The Impact of Humidity in the Thermal Ageing of Celluloid: An Inter-Scale Investigation
Abstract
1. Introduction
2. Materials and Methods
2.1. Choice of the Samples and Sample Preparation
2.2. Accelerated Ageing
2.3. Analytical Techniques
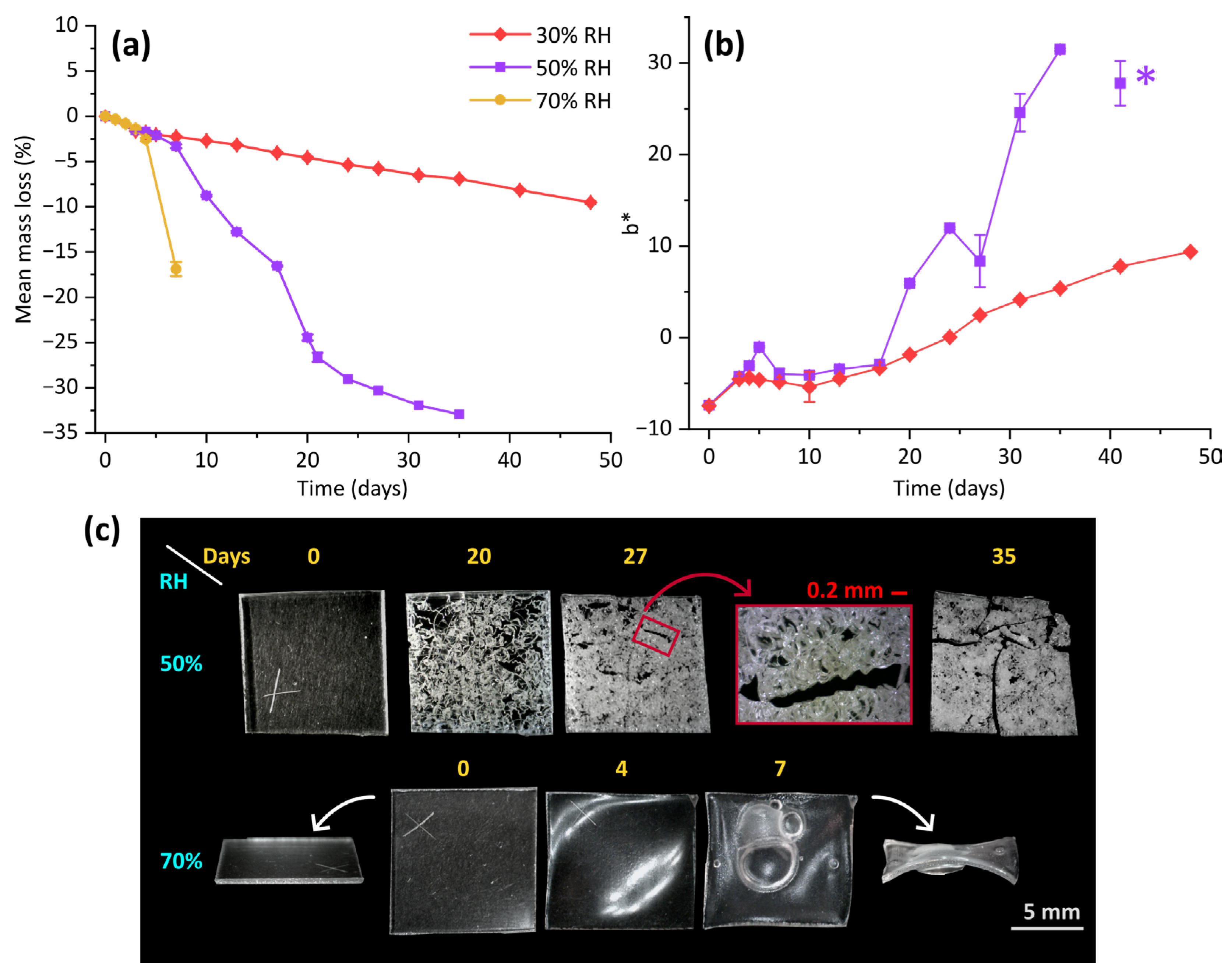
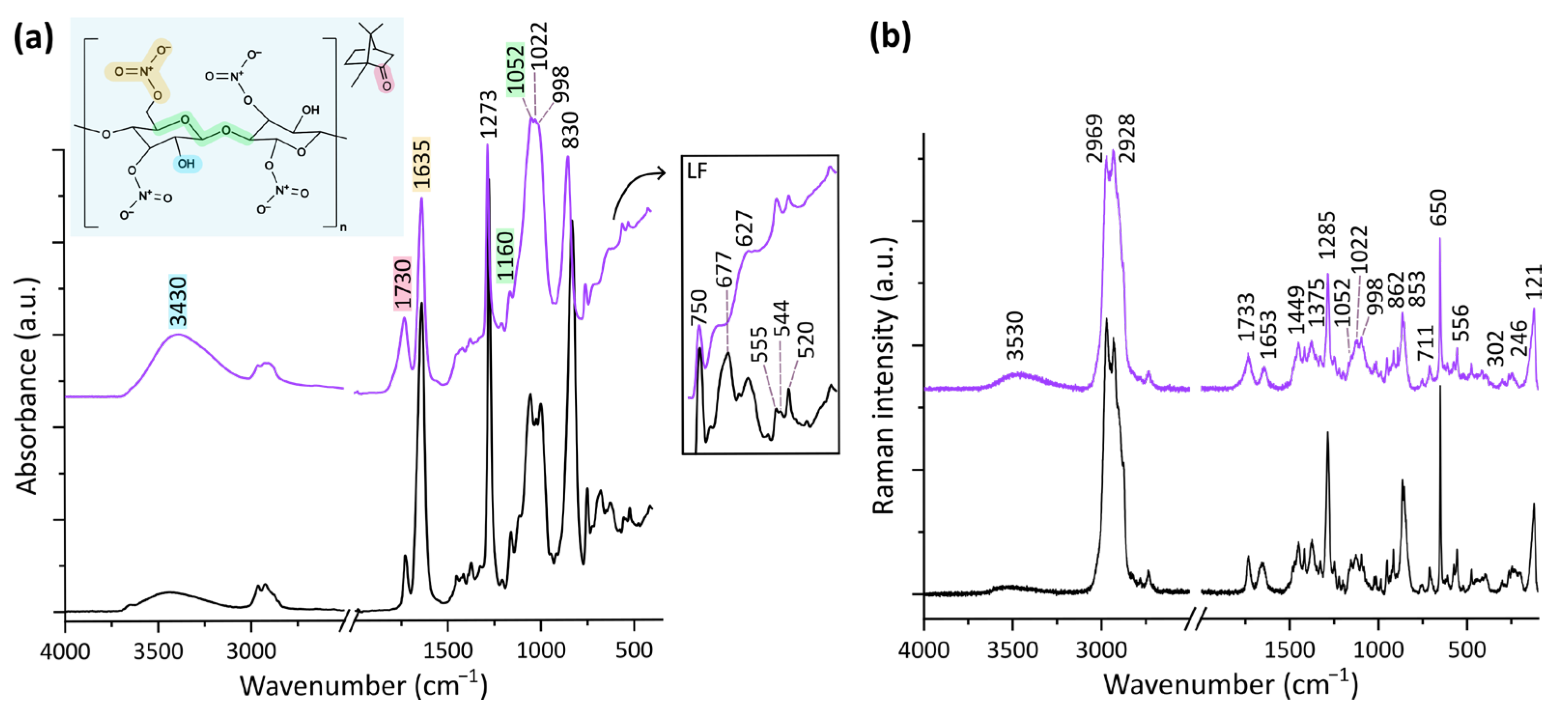
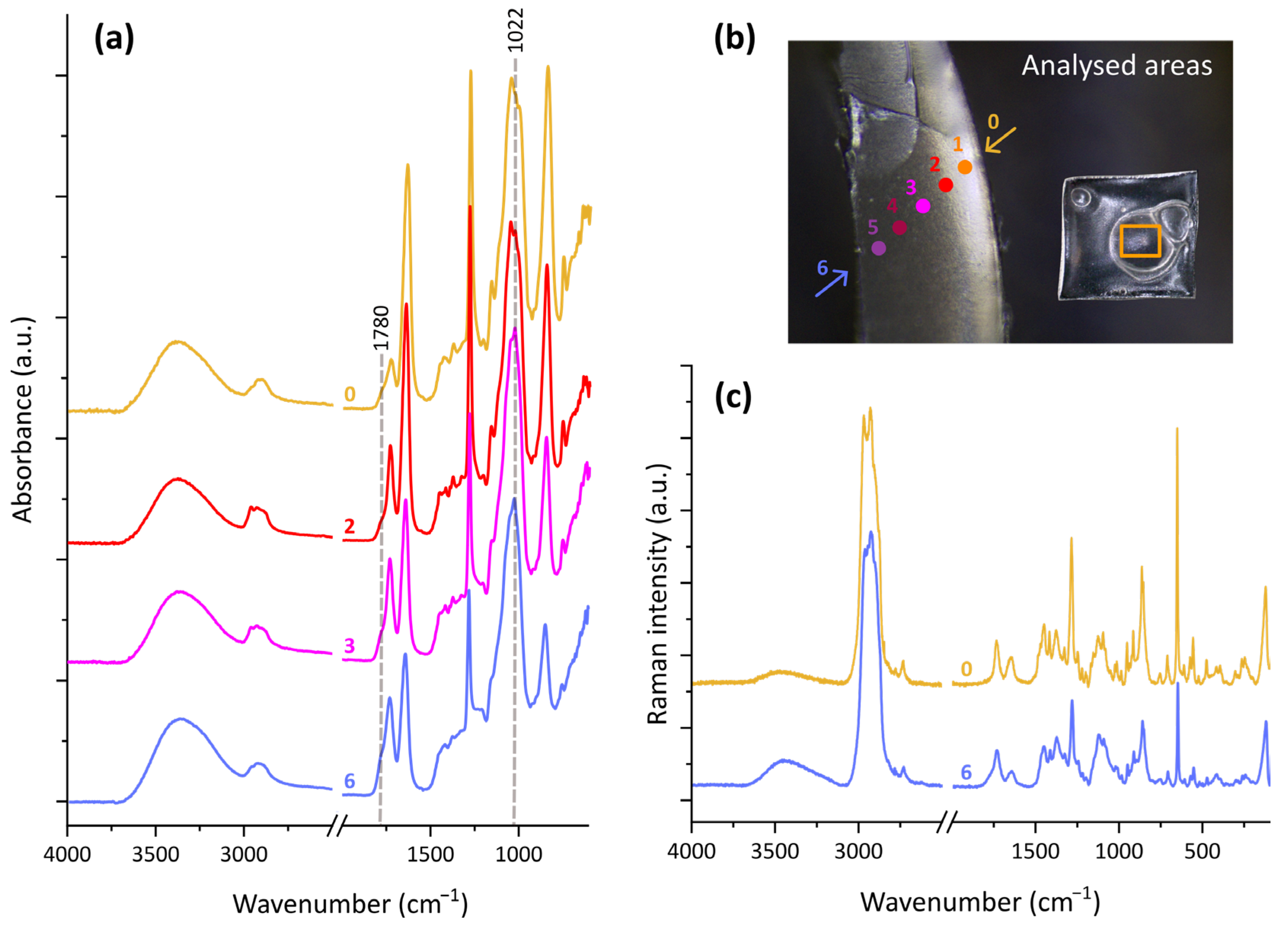
3. Results
3.1. Physical and Morphological Changes
3.2. Chemical Modifications
3.2.1. Changes at the Surface
3.2.2. Changes in the Bulk Material
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shashoua, Y. Conservation of Plastics: Materials Science, Degradation and Preservation, 1st ed.; Elsevier: Amsterdam, The Netherlands; Butterworth-Heinemann: Oxford, UK, 2008; ISBN 978-0-7506-6495-0. [Google Scholar]
- Elsässer, C.; Micheluz, A.; Pamplona, M.; Kavda, S.; Montag, P. Selection of Thermal, Spectroscopic, Spectrometric, and Chromatographic Methods for Characterizing Historical Celluloid. J. Appl. Polym. Sci. 2021, 138, 50477. [Google Scholar] [CrossRef]
- Chandler, C.F. Presentation Address. J. Ind. Eng. Chem. 1914, 6, 156–158. [Google Scholar] [CrossRef]
- Hyatt, J.W. Address of Acceptance. J. Ind. Eng. Chem. 1914, 6, 158–161. [Google Scholar] [CrossRef]
- Springate, M.E. Cellulose Nitrate Plastic (Celluloid) in Archaeological Assemblages: Identification and Care. NEHA 1997, 26, 63–72. [Google Scholar] [CrossRef]
- Neves, A.; Friedel, R.; Callapez, M.E.; Swank, S.D. Safeguarding Our Dentistry Heritage: A Study of the History and Conservation of Nineteenth–Twentieth Century Dentures. Herit. Sci. 2023, 11, 142. [Google Scholar] [CrossRef]
- Konstantinidou, K.; Strekopytov, S.; Humphreys-Williams, E.; Kearney, M. Identification of Cellulose Nitrate X-Ray Film for the Purpose of Conservation: Organic Elemental Analysis. Stud. Conserv. 2016, 62, 24–32. [Google Scholar] [CrossRef]
- Izzo, F.C.; Carrieri, A.; Bartolozzi, G.; Keulen, H.V.; Lorenzon, I.; Balliana, E.; Cucci, C.; Grazzi, F.; Picollo, M. Elucidating the Composition and the State of Conservation of Nitrocellulose-Based Animation Cells by Means of Non-Invasive and Micro-Destructive Techniques. J. Cult. Herit. 2019, 35, 254–262. [Google Scholar] [CrossRef]
- Seymour, R.B.; Kauffman, G.B. The Rise and Fall of Celluloid. J. Chem. Educ. 1992, 69, 311. [Google Scholar] [CrossRef]
- Reilly, J.A. Celluloid Objects: Their Chemistry and Preservation. J. Am. Inst. Conserv. 1991, 30, 145–162. [Google Scholar] [CrossRef]
- Sutherland, K.; Schwarzinger, C.; Price, B.A. The Application of Pyrolysis Gas Chromatography Mass Spectrometry for the Identification of Degraded Early Plastics in a Sculpture by Naum Gabo. J. Anal. Appl. Pyrolysis 2012, 94, 202–208. [Google Scholar] [CrossRef]
- Mazurek, J.; Laganà, A.; Dion, V.; Etyemez, S.; Carta, C.; Schilling, M.R. Investigation of Cellulose Nitrate and Cellulose Acetate Plastics in Museum Collections Using Ion Chromatography and Size Exclusion Chromatography. J. Cult. Herit. 2019, 35, 263–270. [Google Scholar] [CrossRef]
- Shashoua, Y. Inhibiting the Inevitable; Current Approaches to Slowing the Deterioration of Plastics. Macromol. Symp. 2006, 238, 67–77. [Google Scholar] [CrossRef]
- Faber, K.T.; Casadio, F.; Masic, A.; Robbiola, L.; Walton, M. Looking Back, Looking Forward: Materials Science in Art, Archaeology, and Art Conservation. Annu. Rev. Mater. Res. 2021, 51, 435–460. [Google Scholar] [CrossRef]
- Edge, M.; Allen, N.S.; Hayes, M.; Riley, P.N.K.; Horie, C.V.; Luc-Gardette, J. Mechanisms of Deterioration in Cellulose Nitrate Base Archival Cinematograph Film. Eur. Polym. J. 1990, 26, 623–630. [Google Scholar] [CrossRef]
- Berthumeyrie, S.; Collin, S.; Bussiere, P.-O.; Therias, S. Photooxidation of Cellulose Nitrate: New Insights into Degradation Mechanisms. J. Hazard. Mater. 2014, 272, 137–147. [Google Scholar] [CrossRef]
- Neves, A.; Angelin, E.M.; Roldão, É.; Melo, M.J. New Insights into the Degradation Mechanism of Cellulose Nitrate in Cinematographic Films by Raman Microscopy. J. Raman Spectrosc. 2019, 50, 202–212. [Google Scholar] [CrossRef]
- Nunes, S.; Ramacciotti, F.; Neves, A.; Angelin, E.M.; Ramos, A.M.; Roldão, É.; Wallaszkovits, N.; Armijo, A.A.; Melo, M.J. A Diagnostic Tool for Assessing the Conservation Condition of Cellulose Nitrate and Acetate in Heritage Collections: Quantifying the Degree of Substitution by Infrared Spectroscopy. Herit. Sci. 2020, 8, 33. [Google Scholar] [CrossRef]
- Chavez Lozano, M.V.; Elsässer, C.; Angelin, E.M.; Pamplona, M. Shedding Light on Degradation Gradients in Celluloid: An ATR-FTIR Study of Artificially and Naturally Aged Specimens. Polymers 2023, 15, 522. [Google Scholar] [CrossRef]
- Bussiere, P.-O.; Gardette, J.-L.; Therias, S. Photodegradation of Celluloid Used in Museum Artifacts. Polym. Degrad. Stab. 2014, 107, 246–254. [Google Scholar] [CrossRef]
- Quye, A.; Littlejohn, D.; Pethrick, R.A.; Stewart, R.A. Accelerated Ageing to Study the Degradation of Cellulose Nitrate Museum Artefacts. Polym. Degrad. Stab. 2011, 96, 1934–1939. [Google Scholar] [CrossRef]
- Neves, A.; Ramos, A.M.; Callapez, M.E.; Friedel, R.; Réfrégiers, M.; Thoury, M.; Melo, M.J. Novel Markers to Early Detect Degradation on Cellulose Nitrate-Based Heritage at the Submicrometer Level Using Synchrotron UV–VIS Multispectral Luminescence. Sci. Rep. 2021, 11, 20208. [Google Scholar] [CrossRef] [PubMed]
- Thérias, S. Altération des objets en celluloïd dans les collections: Étude des mécanismes de vieillissement. Actes Colloq. Sci. Matér. Patrim. Cult. 2012, 2, 68–73. [Google Scholar]
- Trache, D.; Tarchoun, A.F. Differentiation of Stabilized Nitrocellulose during Artificial Aging: Spectroscopy Methods Coupled with Principal Component Analysis. J. Chemom. 2019, 33, e3163. [Google Scholar] [CrossRef]
- Rothko&Frost Clear Transparent Celluloid Sheet. Available online: https://rothkoandfrost.com/products/clear-transparent-celluloid-sheet?_pos=1&_sid=a7ce80034&_ss=r&_fid=4eec40918 (accessed on 11 June 2025).
- Greenspan, L. Humidity Fixed Points of Binary Saturated Aqueous Solutions. J. Res. Natl. Bur. Stan. Sect. A 1977, 81, 89. [Google Scholar] [CrossRef]
- ISO 25178-2:2021; Geometrical Product Specifications (GPS)—Surface Texture: Areal—Part 2: Terms, Definitions and Surface Texture Parameters. International Organization for Standardization: Geneva, Switzerland, 2021.
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J. Spectrometric Identification of Organic Compounds, 7th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2005; ISBN 978-0-471-39362-7. [Google Scholar]
- Mitchell, G.; France, F.; Nordon, A.; Tang, P.L.; Gibson, L.T. Assessment of Historical Polymers Using Attenuated Total Reflectance-Fourier Transform Infra-Red Spectroscopy with Principal Component Analysis. Herit. Sci. 2013, 1, 28. [Google Scholar] [CrossRef]
- Wei, R.; Huang, S.; Wang, Z.; Wang, C.; Zhou, T.; He, J.; Yuen, R.; Wang, J. Effect of Plasticizer Dibutyl Phthalate on the Thermal Decomposition of Nitrocellulose. J. Therm. Anal. Calorim. 2018, 134, 953–969. [Google Scholar] [CrossRef]
- Jutier, J.-J.; Harrison, Y.; Premont, S.; Prud’homme, R.E. A Nonisothermal Fourier Transform Infrared Degradation Study of Nitrocelluloses Derived from Wood and Cotton. J. Appl. Polym. Sci. 1987, 33, 1359–1375. [Google Scholar] [CrossRef]
- Pereira, A.; Candeias, A.; Cardoso, A.; Rodrigues, D.; Vandenabeele, P.; Caldeira, A.T. Non-Invasive Methodology for the Identification of Plastic Pieces in Museum Environment—A Novel Approach. Microchem. J. 2016, 124, 846–855. [Google Scholar] [CrossRef]
- Castro, K.; De Vallejuelo, S.F.; Astondoa, I.; Goñi, F.M.; Madariaga, J.M. Analysis of Confiscated Fireworks Using Raman Spectroscopy Assisted with SEM-EDS and FTIR. J. Raman Spectrosc. 2011, 42, 2000–2005. [Google Scholar] [CrossRef]
- Abbate, S.; Burgi, L.F.; Gangemi, F.; Gangemi, R.; Lebon, F.; Longhi, G.; Pultz, V.M.; Lightner, D.A. Comparative Analysis of IR and Vibrational Circular Dichroism Spectra for a Series of Camphor-Related Molecules. J. Phys. Chem. A 2009, 113, 11390–11405. [Google Scholar] [CrossRef]
- Paris, C.; Coupry, C. Fourier Transform Raman Spectroscopic Study of the First Cellulose-Based Artificial Materials in Heritage. J. Raman Spectrosc. 2005, 36, 77–82. [Google Scholar] [CrossRef]
- Moore, D.S.; McGrane, S.D. Comparative Infrared and Raman Spectroscopy of Energetic Polymers. J. Mol. Struct. 2003, 661–662, 561–566. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; repr. as paperback; Wiley: Chichester, UK, 2001; ISBN 978-0-470-09307-8. [Google Scholar]
- Mukhamadeeva, R.M.; Zhbankov, R.G.; Sopin, V.F.; Marchenko, G.N. Low-Frequency IR Spectroscopy in the Study of the Structures of Cellulose and Cellulose Nitrates. Russ. Chem. Rev. 1993, 62, 323–336. [Google Scholar] [CrossRef]
- Quye, A.; Littlejohn, D.; Pethrick, R.A.; Stewart, R.A. Investigation of Inherent Degradation in Cellulose Nitrate Museum Artefacts. Polym. Degrad. Stab. 2011, 96, 1369–1376. [Google Scholar] [CrossRef]
- Sinyayev, V.A.; Toxeitova, G.A.; Batyrbayeva, A.A.; Sassykova, L.R.; Azhigulova, R.N.; Sakhipov, Y.N. A Comparative Investigtion of the IR Spectra of a Carbohydrate Series. J. Chem. Technol. Metall. 2020, 55, 724–729. [Google Scholar]
- Fan, M.; Dai, D.; Huang, B. Fourier Transform Infrared Spectroscopy for Natural Fibres. In Fourier Transform-Materials Analysis; Salih, S., Ed.; InTech: London, UK, 2012; ISBN 978-953-51-0594-7. [Google Scholar]
- Kavda, S.; Micheluz, A.; Elsässer, C.; Pamplona, M. Development of a Gel Permeation Chromatography Method for Analysing Cellulose Nitrate in Museums. J. Sep. Sci. 2021, 44, 1795–1804. [Google Scholar] [CrossRef]
- Matsumura, M.; Eastop, D.; Gill, K. Monitoring Emissions from Cellulose Nitrate and Cellulose Acetate Costume Accessories: An Evaluation of Ph Indicator Dyes on Paper, Cotton Tape and Cotton Threads. Conservator 2002, 26, 57–69. [Google Scholar] [CrossRef]
- Chin, A.; Ellison, D.S.; Poehlein, S.K.; Ahn, M.K. Investigation of the Decomposition Mechanism and Thermal Stability of Nitrocellulose/Nitroglycerine Based Propellants by Electron Spin Resonance. Prop. Explos. Pyrotech. 2007, 32, 117–126. [Google Scholar] [CrossRef]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose Nanocrystals: Chemistry, Self-Assembly, and Applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef]
- Lubis, M.; Gana, A.; Maysarah, S.; Ginting, M.H.S.; Harahap, M.B. Production of Bioplastic from Jackfruit Seed Starch (Artocarpus heterophyllus) Reinforced with Microcrystalline Cellulose from Cocoa Pod Husk (Theobroma cacao L.) Using Glycerol as Plasticizer. IOP Conf. Ser. Mater. Sci. Eng. 2018, 309, 012100. [Google Scholar] [CrossRef]


| Ageing RH (%) | Duration (Days) | Mass Loss (%) | Macroscopic Changes | Ra Max (µm) |
|---|---|---|---|---|
| 30 | 48 | 10 | Embrittlement (27) | 0.15 |
| 50 | 41 | 32 | Cracking (20) | 0.14 |
| 70 | 7 | 17 | Bubbles (7) | 1.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piccolo, A.; Tomasin, P.; Zoleo, A.; Miorin, E.; Nodari, L. The Impact of Humidity in the Thermal Ageing of Celluloid: An Inter-Scale Investigation. Polymers 2025, 17, 1648. https://doi.org/10.3390/polym17121648
Piccolo A, Tomasin P, Zoleo A, Miorin E, Nodari L. The Impact of Humidity in the Thermal Ageing of Celluloid: An Inter-Scale Investigation. Polymers. 2025; 17(12):1648. https://doi.org/10.3390/polym17121648
Chicago/Turabian StylePiccolo, Anna, Patrizia Tomasin, Alfonso Zoleo, Enrico Miorin, and Luca Nodari. 2025. "The Impact of Humidity in the Thermal Ageing of Celluloid: An Inter-Scale Investigation" Polymers 17, no. 12: 1648. https://doi.org/10.3390/polym17121648
APA StylePiccolo, A., Tomasin, P., Zoleo, A., Miorin, E., & Nodari, L. (2025). The Impact of Humidity in the Thermal Ageing of Celluloid: An Inter-Scale Investigation. Polymers, 17(12), 1648. https://doi.org/10.3390/polym17121648









