Applications of Fluorescence Technology for Rapid Identification of Marine Plastic Pollution
Abstract
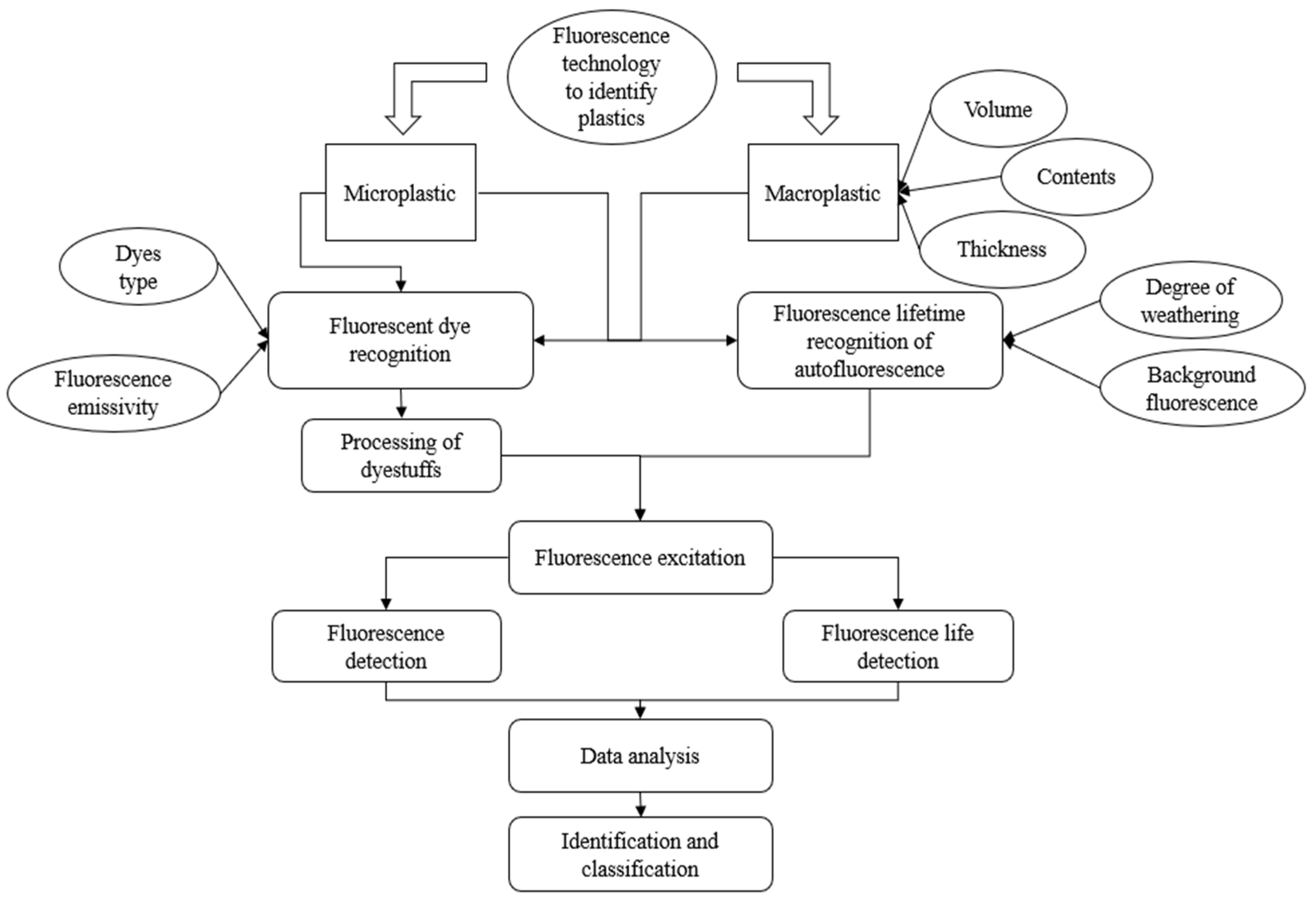
1. Introduction
- (1)
- The foundational principles of fluorescence technology.
- (2)
- Fluorescence spectra used for plastics characterization, including characteristic spectral ranges for various types of plastics.
- (3)
- Fluorescent dyes and staining methods for microplastic detection.
- (4)
- Fluorescence lifetimes of various types of plastics under different backgrounds.
- (5)
- The feasibility of using fluorescence technology for identifying large plastic items and influencing factors.
2. Fluorescent Technology and Fluorescent Substances
2.1. Principles and Types of Fluorescence Spectroscopy
2.2. Comparison of Fluorescence-Based Imaging Technique
2.3. Evaluation Metrics for Fluorescence Spectroscopy
2.4. Characterization of Fluorophores
3. Plastic Identification by Fluorescence Technology
3.1. Microplastic Recognition by Fluorescent Dyes
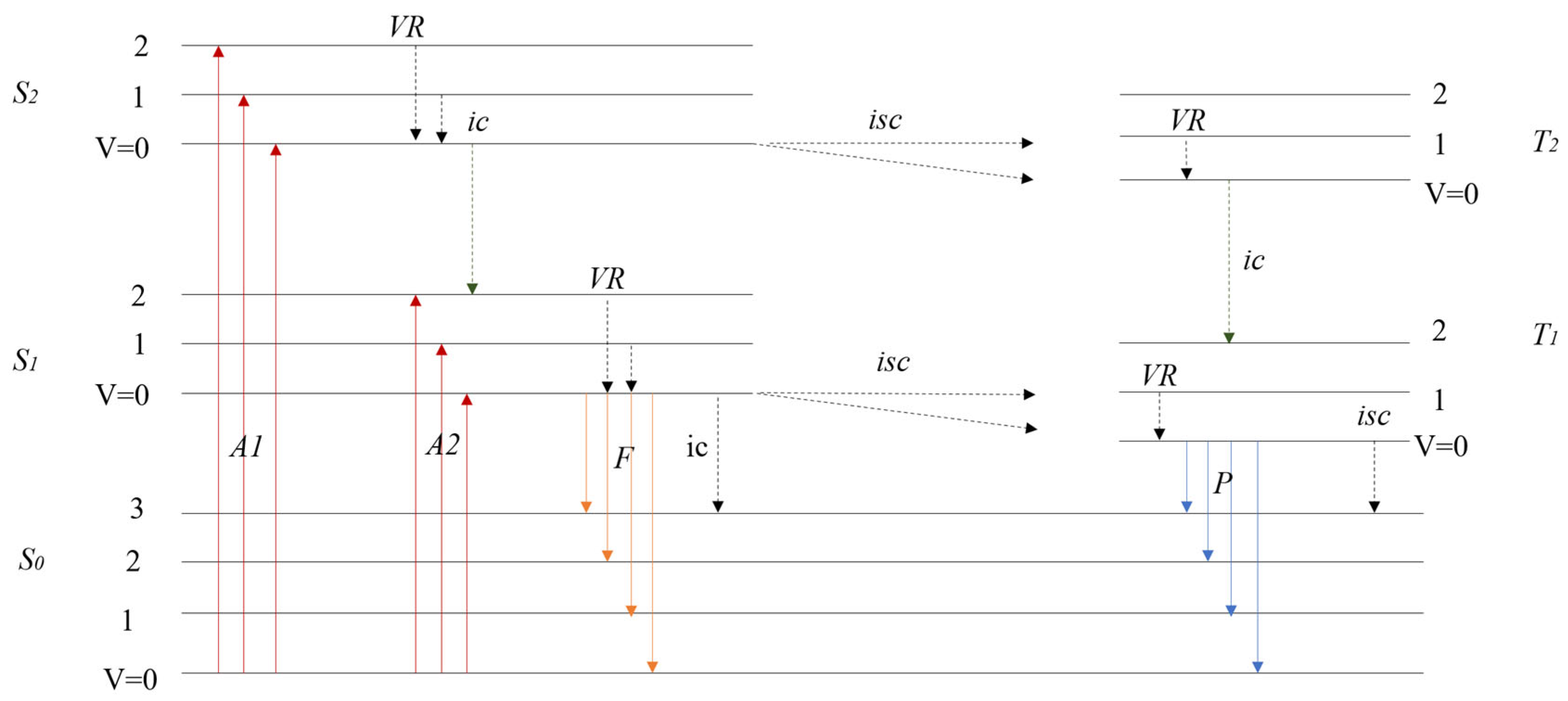
3.2. Plastic Identification of Fluorescence Lifetimes for Autofluorescence
3.2.1. Identification of Microplastics with Fluorescence Lifetime
3.2.2. Identification of Larger Plastics by Fluorescence Technology
4. Prospects
Funding
Acknowledgments
Conflicts of Interest
References
- Real, L.E.P. Plastics Statistics: Production, Recycling, and Market Data. In Recycled Materials for Construction Applications: Plastic Products and Composites; Real, L.E.P., Ed.; Springer International Publishing: Cham, Switzerland, 2023; pp. 103–113. [Google Scholar]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef]
- Soares, S.; Serralha, F.; Paz, M.C.; Carriço, N.; Galatanu, S.-V. Unveiling the data: An analysis of plastic waste with emphasis on the countries of the E3UDRES2 alliance. Heliyon 2024, 10, e28375. [Google Scholar] [CrossRef]
- Gasperi, J.; Dris, R.; Bonin, T.; Rocher, V.; Tassin, B. Assessment of floating plastic debris in surface water along the Seine River. Environ. Pollut. 2014, 195, 163–166. [Google Scholar] [CrossRef]
- Canals, M.; Pham, C.K.; Bergmann, M.; Gutow, L.; Hanke, G.; van Sebille, E.; Angiolillo, M.; Buhl-Mortensen, L.; Cau, A.; Ioakeimidis, C.; et al. The quest for seafloor macrolitter: A critical review of background knowledge, current methods and future prospects. Environ. Res. Lett. 2021, 16, 023001. [Google Scholar] [CrossRef]
- Gillibert, R.; Balakrishnan, G.; Deshoules, Q.; Tardivel, M.; Magazzù, A.; Donato, M.G.; Maragò, O.M.; Lamy de La Chapelle, M.; Colas, F.; Lagarde, F.; et al. Raman tweezers for small microplastics and nanoplastics identification in seawater. Environ. Sci. Technol. 2019, 53, 9003–9013. [Google Scholar] [CrossRef]
- Morales-Caselles, C.; Viejo, J.; Martí, E.; González-Fernández, D.; Pragnell-Raasch, H.; González-Gordillo, J.I.; Montero, E.; Arroyo, G.M.; Hanke, G.; Salvo, V.S.; et al. An inshore–offshore sorting system revealed from global classification of ocean litter. Nat. Sustain. 2021, 4, 484–493. [Google Scholar] [CrossRef]
- Chiba, S.; Saito, H.; Fletcher, R.; Yogi, T.; Kayo, M.; Miyagi, S.; Ogido, M.; Fujikura, K. Human footprint in the abyss: 30 year records of deep-sea plastic debris. Mar. Policy 2018, 96, 204–212. [Google Scholar] [CrossRef]
- Lamb, J.B.; Willis, B.L.; Fiorenza, E.A.; Couch, C.S.; Howard, R.; Rader, D.N.; True, J.D.; Kelly, L.A.; Ahmad, A.; Jompa, J.; et al. Plastic waste associated with disease on coral reefs. Science 2018, 359, 460–462. [Google Scholar] [CrossRef]
- Schuyler, Q.A.; Wilcox, C.; Townsend, K.A.; Wedemeyer-Strombel, K.R.; Balazs, G.; van Sebille, E.; Hardesty, B.D. Risk analysis reveals global hotspots for marine debris ingestion by sea turtles. Glob. Change Biol. 2016, 22, 567–576. [Google Scholar] [CrossRef]
- Mato, Y.; Isobe, T.; Takada, H.; Kanehiro, H.; Ohtake, C.; Kaminuma, T. Plastic resin pellets as a transport medium for toxic chemicals in the marine environment. Environ. Sci. Technol. 2001, 35, 318–324. [Google Scholar] [CrossRef]
- Setälä, O.; Fleming-Lehtinen, V.; Lehtiniemi, M. Ingestion and transfer of microplastics in the planktonic food web. Environ. Pollut. 2014, 185, 77–83. [Google Scholar] [CrossRef]
- Wright, S.L.; Thompson, R.C.; Galloway, T.S. The physical impacts of microplastics on marine organisms: A review. Environ. Pollut. 2013, 178, 483–492. [Google Scholar] [CrossRef]
- Welden, N.A.C.; Cowie, P.R. Long-term microplastic retention causes reduced body condition in the langoustine, Nephrops norvegicus. Environ. Pollut. 2016, 218, 895–900. [Google Scholar] [CrossRef]
- Gray, A.D.; Weinstein, J.E. Size-and shape-dependent effects of microplastic particles on adult daggerblade grass shrimp (Palaemonetes pugio). Environ. Toxicol. Chem. 2017, 36, 3074–3080. [Google Scholar] [CrossRef]
- Barboza, L.; Vethaak, A.; Lavorante, B.; Lundebye, A.-K.; Guilhermino, L. Marine microplastic debris: An emerging issue for food security, food safety and human health. Mar. Pollut. Bull. 2018, 133, 336–348. [Google Scholar] [CrossRef]
- OECD. Global Plastics Outlook; OECD: Paris, France, 2022. [Google Scholar]
- Lebreton, L.; Slat, B.; Ferrari, F.; Sainte-Rose, B.; Aitken, J.; Marthouse, R.; Hajbane, S.; Cunsolo, S.; Schwarz, A.; Levivier, A.; et al. Evidence that the Great Pacific Garbage Patch is rapidly accumulating plastic. Sci. Rep. 2018, 8, 4666. [Google Scholar] [CrossRef]
- Law, K.L.; Thompson, R.C. Microplastics in the seas. Science 2014, 345, 144–145. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- Lee, H.; Shim, W.J.; Kwon, J.-H. Sorption capacity of plastic debris for hydrophobic organic chemicals. Sci. Total. Environ. 2014, 470–471, 1545–1552. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef] [PubMed]
- Desforges, J.-P.W.; Galbraith, M.; Dangerfield, N.; Ross, P.S. Widespread distribution of microplastics in subsurface seawater in the NE Pacific Ocean. Mar. Pollut. Bull. 2014, 79, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Porz, L.; Yilmaz, R.; Wallmann, K.; Spiegel, T.; Neumann, A.; Holtappels, M.; Kasten, S.; Kuhlmann, J.; Ziebarth, N.; et al. Long-term carbon storage in shelf sea sediments reduced by intensive bottom trawling. Nat. Geosci. 2024, 17, 1268–1276. [Google Scholar] [CrossRef]
- Harrison, J.P.; Ojeda, J.J.; Romero-González, M.E. The applicability of reflectance micro-Fourier-transform infrared spectroscopy for the detection of synthetic microplastics in marine sediments. Sci. Total. Environ. 2012, 416, 455–463. [Google Scholar] [CrossRef]
- Vianello, A.; Boldrin, A.; Guerriero, P.; Moschino, V.; Rella, R.; Sturaro, A.; Da Ros, L. Microplastic particles in sediments of Lagoon of Venice, Italy: First observations on occurrence, spatial patterns and identification. Estuar. Coast. Shelf Sci. 2013, 130, 54–61. [Google Scholar] [CrossRef]
- Van Cauwenberghe, L.; Vanreusel, A.; Mees, J.; Janssen, C.R. Microplastic pollution in deep-sea sediments. Environ. Pollut. 2013, 182, 495–499. [Google Scholar] [CrossRef]
- Van Cauwenberghe, L.; Claessens, M.; Vandegehuchte, M.B.; Mees, J.; Janssen, C.R. Assessment of marine debris on the Belgian Continental Shelf. Mar. Pollut. Bull. 2013, 73, 161–169. [Google Scholar] [CrossRef]
- Calvert, R.; McAllister, M.; Whittaker, C.; Raby, A.; Borthwick, A.; van den Bremer, T. The increased wave-induced drift of floating marine litter: A mechanism for the increased wave-induced drift of floating marine litter. arXiv 2021, arXiv:2102.09836. [Google Scholar]
- Suara, K.; Khanarmuei, M.; Ghosh, A.; Yu, Y.; Zhang, H.; Soomere, T.; Brown, R.J. Material and debris transport patterns in Moreton Bay, Australia: The influence of Lagrangian coherent structures. Sci. Total. Environ. 2020, 721, 137715. [Google Scholar] [CrossRef] [PubMed]
- Kane, I.A.; Clare, M.A.; Miramontes, E.; Wogelius, R.; Rothwell, J.J.; Garreau, P.; Pohl, F. Seafloor microplastic hotspots controlled by deep-sea circulation. Science 2020, 368, 1140–1145. [Google Scholar] [CrossRef]
- Vázquez-Rowe, I.; Ita-Nagy, D.; Kahhat, R. Microplastics in fisheries and aquaculture: Implications to food sustainability and safety. Curr. Opin. Green Sustain. Chem. 2021, 29, 100464. [Google Scholar] [CrossRef]
- Dümichen, E.; Barthel, A.-K.; Braun, U.; Bannick, C.G.; Brand, K.; Jekel, M.; Senz, R. Analysis of polyethylene microplastics in environmental samples, using a thermal decomposition method. Water Res. 2015, 85, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Fries, E.; Dekiff, J.H.; Willmeyer, J.; Nuelle, M.-T.; Ebert, M.; Remy, D. Identification of polymer types and additives in marine microplastic particles using pyrolysis-GC/MS and scanning electron microscopy. Environ. Sci. Process. Impacts 2013, 15, 1949–1956. [Google Scholar] [CrossRef]
- Shim, W.J.; Hong, S.H.; Eo, S.E. Identification methods in microplastic analysis: A review. Anal. Methods 2017, 9, 1384–1391. [Google Scholar] [CrossRef]
- Huppertsberg, S.; Knepper, T.P. Instrumental analysis of microplastics—Benefits and challenges. Anal. Bioanal. Chem. 2018, 410, 6343–6352. [Google Scholar] [CrossRef] [PubMed]
- Wolf, M.; Riess, M.; Heitmann, D.; Schreiner, M.; Thoma, H.; Vierle, O.; van Eldik, R. Application of a purge and trap TDS-GC/MS procedure for the determination of emissions from flame retarded polymers. Chemosphere 2000, 41, 693–699. [Google Scholar] [CrossRef]
- Sequeiros, A.; Labidi, J. Characterization and determination of the S/G ratio via Py-GC/MS of agricultural and industrial residues. Ind. Crop. Prod. 2017, 97, 469–476. [Google Scholar] [CrossRef]
- Isah, U.A.; Rashid, M.I.; Lee, S.; Kiman, S.; Iyodo, H.M. Correlations of coal rank with the derived Fourier Transform Infra-Red (FTIR) spectroscopy structural parameters: A review. Infrared Phys. Technol. 2024, 141, 105456. [Google Scholar] [CrossRef]
- Käppler, A.; Windrich, F.; Löder, M.G.; Malanin, M.; Fischer, D.; Labrenz, M.; Eichhorn, K.-J.; Voit, B. Identification of microplastics by FTIR and Raman microscopy: A novel silicon filter substrate opens the important spectral range below 1300 cm−1 for FTIR transmission measurements. Anal. Bioanal. Chem. 2015, 407, 6791–6801. [Google Scholar] [CrossRef]
- Kovach, R.; Peterson, W. The measurement of sensitivity in fluorescence spectroscopy. Am. Lab. 1994, 26, 32G. [Google Scholar]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Liu, S.; Shang, E.; Liu, J.; Wang, Y.; Bolan, N.; Kirkham, M.; Li, Y. What have we known so far for fluorescence staining and quantification of microplastics: A tutorial review. Front. Environ. Sci. Eng. 2022, 16, 8. [Google Scholar] [CrossRef]
- Xue, J.; Pu, Y.; Smith, J.; Gao, X.; Wang, C.; Wu, B. Identifying metastatic ability of prostate cancer cell lines using native fluorescence spectroscopy and machine learning methods. Sci. Rep. 2021, 11, 2282. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Andrade, G.F.; Brolo, A.G. A review on recent advances in the applications of surface-enhanced Raman scattering in analytical chemistry. Anal. Chim. Acta 2020, 1097, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Kassem, A.; Abbas, L.; Coutinho, O.; Opara, S.; Najaf, H.; Kasperek, D.; Pokhrel, K.; Li, X.; Tiquia-Arashiro, S. Applications of Fourier Transform-Infrared spectroscopy in microbial cell biology and environmental microbiology: Advances, challenges, and future perspectives. Front. Microbiol. 2023, 14, 1304081. [Google Scholar]
- Albani, J.R. Principles and Applications of Fluorescence Spectroscopy; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Li, Z.; Xie, Y.; Zeng, Y.; Zhang, Z.; Song, Y.; Hong, Z.; Ma, L.; He, M.; Ma, H.; Cui, F. Plastic leachates lead to long-term toxicity in fungi and promote biodegradation of heterocyclic dye. Sci. Total. Environ. 2022, 806, 150538. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Hong, S.; Hur, J. Copper-binding properties of microplastic-derived dissolved organic matter revealed by fluorescence spectroscopy and two-dimensional correlation spectroscopy. Water Res. 2021, 190, 116775. [Google Scholar] [CrossRef]
- Akoueson, F.; Chbib, C.; Monchy, S.; Paul-Pont, I.; Doyen, P.; Dehaut, A.; Duflos, G. Identification and quantification of plastic additives using pyrolysis-GC/MS: A review. Sci. Total. Environ. 2021, 773, 145073. [Google Scholar] [CrossRef]
- Valeur, B.; Berberan-Santos, M.N. Molecular Fluorescence: Principles and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Lakowicz, J.R. Fluorescence spectroscopy; principles and application to biological macromolecules. In New Comprehensive Biochemistry; Elsevier: Amsterdam, The Netherlands, 1985; Volume 11, pp. 1–26. [Google Scholar]
- Harling, J. The attainment of high potentials by the use of radium. Proc. R. Soc. A Math. Phys. Eng. Sci. 1913, 88, 471–476. [Google Scholar]
- Yang, T.; Fan, X.; Zhou, J. Total Reflection X-Ray Fluorescence Spectroscopy. Open Access Libr. J. 2020, 7, 1. [Google Scholar] [CrossRef]
- Zacharioudaki, D.E.; Fitilis, I.; Kotti, M. Review of Fluorescence Spectroscopy in Environmental Quality Applications. Molecules 2022, 27, 4801. [Google Scholar] [CrossRef]
- Chen, R.F. Fluorescence Quantum Yields of Tryptophan and Tyrosine. Anal. Lett. 1967, 1, 35–42. [Google Scholar] [CrossRef]
- Locquet, N.; Aït-Kaddour, A.; Cordella, C.B.Y. 3D Fluorescence Spectroscopy and Its Applications. In Encyclopedia of Analytical Chemistry; Wiley: Hoboken, NJ, USA, 2018; pp. 1–39. [Google Scholar]
- Senga, Y.; Minami, S. Excitation-Emission Matrix Scanning Spectrofluorometer. Appl. Spectrosc. 1991, 45, 1721–1725. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, Z.; Xiong, K.; Lyu, X.; Hu, C.; Wang, X.; Cheng, K. Analysis of the Three-Dimensional Fluorescence Spectroscopy Characteristics of Dissolved Organic Matter in Groundwater from a Subtropical Cave in Dry Season—Daxiao Cave in South China Karst. Water 2021, 13, 3574. [Google Scholar] [CrossRef]
- Li, L.; Wang, Y.; Zhang, W.; Yu, S.; Wang, X.; Gao, N. New advances in fluorescence excitation-emission matrix spectroscopy for the characterization of dissolved organic matter in drinking water treatment: A review. Chem. Eng. J. 2020, 381, 122676. [Google Scholar] [CrossRef]
- Albani, J.R. Chapter 2—Fluorescence: Principles and Observables. In Structure and Dynamics of Macromolecules: Absorption and Fluorescence Studies; Albani, J.R., Ed.; Elsevier Science: Amsterdam, The Netherlands, 2004; pp. 55–98. [Google Scholar]
- Marshall, J.; Johnsen, S. Fluorescence as a means of colour signal enhancement. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017, 372, 20160335. [Google Scholar] [CrossRef]
- Yang, R.; Dong, G.; Sun, X.; Yang, Y.; Yu, Y.; Liu, H.; Zhang, W. Feasibility of the simultaneous determination of polycyclic aromatic hydrocarbons based on two-dimensional fluorescence correlation spectroscopy. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2018, 190, 342–346. [Google Scholar] [CrossRef]
- Lythgoe, J.N. The Ecology of Vision; Oxford University Press: New York, NY, USA, 1979. [Google Scholar]
- Shivani, K.; Padhy, A.A.; Sahoo, S.; Kumari, V.; Mishra, P. Chapter 14—Spectroscopic methods to detect and analyze protein oligomerization, aggregation, and fibrillation. In Advanced Spectroscopic Methods to Study Biomolecular Structure and Dynamics; Saudagar, P., Tripathi, T., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 415–458. [Google Scholar]
- Monteleone, A. Development of a Novel Method for the Identification and Differentiation of Micro-and Nanoplastics Based on Time Resolved Fluorescence Processes. 2021. Available online: https://www.researchgate.net/publication/354598301_Development_of_a_novel_method_for_the_identification_and_differentiation_of_micro-_and_nanoplastics_based_on_time_resolved_fluorescence_processes (accessed on 11 May 2025).
- Vo-Dinh, T. Multicomponent analysis by synchronous luminescence spectrometry. Anal. Chem. 1978, 50, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Wheatley, L.; Levendis, Y.A.; Vouros, P. Exploratory study on the combustion and PAH emissions of selected municipal waste plastics. Environ. Sci. Technol. 1993, 27, 2885–2895. [Google Scholar] [CrossRef]
- Giessing, A.M.B.; Mayer, L.M.; Forbes, T.L. Synchronous fluorescence spectrometry of 1-hydroxypyrene: A rapid screening method for identification of PAH exposure in tissue from marine polychaetes. Mar. Environ. Res. 2003, 56, 599–615. [Google Scholar] [CrossRef]
- Alghamdi, M.; Abdallah, M.A.-E.; Harrad, S. The utility of X-Ray fluorescence spectrometry as a tool for monitoring compliance with limits on concentrations of halogenated flame retardants in waste polymers: A critical review. Emerg. Contam. 2022, 8, 9–20. [Google Scholar] [CrossRef]
- Bezati, F.; Froelich, D.; Massardier, V.; Maris, E. Addition of tracers into the polypropylene in view of automatic sorting of plastic wastes using X-ray fluorescence spectrometry. Waste Manag. 2010, 30, 591–596. [Google Scholar] [CrossRef]
- Turner, A. In situ elemental characterisation of marine microplastics by portable XRF. Mar. Pollut. Bull. 2017, 124, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Abubaker, S.A.; Taha, A.H. Identification and characterization of different types of plastics wastes using X-ray diffraction and X-ray fluorescence techniques. ARO-Sci. J. Koya Univ. 2021, 9, 22–25. [Google Scholar] [CrossRef]
- Li, X.; Liu, J.; Meng, H.; Huang, Y.; Xie, W.; Liang, Y.; Wang, J. Application of fluorescent tracing technology for investigating the effect of microplastics and nanoplastics on biological organisms. Trac Trends Anal. Chem. 2024, 181, 118039. [Google Scholar] [CrossRef]
- Zhou, F.; Wang, X.; Wang, G.; Zuo, Y. A rapid method for detecting microplastics based on fluorescence lifetime imaging technology (FLIM). Toxics 2022, 10, 118. [Google Scholar] [CrossRef]
- Lichtman, J.W.; Conchello, J.-A. Fluorescence microscopy. Nat. Methods 2005, 2, 910–919. [Google Scholar] [CrossRef]
- Becker, W. Fluorescence lifetime imaging–techniques and applications. J. Microsc. 2012, 247, 119–136. [Google Scholar] [CrossRef]
- Rees, P.; Summers, H.D.; Filby, A.; Carpenter, A.E.; Doan, M. Imaging flow cytometry. Nat. Rev. Methods Primers 2022, 2, 86. [Google Scholar] [CrossRef]
- Erdbrügger, U.; Rudy, C.K.; Etter, M.E.; Dryden, K.A.; Yeager, M.; Klibanov, A.L.; Lannigan, J. Imaging flow cytometry elucidates limitations of microparticle analysis by conventional flow cytometry. Cytom. Part A 2014, 85, 756–770. [Google Scholar] [CrossRef]
- Helmchen, F.; Denk, W. Deep tissue two-photon microscopy. Nat. Methods 2005, 2, 932–940. [Google Scholar] [CrossRef]
- Rubart, M. Two-photon microscopy of cells and tissue. Circ. Res. 2004, 95, 1154–1166. [Google Scholar] [CrossRef]
- Senesi, N.; D’Orazio, V. Fluorescence Spectroscopy. In Encyclopedia of Soils in the Environment; Hillel, D., Ed.; Elsevier: Oxford, UK, 2005; pp. 35–52. [Google Scholar]
- Korak, J.A.; Dotson, A.D.; Summers, R.S.; Rosario-Ortiz, F.L. Critical analysis of commonly used fluorescence metrics to characterize dissolved organic matter. Water Res. 2014, 49, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R.; Weber, G. Quenching of fluorescence by oxygen. Probe for structural fluctuations in macromolecules. Biochemistry 1973, 12, 4161–4170. [Google Scholar] [CrossRef]
- Wilkinson, F. Quenching of electronically excited states by molecular oxygen in fluid solution. Pure Appl. Chem. 1997, 69, 851–856. [Google Scholar] [CrossRef]
- Froehlich, P.M.; Nelson, K. Fluorescence quenching of indoles by amides. J. Phys. Chem. 1978, 82, 2401–2403. [Google Scholar] [CrossRef]
- Wang, G.; Wang, A.-J.; Hu, K.-S. Tryptophan fluorescence quenching by alkaline earth metal cations in deionized bacteriorhodopsin. J. Photochem. Photobiol. B Biol. 2000, 59, 38–41. [Google Scholar] [CrossRef] [PubMed]
- Masilamani, V.; Ghaithan, H.M.; Aljaafreh, M.J.; Ahmed, A.; al Thagafi, R.; Prasad, S.; Alsalhi, M.S. Using a Spectrofluorometer for Resonance Raman Spectra of Organic Molecules. J. Spectrosc. 2017, 2017, 4289830. [Google Scholar] [CrossRef]
- Eilers, P.H.C.; Kroonenberg, P.M. Modeling and correction of Raman and Rayleigh scatter in fluorescence landscapes. Chemom. Intell. Lab. Syst. 2014, 130, 1–5. [Google Scholar] [CrossRef]
- Clarke, R.; Oprysa, A. Fluorescence and Light Scattering. J. Chem. Educ. 2004, 81, 705. [Google Scholar] [CrossRef]
- Servaas, P.C.; Van Dijk, H.K.; Snoeck, T.L.; Stufkens, D.J.; Oskam, A. Relationship between the emission spectra and resonance Raman excitation profiles of W(CO)4(.alpha.-diimine) complexes. Inorg. Chem. 1985, 24, 4494–4498. [Google Scholar] [CrossRef]
- Myers, A.B. Relating absorption, emission, and resonance Raman spectra with electron transfer rates in photoinduced charge transfer systems: Promises and pitfalls. Chem. Phys. 1994, 180, 215–230. [Google Scholar] [CrossRef]
- Panchompoo, J.; Aldous, L.; Baker, M.; Wallace, M.; Compton, R. One-step synthesis of fluorescein modified nano-carbon for Pd(II) detection via fluorescence quenching. Analyst 2012, 137, 2054–2062. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, R. 3-Principle and Application of Fluorescence Lifetime Imaging for Neuroscience: Monitoring Biochemical Signaling in Single Synapses Using Fluorescence Lifetime Imaging. In Neurophotonics and Biomedical Spectroscopy; Alfano, R.R., Shi, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 53–64. [Google Scholar]
- Yasuda, R.; Harvey, C.D.; Zhong, H.; Sobczyk, A.; van Aelst, L.; Svoboda, K. Supersensitive Ras activation in dendrites and spines revealed by two-photon fluorescence lifetime imaging. Nat. Neurosci. 2006, 9, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Yan, W.; Ho, D. Recent Advances in Fluorescence Lifetime Analytical Microsystems: Contact Optics and CMOS Time-Resolved Electronics. Sensors 2017, 17, 2800. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.; Walker, R.; Grant, L.; Stoppa, D.; Borghetti, F.; Charbon, E.; Gersbach, M.; Henderson, R.K. A 32 × 32 50 ps resolution 10 bit time to digital converter array in 130 nm CMOS for time correlated imaging. In Proceedings of the 2009 IEEE Custom Integrated Circuits Conference, San Jose, CA, USA, 30 December 2008; pp. 77–80. [Google Scholar]
- Bruschini, C.; Homulle, H.; Antolovic, I.M.; Burri, S.; Charbon, E. Single-photon avalanche diode imagers in biophotonics: Review and outlook. Light. Sci. Appl. 2019, 8, 87. [Google Scholar] [CrossRef]
- Szapoczka, W.K.; Truskewycz, A.L.; Skodvin, T.; Holst, B.; Thomas, P.J. Fluorescence intensity and fluorescence lifetime measurements of various carbon dots as a function of pH. Sci. Rep. 2023, 13, 10660. [Google Scholar] [CrossRef]
- Monteleone, A.; Wenzel, F.; Langhals, H.; Dietrich, D. New application for the identification and differentiation of microplastics based on fluorescence lifetime imaging microscopy (FLIM). J. Environ. Chem. Eng. 2021, 9, 104769. [Google Scholar] [CrossRef]
- Ashoka, A.H.; Aparin, I.O.; Reisch, A.; Klymchenko, A.S. Brightness of fluorescent organic nanomaterials. Chem. Soc. Rev. 2023, 52, 4525–4548. [Google Scholar] [CrossRef]
- Joung, J.F.; Han, M.; Jeong, M.-Y.; Park, S. Experimental database of optical properties of organic compounds. Sci. Data 2020, 7, 295. [Google Scholar] [CrossRef]
- Fu, W.; Min, J.; Jiang, W.; Li, Y.; Zhang, W. Separation, characterization and identification of microplastics and nanoplastics in the environment. Sci. Total. Environ. 2020, 721, 137561. [Google Scholar] [CrossRef]
- Karakolis, E.G.; Nguyen, B.; You, J.B.; Rochman, C.M.; Sinton, D. Fluorescent dyes for visualizing microplastic particles and fibers in laboratory-based studies. Environ. Sci. Technol. Lett. 2019, 6, 334–340. [Google Scholar] [CrossRef]
- Morgana, S.; Casentini, B.; Tirelli, V.; Grasso, F.; Amalfitano, S. Fluorescence-based detection: A review of current and emerging techniques to unveil micro/nanoplastics in environmental samples. Trac Trends Anal. Chem. 2024, 172, 117559. [Google Scholar] [CrossRef]
- Maes, T.; Jessop, R.; Wellner, N.; Haupt, K.; Mayes, A.G. A rapid-screening approach to detect and quantify microplastics based on fluorescent tagging with Nile Red. Sci. Rep. 2017, 7, 44501. [Google Scholar] [CrossRef] [PubMed]
- Shruti, V.; Pérez-Guevara, F.; Roy, P.D.; Kutralam-Muniasamy, G. Analyzing microplastics with Nile Red: Emerging trends, challenges, and prospects. J. Hazard. Mater. 2022, 423, 127171. [Google Scholar] [CrossRef]
- Fowler, S.D.; Greenspan, P. Application of Nile red, a fluorescent hydrophobic probe, for the detection of neutral lipid deposits in tissue sections: Comparison with oil red O. J. Histochem. Cytochem. 1985, 33, 833–836. [Google Scholar] [CrossRef]
- Aygün, S.; Beşer, B.M.; Acar, M.; Meral, K. Photophysical properties of some fluorescent dyes in SDS micellar solutions. J. Fluoresc. 2020, 30, 849–857. [Google Scholar] [CrossRef]
- Minò, A.; Cinelli, G.; Lopez, F.; Ambrosone, L. Optical behavior of nile red in organic and aqueous media environments. Appl. Sci. 2023, 13, 638. [Google Scholar] [CrossRef]
- Shim, W.J.; Song, Y.K.; Hong, S.H.; Jang, M. Identification and quantification of microplastics using Nile Red staining. Mar. Pollut. Bull. 2016, 113, 469–476. [Google Scholar] [CrossRef]
- Stanton, T.; Johnson, M.; Nathanail, P.; Gomes, R.L.; Needham, T.; Burson, A. Exploring the efficacy of Nile red in microplastic quantification: A costaining approach. Environ. Sci. Technol. Lett. 2019, 6, 606–611. [Google Scholar] [CrossRef]
- Gendron, P.-O.; Avaltroni, F.; Wilkinson, K. Diffusion coefficients of several rhodamine derivatives as determined by pulsed field gradient–nuclear magnetic resonance and fluorescence correlation spectroscopy. J. Fluoresc. 2008, 18, 1093–1101. [Google Scholar] [CrossRef]
- Hermanson, G.T. Bioconjugate Techniques; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Xu, C.; Wu, H.; Gu, F.L. Efficient adsorption and photocatalytic degradation of Rhodamine B under visible light irradiation over BiOBr/montmorillonite composites. J. Hazard. Mater. 2014, 275, 185–192. [Google Scholar] [CrossRef]
- Tong, H.; Jiang, Q.; Zhong, X.; Hu, X. Rhodamine B dye staining for visualizing microplastics in laboratory-based studies. Environ. Sci. Pollut. Res. 2021, 28, 4209–4215. [Google Scholar] [CrossRef] [PubMed]
- Janaki, V.; Oh, B.-T.; Shanthi, K.; Lee, K.-J.; Ramasamy, A.; Kamala-Kannan, S. Efficiency of various semiconductor catalysts for photodegradation of Safranin-T. Res. Chem. Intermed. 2012, 38, 1431–1442. [Google Scholar] [CrossRef]
- Korkmaz, K.; Beşer, B.M.; Şenol, A.M.; Onganer, Y. Safranin T-SDS-GO ternary system: A fluorescent pH sensor. Colloids Surf. B Biointerfaces 2021, 206, 111977. [Google Scholar] [CrossRef]
- Lv, L.; Qu, J.; Yu, Z.; Chen, D.; Zhou, C.; Hong, P.; Sun, S.; Li, C. A simple method for detecting and quantifying microplastics utilizing fluorescent dyes-Safranine T, fluorescein isophosphate, Nile red based on thermal expansion and contraction property. Environ. Pollut. 2019, 255, 113283. [Google Scholar] [CrossRef] [PubMed]
- Prata, J.C.; Reis, V.; Matos, J.T.; da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T. A new approach for routine quantification of microplastics using Nile Red and automated software (MP-VAT). Sci. Total. Environ. 2019, 690, 1277–1283. [Google Scholar] [CrossRef]
- Fischer, A.H.; Jacobson, K.A.; Rose, J.; Zeller, R. Hematoxylin and eosin staining of tissue and cell sections. Cold Spring Harb. Protoc. 2008, 2008, pdb.prot4986. [Google Scholar] [CrossRef]
- Chouchene, K.; da Costa, J.P.; Wali, A.; Girão, A.V.; Hentati, O.; Duarte, A.C.; Rocha-Santos, T.; Ksibi, M. Microplastic pollution in the sediments of Sidi Mansour Harbor in Southeast Tunisia. Mar. Pollut. Bull. 2019, 146, 92–99. [Google Scholar] [CrossRef]
- Zhang, J.; Bai, C.B.; Chen, M.Y.; Yue, S.Y.; Qin, Y.X.; Liu, X.Y.; Xu, M.Y.; Zheng, Q.J.; Zhang, L.; Li, R.Q.; et al. Novel Fluorescent Probe toward Fe(3+) Based on Rhodamine 6G Derivatives and Its Bioimaging in Adult Mice, Caenorhabditis elegans, and Plant Tissues. ACS Omega 2021, 6, 8616–8624. [Google Scholar] [CrossRef]
- Gong, J.; Liu, C.; Jiao, X.; He, S.; Zhao, L.; Zeng, X. Novel rhodamine dye with large Stokes shifts by fusing the 1, 4-diethylpiperazine moiety and its applications in fast detection of Cu 2+. RSC Adv. 2020, 10, 38038–38044. [Google Scholar] [CrossRef]
- Jaworska, A.; Wojcik, T.; Malek, K.; Kwolek, U.; Kepczynski, M.; Ansary, A.A.; Chlopicki, S.; Baranska, M. Rhodamine 6G conjugated to gold nanoparticles as labels for both SERS and fluorescence studies on live endothelial cells. Microchim. Acta 2015, 182, 119–127. [Google Scholar] [CrossRef]
- Croce, S.; Chibon, F. MED12 and uterine smooth muscle oncogenesis: State of the art and perspectives. Eur. J. Cancer 2015, 51, 1603–1610. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Nguyen, H.N.; Nghiem, T.H.L.; Do, X.-H.; To, T.T.; Do, T.X.P.; Do, D.L.; Nguyen, H.G.; Nguyen, H.M.; Nguyen, N.D.; et al. High biocompatible FITC-conjugated silica nanoparticles for cell labeling in both in vitro and in vivo models. Sci. Rep. 2024, 14, 6969. [Google Scholar] [CrossRef]
- Salari, M.; Bitounis, D.; Bhattacharya, K.; Pyrgiotakis, G.; Zhang, Z.; Purington, E.; Gramlich, W.; Grondin, Y.; Rogers, R.; Bousfield, D.; et al. Development & Characterization of Fluorescently Tagged Nanocellulose for Nanotoxicological Studies. Environ. Sci. Nano 2019, 6, 1516–1526. [Google Scholar] [CrossRef] [PubMed]
- Nishi, K.; Isobe, S.; Zhu, Y.; Kiyama, R. Fluorescence-based bioassays for the detection and evaluation of food materials. Sensors 2015, 15, 25831–25867. [Google Scholar] [CrossRef]
- Erni-Cassola, G.; Gibson, M.I.; Thompson, R.C.; Christie-Oleza, J.A. Lost, but Found with Nile Red: A Novel Method for Detecting and Quantifying Small Microplastics (1 mm to 20 μm) in Environmental Samples. Environ. Sci. Technol. 2017, 51, 13641–13648. [Google Scholar] [CrossRef] [PubMed]
- Prata, J.C.; Alves, J.R.; da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T. Major factors influencing the quantification of Nile Red stained microplastics and improved automatic quantification (MP-VAT 2.0). Sci. Total. Environ. 2020, 719, 137498. [Google Scholar] [CrossRef]
- Meyers, N.; Catarino, A.I.; Declercq, A.M.; Brenan, A.; Devriese, L.; Vandegehuchte, M.; De Witte, B.; Janssen, C.; Everaert, G. Microplastic detection and identification by Nile red staining: Towards a semi-automated, cost- and time-effective technique. Sci. Total. Environ. 2022, 823, 153441. [Google Scholar] [CrossRef]
- Le Quoc, P.; Fokina, M.I.; Martynova, D.M.; Olekhnovich, R.O.; Uspenskaya, M.V. Method of manufacturing and staining microplastics for using in the biological experiments. Environ. Sci. Pollut. Res. 2022, 29, 67450–67455. [Google Scholar] [CrossRef]
- Li, W.; Wu, C.; Xiong, Z.; Liang, C.; Li, Z.; Liu, B.; Cao, Q.; Wang, J.; Tang, J.; Li, D. Self-driven magnetorobots for recyclable and scalable micro/nanoplastic removal from nonmarine waters. Sci. Adv. 2022, 8, eade1731. [Google Scholar] [CrossRef]
- Cingolani, M.; Rampazzo, E.; Zaccheroni, N.; Genovese, D.; Prodi, L. Fluorogenic hyaluronan nanogels for detection of micro-and nanoplastics in water. Environ. Sci. Nano 2022, 9, 582–588. [Google Scholar] [CrossRef]
- Granda-Ramírez, C.F.; Hincapié-Mejía, G.M.; Serna-Galvis, E.A.; Torres-Palma, R.A. Degradation of Recalcitrant Safranin T Through an Electrochemical Process and Three Photochemical Advanced Oxidation Technologies. Water Air Soil Pollut. 2017, 228, 425. [Google Scholar] [CrossRef]
- Singh, S.; Mahalingam, H.; Singh, P.K. Polymer-supported titanium dioxide photocatalysts for environmental remediation: A review. Appl. Catal. A Gen. 2013, 462–463, 178–195. [Google Scholar] [CrossRef]
- Ribeiro, F.; Duarte, A.C.; da Costa, J.P. Staining methodologies for microplastics screening. Trac Trends Anal. Chem. 2024, 172, 117555. [Google Scholar] [CrossRef]
- Monteleone, A.; Schary, W.; Wenzel, F.; Langhals, H.; Dietrich, D.R. Label-free identification and differentiation of different microplastics using phasor analysis of fluorescence lifetime imaging microscopy (FLIM)-generated data. Chem.-Biol. Interact. 2021, 342, 109466. [Google Scholar] [CrossRef]
- Henderson, R.K.; Rae, B.R.; Li, D.U. 11-Complementary metal-oxide-semiconductor (CMOS) sensors for fluorescence lifetime imaging (FLIM). In High Performance Silicon Imaging; Durini, D., Ed.; Woodhead Publishing: Cambridge, UK, 2014; pp. 312–347. [Google Scholar]
- Hinde, E.; Digman, M.A.; Hahn, K.M.; Gratton, E. Millisecond spatiotemporal dynamics of FRET biosensors by the pair correlation function and the phasor approach to FLIM. Proc. Natl. Acad. Sci. USA 2013, 110, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Langhals, H.; Zgela, D.; Schlücker, T. Improved High Performance Recycling of Polymers by Means of Bi-Exponential Analysis of Their Fluorescence Lifetimes. Green Sustain. Chem. 2015, 5, 92–100. [Google Scholar] [CrossRef]
- Gies, S.; Schömann, E.M.; Anna Prume, J.; Koch, M. Exploring the Potential of Time-Resolved Photoluminescence Spectroscopy for the Detection of Plastics. Appl. Spectrosc. 2020, 74, 1161–1166. [Google Scholar] [CrossRef]
- Gadella, T.W.J.; Jovin, T.M.; Clegg, R.M. Fluorescence lifetime imaging microscopy (FLIM): Spatial resolution of microstructures on the nanosecond time scale. Biophys. Chem. 1993, 48, 221–239. [Google Scholar] [CrossRef]
- Wohlschläger, M.; Versen, M.; Löder, M.G.J.; Laforsch, C. Identification of different plastic types and natural materials from terrestrial environments using fluorescence lifetime imaging microscopy. Anal. Bioanal. Chem. 2024, 416, 3543–3554. [Google Scholar] [CrossRef]
- Digman, M.A.; Caiolfa, V.R.; Zamai, M.; Gratton, E. The Phasor Approach to Fluorescence Lifetime Imaging Analysis. Biophys. J. 2008, 94, L14–L16. [Google Scholar] [CrossRef]
- Hinde, E.; Digman, M.A.; Welch, C.; Hahn, K.M.; Gratton, E. Biosensor Förster resonance energy transfer detection by the phasor approach to fluorescence lifetime imaging microscopy. Microsc. Res. Tech. 2012, 75, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Peter, M.; Ameer-Beg, S.M. Imaging molecular interactions by multiphoton FLIM. Biol. Cell 2004, 96, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Peter, M.; Ameer-Beg, S.M.; Hughes, M.K.Y.; Keppler, M.D.; Prag, S.; Marsh, M.; Vojnovic, B.; Ng, T. Multiphoton-FLIM Quantification of the EGFP-mRFP1 FRET Pair for Localization of Membrane Receptor-Kinase Interactions. Biophys. J. 2005, 88, 1224–1237. [Google Scholar] [CrossRef]
- Becker, W.; Bergmann, A.; Hink, M.A.; König, K.; Benndorf, K.; Biskup, C. Fluorescence Lifetime Imaging by Time-Correlated Single-Photon Counting. Microsc. Res. Tech. 2004, 63, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Weber, G. Resolution of the fluorescence lifetimes in a heterogeneous system by phase and modulation measurements. J. Phys. Chem. 1981, 85, 949–953. [Google Scholar] [CrossRef]
- Scipioni, L.; Rossetta, A.; Tedeschi, G.; Gratton, E. Phasor S-FLIM: A new paradigm for fast and robust spectral fluorescence lifetime imaging. Nat. Methods 2021, 18, 542–550. [Google Scholar] [CrossRef]
- Wohlschläger, M.; Versen, M.; Löder, M.G.; Laforsch, C. A promising method for fast identification of microplastic particles in environmental samples: A pilot study using fluorescence lifetime imaging microscopy. Heliyon 2024, 10, e25133. [Google Scholar] [CrossRef]
- Xiao, S.; Filippini, A.; Casadei, M.; Caracciolo, G.; Digiacomo, L.; Rossetta, A. Fast and portable fluorescence lifetime analysis for early warning detection of micro- and nanoplastics in water. Environ. Res. 2024, 244, 117936. [Google Scholar] [CrossRef]
- DIN EN ISO 1043:2021; Kunststoffe—Bezeichnungen von polymeren Grundstoffen. Deutsches Institut für Normung: Berlin, Germany, 2021.
- ASTM D638-21; Standard Test Method for Tensile Properties of Plastics. ASTM International: West Conshohocken, PA, USA, 2021.
- Ranjan Gartia, M.; Eichorst, J.P.; Clegg, R.M.; Logan Liu, G. Lifetime imaging of radiative and non-radiative fluorescence decays on nanoplasmonic surface. Appl. Phys. Lett. 2012, 101, 023118. [Google Scholar] [CrossRef]
- Ruedas-Rama, M.J.; Orte, A.; Hall, E.A.; Alvarez-Pez, J.M.; Talavera, E.M. Effect of surface modification on semiconductor nanocrystal fluorescence lifetime. ChemPhysChem 2011, 12, 919–929. [Google Scholar] [CrossRef]
- Wohlschläger, M.; Leiter, N.; Dietlmeier, M.; Löder, M.G.J.; Versen, M.; Laforsch, C. Comparison of Two Classification Methods Trained with FD-FLIM Data to Identify and Distinguish Plastics from Environmental Materials. In Proceedings of the 2023 International Joint Conference on Neural Networks (IJCNN), Gold Coast, Australia, 18–23 June 2023; pp. 1–9. [Google Scholar]
- Piruska, A.; Nikcevic, I.; Lee, S.H.; Ahn, C.; Heineman, W.R.; Limbach, P.A.; Seliskar, C.J. The autofluorescence of plastic materials and chips measured under laser irradiation. Lab. A Chip 2005, 5, 1348–1354. [Google Scholar] [CrossRef] [PubMed]
- Stringari, C.; Cinquin, A.; Cinquin, O.; Digman, M.A.; Donovan, P.J.; Gratton, E. Phasor approach to fluorescence lifetime microscopy distinguishes different metabolic states of germ cells in a live tissue. Proc. Natl. Acad. Sci. USA 2011, 108, 13582–13587. [Google Scholar] [CrossRef] [PubMed]
- ISO 21073:2019; Microscopes—Confocal Microscopes—Optical Data of Fluorescence Confocal Microscopes for Biological Imaging. International Organization for Standardization: Geneva, Switzerland, 2019.
- Silva, A.B.; Bastos, A.S.; Justino, C.I.; da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T.A. Microplastics in the environment: Challenges in analytical chemistry-A review. Anal. Chim. Acta 2018, 1017, 1–19. [Google Scholar] [CrossRef]
- Liu, W.; Zhao, Y.; Shi, Z.; Li, Z.; Liang, X. Ecotoxicoproteomic assessment of microplastics and plastic additives in aquatic organisms: A review. Comp. Biochem. Physiol. Part. D Genom. Proteom. 2020, 36, 100713. [Google Scholar] [CrossRef]
- Hoffmann, K.; Resch-Genger, U.; Mix, R.; Friedrich, J.F. Fluorescence spectroscopic studies on plasma-chemically modified polymer surfaces with fluorophore-labeled functionalities. J. Fluoresc. 2006, 16, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Sonnenschein, M.F.; Roland, C.M. Absorption and fluorescence spectra of poly(ethylene terephthalate) dimers. Polymer 1990, 31, 2023–2026. [Google Scholar] [CrossRef]
- Shi, H.; Frias, J.; Sayed, A.E.-D.H.; De-la-Torre, G.E.; Choo, J.M.; Uddin, S.A.; Rajaram, R.; Chavanich, S.; Najii, A.; Fernández-Severini, M.D. Small plastic fragments: A bridge between large plastic debris and micro-& nano-plastics. TrAC Trends Anal. Chem. 2023, 168, 117308. [Google Scholar]
- Wohlschläger, M.; Versen, M.; Langhals, H. A method for sorting of plastics with an apparatus specific quantum efficiency approach. In Proceedings of the 2019 IEEE Sensors Applications Symposium (SAS), Sophia Antipolis, France, 11–13 March 2019; pp. 1–6. [Google Scholar]
- Wohlschläger, M.; Versen, M. Detection of plastics in water based on their fluorescence behavior. J. Sens. Sens. Syst. 2020, 9, 337–343. [Google Scholar] [CrossRef]
- Ali, S.S.; Elsamahy, T.; Koutra, E.; Kornaros, M.; El-Sheekh, M.; Abdelkarim, E.A.; Zhu, D.; Sun, J. Degradation of conventional plastic wastes in the environment: A review on current status of knowledge and future perspectives of disposal. Sci. Total. Environ. 2021, 771, 144719. [Google Scholar] [CrossRef]
- Andrady, A.L. Weathering and fragmentation of plastic debris in the ocean environment. Mar. Pollut. Bull. 2022, 180, 113761. [Google Scholar] [CrossRef]
- White, K.M.; Koo, H.-J.; Kanuga, K.; Battiste, J.L. Chapter 7—Assessing the Effects of Accelerated Weathering Stresses Used to Predict Service Life. In Service Life Prediction of Polymers and Plastics Exposed to Outdoor Weathering; White, C.C., White, K.M., Pickett, J.E., Eds.; William Andrew Publishing: Norwich, NY, USA, 2018; pp. 95–115. [Google Scholar]
- Philip, M.; Al-Azzawi, F. Effects of Natural and Artificial Weathering on the Physical Properties of Recycled Poly(ethylene terephthalate). J. Polym. Environ. 2018, 26, 3139–3148. [Google Scholar] [CrossRef]
- Idehara, W.; Haga, Y.; Tsujino, H.; Ikuno, Y.; Manabe, S.; Hokaku, M.; Asahara, H.; Higashisaka, K.; Tsutsumi, Y. Exploring Nile Red staining as an analytical tool for surface-oxidized microplastics. Environ. Res. 2025, 269, 120934. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zhan, X.; Wu, X.; Li, J.; Wang, H.; Gao, S. Effect of weathering on environmental behavior of microplastics: Properties, sorption and potential risks. Chemosphere 2020, 242, 125193. [Google Scholar] [CrossRef]
- Htun, T.; Klein, U.K.A. Laser-induced fluorescence decays of polyethylene films. J. Lumin. 2010, 130, 1275–1279. [Google Scholar] [CrossRef]
- Berezin, M.Y.; Achilefu, S. Fluorescence Lifetime Measurements and Biological Imaging. Chem. Rev. 2010, 110, 2641–2684. [Google Scholar] [CrossRef]
- Duan, J.; Bolan, N.; Li, Y.; Ding, S.; Atugoda, T.; Vithanage, M.; Sarkar, B.; Tsang, D.C.W.; Kirkham, M.B. Weathering of microplastics and interaction with other coexisting constituents in terrestrial and aquatic environments. Water Res. 2021, 196, 117011. [Google Scholar] [CrossRef]
- Ivleva, N.P.; Wiesheu, A.C.; Niessner, R. Microplastic in aquatic ecosystems. Angew. Chem. Int. Ed. 2017, 56, 1720–1739. [Google Scholar] [CrossRef] [PubMed]
- Stedmon, C.A.; Markager, S.; Bro, R. Tracing dissolved organic matter in aquatic environments using a new approach to fluorescence spectroscopy. Mar. Chem. 2003, 82, 239–254. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, B.; Chen, H.; Yuan, R.; Wang, F. Distribution, biological effects and biofilms of microplastics in freshwater systems-a review. Chemosphere 2022, 299, 134370. [Google Scholar] [CrossRef]
- Cadondon, J.; Vallar, E.; Shiina, T.; Galvez, M.C. Experimental detection of marine plastic litter in surface waters by 405 nm LD-based fluorescence lidar. Mar. Pollut. Bull. 2024, 207, 116842. [Google Scholar] [CrossRef]
- Chamas, A.; Moon, H.; Zheng, J.; Qiu, Y.; Tabassum, T.; Jang, J.H.; Abu-Omar, M.; Scott, S.L.; Suh, S. Degradation Rates of Plastics in the Environment. ACS Sustain. Chem. Eng. 2020, 8, 3494–3511. [Google Scholar] [CrossRef]
- Provencher, J.F.; Bond, A.L.; Avery-Gomm, S.; Borrelle, S.B.; Rebolledo, E.L.B.; Hammer, S.; Kühn, S.; Lavers, J.L.; Mallory, M.L.; Trevail, A. Quantifying ingested debris in marine megafauna: A review and recommendations for standardization. Anal. Methods 2017, 9, 1454–1469. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Lee, J.J.; Lee, S.; Kang, J.; Kim, K.-T.; Kim, C. A cost-effective and efficient fluorescence staining agent for the identification of microplastics in environmental samples and zebrafish (Danio rerio). J. Hazard. Mater. 2025, 493, 138365. [Google Scholar] [CrossRef] [PubMed]
- Facchetti, S.V.; La Spina, R.; Fumagalli, F.; Riccardi, N.; Gilliland, D.; Ponti, J. Detection of metal-doped fluorescent PVC microplastics in freshwater mussels. Nanomaterials 2020, 10, 2363. [Google Scholar] [CrossRef]
- Hu, J.; Ye, F.; Zhang, S.; Li, H.; Bao, Q.; Gan, J.; Ye, Q.; Wang, W. Multi-dimensional visualization of ingestion, biological effects and interactions of microplastics and a representative POP in edible jellyfish. Environ. Int. 2023, 178, 108028. [Google Scholar] [CrossRef] [PubMed]
- Kühn, S.; Van Franeker, J.A. Quantitative overview of marine debris ingested by marine megafauna. Mar. Pollut. Bull. 2020, 151, 110858. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Fileman, E.; Halsband, C.; Goodhead, R.; Moger, J.; Galloway, T.S. Microplastic ingestion by zooplankton. Environ. Sci. Technol. 2013, 47, 6646–6655. [Google Scholar] [CrossRef]
| Technique | Resolution | Detection Limit | Throughput | Key Advantages | Limitations | Reference |
|---|---|---|---|---|---|---|
| Confocal Laser Scanning Microscopy (CLSM) | Lateral: ~200–250 nm Axial: ~500–700 nm | ≥1 μm | Low | High spatial resolution; optical sectioning; 3D imaging | Photobleaching; limited depth; slow scanning maintenance | [74,76] |
| Fluorescence Lifetime Imaging Microscopy (FLIM) | Depends on platform (typically ~300 nm) | ~0.5–2 μm (lifetime contrast) | Medium | Quantitative contrast independent of intensity; detects environmental effects | Complex setup; long acquisition time | [75,77] |
| Widefield Fluorescence Microscopy | ~250–300 nm | ≥1 μm | High | Fast imaging; simple setup | Poor axial resolution; high background noise | [76] |
| Imaging Flow Cytometry | ~500–1000 nm | ~2–20 μm | Very High | High-throughput analysis of particles in flow; Acquisition of fluorescence and structural data | Requires suspended particles; lower spatial resolution | [78,79] |
| Two-Photon Microscopy | ~300–500 nm lateral | ≥0.5 μm | Low | Deep penetration; minimal photodamage; suitable for in vivo imaging | High cost; slow scanning; needs pulsed laser | [80,81] |
| Plastic Type | Temperature (°C) | Heat Treatment Time (h) | Fluorescence Lifetime Change | Fluorescence Intensity Changes |
|---|---|---|---|---|
| Acrylonitrile-butadiene-styrene copolymer (ABS) | 140 | 12 | −0.178 | Slightly enhanced |
| Poly(p-phenylene oxide (PPO) | 160 | 12 | −2.618 | Significantly enhanced |
| Polyamide 6 (PA) | 160 | 12 | Higher photon yield | |
| Polyethylene terephthalate (PET) | 210~220 | 12 | −0.02 | Slightly enhanced |
| Polylactide (PLA) | 140 | 12 | ||
| Polyurethane (PU) | 160 | 12 |
| Dyes | Target Polymers | Advantage | Limitation | Reference | |
|---|---|---|---|---|---|
| Nile Red | In nonpolar lipids: 460 nm/620 nm In polar lipids: 543 nm/620 nm 560 nm/635 nm | PE, PP, PVC, EVA, PVA, PTFE, PET, PS, PA, acrylic, PU |
|
| [104,106,107,108,109,110,111,112] |
| Rhodamine B | In ethanol: 540 nm/565 nm In methanol: 556 nm/580 nm | PE, PP, PU, PVC, PMMA |
|
| [113,114,115,116] |
| Safranine T | In water: 520 nm/563 nm | PE, PP, PU, PVC |
|
| [117,118,119,120] |
| Eosin B | In water: 521 nm/544 nm In ethanol: 527 nm/550 nm | PE, PP |
|
| [121,122] |
| Rhodamine 6G | In polar lipids: 527 nm/555 nm | HDPE |
|
| [123,124,125,126] |
| Fluorescein Isothiocyanate (FITC) | In polar lipids: 488 nm/517 nm | PS, PVC, PE, PET |
|
| [127,128,129] |
| Author | Band | Plastic-Type | Status | Average Fluorescence Lifetime (ns) | Experimental Equipment |
|---|---|---|---|---|---|
| Adrian Monteleone et al., 2021 [100] | 470 nm 440 nm | PLA | * DIN Heating 12 h | 2.864 (±0.035) | Fluorescence Lifetime Imaging Microscopy (FLIM) System A modular Leica TCS SP8 FALCON (FAst Lifetime CONtrast) system (Leica Microsystems GmbH, Wetzlar, Germany) equipped with an HC PL APO 20×/0.75 Dry CS2 objective lens was employed for fluorescence lifetime imaging of microplastic particles. Image resolution: 512 × 512 |
| PPE | * DIN Ambient/Heating 12 h | 8.143 (±0.060) | |||
| PA6 | * DIN Heating12 h | 4.529 (±0.008) | |||
| ABS | * DIN Ambient/Heating 12 h | 3.850 (±0.033) | |||
| PU | * DIN Ambient | 4.224 (±0.010) | |||
| PET | * DIN Ambient/** ASTM Ambient | 3.519 (±0.090)/3.564 (±0.126) | |||
| M Wohlschläger et al., 2024 [153] | 488 nm | HDPE | Optical LP Filters Single Material FD-FLIM | 1.68 (±0.07) | A frequency-domain fluorescence lifetime imaging (FD-FLIM) camera system, model pco.flim from Excelitas PCO GmbH, Kelheim, Germany Image resolution: 1008 × 1008 pixels |
| Optical BP Filter Single Material FD-FLIM | 3.52 (±0.21) | ||||
| Optical BP Filter multi-material FD-FLIM | 3.24 (±0.60) | ||||
| Siyao Xiao et al., 2024 [154] | 445 nm | PS | COOH-PS | 3.52 (±0.23) | Fluorescence Lifetime Analysis System (FLA Kit) Developer: FLIM LABS (Rome, Italy) The system has a limit measurement of 0.01 mg/mL |
| NH2-PS | 1.98 (±0.07) | ||||
| Micro PS | 2.28 (±0.12) | ||||
| Nano PS | 2.39 (±0.16) |
| Component | Color | Fluorescence Intensity | Excitation Wavelength (nm) | Fluorescence Wavelength (nm) |
|---|---|---|---|---|
| Lemonade PET bottle | Blue | 500 | 380 | 495 |
| 355 | 460 | 495 | ||
| Water (PET 100%, 500 mL) | Bright Blue | 762 | 335 | 435 |
| Water (PET 25%, 600 mL) | Bright Blue | 1385 | 345 | 415 |
| Citrus Lemonade Bottle (1500 mL) | Blue | 3732 | 370 | 445 |
| Author | Band | Plastic-Type | Status | Average Fluorescence Lifetime (ns) | Installations |
|---|---|---|---|---|---|
| Heinz Langhals et al., 2015 [142] | 403 nm | PMMA | 0.841 | Fluorescence lifetime measurement equipment: PicoQuant (Berlin, Germany) FluoTime 300; Pico Quant PicoHarp 300 (PC-405 laser; 403 nm). | |
| PS | 3.290 | ||||
| PC | 1.038 | ||||
| PET | Soft Drink Bottles | 1.840 | |||
| Plates | 4.466 | ||||
| PE | LDPE | 2.19 | |||
| HDPE | <0.2 | ||||
| EHDPE | 1.58 | ||||
| Silicone | Binder Sn | 3.078 | |||
| Binder Pt | 3.162 | ||||
| Binder Pt (50) | 3.114 | ||||
| Hose | 4.333 | ||||
| Delrin® (POM) | DuPont’s polyformaldehyde | 4.024 | |||
| Luran® (ASA) | Styrene-polyacrylonitrile copolymer from BASF | 3.976 | |||
| Ultramid® (PA) | Polyamide with glass fibers from BASF | 3.784 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Li, Y.; Zhu, L.; Song, X.; Ren, C.; Guo, B.; Gu, Y. Applications of Fluorescence Technology for Rapid Identification of Marine Plastic Pollution. Polymers 2025, 17, 1679. https://doi.org/10.3390/polym17121679
Zhang H, Li Y, Zhu L, Song X, Ren C, Guo B, Gu Y. Applications of Fluorescence Technology for Rapid Identification of Marine Plastic Pollution. Polymers. 2025; 17(12):1679. https://doi.org/10.3390/polym17121679
Chicago/Turabian StyleZhang, Haoyu, Yanjun Li, Lixin Zhu, Xindi Song, Changbin Ren, Buyu Guo, and Yanzhen Gu. 2025. "Applications of Fluorescence Technology for Rapid Identification of Marine Plastic Pollution" Polymers 17, no. 12: 1679. https://doi.org/10.3390/polym17121679
APA StyleZhang, H., Li, Y., Zhu, L., Song, X., Ren, C., Guo, B., & Gu, Y. (2025). Applications of Fluorescence Technology for Rapid Identification of Marine Plastic Pollution. Polymers, 17(12), 1679. https://doi.org/10.3390/polym17121679







