M.; Palladino, F.; Nucci, E.R.; Machado, A.R.T.; Rosa, C.A.; Santos, I.J.B. Bacterial nanocellulose produced as a by-product of the brewing industry and used as an adsorbent for synthetic solutions of Co(II), Cu(II), Ni(II) AND Fe(III). J. Polym. Environ. 2024, 32, 6803–6819. [Google Scholar] [CrossRef]
- Madhogaria, B.; Banerjee, S.; Chakraborty, S.; Dhak, P.; Kundu, A. Alleviation of heavy metals chromium, cadmium and lead and plant growth promotion in Vigna radiata L. plant using isolated Pseudomonas geniculata. Int. Microbiol. 2024, 28, 133–149. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.; Dey, S.; Ram, D.K.; Das, A.P. Hexavalent chromium pollution and its sustainable management through bioremediation. Geomicrobiol. J. 2023, 41, 324–334. [Google Scholar] [CrossRef]
- Mohebi, M.; Parashkoohi, M.G.; Mohammadi, A. A comparison of biofilm and suspension methods in removing heavy metals (chromium) from industrial wastewater with Scenedesmus obliquus and Chlorella vulgaris. Results Eng. 2024, 24, 103397. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, K.; Zhao, Y.; Li, D.; Sun, X.; Lin, L.; Feng, H.; Huang, Q.; Zhu, Z. Screening of cadmium-chromium-tolerant strains and synergistic remediation of heavy metal-contaminated soil using king grass combined with highly efficient microbial strains. Sci. Total Environ. 2023, 912, 168990. [Google Scholar] [CrossRef]
- Chau, T.P.; Samdani, M.S.; Jhanani, G.K.; Sathiyamoorthi, E.; Lee, J. Metal accumulation and genetic adaptation of Oryza sativa to Cadmiun and Chromium heavy metal stress: A hydroponic and RAPD analyses. Environ. Res. 2024, 242, 117793. [Google Scholar] [CrossRef]
- Mir, I.S.; Riaz, A.; Roy, J.S.; Fréchette, J.; Morency, S.; Gomes, O.P.; Dumée, L.F.; Greener, J.; Messaddeq, Y. Removal of cadmium and chromium heavy metals from aqueous medium using composite bacterial cellulose membrane. Chem. Eng. J. 2024, 490, 151665. [Google Scholar] [CrossRef]
- Basit, A.; Andleeb, S.; Liaqat, I.; Ashraf, N.; Ali, S.; Naseer, A.; Nazir, A.; Kiyani, F. Characterization of heavy metal-associated bacteria from petroleum-contaminated soil and their resistogram and antibiogram analysis. Folia Microbiol. 2024, 69, 975–991. [Google Scholar] [CrossRef]
- Carreño-Sayago, U.F. Development of microspheres using water hyacinth (Eichhornia crassipes) for treatment of contaminated water with Cr(VI). Environ. Dev. Sustain. 2021, 23, 4735–4746. [Google Scholar] [CrossRef]
- Sayago, U.F.C.; Castro, Y.P. Development of a composite material between bacterial cellulose and E crassipes, for the treatment of water contaminated by chromium (VI). Int. J. Environ. Sci. Technol. 2022, 19, 6285–6298. [Google Scholar] [CrossRef]
- Dong, F.X.; Yan, L.; Zhou, X.H.; Huang, S.T.; Liang, J.Y.; Zhang, W.X.; Guo, Z.W.; Guo, P.R.; Qian, W.; Kong, L.J.; et al. Simultaneous adsorption of Cr (VI) and phenol by biochar-based iron oxide composites in water: Performance, kinetics and mechanism. J. Hazard. Mater. 2021, 416, 125930. [Google Scholar] [CrossRef] [PubMed]
- Sayago, U.F.C. Design and development of a biotreatment of E. crassipes for the decontamination of water with Chromium (VI). Sci. Rep. 2021, 11, 9326. [Google Scholar] [CrossRef] [PubMed]
- Carreno Sayago, U.F. Design, scaling, and development of biofilters with e crassipes for treatment of water contaminated with Cr (VI). Water 2021, 13, 1317. [Google Scholar] [CrossRef]
- Tovar, C.N.T.; López, L.C.N.; Lara, C.E.C. Breakthrough and thermodynamic adsorption of Cr (VI) y Ni (II) bioadsorption in continuous system. Sci. Tech. 2021, 26, 72–81. [Google Scholar] [CrossRef]
- Rodríguez, I.A.; González, J.C.; Zárate, M.M.; Oviedo, J.T.; Nava, M.A.; Juárez, V.M.; Castillo, F.N. Biosorption of Chromium (VI) by Cucumis melo Shell. J. Multidiscip. Eng. Sci. Technol. 2015, 2, 988–993. [Google Scholar]
- Wang, Y.; Wang, C.; Huang, X.; Zhang, Q.; Wang, T.; Guo, X. Guideline for modeling solid-liquid adsorption: Kinetics, isotherm, fixed bed, and thermodynamics. Chemosphere 2024, 349, 140736. [Google Scholar] [CrossRef]
- Yusop, M.F.M.; Abdullah, A.Z.; Ahmad, M.A. Amoxicillin adsorption from aqueous solution by Cu (II) modified lemon peel based activated carbon: Mass transfer simulation, surface area prediction and F-test on isotherm and kinetic models. Powder Technol. 2024, 438, 119589. [Google Scholar] [CrossRef]
- Sayago, U.F.C.; Ballesteros, B. Development of a treatment for water contaminated with Cr (VI) using cellulose xanthogenate from E. crassipes on a pilot scale. Sci. Rep. 2023, 13, 1970. [Google Scholar] [CrossRef]
- Chu, K.H.; Hashim, M.A.; Santos, Y.T.d.C.; Debord, J.; Harel, M.; Bollinger, J.-C. The Redlich–Peterson isotherm for aqueous phase adsorption: Pitfalls in data analysis and interpretation. Chem. Eng. Sci. 2023, 285, 119573. [Google Scholar] [CrossRef]
- Tenea, A.-G.; Dinu, C.; Rus, P.A.; Ionescu, I.A.; Gheorghe, S.; Iancu, V.I.; Vasile, G.G.; Pascu, L.F.; Chiriac, F.L. Exploring adsorption dynamics of heavy metals onto varied commercial microplastic substrates: Isothermal models and kinetics analysis. Heliyon 2024, 10, e35364. [Google Scholar] [CrossRef]
- Zain, N.S.; Mahmoud, M.; Khan, M.I.; Zafar, F.; Manzoor, S.; Akhtar, N.; El Azab, I.H.; El-Bahy, Z.M. Machine learning-assisted optimization and evaluation of methylene blue adsorption kinetics on Citrus aurantifolia leaves: Insights from isotherm and thermodynamic studies. J. Taiwan Inst. Chem. Eng. 2024, 164, 105696. [Google Scholar] [CrossRef]
- AlJaberi, F.Y. Extensive Study of Electrocoagulation-Based Adsorption Process of Real Groundwater Treatment: Isotherm Modeling, Adsorption Kinetics, and Thermodynamics. Water 2024, 16, 619. [Google Scholar] [CrossRef]
- Alshammari, N.A.H.; Alnawmasi, J.S.; Alotaibi, A.M.; Alshammari, O.A.; Abomuti, M.A.; Elsayed, N.H.; El-Bindary, A.A. Efficient adsorption of fluorescein dye from aqueous solutions by Al/Th-MOF bimetal-organic frameworks: Adsorption isotherm, kinetics, DFT computation, and optimization via Box-Behnken design. Process. Saf. Environ. Prot. 2024, 190, 353–371. [Google Scholar] [CrossRef]
- de Oliveira, J.T.; Nunes, K.G.P.; Estumano, D.C.; Féris, L.A. Applying the Bayesian Technique, Statistical Analysis, and the Maximum Adsorption Capacity in a Deterministic Way for Caffeine Removal by Adsorption: Kinetic and Isotherm Modeling. Ind. Eng. Chem. Res. 2024, 63, 1530–1545. [Google Scholar] [CrossRef]
- Sayago, U.F.C.; Gómez-Caicedo, M.I.; Suárez, Á.L.M. Design of a sustainable system for wastewater treatment and generation of biofuels based on the biomass of the aquatic plant Eichhornia crassipes. Sci. Rep. 2024, 14, 11068. [Google Scholar] [CrossRef]
- Sayago, U.F.C. Design of a sustainable development process between phytoremediation and production of bioethanol with Eichhornia crassipes. Environ. Monit. Assess. 2019, 191, 221. [Google Scholar] [CrossRef]
- Sayago, U.F.C.; Ballesteros, V.A.B. The Design of a Process for Adsorbing and Eluting Chromium (VI) Using Fixed-Bed Columns of E. crassipes with Sodium Tripolyphosphate (TPP). Water 2024, 16, 952. [Google Scholar] [CrossRef]
- Sayago, U.F.C.; Castro, Y.P.; Rivera, L.R.C.; Mariaca, A.G. Estimation of equilibrium times and maximum capacity of adsorption of heavy metals by E. crassipes (review). Environ. Monit. Assess. 2020, 192, 141. [Google Scholar] [CrossRef]
- Sayago, U.F.C.; Ballesteros, V.B.; Aguilar, A.M.L. Bacterial Cellulose-Derived Sorbents for Cr (VI) Remediation: Adsorption, Elution, and Reuse. Polymers 2024, 16, 2605. [Google Scholar] [CrossRef]
- Muthu, S.S.; Rathinamoorthy, R. Bacterial cellulose. In Bacterial Cellulose: Sustainable Material for Textiles; Muthu, S.S., Rathinamoorthy, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2021; pp. 19–60. [Google Scholar]
- Carreño Pineda, L.D.; Caicedo Mesa, L.A.; Martínez Riascos, C.A. Técnicas de fermentación y aplicaciones de la celulosa bacteriana: Una revisión. Ing. Cienc. 2012, 8, 307–335. [Google Scholar] [CrossRef]
- Tran, G.T.; Nguyen, T.T.T.; Nguyen, D.T.C.; Van Tran, T. Bacterial cellulose and composites for the treatment of water pollution: A review. Environ. Chem. Lett. 2025, 23, 707–732. [Google Scholar] [CrossRef]
- Jamshaid, A.; Hamid, A.; Muhammad, N.; Naseer, A.; Ghauri, M.; Iqbal, J.; Rafiq, S.; Shah, N.S. Cellulose-based materials for the removal of heavy metals from wastewater—An overview. ChemBioEng Rev. 2017, 4, 240–256. [Google Scholar] [CrossRef]
- Worch, E. Adsorption Technology in Water Treatment: Fundamentals, Processes, and Modeling; Walter de Gruyter: Berlin, Germany, 2012. [Google Scholar]
- Rico Iváñez, M. Diseño de un Sistema de Adsorción para la Eliminación de Fenol Presente en Disolución Acuosa. Ph.D. Thesis, Universitat Politècnica de València, Valencia, Spain, 2017. [Google Scholar]
- Sayago, U.F.C.; Ballesteros, V.B.; Lozano, A.M. Development of a Treatment System of Water with Cr (VI) Through Models Using E. crassipes Biomass with Iron Chloride. Toxics 2025, 13, 230. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Jia, S.; Wan, T.; Jia, Y.; Yang, H.; Li, J.; Yan, L.; Zhong, C. Biosynthesis of spherical Fe3O4/bacterial cellulose nanocomposites as adsorbents for heavy metal ions. Carbohydr. Polym. 2011, 86, 1558–1564. [Google Scholar] [CrossRef]
- Hamimed, S.; Abdeljelil, N.; Landoulsi, A.; Chatti, A.; Aljabali, A.A.; Barhoum, A. Bacterial cellulose nanofibers: Biosynthesis, unique properties, modification, and emerging applications. In Handbook of Nanocelluloses: Classification, Properties, Fabrication, and Emerging Applications; Springer: Berlin/Heidelberg, Germany, 2022; pp. 297–334. [Google Scholar]
- Gregory, D.A.; Tripathi, L.; Fricker, A.T.; Asare, E.; Orlando, I.; Raghavendran, V.; Roy, I. Bacterial cellulose: A smart biomaterial with diverse applications. Mater. Sci. Eng. R Rep. 2021, 145, 100623. [Google Scholar] [CrossRef]
- Sayago, U.F.C. The Design of a Sustainable Industrial Wastewater Treatment System and The Generation of Biohydrogen from E. crassipes. Polymers 2024, 16, 893. [Google Scholar] [CrossRef]
- Zeb, B.S.; Mahmood, Q.; Irshad, M.; Zafar, H.; Wang, R. Sustainable treatment of combined industrial wastewater: Synergistic phytoremediation with Eichhornia crassipes, Pistia stratiotes, and Arundo donax in biofilm wetlands. Int. J. Phytoremediation 2025, 27, 128–134. [Google Scholar] [CrossRef]
- Eid, E.M.; Shaltout, K.H.; Almuqrin, A.H.; Aloraini, D.A.; Khedher, K.M.; Taher, M.A.; Alfarhan, A.H.; Picó, Y.; Barcelo, D. Uptake prediction of nine heavy metals by Eichhornia crassipes grown in irrigation canals: A biomonitoring approach. Sci. Total. Environ. 2021, 782, 146887. [Google Scholar] [CrossRef]
- Adornado, A.P.; Soriano, A.N.; Orfiana, O.N.; Pangon, M.B.J.; Nieva, A.D. Simulated biosorption of Cd(II) and Cu(II) in single and binary metal systems by water hyacinth (Eichhornia crassipes) using aspen adsorption. ASEAN J. Chem. Eng. 2017, 16, 21–43. [Google Scholar] [CrossRef]
- Quintuña, D.J. Biosorption of CD2+ and PB2+ with Cocoa Bark: Experimentation, Mathematical Modeling and Simulation Numerical. ESPOCH Congr. Ecuadorian J. STEAM. 2021, 1, 942–952. [Google Scholar] [CrossRef]
- de Quadros Melo, D.; Vidal, C.B.; da Silva, A.L.; Raulino, G.S.C.; da Luz, A.D.; da Luz, C.; Fechine, P.B.A.; Mazzeto, S.E.; do Nascimento, R.F. Removal of toxic metal ions using modified lignocellulosic fibers as eco-friendly biosorbents: Mathematical modeling and numerical simulation. Int. J. Civ. Environ. Eng. 2015, 15, 14–25. [Google Scholar]
- Hong, M.; Yu, L.; Wang, Y.; Zhang, J.; Chen, Z.; Dong, L.; Zan, Q.; Li, R. Heavy metal adsorption with zeolites: The role of hierarchical pore architecture. Chem. Eng. J. 2019, 359, 363–372. [Google Scholar] [CrossRef]
- Bañón Juares, H. Diseño de un Sistema de Adsorción en Carbón Activado Para la Eliminación de Cromo Hexavalente en Disolución Acuosa. Ph.D. Thesis, Universitat Politècnica de València, Valencia, Spain, 2018. [Google Scholar]
- Zhang, R.; Tian, Y. Characteristics of natural biopolymers and their derivative as sorbents for chromium adsorption: A review. J. Leather Sci. Eng. 2020, 2, 24. [Google Scholar] [CrossRef]
- Liu, C.; Jin, R.-N.; Ouyang, X.-K.; Wang, Y.-G. Adsorption behavior of carboxylated cellulose nanocrystal—Polyethyleneimine composite for removal of Cr(VI) ions. Appl. Surf. Sci. 2017, 408, 77–87. [Google Scholar] [CrossRef]
- Li, Z.-Y.; Dong, J.-J.; Azi, F.; Feng, X.; Ge, Z.-W.; Yang, S.; Sun, Y.-X.; Guan, X.-Q.; Dong, M.-S. Mechanism of Cr(VI) removal by polyphenols-rich bacterial cellulose gel produced from fermented wine pomace. NPJ Clean Water 2024, 7, 21. [Google Scholar] [CrossRef]
- Kaur, J.; Sengupta, P.; Mukhopadhyay, S. Critical review of bioadsorption on modified cellulose and removal of divalent heavy metals (Cd, Pb, and Cu). Ind. Eng. Chem. Res. 2022, 61, 1921–1954. [Google Scholar] [CrossRef]
- Allah, M.A.A.H.; Alshamsi, H.A. Green synthesis of ZnO NPs using Pontederia crassipes leaf extract: Characterization, their adsorption behavior and anti-cancer property. Biomass- Convers. Biorefinery 2024, 14, 10487–10500. [Google Scholar] [CrossRef]
- Tran, T.K.; Le, Q.D.; Vo, T.M.; Diep, M.Q.; Nguyen, T.D.; Huynh, H.H.; Ly, Q.K.; Kim, N.; Kooh, M.R.R. The preparation of eco-friendly magnetic adsorbent from wild water hyacinth (Eichhornia crassipes): The Application for removing lead ions from industrial wastewater. Adsorpt. Sci. Technol. 2022, 2022, 5427851. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, K.; Yao, X.; Jiang, J.; Wang, M.; Yuan, S. Ultrasound-assisted preparation of Fe(OH)3@bacterial cellulose aerogel for efficient removal of organic contamination in water. Appl. Surf. Sci. 2023, 607, 154959. [Google Scholar] [CrossRef]
- Lamm, M.E.; Johnson, D.A.; Copenhaver, K.; Bhagia, S.; Hubbard, A.M.; Walker, C.C.; Doyle, K.; Ozcan, S. Exploiting the Properties of Non-Wood Feedstocks to Produce Tailorable Lignin-Containing Cellulose Nanofibers. Polymers 2024, 16, 2598. [Google Scholar] [CrossRef]
- Gu, Y.; Yao, F.; Gong, R.; Di, Y.; Srinivasan, V.; Hu, X.; Liu, B.; Min, D.; Lian, C.; Dong, X.; et al. A Full Green, Sustainable Paper-Based Packaging Material with High-Strength, Water Resistance, and Thermal Insulation. Polymers 2024, 17, 6. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Yang, M.; Shi, Y.; Sun, L.; Zheng, H.; Wu, M.; Gao, G.; Ma, T.; Li, G. Multifunctional bacterial cellulose-bentonite@polyethylenimine composite membranes for enhanced water treatment: Sustainable dyes and metal ions adsorption and antibacterial properties. J. Hazard. Mater. 2024, 477, 135267. [Google Scholar] [CrossRef]
- Sayago, U.F.C.; Ballesteros, V.B.; Aguilar, A.M.L. Designing, Modeling and Developing Scale Models for the Treatment of Water Contaminated with Cr (VI) through Bacterial Cellulose Biomass. Water 2024, 16, 2524. [Google Scholar] [CrossRef]
- Rattanawongwiboon, T.; Khongbunya, N.; Namvijit, K.; Lertsarawut, P.; Laksee, S.; Hemvichian, K.; Madrid, J.F.; Ummartyotin, S. Eco-Friendly Dye Adsorbent from Poly(vinyl amine) Grafted Onto Bacterial Cellulose Sheet by Using Gamma Radiation-Induced Simultaneous Grafting and Base Hydrolysis. J. Polym. Environ. 2023, 32, 3048–3060. [Google Scholar] [CrossRef]
- Sayago, U.F.C.; Ballesteros Ballesteros, V. Recent advances in the treatment of industrial wastewater from different celluloses in continuous systems. Polymers 2023, 15, 3996. [Google Scholar] [CrossRef]
- Gao, Y.; He, D.; Wu, L.; Wang, Z.; Yao, Y.; Huang, Z.-H.; Yang, H.; Wang, M.-X. Porous and ultrafine nitrogen-doped carbon nanofibers from bacterial cellulose with superior adsorption capacity for adsorption removal of low-concentration 4-chlorophenol. Chem. Eng. J. 2021, 420, 127411. [Google Scholar] [CrossRef]
- Tariq, A.; Yahaya, N.; Sajid, M. Low cost adsorbents derived from vegetables and fruits: Synthesis, properties, and applications in removal of heavy metals from water. Desalination Water Treat. 2024, 320, 100626. [Google Scholar] [CrossRef]
- Lin, S.; Huang, W.; Yang, H.; Sun, S.; Yu, J. Recycling application of waste long-root Eichhornia crassipes in the heavy metal removal using oxidized biochar derived as adsorbents. Bioresour. Technol. 2020, 314, 123749. [Google Scholar] [CrossRef]
- Lin, S.; Yang, H.; Na, Z.; Lin, K. A novel biodegradable arsenic adsorbent by immobilization of iron oxyhydroxide (FeOOH) on the root powder of long-root Eichhornia crassipes. Chemosphere 2018, 192, 258–266. [Google Scholar] [CrossRef]
- Bin-Shafique, S.; Huang, J.; Malla, S.; Mitra, M.C.; Rehman, S. Stabilization of heavy metals in fly ash and its effect on strength. Int. J. Geosynth. Ground Eng. 2022, 8, 63. [Google Scholar] [CrossRef]
- Ai, S.; Gao, K.; Yu, W.; Liu, L. Fabrication of cellulose-based carboxylate-functionalized materials via cosolubilization-crystallization for reversible Pb2+ adsorption. Environ. Technol. Innov. 2025, 37, 104058. [Google Scholar] [CrossRef]
- Roosen, J.; Binnemans, K. Adsorption and chromatographic separation of rare earths with EDTA- and DTPA-functionalized chitosan biopolymers. J. Mater. Chem. A 2014, 2, 1530–1540. [Google Scholar] [CrossRef]
- Mittal, S.; Kumar, V.; Sharma, R.K. Solid phase extraction studies on cellulose based chelating resin for separation, pre-concentration and estimation of Cu2+and Ni2+. J. Indian Chem. Soc. 2022, 99, 100481. [Google Scholar] [CrossRef]
- Khan, T.A.; Mukhlif, A.A.; Khan, E.A. Uptake of Cu2+ and Zn2+ from simulated wastewater using muskmelon peel biochar: Isotherm and kinetic studies. Egypt. J. Basic Appl. Sci. 2017, 4, 236–248. [Google Scholar] [CrossRef]
- Hossain, M.D.F.; Akther, N.; Zhou, Y. Recent advancements in graphene adsorbents for wastewater treatment: Current status and challenges. Chin. Chem. Lett. 2020, 31, 2525–2538. [Google Scholar] [CrossRef]
- Amuda, O.; Giwa, A.; Bello, I. Removal of heavy metal from industrial wastewater using modified activated coconut shell carbon. Biochem. Eng. J. 2007, 36, 174–181. [Google Scholar] [CrossRef]
- Doyo, A.N.; Kumar, R.; Barakat, M. Recent advances in cellulose, chitosan, and alginate based biopolymeric composites for adsorption of heavy metals from wastewater. J. Taiwan Inst. Chem. Eng. 2023, 151, 105095. [Google Scholar] [CrossRef]
- Ricou, P.; Lécuyer, I.; Le Cloirec, P. Removal of CU2+, ZN2+ and PB2+ by adsorption onto fly ash and fly ash/lime mixing. Water Sci. Technol. 1999, 39, 239–247. [Google Scholar] [CrossRef]
- Ngah, W.S.W.; Kamari, A.; Fatinathan, S.; Ng, P.W. Adsorption of chromium from aqueous solution using chitosan beads. Adsorption 2006, 12, 249–257. [Google Scholar] [CrossRef]
- Hosseini, H.; Mousavi, S.M. Bacterial cellulose/polyaniline nanocomposite aerogels as novel bioadsorbents for removal of hexavalent chromium: Experimental and simulation study. J. Clean. Prod. 2021, 278, 123817. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Ye, M.; Zhang, X.; Zhang, H.; Wang, G.; Zhang, Y. Hierarchically porous poly(amidoxime)/bacterial cellulose composite aerogel for highly efficient scavenging of heavy metals. J. Colloid Interface Sci. 2021, 600, 752–763. [Google Scholar] [CrossRef] [PubMed]
- Peiravi-Rivash, O.; Mashreghi, M.; Baigenzhenov, O.; Hosseini-Bandegharaei, A. Producing bacterial nano-cellulose and keratin from wastes to synthesize keratin/cellulose nanobiocomposite for removal of dyes and heavy metal ions from waters and wastewaters. Colloids Surfaces A 2023, 656, 130355. [Google Scholar] [CrossRef]
- Abu-Danso, E.; Peräniemi, S.; Leiviskä, T.; Kim, T.; Tripathi, K.M.; Bhatnagar, A. Synthesis of clay-cellulose biocomposite for the removal of toxic metal ions from aqueous medium. J. Hazard. Mater. 2020, 381, 120871. [Google Scholar] [CrossRef] [PubMed]
- Croitoru, A.-M.; Ficai, A.; Ficai, D.; Trusca, R.; Dolete, G.; Andronescu, E.; Turculet, S.C. Chitosan/graphene oxide nanocomposite membranes as adsorbents with applications in water purification. Materials 2020, 13, 1687. [Google Scholar] [CrossRef]
- Peralta, M.E.; Nisticò, R.; Franzoso, F.; Magnacca, G.; Fernandez, L.; Parolo, M.E.; León, E.G.; Carlos, L. Highly efficient removal of heavy metals from waters by magnetic chitosan-based composite. Adsorption 2019, 25, 1337–1347. [Google Scholar] [CrossRef]






| Model Isotherm | |||
| Capacity of adsorption | (1) | = | qe: (mg/g) adsorption capacity at equilibrium; V: volume (mL); m: biomass (g); Ci: initial concentration mg/L; Cf/L: final concentration. |
| Freundlich equation | (2) | qe: (mg/g) adsorption capacity; Cs: (mg/L) equilibrium concentration of adsorbents in solution; Kf: (L/mg) constant for Freundlich. | |
| Langmuir equation | (3) | = | qe: (mg/g) adsorption capacity at equilibrium; Ce: (mg/L) equilibrium concentration of adsorbents in solutio; qm: (mg/g) maximum adsorption capacity; Kl: (mg/g) Langmuir constant. |
| Model Kinetic | |||
| Pseudo first order | (4) | qt and qe (mg/g) are uptake amount of pollutions at equilibrium and time t (h); K1(min−1) is adsorption rate constant of pseudo-first order. | |
| Pseudo Second order | (5) | qt and qe (mg/g) are uptake amount of pollution at equilibrium and time t (h); K2 second-order model. |
| Biomass | Biomass m (g) | Volume (mL) | Density Biomass | Particle (mg) | Volume Particle (mm) | ||
|---|---|---|---|---|---|---|---|
| BC | 0.3 | 0.48 | 0.62 | 0.01 | 0.005 | 1.9 | 0.68 |
| EC | 0.3 | 0.35 | 0.85 | 0.01 | 0.005 | 2 | 0.57 |
| EC(25)+BC(75) | 0.3 | 0.46 | 0.65 | 0.01 | 0.005 | 2 | 0.67 |
| EC(50)+BC(50) | 0.3 | 0.40 | 0.75 | 0.01 | 0.005 | 1.9 | 0.62 |
| EC(75)+BC(25) | 0.3 | 0.38 | 0.78 | 0.01 | 0.005 | 2 | 0.60 |
| Element | Weight | Percentages % |
|---|---|---|
| Carbon | 49.2 | 48.7 |
| Oxygen | 38.2 | 37.2 |
| Chromium | 4.2 | 4.1 |
| Element | Weight | Percentages % |
|---|---|---|
| Carbon | 45.1 | 44.1 |
| Oxygen | 41.3 | 40.2 |
| Chromium | 10.0 | 9.1 |
| Experiment 600 mg/L | EC(75)+BC(25) | EC(50)+BC(50) | EC(25)+BC(75) |
|---|---|---|---|
| Time of rupture (min) | 120 | 160 | 200 |
| Capacity of adsorptions (mg/g) | 99 ± 6 | 116 ± 5 | 123 ± 6 |
| Isotherm | Constante | R2 | |
|---|---|---|---|
| EC(25)+BC(75) | Langmuir | Kl = 1.1; qms; 123 | 0.99 |
| Freundlich | Kf = 0.16 | 0.91 | |
| EC(50)+BC(50) | Langmuir | Kl = 0.9; qms; 117 | 0.99 |
| Freundlich | Kf = 0.14 | 0.91 | |
| EC(75)+BC(25) | Langmuir | Kl = 0.7; qms; 99 | 0.99 |
| Freundlich | Kf = 0.12 | 0.91 |
| Pseudo First Order | Pseudo Second Order | |||||
|---|---|---|---|---|---|---|
| Samples | qt (mg/g) | K1 (min) | R2 | qt (mg/g) | K2 g/(mg*min) | R2 |
| BC | 140 | 0.93 | 0.94 | 141 | 1.22 | 0.99 |
| BC(75)+EC(25) | 123 | 0.94 | 0.96 | 124 | 1.20 | 0.98 |
| BC(50)+EC(50) | 117 | 0.94 | 0.97 | 116 | 1.16 | 0.96 |
| BC(25)+EC(75) | 101 | 0.95 | 0.99 | 101 | 1.01 | 0.9 |
| EC | 45 | 0.99 | 0.99 | 46 | 0.75 | 0.9 |
| Cost | BC | EC(25)+BC(75) | EC(50)+BC(50) | EC(75)+BC(25) | EC |
|---|---|---|---|---|---|
| Capacity of adsorptions (g Cr/kg material) Cost (USD) 1 kg material | 500 ± 9 20 | 520 ± 11 18 | 600 ± 11 14 | 420 ± 10 12 | 178 ± 14 7 |
| g Cr/(USD) | 25 | 28 | 42 | 35 | 25 |
| Contaminant | Capacities of Adsorption mg/g | Cost (USD) 1 kg Material | g /(USD) | Reference | |
|---|---|---|---|---|---|
| EC(50)+BC(50) | Cr (VI) | 600 | 14 | 42 | |
| Musk melon | Cu (II) | 120 | 10 | 12 | [69] |
| Banana peel | Cu (II) | 25 | 5 | 5 | [70] |
| Coconut shell carbon | Zn (II) | 45 | 10 | 5 | [71] |
| Alginate | Cr (VI) | 238 | 25 | 10 | [72] |
| Fly ash | Cu (II) | 207 | 20 | 10 | [73] |
| Chitosan beads | Cr (VI) | 76 | 5 | 15 | [74] |
| Bacterial cellulose/polyaniline | Cr (VI) | 755 | 25 | 30 | [75] |
| Poly(amidoxime)/bacterial | Pb (II) | 1500 | 60 | 25 | [76] |
| keratin from wastes to synthesize keratin/cellulose nanobiocomposite | Cd (II) | 695 | 60 | 12 | [77] |
| Clay_cellulose biocomposite | Pb (II) | 389 | 30 | 13 | [78] |
| BC/EDTA/ GO nano_composite membrane | Pb (II) | 970 | 45 | 22 | [79] |
| Magnetic chitosan composite (MCC) | Pb (II) | 220 | 25 | 9 | [80] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sayago, U.F.C.; Ballesteros, V.B.; Lozano, A.M. Design of Biomass Adsorbents Based on Bacterial Cellulose and E. crassipes for the Removal of Cr (VI). Polymers 2025, 17, 1712. https://doi.org/10.3390/polym17121712
Sayago UFC, Ballesteros VB, Lozano AM. Design of Biomass Adsorbents Based on Bacterial Cellulose and E. crassipes for the Removal of Cr (VI). Polymers. 2025; 17(12):1712. https://doi.org/10.3390/polym17121712
Chicago/Turabian StyleSayago, Uriel Fernando Carreño, Vladimir Ballesteros Ballesteros, and Angelica María Lozano. 2025. "Design of Biomass Adsorbents Based on Bacterial Cellulose and E. crassipes for the Removal of Cr (VI)" Polymers 17, no. 12: 1712. https://doi.org/10.3390/polym17121712
APA StyleSayago, U. F. C., Ballesteros, V. B., & Lozano, A. M. (2025). Design of Biomass Adsorbents Based on Bacterial Cellulose and E. crassipes for the Removal of Cr (VI). Polymers, 17(12), 1712. https://doi.org/10.3390/polym17121712








