Physicochemical Characterization and Properties of Cassava Starch: A Review
Abstract
1. Introduction
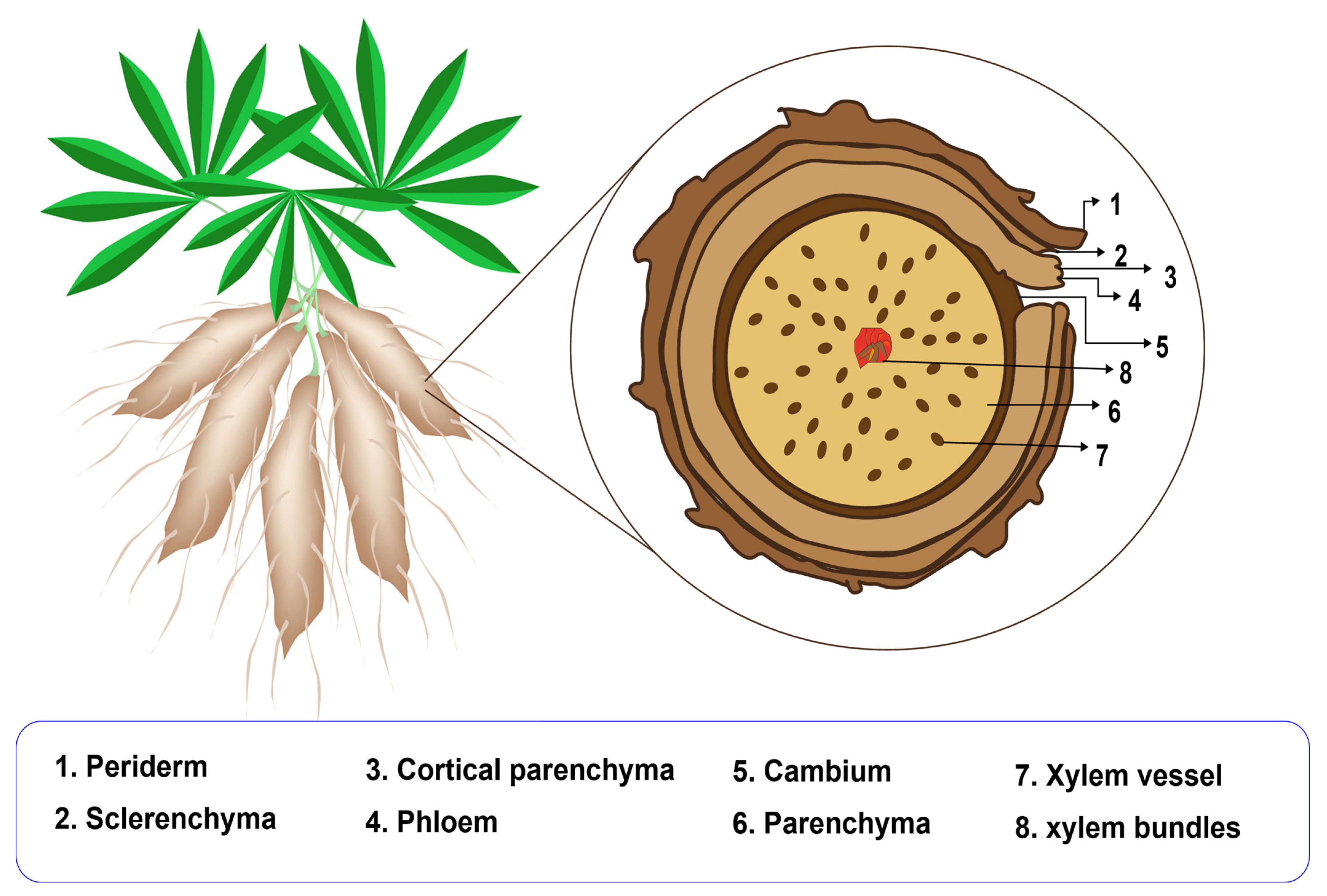
2. Cassava Starch Structure
3. Morphology and Characterization of Physical Cassava Starch Changes
3.1. Cassava Starch Granule Morphology
3.2. Molecular Mass of Cassava Starch
| Dissolution Process | Method | Mw (g mol−1) | Rg (nm) | RH (nm) | Ref. |
|---|---|---|---|---|---|
| 90% DMSO/H2O heated in boiling water-bath for 1 h with intermittent vortex mixing. | SEC/MALLS | 4.76 × 107 (AP) 2.52 × 106 (AM) | 118.6 ± 0.41 | -- | [32] |
| DMSO/LiBr heated, degassed with ultrasound in a boiling water bath for 1 h with continuous shaking. | GPC/MALLS | 1–3 × 107 | 95–135 | -- | [54] |
| 95% DMSO/H2O and solubilised in water by microwave heating under pressure. | HPSEC/MALLS/DRI | 1.30 × 108 | 186 ± 2 | [55] | |
| DMSO/water (95/5) mixture and then solubilized in water by microwave heating under pressure. | A4F/MALLS | 2.71–5.51 × 108 (AP) | 229–286 | - | [56] |
| DMSO /LiBr heated at 95 °C in boiling water-bath while stirring for 15 min, and 18 h at room temperature while continuously stirring. | SEC/MALLS | 5.7 × 107 | -- | 283–138 | [59] |
| 90% DMSO and heated in a boiling water bath for 1 h with constant stirring. | HPSEC/MALLS/DRI | 2.0 × 108 | 171.1 ± 18.5 | - | [61] |
| Starch samples were heated in a boiling hot water bath for 20 min with continuous stirring. | HPSEC/MALLS/R | 2.72 × 108 | 171.2 ± 3.67 | - | [62] |
| Water by microwave heating under pressure. | HPSEC/MALLS/VS/DRI | 4.10 × 107–2.17 × 107 | 130–132 | 89–97 | [63] |
| Sample solved in DMSO with concentration between 1–3 g mL−1. | SLS | 1.0 × 108 | - | - | [64] |
| Starches (25 mg) were solubilized in 1 M KOH (0.5 mL) for 3 days at 4 °C under gentle magnetic stirring, and 4.5 mL pure water and 0.1 M HCl (5 mL). | A4F/MALLS and HPSEC/MALLS | 4.08 × 108–5.20 × 108 | 277–285 | - | [65] |
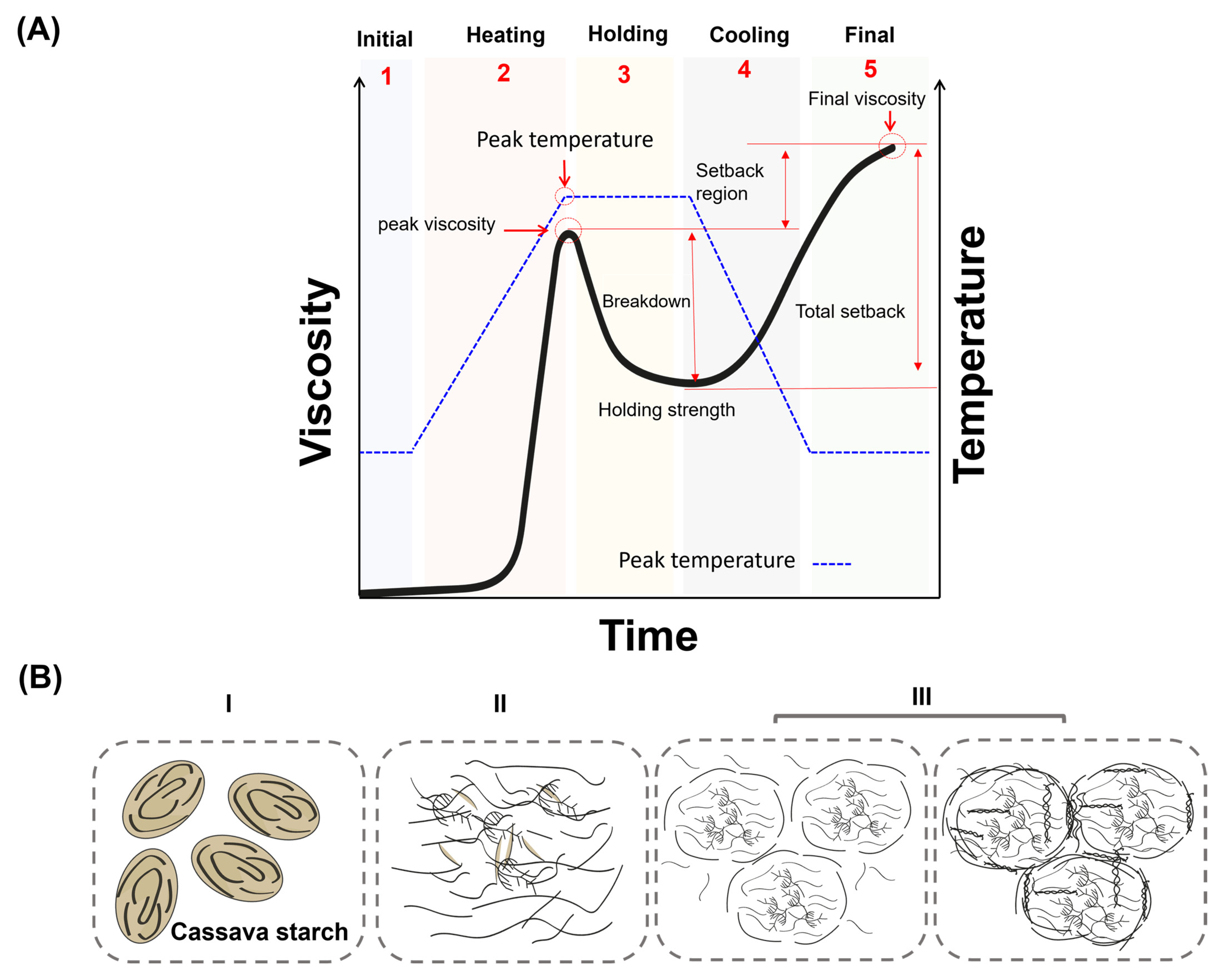
3.3. Starch Gelatinization
- (i)
- Initial hydration: Water is reversibly adsorbed on the surface of the granules, causing them to swell.
- (ii)
- Granule expansion and chain release: As more water is absorbed, the granules significantly increase in volume, which leads to a rapid rise in system viscosity and loss of birefringence. Simultaneously, the AM and AP chains are released and form a viscous solution through hydrogen bonding with water [73]. Gelatinization is thus a physical process that begins in the amorphous regions of the granule, where intermolecular forces are relatively weak [75]. This process results in the irreversible loss of molecular order, representing an order–disorder phase transition. From a thermodynamic perspective, it corresponds to an increase in the configurational entropy of the polymer chains (Figure 4A) [73].
- (iii)
- Granule disintegration: As the system surpasses the critical temperature, the granules lose their structural integrity, becoming formless sacs that disintegrate and disperse in hot water [76].

3.4. Pasting
3.5. Retrogradation
4. Spectroscopic Characterization of Cassava Starch
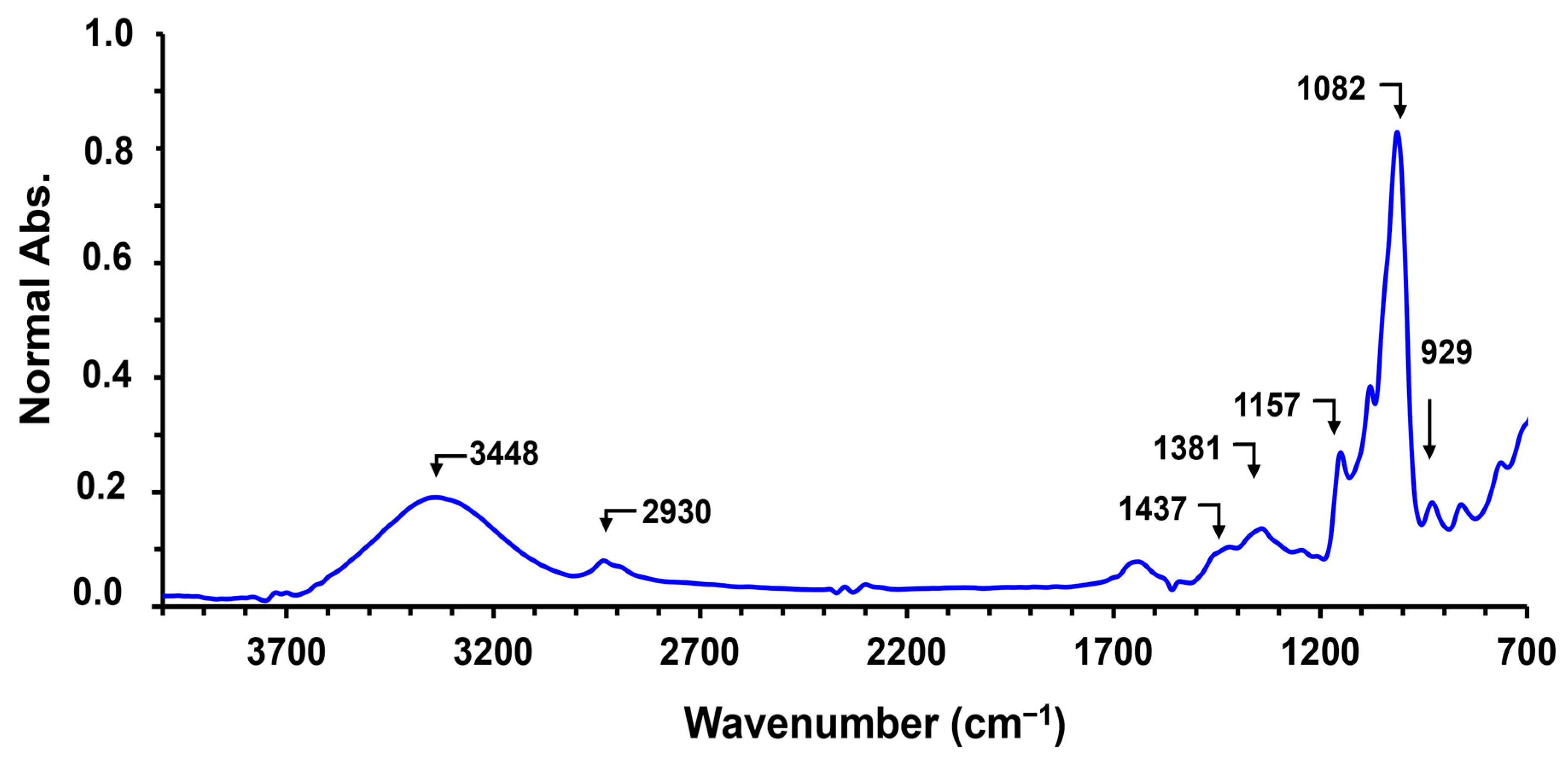
4.1. Fourier-Transform Infrared Spectroscopy (FTIR)
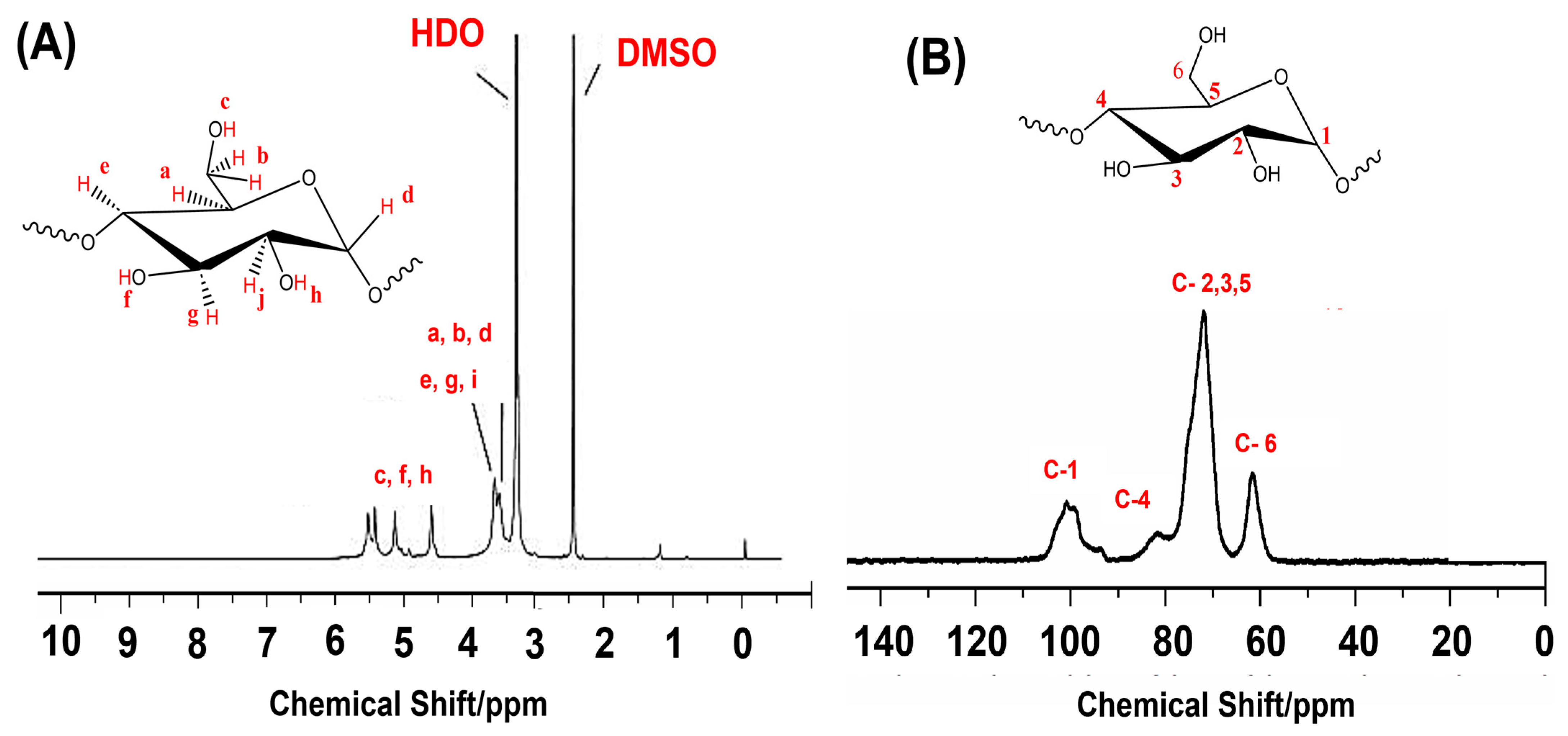
4.2. Nuclear Magnetic Resonance (NMR) Spectroscopy
4.3. Raman Spectroscopy
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AM | amylose |
| AP | amylopectin |
| SEM | scanning electron microscopy |
| AFM | atomic force microscopy |
| Mw | molecular weight |
| Rg | apparent radius of gyration |
| RH | apparent hydrodynamic radius |
| SEC | size-exclusion chromatography |
| GPC | gel permeation chromatography |
| HPSEC | high-performance size exclusion chromatography |
| MALLS | multi-angle laser light scattering |
| DRI | differential refractive index |
| A4F | asymmetrical flow field-flow fractionation |
| HPLC | high-performance liquid chromatography |
| DMSO | dimethyl sulfoxide |
| PDI | polydispersity index |
| Dt | translational diffusion coefficients |
| XRD | X-ray diffraction |
| DSC | differential scanning calorimetry |
| Tg | glass transition temperature |
| Tm | melting endotherm |
| ΔH | enthalpy change |
| RVA | Rapid Visco Analyzer |
| NaCl | sodium chloride |
| FTIR | Fourier-transform infrared spectroscopy |
| NMR | nuclear magnetic resonance spectroscopy |
| PCA | principal component analysis |
| PLS | partial least squares |
References
- Chisenga, S.M.; Workneh, T.S.; Bultosa, G.; Alimi, B.A. Progress in research and applications of cassava flour and starch: A review. J. Food Sci. Technol. 2019, 56, 2799–2813. [Google Scholar] [CrossRef] [PubMed]
- Howeler, R.H.; Hershey, C.H. Cassava in Asia: Research and development to increase its potential use in food, feed and industry: A Thai example. In Proceedings of the Research and Development of Cassava Production to Increase Its Potential for Processing, Animal Feed and Ethanol, Bangkok, Thailand, 16 January 2002; Centro Internacional de Agricultura Tropical (CIAT): Bangkok, Thailand, 2002. [Google Scholar]
- Adebayo, W.G. Cassava production in africa: A panel analysis of the drivers and trends. Heliyon 2023, 9, e19939. [Google Scholar] [CrossRef] [PubMed]
- Edhirej, A.; Sapuan, S.M.; Jawaid, M.; Zahari, N.I. Cassava: Its polymer, fiber, composite, and application. Polym. Compos. 2017, 38, 555–570. [Google Scholar] [CrossRef]
- Panghal, A.; Munezero, C.; Sharma, P.; Chhikara, N. Cassava toxicity, detoxification, and its food applications: A review. Toxin Rev. 2019, 40, 1–15. [Google Scholar] [CrossRef]
- Breuninger, W.F.; Piyachomkwan, K.; Sriroth, K. Tapioca/cassava starch: Production and use. In Starch; Academic Press: San Diego, CA, USA, 2009; pp. 541–568. [Google Scholar] [CrossRef]
- Bradbury, J.H.; Holloway, W.D. Chemistry of Tropical Root Crops: Significance for Nutrition and Agriculture in the Pacific; No. 435-2016-33750; Australian Centre for International Agricultural Research (ACIAR): Bruce, Australia, 1988.
- Daud, Z.; Awang, H.; Kassim, A.S.M.; Hatta, M.Z.M.; Aripin, A.M. Comparison of pineapple leaf and cassava peel by chemical properties and morphology characterization. Adv. Mater. Res. 2014, 974, 384–388. [Google Scholar] [CrossRef]
- Mehdi, R.; Lamm, C.E.; Bodampalli, A.R.; Müdsam, C.; Saeed, M.; Klima, J.; Kraner, M.E.; Ludewig, F.; Knoblauch, M.; Gruissem, W.; et al. Symplasmic phloem unloading and radial post-phloem transport via vascular rays in tuberous roots of Manihot esculenta. J. Exp. Bot. 2019, 70, 5559–5573. [Google Scholar] [CrossRef]
- Saengchan, K.; Nopharatana, M.; Lerdlattaporn, R.; Songkasiri, W. Enhancement of starch-pulp separation in centrifugal-filtration process: Effects of particle size and variety of cassava root on free starch granule separation. Food Bioprod. Process. 2015, 95, 208–217. [Google Scholar] [CrossRef]
- Tharanathan, R.N. Starch—Value addition by modification. Crit. Rev. Food Sci. Nutr. 2005, 45, 371–384. [Google Scholar] [CrossRef]
- Pokhrel, S. A review on introduction and applications of starch and its biodegradable polymers. Int. J. Environ. 2015, 4, 114–125. [Google Scholar] [CrossRef]
- Tappiban, P.; Smith, D.R.; Triwitayakorn, K.; Bao, J. Recent understanding of starch biosynthesis in cassava for quality improvement: A review. Trends Food Sci. Technol. 2019, 83, 167–180. [Google Scholar] [CrossRef]
- Lu, D.R.; Xiao, C.M.; Xu, S.J. Starch-based completely biodegradable polymer materials. Express Polym. Lett. 2009, 3, 366–375. [Google Scholar] [CrossRef]
- Pfister, B.; Zeeman, S.C. Formation of starch in plant cells. Cell. Mol. Life Sci. 2016, 73, 2781–2807. [Google Scholar] [CrossRef] [PubMed]
- Bonechi, C.; Consumi, M.; Donati, A.; Leone, G.; Magnani, A. Biomass: An overview. In Bioenergy Systems for the Future; Woodhead Publishing: Cambridge, UK, 2017; pp. 3–42. [Google Scholar] [CrossRef]
- Imberty, A.; Buléon, A.; Tran, V.; Péerez, S. Recent advances in knowledge of starch structure. Starch-Stärke 1991, 43, 375–384. [Google Scholar] [CrossRef]
- Wang, S.; Li, C.; Copeland, L.; Niu, Q.; Wang, S. Starch retrogradation: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 568–585. [Google Scholar] [CrossRef]
- Masina, N.; Choonara, Y.E.; Kumar, P.; du Toit, L.C.; Govender, M.; Indermun, S.; Pillay, V. A review of the chemical modification techniques of starch. Carbohydr. Polym. 2017, 157, 1226–1236. [Google Scholar] [CrossRef]
- Zhang, X.; Szydlowski, N.; Delvallé, D.; d’Hulst, C.; James, M.G.; Myers, A.M. Overlapping functions of the starch synthases SSII and SSIII in amylopectin biosynthesis in Arabidopsis. BMC Plant Biol. 2008, 8, 96. [Google Scholar] [CrossRef]
- Biliaderis, C.G. Structural transitions and related physical properties of starch. In Starch; Academic Press: San Diego, CA, USA, 2009; pp. 293–372. [Google Scholar] [CrossRef]
- Seung, D. Amylose in starch: Towards an understanding of biosynthesis, structure and function. New Phytol. 2020, 228, 1490–1504. [Google Scholar] [CrossRef]
- Chinma, C.E.; Ariahu, C.C.; Abu, J.O. Chemical composition, functional and pasting properties of cassava starch and soy protein concentrate blends. J. Food Sci. Technol. 2013, 50, 1179–1185. [Google Scholar] [CrossRef]
- Sriroth, K.; Santisopasri, V.; Petchalanuwat, C.; Kurotjanawong, K.; Piyachomkwan, K.; Oates, C. Cassava starch granule structure–function properties: Influence of time and conditions at harvest on four cultivars of cassava starch. Carbohydr. Polym. 1999, 38, 161–170. [Google Scholar] [CrossRef]
- Freitas, R.A.; Paula, R.C.; Feitosa, J.P.A.; Rocha, S.; Sierakowski, M.R. Amylose contents, rheological properties and gelati-nization kinetics of yam (Dioscorea alata) and cassava (Manihot utilissima) starches. Carbohydr. Polym. 2004, 55, 3–8. [Google Scholar] [CrossRef]
- Numfor, F.A.; Walter, W.M., Jr.; Schwartz, S.J. Physicochemical changes in cassava starch and flour associated with fer-mentation: Effect on textural properties. Starch-Stärke 1995, 47, 86–91. [Google Scholar] [CrossRef]
- Asaoka, M.; Blanshard, J.M.V.; Rickard, J.E. Seasonal effects on the physico-chemical properties of starch from four cultivars of cassava. Starch-Stärke 1991, 43, 455–459. [Google Scholar] [CrossRef]
- Nuwamanya, E.; Baguma, Y.; Wembabazi, E.; Rubaihayo, P. A comparative study of the physicochemical properties of starches from root, tuber and cereal crops. Afr. J. Biotechnol. 2011, 10, 12018–12030. [Google Scholar] [CrossRef]
- Oyeyinka, S.A.; Adeloye, A.A.; Smith, S.A.; Adesina, B.O.; Akinwande, F.F. Physicochemical properties of flour and starch from two cassava varieties. Agrosearch 2019, 19, 28–45. [Google Scholar] [CrossRef]
- Aryee, F.N.A.; Oduro, I.; Ellis, W.O.; Afuakwa, J.J. The physicochemical properties of flour samples from the roots of 31 varieties of cassava. Food Control 2006, 17, 916–922. [Google Scholar] [CrossRef]
- Farias, F.D.A.C.; de Souza Moretti, M.M.; Costa, M.S.; Bordignon Junior, S.E.; Cavalcante, K.B.; Boscolo, M.; Gomes, E.; Franco, C.M.L.; da Silva, R. Structural and physicochemical characteristics of taioba starch in comparison with cassava starch and its potential for ethanol production. Ind. Crops Prod. 2020, 157, 112825. [Google Scholar] [CrossRef]
- Yuliarti, O.; Gusti, E.; Chiang, J.H.; Teo, P.X.; Ng, J.Y. Rheological and microstructural properties of native cassava starch-low methoxyl pectin in a fruit filling gel system. LWT 2021, 146, 111568. [Google Scholar] [CrossRef]
- Kayode, B.I.; Kayode, R.M.; Salami, K.O.; Obilana, A.O.; George, T.T.; Dudu, O.E.; Adebo, O.A.; Njobeh, P.B.; Diarra, S.S.; Oyeyinka, S.A. Morphology and physicochemical properties of starch isolated from frozen cassava root. LWT 2021, 147, 111546. [Google Scholar] [CrossRef]
- Alvis, A.; Vélez, C.A.; Villada, H.S.; Rada-Mendoza, M. Análisis físico-químico y morfológico de almidones de ñame, yuca y papa y determinación de la viscosidad de las pastas. Inf. Tecnol. 2008, 19, 19–28. [Google Scholar] [CrossRef]
- Zhang, Y.; Nie, L.; Sun, J.; Hong, Y.; Yan, H.; Li, M.; You, X.; Zhu, L.; Fang, F. Impacts of environmental factors on pasting properties of cassava flour mediated by its macronutrients. Front. Nutr. 2020, 7, 272. [Google Scholar] [CrossRef]
- Hoover, R. Composition, molecular structure, and physicochemical properties of tuber and root starches: A review. Carbohydr. Polym. 2001, 45, 253–267. [Google Scholar] [CrossRef]
- Szymońska, J.; Targosz-Korecka, M.; Krok, F. Characterization of starch nanoparticles. J. Phys. Conf. Ser. 2009, 146, 012027. [Google Scholar] [CrossRef]
- Santisopasri, V.; Kurotjanawong, K.; Chotineeranat, S.; Piyachomkwan, K.; Sriroth, K.; Oates, C.G. Impact of water stress on yield and quality of cassava starch. Ind. Crops Prod. 2001, 13, 115–129. [Google Scholar] [CrossRef]
- Janket, A.; Vorasoot, N.; Toomsan, B.; Kaewpradit, W.; Jogloy, S.; Theerakulpisut, P.; Holbrook, C.C.; Kvien, C.K.; Banterng, P. Starch accumulation and granule size distribution of cassava cv. Rayong 9 grown under irrigated and rainfed conditions using different growing seasons. Agronomy 2020, 10, 412. [Google Scholar] [CrossRef]
- Leonel, M.; de Souza Fernandes, D.; Dos Santos, T.P.R. Unmodified cassava starches with high phosphorus content. Int. J. Biol. Macromol. 2021, 187, 113–118. [Google Scholar] [CrossRef]
- Kasemwong, K.; Ruktanonchai, U.R.; Srinuanchai, W.; Itthisoponkul, T.; Sriroth, K. Effect of high-pressure microfluidization on the structure of cassava starch granule. Starch-Stärke 2011, 63, 160–170. [Google Scholar] [CrossRef]
- Teerawanichpan, P.; Lertpanyasampatha, M.; Netrphan, S.; Varavinit, S.; Boonseng, O.; Narangajavana, J. Influence of cassava storage root development and environmental conditions on starch granule size distribution. Starch-Stärke 2008, 60, 696–705. [Google Scholar] [CrossRef]
- Luchese, C.L.; Spada, J.C.; Tessaro, I.C. Starch content affects physicochemical properties of corn and cassava starch-based films. Ind. Crops Prod. 2017, 109, 619–626. [Google Scholar] [CrossRef]
- Moorthy, S.N.; Ramanujam, T. Variation in properties of starch in cassava varieties in relation to age of the crop. Starch-Stärke 1986, 38, 58–61. [Google Scholar] [CrossRef]
- Janket, A.; Vorasoot, N.; Toomsan, B.; Kaewpradit, W.; Theerakulpisut, P.; Holbrook, C.C.; Kvien, C.K.; Jogloy, S.; Banterng, P. Accumulation dynamics of starch and its granule size distribution of cassava genotypes at different growing seasons. Agriculture 2020, 10, 380. [Google Scholar] [CrossRef]
- Li, M.; Daygon, V.D.; Solah, V.; Dhital, S. Starch granule size: Does it matter? Crit. Rev. Food Sci. Nutr. 2023, 63, 3683–3703. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.; Hong, Y.; Zhang, Y.; Gu, Z.; Cheng, L.; Li, Z.; Li, C. Effect of cassava starch structure on scalding of dough and baking expansion ability. Food Chem. 2021, 352, 129350. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.T.; Jane, J.L.; Rajagopalan, S.; Seib, P.A. Effect of starch granule size on physical properties of starch-filled polyethylene film. Biotechnol. Progr. 1992, 8, 51–57. [Google Scholar] [CrossRef]
- Uchechukwu-Agua, A.D.; Caleb, O.J.; Opara, U.L. Postharvest handling and storage of fresh cassava root and products: A review. Food Bioprocess Technol. 2015, 8, 729–748. [Google Scholar] [CrossRef]
- Tan, X.; Gu, B.; Li, X.; Xie, C.; Chen, L.; Zhang, B. Effect of growth period on the multi-scale structure and physicochemical properties of cassava starch. Int. J. Biol. Macromol. 2017, 101, 9–15. [Google Scholar] [CrossRef]
- Gu, B.; Yao, Q.; Li, K.; Chen, S. Change in physicochemical traits of cassava roots and starches associated with gentypes and environmental factors. Starch-Stärke 2013, 65, 253–263. [Google Scholar] [CrossRef]
- Jindaluang, W.; Somarsa, W.; Darunsontaya, T.; Anusontpornperm, S.; Jaroenchasri, R. Effect of Chicken Manure and Cassava Starch Manufacturing Wastes on Aggregate Stability and Yield of Cassava Grown on Sandy Soil. J. Soil Sci. Plant Nutr. 2025, 25, 291–302. [Google Scholar] [CrossRef]
- Wongnoi, S.; Banterng, P.; Vorasoot, N.; Jogloy, S.; Theerakulpisut, P. Physiology, growth and yield of different cassava genotypes planted in upland with dry environment during high storage root accumulation stage. Agronomy 2020, 10, 576. [Google Scholar] [CrossRef]
- Liu, K.; Zu, Y.; Chi, C.; Gu, B.; Chen, L.; Li, X. Modulation of the digestibility and multi-scale structure of cassava starch by controlling the cassava growth period. Int. J. Biol. Macromol. 2018, 120, 346–353. [Google Scholar] [CrossRef]
- Tetchi, F.A.; Rolland-Sabaté, A.; Amani, G.N.G.; Colonna, P. Molecular and physicochemical characterisation of starches from yam, cocoyam, cassava, sweet potato and ginger produced in the Ivory Coast. J. Sci. Food Agric. 2007, 87, 1906–1916. [Google Scholar] [CrossRef]
- Morante, N.; Ceballos, H.; Sánchez, T.; Rolland-Sabaté, A.; Calle, F.; Hershey, C.; Gibert, O.; Dufour, D. Discovery of new spontaneous sources of amylose-free cassava starch and analysis of their structure and techno-functional properties. Food Hydrocoll. 2016, 56, 383–395. [Google Scholar] [CrossRef]
- Rolland-Sabaté, A.; Sánchez, T.; Buléon, A.; Colonna, P.; Ceballos, H.; Zhao, S.-S.; Zhang, P.; Dufour, D. Molecular and supra-molecular structure of waxy starches developed from cassava (Manihot esculenta Crantz). Carbohydr. Polym. 2013, 92, 1451–1462. [Google Scholar] [CrossRef] [PubMed]
- Cave, R.A.; Seabrook, S.A.; Gidley, M.J.; Gilbert, R.G. Characterization of starch by size-exclusion chromatography: The limitations imposed by shear scission. Biomacromolecules 2009, 10, 2245–2253. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, W.; Renner-Nantz, J.J.; Shoemaker, C.F. Starch molecular mass and size by size-exclusion chromatography in DMSO-LiBr coupled with multiple angle laser light scattering. Cereal Chem. 1998, 75, 530–535. [Google Scholar] [CrossRef]
- Ruebsam, H.; Krottenthaler, M.; Gastl, M.; Becker, T. An overview of separation methods in starch analysis: The importance of size exclusion chromatography and field flow fractionation. Starch-Stärke 2012, 64, 683–695. [Google Scholar] [CrossRef]
- Castanha, N.; Miano, A.C.; Jones, O.G.; Reuhs, B.L.; Campanella, O.H.; Augusto, P.E. Starch modification by ozone: Correlating molecular structure and gel properties in different starch sources. Food Hydrocoll. 2020, 108, 106027. [Google Scholar] [CrossRef]
- Mufumbo, R.; Yona, B.; Stephen, K.; Nuwamanya, E.; Patrick, R.; Mukasa, S.B.; Bruce, H.; Kyamanywa, S. Amylopectin molecular structure and functional properties of starch from three Ugandan cassava varieties. J. Plant Breed. Crop Sci. 2011, 3, 195–202. [Google Scholar] [CrossRef]
- Boonna, S.; Rolland-Sabaté, A.; Lourdin, D.; Tongta, S. Macromolecular characteristics and fine structure of amylomaltase-treated cassava starch. Carbohydr. Polym. 2019, 205, 143–150. [Google Scholar] [CrossRef]
- Clasen, S.H.; Müller, C.M.; Parize, A.L.; Pires, A.T.S. Synthesis and characterization of cassava starch with maleic acid derivatives by etherification reaction. Carbohydr. Polym. 2018, 180, 348–353. [Google Scholar] [CrossRef]
- Rolland-Sabaté, A.; Sánchez, T.; Buléon, A.; Colonna, P.; Jaillais, B.; Ceballos, H.; Dufour, D. Structural characterization of novel cassava starches with low and high-amylose contents in comparison with other commercial sources. Food Hydrocoll. 2012, 27, 161–174. [Google Scholar] [CrossRef]
- Tappiban, P.; Ying, Y.; Pang, Y.; Sraphet, S.; Srisawad, N.; Smith, D.R.; Wu, P.; Triwitayakorn, K.; Bao, J. Gelatinization, pasting and retrogradation properties and molecular fine structure of starches from seven cassava cultivars. Int. J. Biol. Macromol. 2020, 150, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Sillanpää, M.; Matilainen, A.; Lahtinen, T. Characterization of NOM. Nat. Org. Matter Water 2015, 2, 17–53. [Google Scholar] [CrossRef]
- Guo, L.; Li, J.; Gui, Y.; Zhu, Y.; Cui, B. Improving waxy rice starch functionality through branching enzyme and glucoamylase: Role of amylose as a viable substrate. Carbohydr. Polym. 2020, 230, 115712. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Miao, M.; Jiang, H.; Xue, J.; Jiang, B.; Zhang, T.; Gao, Y.; Jia, Y. Partial branching enzyme treatment increases the low glycaemic property and α-1,6 branching ratio of maize starch. Food Chem. 2014, 164, 502–509. [Google Scholar] [CrossRef]
- Lemos, P.V.F.; Barbosa, L.S.; Ramos, I.G.; Coelho, R.E.; Druzian, J.I. Characterization of amylose and amylopectin fractions separated from potato, banana, corn, and cassava starches. Int. J. Biol. Macromol. 2019, 132, 32–42. [Google Scholar] [CrossRef]
- Yohannes, G.; Jussila, M.; Hartonen, K.; Riekkola, M.L. Asymmetrical flow field-flow fractionation technique for separation and characterization of biopolymers and bioparticles. J. Chromatogr. A 2011, 1218, 4104–4116. [Google Scholar] [CrossRef]
- Juna, S.; Huber, A. Determination of molar mass distribution of tapioca starch using asymmetrical flow field flow fractionation. Starch-Stärke 2012, 64, 87–96. [Google Scholar] [CrossRef]
- BeMiller, J.N. Starches: Molecular and granular structures and properties. Carbohydr. Chem. Food Sci. 2019, 3, 159–189. [Google Scholar] [CrossRef]
- Schmiele, M.; Sampaio, U.M.; Gomes, P.T.G.; Clerici, M.T.P.S. Physical modifications of starch. In Starches for Food Application; Academic Press: San Diego, CA, USA, 2019; pp. 223–269. [Google Scholar] [CrossRef]
- Ratnayake, W.S.; Jackson, D.S. Starch gelatinization. Adv. Food Nutr. Res. 2008, 55, 221–268. [Google Scholar] [CrossRef]
- French, D. Fine structure of starch and its relationship to the organization of starch granules. J. Jpn. Soc. Starch Sci. 1972, 19, 8–25. [Google Scholar] [CrossRef]
- Marchant, J.L.; Blanshard, J.M.V. Studies of the dynamics of the gelatinization of starch granules employing a small angle light scattering system. Starch-Stärke 1978, 30, 257–264. [Google Scholar] [CrossRef]
- Zhu, F. Composition, structure, physicochemical properties, and modifications of cassava starch. Carbohydr. Polym. 2015, 122, 456–480. [Google Scholar] [CrossRef] [PubMed]
- Altay, F.; Gunasekaran, S. Influence of drying temperature, water content, and heating rate on gelatinization of corn starches. J. Agric. Food Chem. 2006, 54, 4235–4245. [Google Scholar] [CrossRef] [PubMed]
- Dome, K.; Podgorbunskikh, E.; Bychkov, A.; Lomovsky, O. Changes in the crystallinity degree of starch having different types of crystal structure after mechanical pretreatment. Polymers 2020, 12, 641. [Google Scholar] [CrossRef]
- Perotti, G.F.; Tronto, J.; Bizeto, M.A.; Izumi, C.; Temperini, M.L.; Lugão, A.B.; Parra, D.F.; Constantino, V.R. Biopolymer-clay nanocomposites: Cassava starch and synthetic clay cast films. J. Brazil. Chem. Soc. 2014, 25, 320–330. [Google Scholar] [CrossRef]
- Lin, R.H.; Fan, Y.Y.; Liu, T.; Yang, H.; Ma, L.J.; Huang, X.; Liu, Y. Structural characterization of controlled decrystallization of cassava starch. Starch-Stärke 2020, 72, 1900049. [Google Scholar] [CrossRef]
- Huang, Z.Q.; Lu, J.P.; Li, X.H.; Tong, Z.F. Effect of mechanical activation on physico-chemical properties and structure of cassava starch. Carbohydr. Polym. 2007, 68, 128–135. [Google Scholar] [CrossRef]
- Nuwamanya, E.; Baguma, Y.; Emmambux, M.N.; Rubaihayo, P. Crystalline and pasting properties of cassava starch are influenced by its molecular properties. Afr. J. Food Sci. 2010, 41, 8–15. [Google Scholar]
- Defloor, I.; Dehing, I.; Delcour, J.A. Physico-chemical properties of cassava starch. Starch-Stärke 1998, 50, 58–64. [Google Scholar] [CrossRef]
- Tô, H.T.; Karrila, S.J.; Nga, L.H.; Karrila, T.T. Effect of blending and pregelatinizing order on properties of pregelatized starch from rice and cassava. Food Res. 2020, 4, 102–112. [Google Scholar] [CrossRef]
- Zobel, H.F.; Young, S.N.; Rocca, L.A. Starch gelatinization: An X-ray diffraction study. Cereal Chem. 1988, 65, 443–446. [Google Scholar]
- Hornung, P.S.; do Prado Cordoba, L.; da Silveira Lazzarotto, S.R.; Schnitzler, E.; Lazzarotto, M.; Ribani, R.H. Brazilian Dioscore-aceas starches. J. Therm. Anal. Calorim. 2017, 127, 1869–1877. [Google Scholar] [CrossRef]
- Freire, E. Differential scanning calorimetry. In Protein Stability and Folding; Human Press: Totowa, NJ, USA, 1995; pp. 191–218. [Google Scholar] [CrossRef]
- Biliaderis, C.G.; Maurice, T.J.; Vose, J.R. Starch gelatinization phenomena studied by differential scanning calorimetry. J. Food Sci. 1980, 45, 1669–1674. [Google Scholar] [CrossRef]
- Zaidul, I.S.M.; Absar, N.; Kim, S.J.; Suzuki, T.; Karim, A.A.; Yamauchi, H.; Noda, T. DSC study of mixtures of wheat flour and potato, sweet potato, cassava, and yam starches. J. Food Eng. 2008, 86, 68–73. [Google Scholar] [CrossRef]
- Beninca, C.; Colman, T.A.D.; Lacerda, L.G.; da Silva Carvalho Filho, M.A.; Demiate, I.M.; Bannach, G.; Schnitzler, E. Thermal, rheological, and structural behaviors of natural and modified cassava starch granules, with sodium hypochlorite solutions. J. Therm. Anal. Calorim. 2013, 111, 2217–2222. [Google Scholar] [CrossRef]
- Liu, T.Y.; Ma, Y.; Yu, S.F.; Shi, J.; Xue, S. The effect of ball milling treatment on structure and porosity of maize starch granule. Innov. Food Sci. Emerg. Technol. 2011, 12, 586–593. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, J.; Luo, S.; Li, C.; Ye, J.; Liu, C.; Gilbert, R.G. Physicochemical and structural properties of pregelatinized starch prepared by improved extrusion cooking technology. Carbohydr. Polym. 2017, 175, 265–272. [Google Scholar] [CrossRef]
- Pérez, E.E.; Breene, W.M.; Bahnassey, Y.A. Variations in the gelatinization profiles of cassava, Sagu, and arrowroot native starches as measured with different thermal and mechanical methods. Starch/Stärke 1998, 50, 70–72. [Google Scholar] [CrossRef]
- Sajeev, M.S.; Moorthy, S.N.; Kailappan, R.; Rani, V.S. Gelatinization characteristics of cassava starch settled in the presence of different chemicals. Starch/Stärke 2003, 55, 213–221. [Google Scholar] [CrossRef]
- Garcia, M.C.; Franco, C.M.L.; Júnior, M.S.S.; Caliari, M. Structural characteristics and gelatinization properties of sour cassava starch. J. Therm. Anal. Calorim. 2016, 123, 919–926. [Google Scholar] [CrossRef]
- Atichokudomchai, N.; Varavinit, S.; Chinachoti, P. Gelatinization transitions of acid-modified tapioca starches by differential scanning calorimetry (DSC). Starch/Stärke 2002, 54, 296–302. [Google Scholar] [CrossRef]
- Sangseethong, K.; Termvejsayanon, N.; Sriroth, K. Characterization of physicochemical properties of hypochlorite- and peroxide-oxidized cassava starches. Carbohydr. Polym. 2010, 82, 446–453. [Google Scholar] [CrossRef]
- Temsiripong, T.; Pongsawatmanit, R.; Ikeda, S.; Nishinari, K. Influence of xyloglucan on gelatinization and retro-gradation of tapioca starch. Food Hydrocoll. 2005, 19, 1054–1063. [Google Scholar] [CrossRef]
- Andrade, M.M.P.; Oliveira, C.S.; Colman, T.A.D.; Costa, F.J.O.G.; Schnitzler, E. Effects of heat–moisture treatment on organic cassava starch. J. Therm. Anal. Calorim. 2014, 115, 2115–2122. [Google Scholar] [CrossRef]
- Cornejo, F.; Maldonado-Alvarado, P.; Palacios-Ponce, S.; Hugo, D.; Rosell, C.M. Impact of cassava starch varieties on the physiochemical change during enzymatic hydrolysis. Molecules 2022, 27, 6098. [Google Scholar] [CrossRef]
- Wang, S.; Chao, C.; Cai, J.; Niu, B.; Copeland, L.; Wang, S. Starch–lipid and starch–lipid–protein complexes: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1056–1079. [Google Scholar] [CrossRef]
- Keeratiburana, T.; Hansen, A.R.; Soontaranon, S.; Blennow, A.; Tongta, S. Porous high amylose rice starch modified by amyloglucosidase and maltogenic α-amylase. Carbohydr. Polym. 2020, 230, 115611. [Google Scholar] [CrossRef]
- Garcia, V.; Colonna, P.; Lourdin, D.; Buleon, A.; Bizot, H.; Ollivon, M. Thermal transitions of cassava starch at intermediate water contents. J. Therm. Anal. Calorim. 1996, 47, 1213–1228. [Google Scholar] [CrossRef]
- Sandoval, A.A.; Rodríguez, S.E.; Fernández, Q.A. Application of analysis by differential scanning calorimetry (DSC) for the characterization of the modifications of the starch. DYNA 2005, 72, 45–53. [Google Scholar]
- Chatakanonda, P.; Chinachoti, P.; Sriroth, K.; Piyachomkwan, K.; Chotineeranat, S.; Tang, H.-R.; Hills, B. The influence of time and conditions of harvest on the functional behaviour of cassava starch—A proton NMR relaxation study. Carbohydr. Polym. 2003, 53, 233–240. [Google Scholar] [CrossRef]
- Englyst, H.N.; Kingman, S.M.; Cummings, J.H. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 1992, 46, S33–S50. [Google Scholar] [PubMed]
- Pereira, B.L.B.; Leonel, M. Resistant starch in cassava products. Food Sci. Technol. 2014, 34, 298–302. [Google Scholar] [CrossRef][Green Version]
- Wang, Y.; Ou, X.; Al-Maqtari, Q.A.; He, H.J.; Othman, N. Evaluation of amylose content: Structural and functional properties, analytical techniques, and future prospects. Food Chem. 2024, 24, 101830. [Google Scholar] [CrossRef] [PubMed]
- Mejía-Agüero, L.E.; Galeno, F.; Hernández-Hernández, O.; Matehus, J.; Tovar, J. Starch determination, amylose content and susceptibility to in vitro amylolysis in flours from the roots of 25 cassava varieties. J. Sci. of Food Agric. 2012, 92, 673–678. [Google Scholar] [CrossRef]
- Chatpapamon, C.; Wandee, Y.; Uttapap, D.; Puttanlek, C.; Rungsardthong, V. Pasting properties of cassava starch modified by heat-moisture treatment under acidic and alkaline pH environments. Carbohydr. Polym. 2019, 215, 338–347. [Google Scholar] [CrossRef]
- Balet, S.; Guelpa, A.; Fox, G.; Manley, M. Rapid visco analyser (RVA) as a tool for measuring starch-related physiochemical properties in cereals: A review. Food Anal. Methods 2019, 12, 2344–2360. [Google Scholar] [CrossRef]
- Defenbaugh, L.B.; Walker, C.E. Comparison of starch pasting properties in the Brabender viscoamylograph and the rapid visco-analyzer. Cereal Chem. 1989, 66, 493–499. [Google Scholar]
- Kaur, L.; Singh, J.; McCarthy, O.J.; Singh, H. Physico-chemical, rheological and structural properties of fractionated potato starches. J. Food Eng. 2007, 82, 383–394. [Google Scholar] [CrossRef]
- Suh, D.S.; Jane, J.L. Comparison of starch pasting properties at various cooking conditions using the micro visco-amylo-graph and the rapid visco analyser. Cereal Chem. 2003, 80, 745–749. [Google Scholar] [CrossRef]
- Hossen, M.S.; Sotome, I.; Takenaka, M.; Isobe, S.; Nakajima, M.; Okadome, H. Effect of particle size of different crop starches and their flours on pasting properties. Jpn. J. Food Eng. 2011, 12, 29–35. [Google Scholar] [CrossRef]
- Singh, S.; Singh, N.; Isono, N.; Noda, T. Relationship of granule size distribution and amylopectin structure with pasting, thermal, and retrogradation properties in wheat starch. J. Agric Food Chem. 2010, 58, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Nastasi, J.R.; Alagappan, S.; Cozzolino, D. The Combination of Machine Learning Tools with the Rapid Visco Analyser (RVA) to Enhance the Analysis of Starchy Food Ingredients and Products. Appl. Sci. 2025, 15, 3376. [Google Scholar] [CrossRef]
- Zaidul, I.S.M.; Norulaini, N.N.; Omar, A.M.; Yamauchi, H.; Noda, T. RVA analysis of mixtures of wheat flour and potato, sweet potato, yam, and cassava starches. Carbohydr. Polym. 2007, 69, 784–791. [Google Scholar] [CrossRef]
- Concepcion, J.C.T.; Ouk, M.; Zhao, D.; Fitzgerald, M.A. The need for new tools and investment to improve the accuracy of selecting for grain quality in rice. Field Crops Res. 2015, 182, 60–67. [Google Scholar] [CrossRef]
- Liu, S.; Yuan, T.Z.; Wang, X.; Reimer, M.; Isaak, C.; Ai, Y. Behaviors of starches evaluated at high heating temperatures using a new model of Rapid Visco Analyzer–RVA 4800. Food Hydrocoll. 2019, 94, 217–228. [Google Scholar] [CrossRef]
- Rodríguez, E.E.; Fernández, A.; Cuvelier, G.; Relkin, P.; Bello, L.A. Starch retrogradation in cassava flour from cooked parenchyma. Starch/Stärke 2008, 60, 174–180. [Google Scholar] [CrossRef]
- Adepeju, A.B.; Oladapo, A.S.; Adepeju, D.M.; Adisa, A.M. Comparative Studies of Starch from Fresh and Dried Cassava Chips. Int. J. Sci. Eng. Sci. 2017, 1, 21–24. [Google Scholar]
- Fredriksson, H.; Silverio, J.; Andersson, R.; Eliasson, A.C.; Åman, P.J.C.P. The influence of amylose and amylopectin characteristics on gelatinization and retrogradation properties of different starches. Carbohydr. Polym. 1998, 35, 119–134. [Google Scholar] [CrossRef]
- Zeleznak, K.J.; Hoseney, R.C. The role of water in the retrogradation of wheat starch gels and bread crumb. Cereal Chem. 1986, 63, 407–411. [Google Scholar]
- Zhou, X.; Wang, R.; Yoo, S.H.; Lim, S.T. Water effect on the interaction between amylose and amylopectin during retrogradation. Carbohydr. Polym. 2011, 86, 1671–1674. [Google Scholar] [CrossRef]
- Christianson, D.D.; Hodge, J.E.; Osborne, D.; Detroy, R.W. Gelatinization of wheat starch as modified by xanthan gum, guar gum, and cellulose gum. Cereal Chem. 1981, 58, 513–517. [Google Scholar]
- Seetapan, N.; Fuongfuchat, A.; Gamonpilas, C.; Methacanon, P.; Pongjaruwat, W.; Limparyoon, N. Effect of modified tapioca starch and xanthan gum on low-temperature texture stability and dough viscoelasticity of a starch-based food gel. J. Food Eng. 2013, 119, 446–453. [Google Scholar] [CrossRef]
- Kohyama, K.; Nishinari, K. Effect of soluble sugars on gelatinization and retrogradation of sweet potato starch. J. Agric. Food Chem. 1991, 39, 1406–1410. [Google Scholar] [CrossRef]
- Donmez, D.; Pinho, L.; Patel, B.; Desam, P.; Campanella, O.H. Characterization of starch–water interactions and their effects on two key functional properties: Starch gelatinization and retrogradation. Curr. Opin. Food Sci. 2021, 39, 103–109. [Google Scholar] [CrossRef]
- Thirathumthavorn, D.; Trisuth, T. Gelatinization and retrogradation properties of native and hydroxypropylated crosslinked tapioca starches with added sucrose and sodium chloride. Int. J. Food Prop. 2008, 11, 858–864. [Google Scholar] [CrossRef][Green Version]
- Berthomieu, C.; Hienerwadel, R. Fourier transform infrared (FTIR) spectroscopy. Photosynth. Res. 2009, 101, 157–170. [Google Scholar] [CrossRef]
- Sacithraa, R.; Mohan, M.; Vijayachitra, S. Quantitative analysis of tapioca starch using FT-IR spectroscopy and partial least squares. Int. J. Comput. Appl. 2013, 975, 8887. [Google Scholar]
- Rengifo, A.F.C.; Arrieta, Á.A.; Palencia, M. Phosphorus slow release from cassava starch-hydroxyapatite composites derived from aquaculture waste. J. Environ. Chem. Eng. 2025, 13, 116651. [Google Scholar] [CrossRef]
- Nybacka, L. FTIR Spectroscopy of Glucose. Bachelor’s Thesis, Uppsala Universitet, Uppsala, Sweden, 2016. [Google Scholar]
- Garces, V.; García-Quintero, A.; Lerma, T.A.; Palencia, M.; Combatt, E.M.; Arrieta, Á.A. Characterization of cassava starch and its structural changes resulting from thermal stress by functionally-enhanced derivative spectroscopy (FEDS). Polysaccharides 2021, 2, 866–877. [Google Scholar] [CrossRef]
- Chamorro, A.F.; Palencia, M.; Combatt, E.M. Biodegradable Cassava Starch/Phosphorite/Citric Acid Based Hydrogel for Slow Release of Phosphorus: In Vitro Study. Gels 2024, 10, 431. [Google Scholar] [CrossRef]
- Chamorro, A.F.; Palencia, M.; Arrieta, Á.A. Development of High-Efficiency Fertilizer by Hydrogels Obtained from Cassava Starch and Citric Acid for Slow Release of Ammonium and Potassium. Gels 2024, 10, 434. [Google Scholar] [CrossRef] [PubMed]
- Bergo, P.; Sobral, P.J.A.; Prison, J.M. Effect of glycerol on physical properties of cassava starch films. J. Food Process. Preserv. 2010, 34, 401–410. [Google Scholar] [CrossRef]
- Méndez, P.A.; Méndez, Á.M.; Martínez, L.N.; Vargas, B.; López, B.L. Cassava and banana starch modified with maleic anhydride-poly (ethylene glycol) methyl ether (Ma-mPEG): A comparative study of their physicochemical properties as coatings. Int. J. Biol. Macromol. 2022, 205, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Vergara, L.D.; Cifuentes, M.T.; Franco, A.P.; Pérez, C.E.; Andrade, R.D. Development and characterization of edible films based on native cassava starch, beeswax, and propolis. NFS J. 2020, 21, 39–49. [Google Scholar] [CrossRef]
- Abdullah, A.H.D.; Chalimah, S.; Primadona, I.; Hanantyo, M.H.G. Physical and chemical properties of corn, cassava, and potato starchs. IOP Conf. Ser. Earth Environ. Sci. 2018, 160, 012003. [Google Scholar] [CrossRef]
- Nikonenko, N.A.; Buslov, D.K.; Sushiko, N.I.; Zhbankov, R.G. Investigation of Stretching Vibrations of Glycosidic Linkages in Disaccharides and Polysaccharides with Use of IR Spectra Deconvolution. Biopolymers 2000, 57, 257–262. [Google Scholar] [CrossRef]
- Mina, J.; Valadez-González, A.; Herrera-Franco, P.; Zuluaga, F.; Delvasto, S. Physicochemical characterization of natural and acetylated thermoplastic cassava starch. Dyna 2011, 78, 166–173. [Google Scholar]
- Jyothi, A.N.; Moorthy, S.N.; Rajasekharan, K.N. Effect of cross-linking with epichlorohydrin on the properties of cassava (Manihot esculenta Crantz) starch. Starch-Stärke 2006, 58, 292–299. [Google Scholar] [CrossRef]
- Han, H.L.; Sosulski, F.W. Cationization of potato and tapioca starches using an aqueous alcoholic-alkaline process. Starch-Stärke 1998, 50, 487–492. [Google Scholar] [CrossRef]
- Mbougueng, P.D.; Tenin, D.; Scher, J.; Tchiégang, C. Influence of acetylation on physicochemical, functional and thermal properties of potato and cassava starches. J. Food Eng. 2012, 108, 320–326. [Google Scholar] [CrossRef]
- Hoover, R.; Hannouz, D.; Sosulski, F.W. Effects of hydroxypropylation on thermal properties, starch digestibility and freeze-thaw stability of field pea (Pisum sativum cv Trapper) starch. Starch-Stärke 1988, 40, 383–387. [Google Scholar] [CrossRef]
- Palencia, M. Functional transformation of Fourier-transform mid-infrared spectrum for improving spectral specificity by simple algorithm based on wavelet-like functions. J. Adv. Res. 2018, 14, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, H.; Song, J.; Zhang, Y.; Zhang, H. Understanding the structural characteristics, pasting and rheological behaviours of pregelatinised cassava starch. Int. J. Food Sci. Technol. 2018, 53, 2173–2180. [Google Scholar] [CrossRef]
- Zhang, X.; Li, R.; Kang, H.; Luo, D.; Fan, J.; Zhu, W.; Liu, X.; Tong, Q. Effects of low molecular sugars on the retrogradation of tapioca starch gels during storage. PLoS ONE 2017, 12, e0190180. [Google Scholar] [CrossRef]
- Deeyai, P.; Suphantharika, M.; Wongsagonsup, R.; Dangtip, S. Characterization of modified tapioca starch in atmospheric argon plasma under diverse humidity by FTIR spectroscopy. Chin. Phys. Lett. 2013, 30, 018103. [Google Scholar] [CrossRef]
- Klein, B.; Vanier, N.L.; Moomand, K.; Pinto, V.Z.; Colussi, R.; Zavareze, E.d.R.; Dias, A.R.G. Ozone oxidation of cassava starch in aqueous solution at different pH. Food Chem. 2014, 155, 167–173. [Google Scholar] [CrossRef]
- Mei, J.Q.; Zhou, D.N.; Jin, Z.Y.; Xu, X.M.; Chen, H.Q. Effects of citric acid esterification on digestibility, structural and physicochemical properties of cassava starch. Food Chem. 2015, 187, 378–384. [Google Scholar] [CrossRef]
- Nuwamanya, E.; Baguma, Y.; Kawuki, R.S.; Rubalhayo, P.R. Quantification of starch physicochemical characteristics in a cassava segregating population. Afr. Crop Sci. J. 2009, 16, 191–202. [Google Scholar] [CrossRef]
- Htoon, A.; Shrestha, A.K.; Flanagan, B.M.; Lopez-Rubio, A.; Bird, A.R.; Gilbert, E.; Gidley, M. Effects of processing high amylose maize starches under controlled conditions on structural organisation and amylase digestibility. Carbohydr. Polym. 2009, 75, 236–245. [Google Scholar] [CrossRef]
- Tongta, S.; Kiatponglap, W.; Sriroth, K. Effect on aging temperature on retrogradation of concentrated cassava starch gel. In Starch: Progress in Structural Studies, Modifications, and Applications; Polish Society of Food Technologist: Warszawa Poland, 2004; pp. 357–364. [Google Scholar]
- Vázquez-Vuelvas, O.F.; Chávez-Camacho, F.A.; Meza-Velázquez, J.A.; Mendez-Merino, E.; Ríos-Licea, M.M.; Contreras-Esquivel, J.C. A comparative FTIR study for supplemented agavin as functional food. Food Hydrocoll. 2020, 103, 105642. [Google Scholar] [CrossRef]
- Yu, P. Applications of hierarchical cluster analysis (CLA) and principal component analysis (PCA) in feed structure and feed molecular chemistry research, using synchrotron-based Fourier transform infrared (FTIR) microspectroscopy. J. Agric. Food Chem. 2005, 53, 7115–7127. [Google Scholar] [CrossRef] [PubMed]
- Kos, G.; Sieger, M.; McMullin, D.; Zahradnik, C.; Sulyok, M.; Öner, T.; Mizaikoff, B.; Krska, R. A novel chemometric classification for FTIR spectra of mycotoxin-contaminated maize and peanuts at regulatory limits. Food Addit. Contam. Part A 2016, 33, 1596–1607. [Google Scholar] [CrossRef] [PubMed]
- Weller, C.T. Carbohydrates Studied by NMR. In Encyclopedia of Spectroscopy and Spectrometry, 3rd ed.; Academic Press: Amsterdam, The Netherlands, 2017; pp. 158–164. [Google Scholar] [CrossRef]
- Zhu, F. NMR spectroscopy of starch systems. Food Hydrocoll. 2017, 63, 611–624. [Google Scholar] [CrossRef]
- Iulianelli, G.C.; Tavares, M.I.B. Application of solid-state NMR spectroscopy to evaluate cassava genotypes. J. Food Compos. Anal. 2016, 48, 88–94. [Google Scholar] [CrossRef]
- Hong, L.F.; Cheng, L.H.; Lee, C.Y.; Peh, K.K. Characterisation of physicochemical properties of propionylated corn starch and its application as stabiliser. Food Technol. Biotechnol. 2015, 53, 278. [Google Scholar] [CrossRef]
- Xie, X.; Zhang, Y.; Zhu, Y.; Lan, Y. Preparation and drug-loading properties of amphoteric cassava starch nanoparticles. Nanomaterials 2022, 12, 598. [Google Scholar] [CrossRef]
- Bunkerd, R.; Molloy, R.; Somsunan, R.; Punyodom, W.; Topham, P.D.; Tighe, B.J. Synthesis and characterization of chemically-modified cassava starch grafted with poly(2-ethylhexyl acrylate) for blending with poly(lactic acid). Starch-Stärke 2018, 70, 1800093. [Google Scholar] [CrossRef]
- Amaraweera, S.M.; Gunathilake, C.; Gunawardene, O.H.; Fernando, N.M.; Wanninayaka, D.B.; Manamperi, A.; Dassanayake, R.S.; Rajapaksha, S.M.; Gangoda, M.; Fernando, C.A.N.; et al. Preparation and characterization of biodegradable cassava starch thin films for potential food packaging applications. Cellulose 2021, 28, 10531–10548. [Google Scholar] [CrossRef]
- Boruczkowska, H.; Boruczkowski, T.; Gubica, T.; Anioł, M.; Tomaszewska-Ciosk, E. Analysis of the chemical structure of insoluble products of enzymatic esterification of starch and transesterification of acetylated starch with oleic acid by solid-state CP/MAS 13C NMR. Starch-Stärke 2016, 68, 1180–1186. [Google Scholar] [CrossRef]
- Dunn, L.B.; Krueger, W.J. Branching ratios of starch via proton nuclear magnetic resonance and their use in determining amylose/amylopectin content: Evidence for three types of amylopectin. Macromol. Symp. 1999, 140, 179–186. [Google Scholar] [CrossRef]
- Cuenca, P.; Ferrero, S.; Albani, O. Preparation and characterization of cassava starch acetate with high substitution degree. Food Hydrocoll. 2020, 100, 105430. [Google Scholar] [CrossRef]
- Triwises, P.; Ruksakulpiwat, Y.; Ruksakulpiwat, C. The modification of tapioca starch by esterification technique. Suranaree J. Sci. Technol. 2016, 23, 157. [Google Scholar]
- Xu, A.; Seib, P.A. Determination of the level and position of substitution in hydroxypropylated starch by high-resolution 1H-NMR spectroscopy of alpha-limit dextrins. J. Cereal Sci. 1997, 25, 17–26. [Google Scholar] [CrossRef]
- Witono, J.R.; Marsman, J.H.; Noordergraaf, I.W.; Heeres, H.J.; Janssen, L.P. Improved homopolymer separation to enable the application of 1H NMR and HPLC for the determination of the reaction parameters of the graft copolymerization of acrylic acid onto starch. Carbohydr. Res. 2013, 370, 38–45. [Google Scholar] [CrossRef]
- Kiatkamjornwong, S.; Thakeow, P.; Sonsuk, M. Chemical modification of cassava starch for degradable polyethylene sheets. Polym. Degrad. Stab. 2001, 73, 363–375. [Google Scholar] [CrossRef]
- Bello, M.; Ochoa, N.; Balsamo, V.; Lopez-Carrasquero, F.; Coll, S.; Monsalve, A.; González, G. Modified cassava starches as corrosion inhibitors of carbon steel: An electrochemical and morphological approach. Carbohydr. Polym. 2010, 82, 561–568. [Google Scholar] [CrossRef]
- Hou, C.; Chen, Y.; Li, W. Thiocarbamide and microwave-accelerated green methylation of cassava starch with dimethyl carbonate. Carbohydr. Res. 2012, 355, 87–91. [Google Scholar] [CrossRef]
- Fonseca-Florido, H.A.; Soriano-Corral, F.; Yañez-Macías, R.; González-Morones, P.; Hernández-Rodríguez, F.; Aguirre-Zurita, J.; Ávila-Orta, C.; Rodríguez-Velázquez, J. Effects of multiphase transitions and reactive extrusion on in situ thermoplasticization/succination of cassava starch. Carbohydr. Polym. 2019, 225, 115250. [Google Scholar] [CrossRef]
- Zhong, L.; Ding, Y.; Zhang, B.; Wang, Z.; Li, C.; Fu, X.; Huang, Q. Effect of octenylsuccinylation of oxidized cassava starch on grease resistance and waterproofing of food wrapping paper. Starch-Stärke 2019, 71, 1800284. [Google Scholar] [CrossRef]
- Fang, K.; He, W.; Jiang, Y.; Li, K.; Li, J. Preparation, characterization and physicochemical properties of cassava starch-ferulic acid complexes by mechanical activation. Int. J. Biol. Macromol. 2020, 160, 482–488. [Google Scholar] [CrossRef]
- Luo, Z.; Cheng, W.; Chen, H.; Fu, X.; Peng, X.; Luo, F.; Nie, L. Preparation and properties of enzyme-modified cassava starch–zinc complexes. J. Agric. Food Chem. 2013, 61, 4631–4638. [Google Scholar] [CrossRef] [PubMed]
- Atichokudomchai, N.; Varavinit, S.; Chinachoti, P. A study of ordered structure in acid-modified tapioca starch by 13C CP/MAS solid-state NMR. Carbohydr. Polym. 2004, 58, 383–389. [Google Scholar] [CrossRef]
- Veregin, R.P.; Fyfe, C.A.; Marchessault, R.H.; Taylor, M.G. Characterization of the crystalline A and B starch polymorphs and investigation of starch crystallization by high-resolution carbon-13 CP/MAS NMR. Macromolecules 1986, 19, 1030–1034. [Google Scholar] [CrossRef]
- Cooke, D.; Gidley, M.J. Loss of crystalline and molecular order during starch gelatinisation: Origin of the enthalpic transition. Carbohydr. Res. 1992, 227, 103–112. [Google Scholar] [CrossRef]
- Mutungi, C.; Passauer, L.; Onyango, C.; Jaros, D.; Rohm, H. Debranched cassava starch crystallinity determination by Raman spectroscopy: Correlation of features in Raman spectra with X-ray diffraction and 13C CP/MAS NMR spectroscopy. Carbohydr. Polym. 2012, 87, 598–606. [Google Scholar] [CrossRef]
- Sluiter, J.B.; Michel, K.P.; Addison, B.; Zeng, Y.; Michener, W.; Paterson, A.L.; Perras, F.A.; Wolfrum, E.J. Direct determination of cellulosic glucan content in starch-containing samples. Cellulose 2021, 28, 1989–2002. [Google Scholar] [CrossRef]
- Richardson, S.; Nilsson, G.; Cohen, A.; Momcilovic, D.; Brinkmalm, G.; Gorton, L. Enzyme-aided investigation of the substituent distribution in cationic potato amylopectin starch. Anal. Chem. 2003, 75, 6499–6508. [Google Scholar] [CrossRef]
- Richardson, S.; Gorton, L. Characterisation of the substituent distribution in starch and cellulose derivatives. Anal. Chim. Acta 2003, 497, 27–65. [Google Scholar] [CrossRef]
- Mischnick, P.; Heinrich, J.; Gohdes, M.; Wilke, O.; Rogmann, N. Structure analysis of 1, 4-glucan derivatives. Macromol. Chem. Phys. 2000, 201, 1985–1995. [Google Scholar] [CrossRef]
- Agbenyega, J.K.; Ellis, G.; Hendra, P.J.; Maddams, W.F.; Passingham, C.; Willis, H.; Chalmers, J. Applications of Fourier transform Raman spectroscopy in the synthetic polymer field. Spectrochim. Acta Part A 1990, 46, 197–216. [Google Scholar] [CrossRef]
- Rygula, A.; Majzner, K.; Marzec, K.M.; Kaczor, A.; Pilarczyk, M.; Baranska, M. Raman spectroscopy of proteins: A review. J. Raman Spectrosc. 2013, 44, 1061–1076. [Google Scholar] [CrossRef]
- Yuen, S.N.; Choi, S.M.; Phillips, D.L.; Ma, C.Y. Raman and FTIR spectroscopic study of carboxymethylated non-starch polysaccharides. Food Chem. 2009, 114, 1091–1098. [Google Scholar] [CrossRef]
- Czamara, K.; Majzner, K.; Pacia, M.Z.; Kochan, K.; Kaczor, A.; Baranska, M. Raman spectroscopy of lipids: A review. J. Raman Spectrosc. 2015, 46, 4–20. [Google Scholar] [CrossRef]
- Herrero, A.M. Raman spectroscopy for monitoring protein structure in muscle food systems. Crit. Rev. Food Sci. Nutr. 2008, 48, 512–523. [Google Scholar] [CrossRef]
- Santha, N.; Sudha, K.G.; Vijayakumari, K.P.; Nayar, V.U.; Moorthy, S.N. Raman and infrared spectra of starch samples of sweet potato and cassava. Starch 1990, 102, 705–712. [Google Scholar] [CrossRef]
- Almeida, M.R.; Alves, R.S.; Nascimbem, L.B.L.R.; Stephani, R.; Poppi, R.J.; de Oliveira, L.F.C. Determination of amylose content in starch using Raman spectroscopy and multivariate calibration analysis. Anal. Bioanal. Chem. 2010, 397, 2693–2701. [Google Scholar] [CrossRef]
- Sekkal, M.; Dincq, V.; Legrand, P.; Huvenne, J.P. Investigation of the glycosidic linkages in several oligosaccharides using FT-IR and FT Raman spectroscopies. J. Mol. Struct. 1995, 349, 349–352. [Google Scholar] [CrossRef]
- Kizil, R.; Irudayaraj, J.; Seetharaman, K. Characterization of irradiated starches by using FT-Raman and FTIR spectroscopy. J. Agric. Food Chem. 2002, 50, 3912–3918. [Google Scholar] [CrossRef]
- Dupuy, N.; Laureyns, J. Recognition of starches by Raman spectroscopy. Carbohydr. Polym. 2002, 49, 83–90. [Google Scholar] [CrossRef]
- Cardoso, V.G.K.; Poppi, R.J. Cleaner and faster method to detect adulteration in cassava starch using Raman spectroscopy and one-class support vector machine. Food Control 2021, 125, 107917. [Google Scholar] [CrossRef]
- Fan, D.; Ma, W.; Wang, L.; Huang, J.; Zhao, J.; Zhang, H.; Chen, W. Determination of structural changes in microwaved rice starch using Fourier transform infrared and Raman spectroscopy. Starch-Stärke 2012, 64, 598–606. [Google Scholar] [CrossRef]
- Flores, M.A.; Jiménez, E.M.; Mora, E.R. Determination of the structural changes by FT-IR, Raman, and CP/MAS 13C NMR spectroscopy on retrograded starch of maize tortillas. Carbohydr. Polym. 2012, 87, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Shi, J.; Zhang, F.; Zou, X.; Holmes, M.; Zhang, W.; Huang, X.; Cui, X.; Xue, J. Determination of retrogradation degree in starch by mid-infrared and Raman spectroscopy during storage. Food Anal. Methods 2017, 10, 3694–3705. [Google Scholar] [CrossRef]

| Crop | Amylose (%) | Protein (%) | Lipid (%) | a Fiber (%) | Ref. |
|---|---|---|---|---|---|
| Rayong 9 | - | 4.85 ± 0.09 | 0.08 ± 0.01 | 3.44 ± 0.17 | [10] |
| Rayong 11 | - | 4.55 ± 0.06 | 0.16 ± 0.01 | 3.14 ± 0.26 | [10] |
| Cultivar TMS 30470 | 29.5 ± 0.67 | 0.32 ± 0.01 | 0.17 ± 0.00 | 1.2 ± 0.00 | [23] |
| Rayong 1 | 24.1 | 0.17 ± 0.04 | - | - | [24] |
| KU 50 | 21.4 | 0.30 ± 0.04 | - | - | [24] |
| N.R | 23.0 | 0.1 | 1.2 | - | [25] |
| N.R | 23.0 | 0.1 | - | - | [25] |
| N.R | 16.11 ± 1.0 | 0.31 ± 0.01 | 0.20 ± 0.03 | 0.23 ± 0.06 | [26] |
| HMC-1 | - | 0.26 | 0.31 | - | [27] |
| MH97/2962 | - | 0.47 | 0.55 | - | [28] |
| TMS 326 | - | 1.26 ± 0.06 | 1.59 ± 0.13 | 1.95 ± 0.08 | [29] |
| TME 419 | - | 0.51 ± 0.08 | 0.94 ± 0.16 | 2.15 ± 0.14 | [29] |
| M82/00032 | 24.0 ± 0.00 | 1.75 ± 0.00 | - | 1.54 ± 0.21 | [30] |
| I93/0560 | 13.2 ± 0.00 | 1.74 ± 0.00 | - | 2.04 ± 0.12 | [30] |
| N.R | 23.65 ± 0.15 | 0.14 ± 0.22 | 0.11 ± 0.01 | - | [31] |
| Adira-4 | 26.3 | - | 0.013 | - | [32] |
| TMS 98/0581 | 26.41 ± 0.13 | 0.40 ± 0.07 | 0.13 ± 0.001 | - | [33] |
| ICA-C523-7 | 14.67 ± 0.25 | 0.60 ± 0.02 | 0.31 ± 0.00 | 0.04 ± 0.01 | [34] |
| MBra 383 | 14.43 ± 0.51 | 0.60 ± 0.03 | 0.32 ± 0.01 | 0.05 ± 0.01 | [34] |
| N.R | 17.2 ± 0.4 | 1.9 ± 0.2 | - | 2.8 ± 0.3 | [35] |
| Crop | Age (Months) | Region | Starch Content (% Total Cassava) | Size (Average, µm) | Shape | Ref. |
|---|---|---|---|---|---|---|
| Rayong 1 (1957) | 16 | Thailand | - | 8–22 | oval, round, and truncated | [24] |
| TMS 98/0581 | 10 | Nigeria | 87.44–89.64 | 6–22 | irregular, truncated, and oval-shaped granules | [33] |
| Rayong 90 | 12 | Thailand | - | 5–25 | Not described | [38] |
| Rayong 9 | 12 | Thailand | 81.3 | 7–30 | Not described | [39] |
| Crops-2017 | 11 | Brazil | 86.9 | 16–20 | circular with some truncated | [40] |
| - | Thailand | 95 | 10–40 | truncated | [41] | |
| Kasetsart 50 | 12 | Thailand | - | 9–22 | spherical and some were truncated | [42] |
| - | - | - | - | 11.9 | rounded or oval shape | [43] |
| CMR 38-125-77 | 9 | Thailand | 82.16 | 7–29 | Not described | [39] |
| T–Initial (°C) | Tp–Peak (°C) | T–Final (°C) | ΔH (J g−1) | Ref. |
|---|---|---|---|---|
| 62.4 | 69.3 | 84.1 | 4.8 | [95] |
| 66.9 ± 0.2 | 70.1 ± 0.1 | 85.1 ± 0.7 | 15.6 ± 0.5 | [96] |
| 58.6 ± 0.2 | 63.3 ± 0.4 | 69.2 ± 0.5 | 13.8 ± 0.3 | [97] |
| 63.9 ± 0.2 | 70.5 ± 0.1 | 82.7 ± 1.0 | 8.5 ± 0.1 | [98] |
| 64.3 ± 0.0 | 71.7 ± 0.0 | 81.0 ± 0.0 | 17.4 ± 0.1 | [99] |
| 55.1 ± 1.9 | 70.2 ± 0.3 | 80.1 ± 0.9 | 16.3 ± 0.7 | [100] |
| 64.0 ± 0.0 | 70.2 ± 0.1 | 78.3 ± 0.1 | 12.0 ± 0.1 | [41] |
| 60.2 ± 0.1 | 67.5 ± 0.0 | 75.83± 1.0 | 13.5 ± 0.0 | [101] |
| Functional Groups | Vibration | Wave Number (cm−1) | ||
|---|---|---|---|---|
| Cassava | Corn | Potato | ||
| O–H | Stretching | 3448 | 3448 | 3523 |
| C–H | Stretching | 2930 | 2929 | 2927 |
| CH2 | Symmetric deformation | 1437 | 1437 | 1437 |
| CH2 | Symmetric scissoring | 1417 | 1415 | 1419 |
| C–H | Symmetric bending | 1381 | 1381 | 1381 |
| C–O–C | Asymmetric stretching | 1157 | 1157 | 1157 |
| C–O | Stretching | 1082, 1016 | 1082, 1018 | 1082, 993 |
| C–O–C | Ring vibration of carbohydrate | 929, 860, 763 | 929, 860, 763 | 929, 860, 763 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chamorro, A.F.; Palencia, M.; Lerma, T.A. Physicochemical Characterization and Properties of Cassava Starch: A Review. Polymers 2025, 17, 1663. https://doi.org/10.3390/polym17121663
Chamorro AF, Palencia M, Lerma TA. Physicochemical Characterization and Properties of Cassava Starch: A Review. Polymers. 2025; 17(12):1663. https://doi.org/10.3390/polym17121663
Chicago/Turabian StyleChamorro, Andrés Felipe, Manuel Palencia, and Tulio Armando Lerma. 2025. "Physicochemical Characterization and Properties of Cassava Starch: A Review" Polymers 17, no. 12: 1663. https://doi.org/10.3390/polym17121663
APA StyleChamorro, A. F., Palencia, M., & Lerma, T. A. (2025). Physicochemical Characterization and Properties of Cassava Starch: A Review. Polymers, 17(12), 1663. https://doi.org/10.3390/polym17121663








