Biodegradable, Wear-Resistant and Resilient Thermoplastic Polycarbonate-Based Polyurethane with Nanoscale Microphase Structure
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
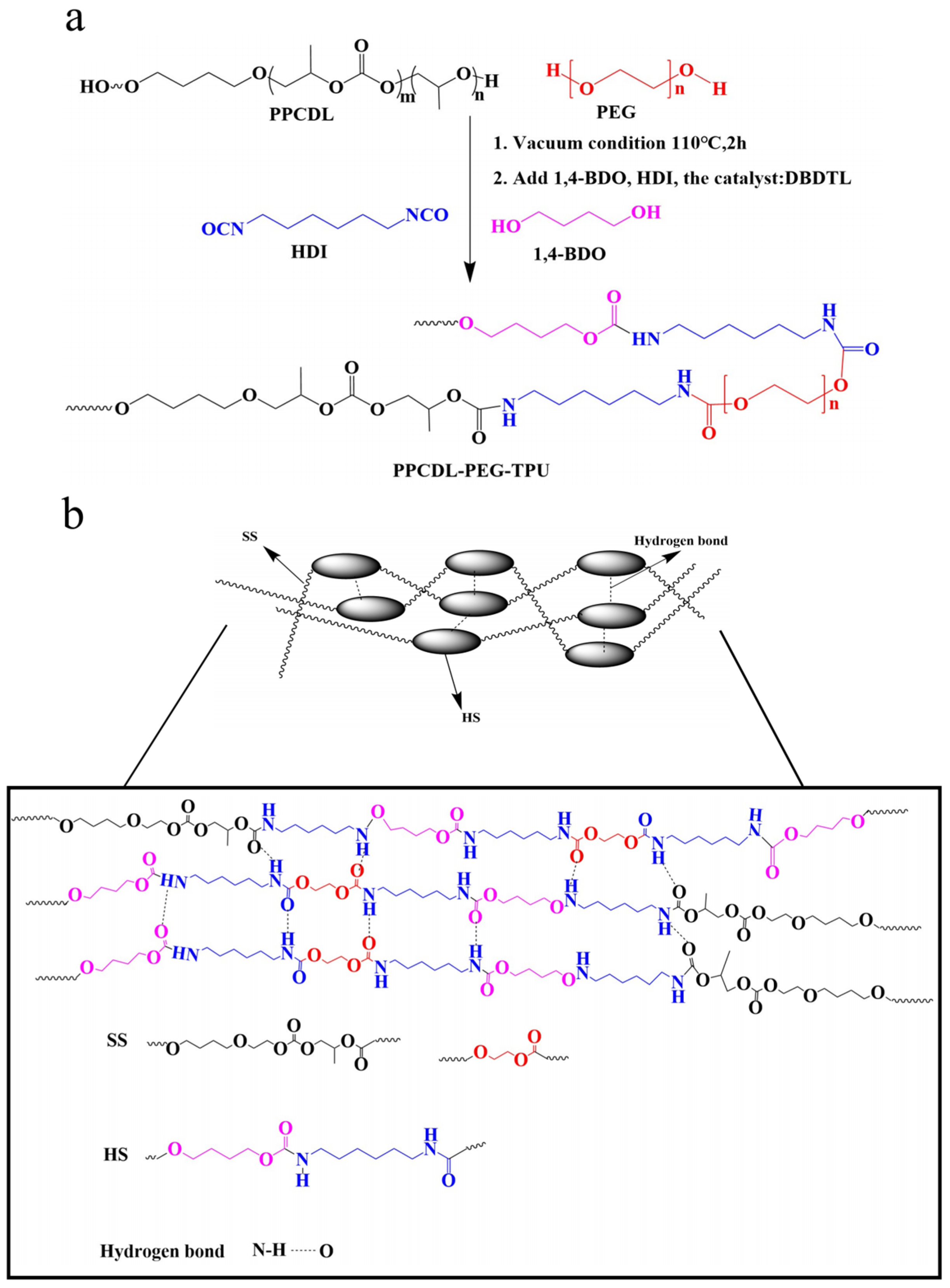
2.2. Synthesis of PPCDL-TPU and PPCDL-PEG-TPU
2.3. Measurement Methods
3. Results and Discussion
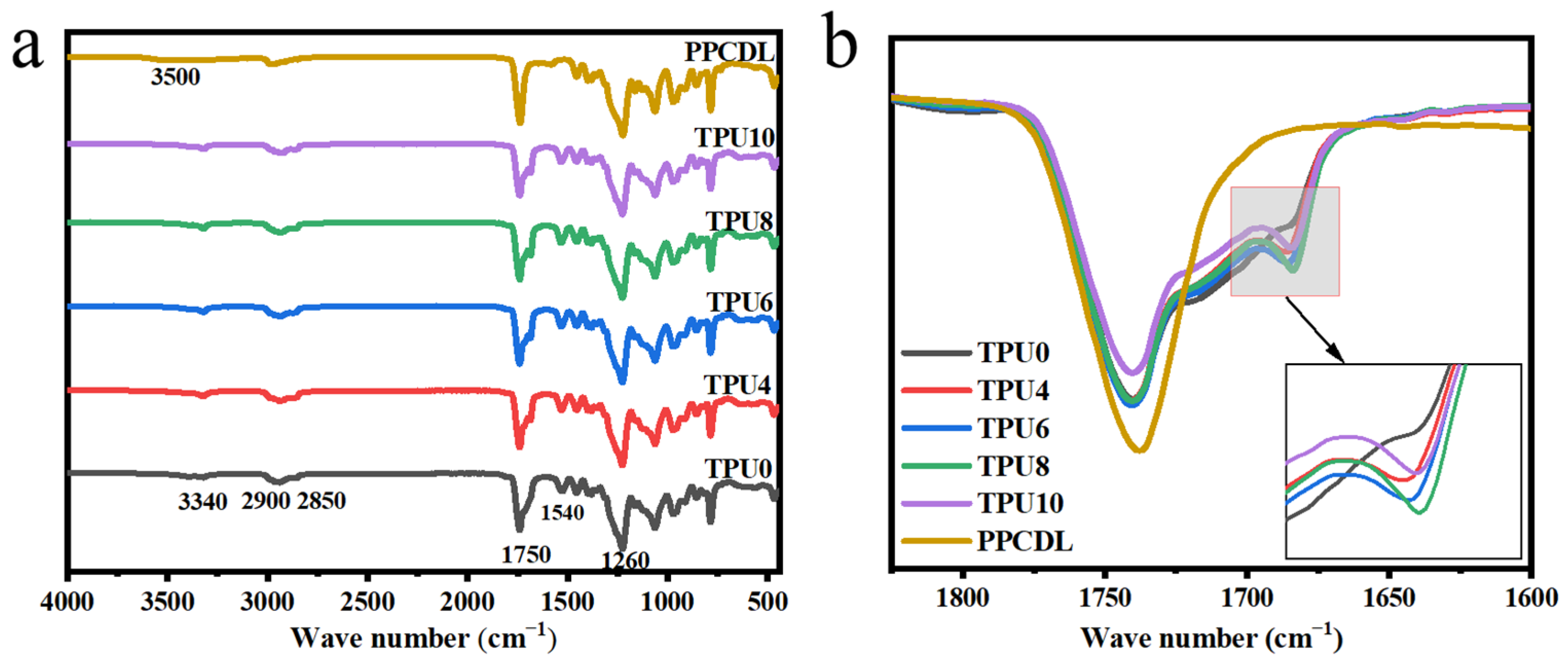
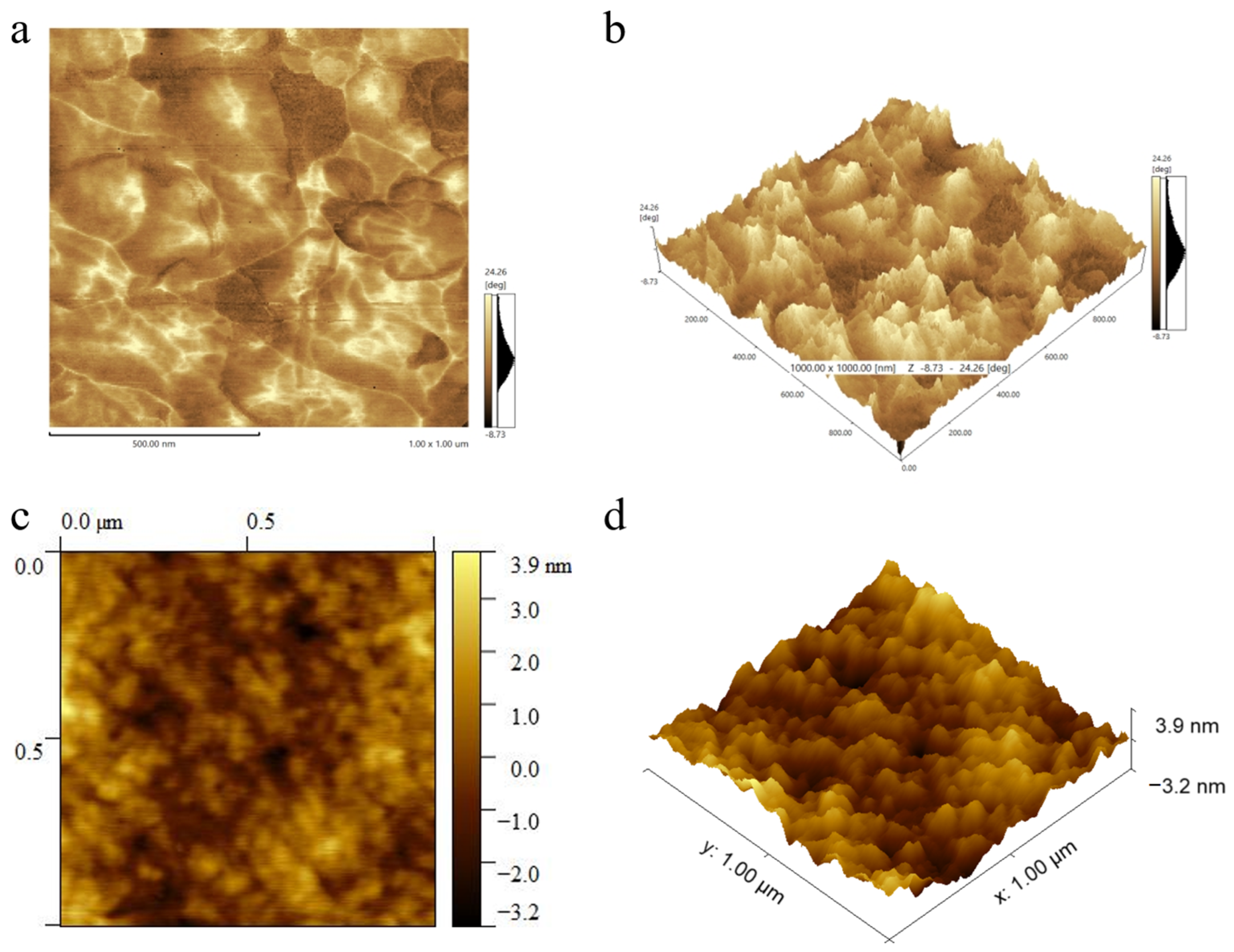
3.1. The Structure of PPCDL-TPU and PPCDL-PEG1000-TPU
3.2. Properties of the Synthesized TPUs
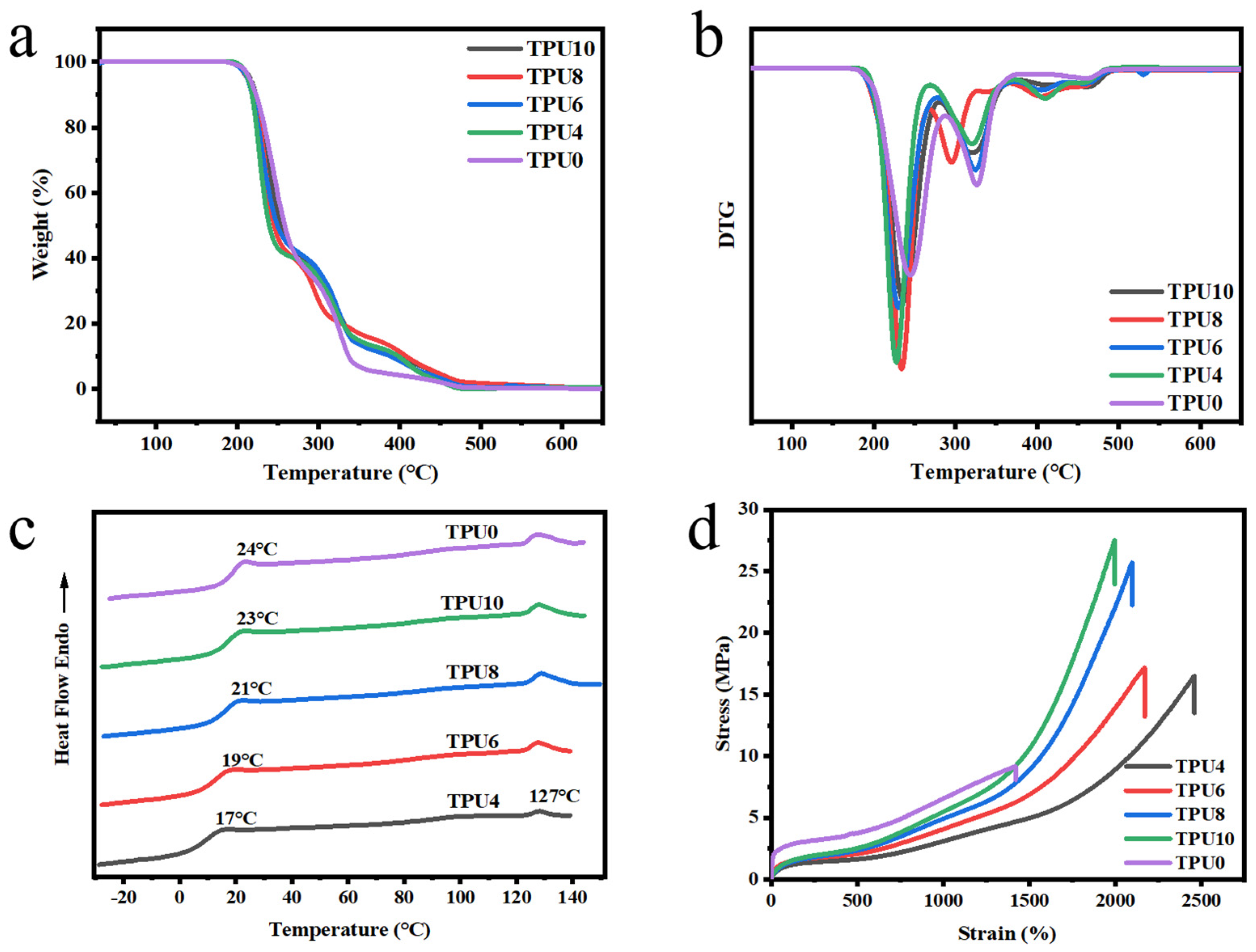
3.2.1. Thermal Properties
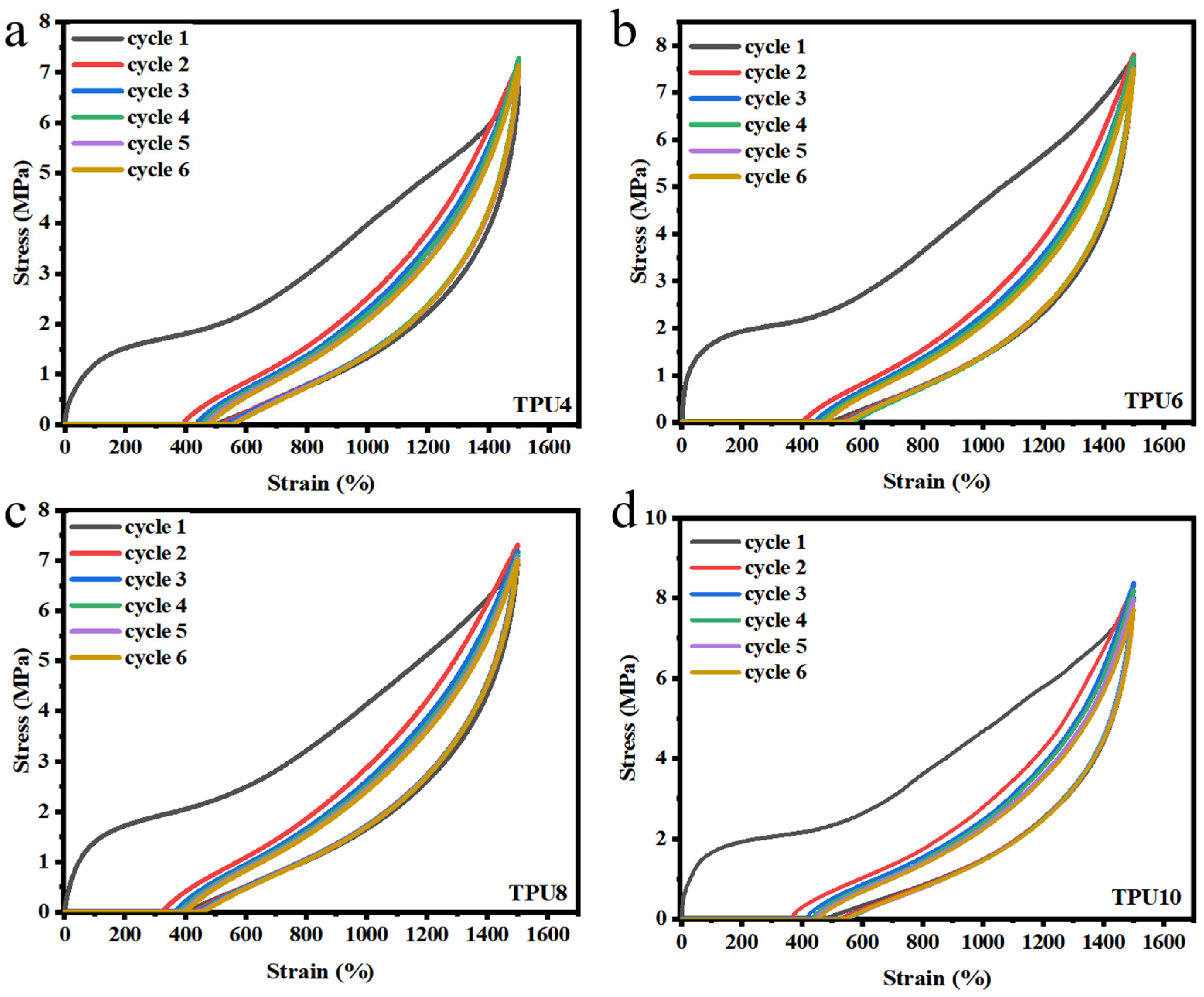
3.2.2. Mechanical Properties
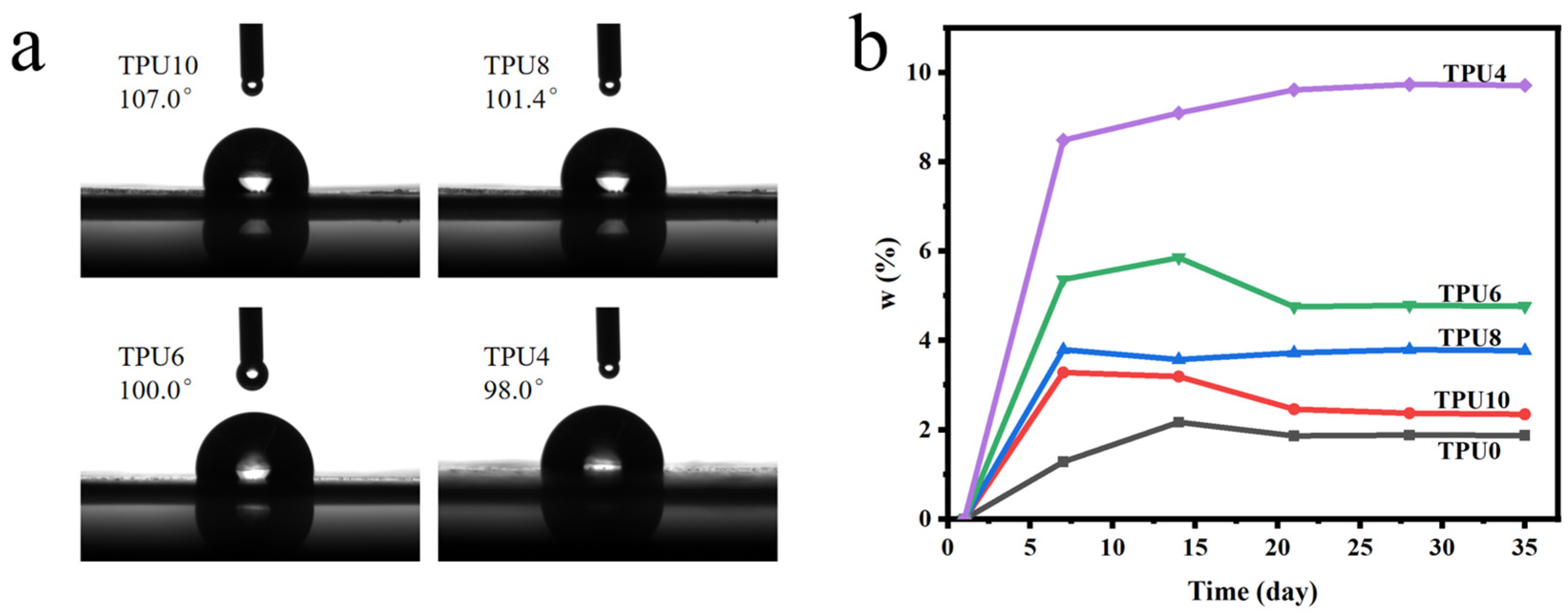
3.2.3. Hydrophobicity and Water Absorption
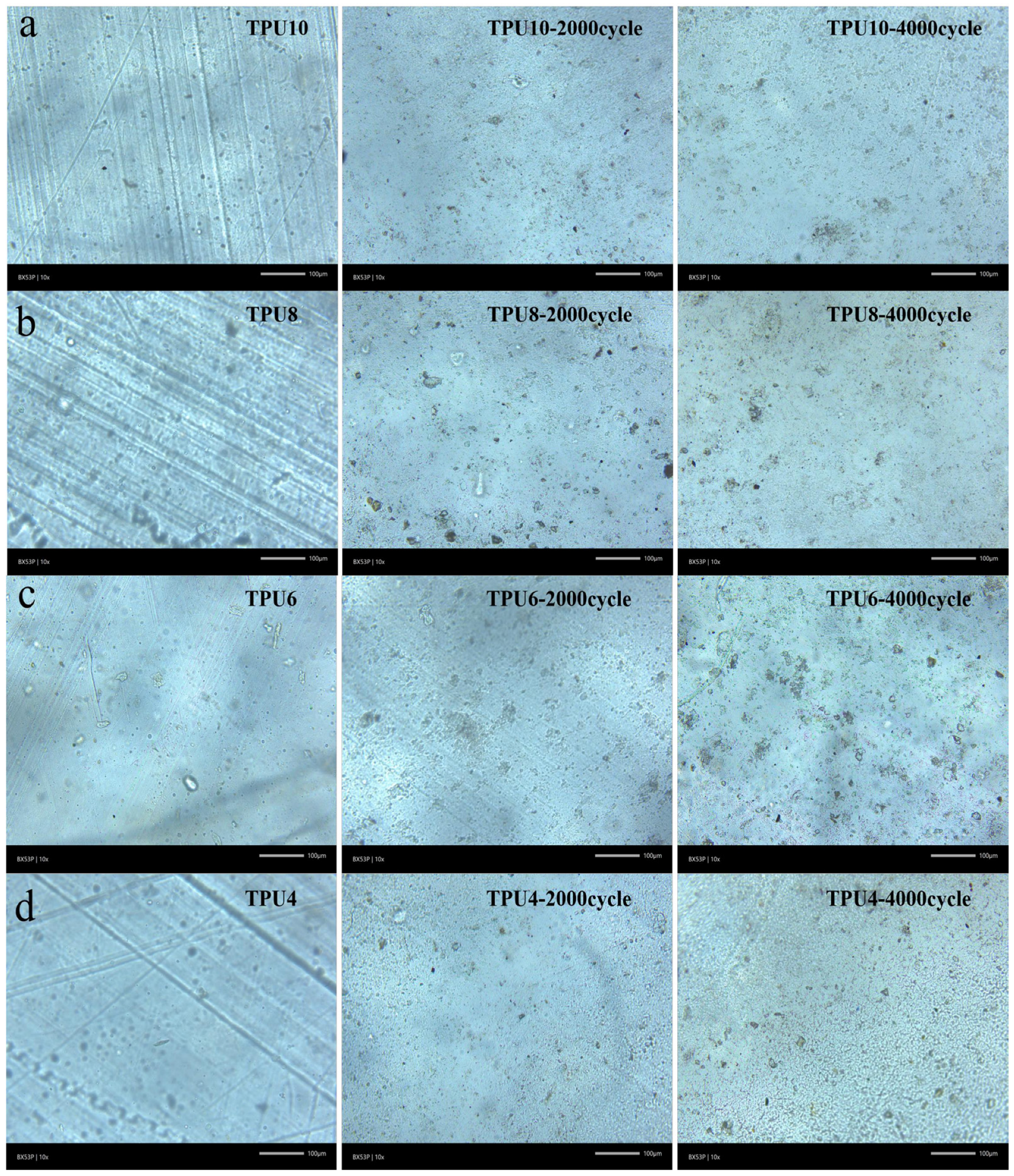
3.2.4. Akron Abrasion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Delavardea, A.; Savin, G.; Derkenne, P.; Boursier, M.; Morales-Cerrada, R.; Nottelet, B.; Pinaud, J.; Caillol, S. Sustainable polyurethanes: Toward new cutting-edge opportunities. Prog. Polym. Sci. 2024, 151, 101805. [Google Scholar] [CrossRef]
- Wang, H.; Li, T.; Li, J.; Zhao, R.; Ding, A.; Xu, F.-J. Structural engineering of polyurethanes for biomedical applications. Prog. Polym. Sci. 2024, 151, 101803. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, S. Crystal size effect on large deformation mechanisms of thermoplastic polyurethane. Extrem. Mech. Lett. 2025, 74, 102275. [Google Scholar] [CrossRef]
- Liu, C.; Chen, K.; Wu, B.; Sun, W.; Ji, L.; Wu, Y. Preparation of a Novel Composite Coating of APP/MMT/APTES on Polyurethane with Improved Flame-Retarding Performance. ACS Omega 2024, 9, 48350–48360. [Google Scholar] [CrossRef]
- Zhao, J.; Zhu, J.; Zhang, J.; Huang, Z.; Qi, D. Review of research on thermoplastic self-healing polyurethanes. React. Funct. Polym. 2024, 199, 105886. [Google Scholar] [CrossRef]
- Valavan, A.; Komolafe, A.; Harris, N.R.; Beeby, S. Vacuum Thermoforming for Packaging Flexible Electronics and Sensors in E-Textiles. IEEE Trans. Compon. Packag. Technol. 2023, 13, 715–723. [Google Scholar] [CrossRef]
- Kohari, A.; Barany, T. Sustainable thermoplastic elastomers based on thermoplastic polyurethane and ground tire rubber. J. Appl. Polym. Sci. 2024, 141, 44. [Google Scholar] [CrossRef]
- Jing, Z.; Huang, X.; Liang, J.; Xu, C.; Li, Y. Enhancement of crystallization and adjustment properties for biocompatible poly(lactide)-based thermoplastic polyurethane via stereocomplexation. J. Appl. Polym. Sci. 2024, 31, 92. [Google Scholar] [CrossRef]
- Giehl, D.; Hansen, E.; Robinson, L.C.; de Aquim, P.M. Reusing finished leather waste to produce pigmented thermoplastic polyurethane composite. Collagen Leather 2024, 6, 5. [Google Scholar] [CrossRef]
- Zhu, M.; Ma, Z.; Liu, L.; Zhang, J.; Huo, S.; Song, P. Recent advances in fire-retardant rigid polyurethane foam. J. Mater. Sci. Technol. 2022, 112, 315–328. [Google Scholar] [CrossRef]
- Ye, S.; Wang, S.; Lin, L.; Xiao, M.; Meng, Y. CO2 derived biodegradable polycarbonates: Synthesis, modification and applications. Adv. Ind. Eng. Polym. Res. 2019, 2, 143–160. [Google Scholar] [CrossRef]
- Xu, Y.; Lin, L.; Xiao, M.; Wang, S.; Smith, A.T.; Sun, L.; Meng, Y. Synthesis and properties of CO2-based plastics: Environmentally-friendly, energy-saving and biomedical polymeric materials. Prog. Polym. Sci. 2018, 80, 163–182. [Google Scholar] [CrossRef]
- Huang, X.; Alferov, K.; Zhao, T.T.; Yin, J.Y.; Wang, S.J.; Han, D.M.; Huang, S.; Huang, Z.H.; Xiao, M.; Meng, Y.Z. Facile and direct synthesis of oligocarbonate diols from carbon dioxide and their application as sustainable feedstock for polyurethane. J. CO2 Util. 2023, 75, 102571. [Google Scholar] [CrossRef]
- Huang, X.; Zhao, T.T.; Wang, S.J.; Han, D.M.; Huang, S.; Guo, H.; Xiao, M.; Meng, Y.Z. Self-Healable, Transparent, Biodegradable, and Shape Memorable Polyurethanes Derived from Carbon Dioxide-Based Diols. Molecules 2024, 29, 4364. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.Y.; Huang, X.; Zhao, T.T.; Xiao, M.; Han, D.M.; Huang, S.; Wang, S.J.; Meng, Y.Z. Efficient and Scalable Synthesis of CO2-Based Polycarbonate Diols with Difunctional Initiator and Proton Exchange Agent. J. Polym. Sci. 2025, 63, 372–382. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, M.; Meng, X.; Liu, A.; Duo, L.a. Waste rubber-Black pollution reframed as a global issue: Ecological challenges and sustainability initiatives. Environ. Pollut. 2024, 356, 124291. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Arora, S. Mitigation of environmental pollution using cellulose-based natural rubber latex composites. Curr. Sci. India 2024, 127, 290–296. [Google Scholar] [CrossRef]
- Jiang, X.; Zhu, B.; Zhu, M. An overview on the recycling of waste poly(vinyl chloride). Green Chem. 2023, 25, 5802–5813. [Google Scholar] [CrossRef]
- Marsura, G.; Bahu, J.O.; Tovar, L.P.; Fernandez-Felisbino, R.; Gomes, E.L. Recycled PVC to eco-friendly materials for footwear industry: Process and mechanical properties. Polimeros 2025, 35, e20240040er. [Google Scholar] [CrossRef]
- Feng, Z.-W.; Dai, Q.-Q.; He, J.-Y.; Bai, C.-X. Biomimetic Synthesis and Properties of Polyisoprene-based Rubber Grafted with Poly(amino acid). Acta Polym. Sin. 2023, 54, 593–600. [Google Scholar]
- Pichaiyut, S.; Uttaro, C.; Ritthikan, K.; Nakason, C. Biodegradable thermoplastic natural rubber based on natural rubber and thermoplastic starch blends. J. Polym. Res. 2023, 30, 23. [Google Scholar] [CrossRef]
- Guo, W.; Liu, Z.; Yang, S.; Li, L. Fabrication of Sustainable Composite Foam from Ethylene Vinyl Acetate-Based Sole Waste via Solid-State Shear Milling and Supercritical Carbon Dioxide Foaming Technologies. ACS Sustain. Chem. Eng. 2023, 11, 13918–13927. [Google Scholar] [CrossRef]
- Rodrigo-Carranza, V.; Hoogkamer, W.; Gonzalez-Rave, J.M.; Horta-Munoz, S.; Serna-Moreno, M.d.C.; Romero-Gutierrez, A.; Gonzalez-Mohino, F. Influence of different midsole foam in advanced footwear technology use on running economy and biomechanics in trained runners. Scand. J. Med. Sci. Sports 2024, 34, e14526. [Google Scholar] [CrossRef]
- Martinez-Martinez, D.; Tiss, B.; Glanzmann, L.N.; Wolthuizen, D.J.; Cunha, L.; Mansilla, C.; De Hosson, J.T.M. Protective films on complex substrates of thermoplastic and cellular elastomers: Prospective applications to rubber, nylon and cork. Surf. Coat. Tech. 2022, 442, 128405. [Google Scholar] [CrossRef]
- Zahmatkesh, S.; Rizvi, R. Evaluating surface texturing technologies of rubber composites on ice using a novel high-velocity tribotest method. Tribol. Int. 2025, 206, 110538. [Google Scholar] [CrossRef]
- Sato, S.; Yamaguchi, T.; Shibata, K.; Nishi, T.; Moriyasu, K.; Harano, K.; Hokkirigawa, K. Dry sliding friction and Wear behavior of thermoplastic polyurethane against abrasive paper. Biotribology 2020, 23, 12–18. [Google Scholar] [CrossRef]
- Van Alsenoy, K.; van der Linden, M.L.; Girard, O.; Ryu, J.H.; Al Raisi, L.; Santos, D. Effects of hybrid custom foot orthoses on running economy, running mechanics and comfort: A double-blinded randomized crossover study. Gait Posture 2025, 118, 45–50. [Google Scholar] [CrossRef]
- Brueckner, K.; Odenwald, S.; Schwanitz, S.; Heidenfelder, J.; Milani, T. Polyurethane-foam Midsoles in Running Shoes-Impact Energy and Damping. In Proceedings of the Engineering of Sport 8—Engineering Emotion—8th Conference of the International Sports Engineering Association (ISEA), Vienna, Austria, 12–16 July 2010; Volume 2, pp. 2789–2793. [Google Scholar]
- Yamauchi, H.; Inayama, S.; Nakabayashi, M.; Hayashi, S. Systematic Order-Made Synthesis of Sequence-Defined Polyurethanes with Length, Types, and Topologies. ACS Macro Lett. 2023, 12, 1264–1271. [Google Scholar] [CrossRef]
- Huang, Z.; Geng, S.; Chen, Y.; Li, Y.; Fei, M.; Qiu, R.; Chen, T.; Liu, W. Biobased comb polyurethane hot-melt adhesives consisting of dangling fatty acid chains and H-bonds for tailoring bonding strength. Eur. Polym. J. 2025, 229, 113880. [Google Scholar] [CrossRef]
- Wang, J.J.; Liu, Z.X.; Qiu, H.J.; Wang, C.X.; Dong, X.Y.; Du, J.H.; Li, X.L.; Yang, X.F.; Fang, H.G.; Ding, Y.S. A robust bio-based polyurethane employed as surgical suture with help to promote skin wound healing. Biomater. Adv. 2025, 166, 214048. [Google Scholar] [CrossRef]
- Yan, D.G.; Feng, B.B.; Zhao, S.L.; Gu, C.Y.; Fan, Y.Q. Robust phase change materials based on PBAT-block-PEG multiblock copolymers with novel thermostability prepared via facile melt transesterification. J. Energy Storage 2024, 84, 110895. [Google Scholar] [CrossRef]
- Noormohammadi, F.; Nourany, M.; Sadeghi, G.M.M.; Wang, P.Y.; Shahsavarani, H. The role of cellulose nanowhiskers in controlling phase segregation, crystallization and thermal stimuli responsiveness in PCL-PEGx-PCL block copolymer-based PU for human tissue engineering applications. Carbohydr. Polym. 2021, 252, 117219. [Google Scholar] [CrossRef]
- Tian, Y.S.; Wei, Y.; Wang, M.; Wang, J.D.; Li, X.F.; Qin, X.; Zhang, L.Q. Ultra-stretchable, tough, and self-healing polyurethane with tunable microphase separation for flexible wearable electronics. Nano Energy 2025, 139, 110908. [Google Scholar] [CrossRef]
- Niemczyk, A.; Piegat, A.; Olalla, A.S.; El Fray, M. New approach to evaluate microphase separation in segmented polyurethanes containing carbonate macrodiol. Eur. Polym. J. 2017, 93, 182–191. [Google Scholar] [CrossRef]
- You, J.-L.; Chin, K.-Y.; Lai, Y.-T.; Cheng, C.-T.; Chang, S.-M. A method for synthesis of waterborne polyurethane using an eco-friendly surfactant. Colloid. Surf. A 2024, 702, 135013. [Google Scholar] [CrossRef]
- Lu, T.H.; Liu, Y.; Xin, B.J.; Zhou, H.T.; Xia, W.L. Crosslinked waterborne polyurethane-acrylate phase change material with excellent flexibility and shape stability. J. Polym. Res. 2025, 32, 144. [Google Scholar] [CrossRef]
- Oprea, S.; Potolinca, V.O. Synthesis and properties of water-dispersible polyurethanes based on various diisocyanates and PEG as the hard segment. J. Appl. Polym. Sci. 2023, 140, 53948. [Google Scholar] [CrossRef]
- Liu, B.; Niu, Z.; Wang, Z.; Wang, Y.; Zou, H.; Zhao, X.; Hu, S. Effect of hard segment content on the phase separation and properties of hydroxyl-terminated polybutadiene thermoplastic polyurethane. Polym. Test. 2025, 143, 108681. [Google Scholar] [CrossRef]
- Klinedinst, D.B.; Yilgor, I.; Yilgor, E.; Zhang, M.; Wilkes, G.L. The effect of varying soft and hard segment length on the structure-property relationships of segmented polyurethanes based on a linear symmetric diisocyanate, 1,4-butanediol and PTMO soft segments. Polymer 2012, 53, 5358–5366. [Google Scholar] [CrossRef]
- Huang, M.; Li, Z.; Xu, W.; He, S.; Liu, W.; Jiang, L.; Liu, H. Visualization and quantification of microphase separation in thermoplastic polyurethanes under different hard segment contents and its effect on the mechanical properties. Polym. Test. 2024, 131, 108329. [Google Scholar] [CrossRef]
- Yang, Z. Preparation and characterization of amphiphilic, biodegradable, waterborne polyurethanes without using organic solvent and catalyst. RSC Adv. 2024, 14, 17306–17317. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Peng, D.; Zhao, N.; Mu, Q.; Li, J. The physical properties of nonionic waterborne polyurethane with a polyether as side chain. J. Appl. Polym. Sci. 2013, 127, 1848–1852. [Google Scholar] [CrossRef]
- Pešić, I.; Durand, J.-O.; Radović, D.V.; Ostojić, S.; Petrović, M.; Radojević, V.; Spasenović, M.; Pergal, M.V. Synthesis and properties of in situ prepared polyurethane/PEG-MXene nanocomposites. Prog. Org. Coat. 2025, 204, 109235. [Google Scholar] [CrossRef]
- Gao, Y.; Sun, L.; Chen, P.; Wu, Y.; Liu, Y.; Gao, C. A stretch hardening self-assembly strategy inspired by natural rubber for degradable, recyclable and self-repairing polyurethane elastomers. Polym. Degrad. Stab. 2023, 215, 110441. [Google Scholar] [CrossRef]
- Gao, X.; Niu, C.; Wang, J.; Wu, Y.; Chen, Y.; Zhao, L.; Hu, D. Micro-crosslinked thermoplastic polyurethane foam with ultra-low density, excellent resilience and recycling by supercritical CO2/N2. J. CO2 Util. 2025, 94, 103061. [Google Scholar] [CrossRef]
- Ren, Y.; Zhang, T.; Wang, S.; Han, D.; Huang, S.; Guo, H.; Xiao, M.; Meng, Y. CO2 derived ABA triblock all-polycarbonate thermoplastic elastomer with ultra-high elastic recovery. J. CO2 Util. 2024, 85, 102853. [Google Scholar] [CrossRef]
- Bakhtiari, M.; Bakhshandeh, E.; Jafari, R.; Momen, G. Enhancing anti-icing efficacy in hybrid polyurethane coatings: Evaluating the significance of molecular weight, chemical structure, and content of PEG/PDMS. Appl. Surf. Sci. 2025, 684, 161951. [Google Scholar] [CrossRef]
- Wang, S.; Huang, R.; Ren, K.; Shen, L.; Li, X.; Lei, G.; Shen, L.; Zhan, Y.; Zheng, Y.; Jiang, L. Structural control of PEG-intercalating Na-bentonite and its influence on the properties of castor oil-based polyurethane coating. Prog. Org. Coat. 2023, 178, 107499. [Google Scholar] [CrossRef]
- Jee, C.; Kang, K.S.; Bae, J.-H.; Min, J.G.; Kim, B.J.; Seo, C.M.; Huh, P. Effect of molecular weight on the properties of polyethylene glycol polyurethane-co-polydimethylsiloxane polyurethane. Polym.-Plast. Technol. Mater. 2020, 59, 1967–1972. [Google Scholar] [CrossRef]
- Francolini, I.; Silvestro, I.; Di Lisio, V.; Martinelli, A.; Piozzi, A. Synthesis, Characterization, and Bacterial Fouling-Resistance Properties of Polyethylene Glycol-Grafted Polyurethane Elastomers. Int. J. Mol. Sci. 2019, 20, 1001. [Google Scholar] [CrossRef]
- Li, S.F.; Dong, C.L.; Yuan, C.Q.; Bai, X.Q. Effects of TiO2 nano-particles on wear-resistance and vibration-reduction properties of a polymer for water-lubricated bearing. Wear 2023, 522, 204713. [Google Scholar] [CrossRef]
- Lee, J.; Chung, S.H.; Kim, B.; Son, J.H.; Lin, Z.H.; Lee, S.; Kim, S. Wear and triboelectric performance of polymers with non-polar lubricants. Tribol. Int. 2023, 178, 108088. [Google Scholar] [CrossRef]
- Buketov, A.; Sapronov, O.; Klevtsov, K.; Kim, B. Functional Polymer Nanocomposites with Increased Anticorrosion Properties and Wear Resistance for Water Transport. Polymers 2023, 15, 3449. [Google Scholar] [CrossRef] [PubMed]
| Samples | Ingredient | Mn (Da) | PDI | |||
|---|---|---|---|---|---|---|
| PPCDL a (g) | PEG1000 b (g) | HDI (g) | BDO (g) | |||
| TPU0 | 20.05 | 0 | 3.91 | 1.09 | 36,586 | 1.53 |
| TPU10 | 20.28 | 1.01 | 4.18 | 1.13 | 102,089 | 1.46 |
| TPU8 | 20.74 | 1.33 | 4.35 | 1.16 | 94,492 | 1.50 |
| TPU6 | 20.03 | 1.69 | 4.29 | 1.13 | 56,077 | 1.54 |
| TPU4 | 20.56 | 2.60 | 4.27 | 1.01 | 64,498 | 1.62 |
| TPU0 | TPU10 | TPU8 | TPU6 | TPU4 | |
|---|---|---|---|---|---|
| Hardness (Shore A) | 90 ± 1 | 85 ± 1 | 83 ± 1 | 75 ± 1 | 72 ± 1 |
| σ (MPa) | 8.9 | 27.5 | 25.6 | 17.1 | 16.5 |
| ε (%) | 1419 | 1995 | 2094 | 2169 | 2458 |
| ε 1500% (MPa) | / | 8.39 | 7.28 | 7.81 | 7.31 |
| Sample | TPU10 | TPU8 | TPU6 | TPU4 |
|---|---|---|---|---|
| 2000 circle Abrasion (cm3) | 0 | 0 | 0 | 0 |
| 4000 circle Abrasion (cm3) | 0.0120 | 0.0123 | 0.0119 | 0.0117 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, S.; Wang, J.; Yan, Q.; Li, A.; Liu, C.; Wu, X.; Meng, Y. Biodegradable, Wear-Resistant and Resilient Thermoplastic Polycarbonate-Based Polyurethane with Nanoscale Microphase Structure. Polymers 2025, 17, 1665. https://doi.org/10.3390/polym17121665
Su S, Wang J, Yan Q, Li A, Liu C, Wu X, Meng Y. Biodegradable, Wear-Resistant and Resilient Thermoplastic Polycarbonate-Based Polyurethane with Nanoscale Microphase Structure. Polymers. 2025; 17(12):1665. https://doi.org/10.3390/polym17121665
Chicago/Turabian StyleSu, Shuang, Jintao Wang, Qi Yan, Anqi Li, Chuang Liu, Xianli Wu, and Yuezhong Meng. 2025. "Biodegradable, Wear-Resistant and Resilient Thermoplastic Polycarbonate-Based Polyurethane with Nanoscale Microphase Structure" Polymers 17, no. 12: 1665. https://doi.org/10.3390/polym17121665
APA StyleSu, S., Wang, J., Yan, Q., Li, A., Liu, C., Wu, X., & Meng, Y. (2025). Biodegradable, Wear-Resistant and Resilient Thermoplastic Polycarbonate-Based Polyurethane with Nanoscale Microphase Structure. Polymers, 17(12), 1665. https://doi.org/10.3390/polym17121665








