Influence of Water Regeneration on Chemical and Process Indices in an Energy-Integrated PVC Production Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Process Description
2.2. Inherent Safety Index
2.3. Inherent Chemical Safety Index
2.4. Inherent Process Safety Index
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ETA | Event Tree Analysis |
| CCA | Cause–Consequence Analysis |
| CAPE | Computer-aided process engineering |
| ISRAM | Information Security Risk Analysis Method |
| FMEA | Failure mode and effects analysis |
| HAZOP | Hazard and Operability study |
| HRA | Health risk assessment |
References
- Beltrán Domínguez, C. Ingeniería Básica de una Planta de Producción de PVC Granular; Trabajo Fin de Grado, Universidad de Sevilla: Sevilla, Spain, 2016; Available online: https://biblus.us.es/bibing/proyectos/abreproy/90856/fichero/2-TFG+PVC.+Memoria+%2812.09.2016%29.pdf (accessed on 31 January 2024).
- Moulay, S. Chemical modification of poly(vinyl chloride)—Still on the run. Prog. Polym. Sci. 2010, 35, 303–331. [Google Scholar] [CrossRef]
- Leadbitter, J. PVC and sustainability. Prog. Polym. Sci. 2002, 27, 2197–2226. [Google Scholar] [CrossRef]
- Thornton, J. Environmental Impacts of Polyvinyl Chloride (PVC) Building Materials; Healthy Building Network: Washington, DC, USA, 2002; Available online: http://mts.sustainableproducts.com/SMaRT/ThorntonRevised.pdf (accessed on 31 January 2025).
- Mittal, A.; Naushad, M.; Sharma, G.; Alothman, Z.A.; Wabaidur, S.M.; Alam, M. Fabrication of MWCNTs/ThO2 nanocomposite and its adsorption behavior for the removal of Pb(II) metal from aqueous medium. Desalin. Water Treat. 2016, 57, 21863–21869. [Google Scholar] [CrossRef]
- Khan, M.A.; Alqadami, A.A.; Wabaidur, S.M.; Siddiqui, M.R.; Jeon, B.-H.; Alshareef, S.A.; Alothman, Z.A.; Hamedelniel, A.E. Oil industry waste based non-magnetic and magnetic hydrochar to sequester potentially toxic post-transition metal ions from water. J. Hazard. Mater. 2020, 400, 123247. [Google Scholar] [CrossRef]
- Wabaidur, S.M.; Khan, M.A.; Siddiqui, M.R.; Otero, M.; Jeon, B.-H.; Alothman, Z.A.; Hakami, A.A.H. Oxygenated functionalities enriched MWCNTs decorated with silica coated spinel ferrite—A nanocomposite for potentially rapid and efficient de-colorization of aquatic environment. J. Mol. Liq. 2020, 317, 113916. [Google Scholar] [CrossRef]
- Narváez-Rincón, P.C.; Serna, J.; Orjuela, Á.; Camargo, M. Sustainability assessment for chemical product and process design during early design stages. In Towards Sustainable Chemical Processes; Ren, J., Wang, Y., He, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 3–41. [Google Scholar] [CrossRef]
- Ministerio de Ambiente y Desarrollo Sostenible. Resolución 1256 de 2021: Por la Cual se Reglamenta el Uso de las Aguas Residuales y se Adoptan Otras Disposiciones; 2021. Available online: https://www.minambiente.gov.co/documento-normativa/resolucion-1256-de-2021/ (accessed on 16 April 2025).
- República de Colombia. Decreto 1076 de 2015: Decreto Único Reglamentario del Sector Ambiente y Desarrollo Sostenible. Available online: https://www.funcionpublica.gov.co/eva/gestornormativo/norma.php?i=78153 (accessed on 16 April 2025).
- El-Halwagi, M.M. Pollution Prevention Through Process Integration: Systematic Design Tools, 1st ed.; Academic Press: San Diego, CA, USA, 1997; ISBN 978-0-12-236845-5. [Google Scholar]
- Sonune, A.; Ghate, R. Developments in wastewater treatment methods. Desalination 2004, 167, 55–63. [Google Scholar] [CrossRef]
- Bestratén, M. NTP 328: Análisis de Riesgos Mediante el Árbol de Sucesos; Instituto Nacional de Seguridad e Higiene en el Trabajo: Madrid, Spain, 1992; Available online: https://www.icao.int/SAM/Documents/2014-ADSAFASS/ntp_328.pdf (accessed on 16 April 2025).
- Cruz de Castro, F. Guía Técnica: Métodos Cuantitativos Para el Análisis de Riesgos; Dirección General de Protección Civil: Madrid, Spain, 1992; Available online: https://gc.scalahed.com/recursos/files/r161r/w25012w/Seguridad_Higiene_Ambiental/Guia_tecnica.pdf (accessed on 16 April 2025).
- Rot, A. IT Risk Assessment: Quantitative and Qualitative Approach. In Proceedings of the World Congress on Engineering and Computer Science 2008 (WCECS 2008), San Francisco, CA, USA, 22–24 October 2008; International Association of Engineers: Hong Kong, China, 2008; pp. 1073–1078. Available online: https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=11fe25b459871f9beac7ec3a35c21955025abfe4 (accessed on 16 April 2025).
- Heikkilä, A.M. Inherent Safety in Process Plant Design: An Index-Based Approach; VTT Publications 384; Technical Research Centre of Finland (VTT): Espoo, Finland, 1999; 129p, ISBN 951-38-5371-3. [Google Scholar]
- González-Delgado, A.D. Ingeniería de Procesos Asistida por Computador (CAPE) Aplicada a las Demandas del Proceso de Extracción de Aceite Crudo de Palma; XIX Conferencia Internacional sobre Palma de Aceite, 2022; Federación Nacional de Cultivadores de Palma de Aceite (Fedepalma): Bogotá, Colombia. Available online: https://fedepalma.org/conferenciainternacional/wp-content/uploads/2022/09/Ingenieria-de-procesos-asistida_compressed.pdf (accessed on 30 April 2025).
- Carroll, W.F.; Johnson, R.W.; Moore, S.S.; Paradis, R.A. Poly(Vinyl Chloride). In Applied Plastics Engineering Handbook; Kutz, M., Ed.; Plastics Design Library; William Andrew Publishing: Oxford, UK, 2011; pp. 61–76. ISBN 978-1-4377-3514-7. [Google Scholar] [CrossRef]
- Summers, A.E. Introduction to Layers of Protection Analysis. J. Hazard. Mater. 2003, 104, 163–168. [Google Scholar] [CrossRef]
- Markowski, A.S.; Mujumdar, A.S. Layer-of-Protection Analysis (dLOPA) for Industrial Drying Risk Assessment. In Proceedings of the 14th International Drying Symposium (IDS 2004), Sao Paulo, Brazil, 22 August 2004; Available online: https://www.researchgate.net/publication/41649943_Layer-of-protection_analysis_dLOPA_for_industrial_drying_risk_assessment (accessed on 30 April 2025).
- Wang, H.; Chen, B.; He, X.; Qiu, T.; Zhao, J. SDG-Based HAZOP Analysis of Operating Mistakes for PVC Process. Process Saf. Environ. Prot. 2009, 87, 40–46. [Google Scholar] [CrossRef]
- Aguilar-Vásquez, E.; Ramos-Olmos, M.; González-Delgado, Á.D. A Joint Computer-Aided Simulation and Water-Energy-Product (WEP) Approach for Technical Evaluation of PVC Production. Sustainability 2023, 15, 8096. [Google Scholar] [CrossRef]
- Bracho-Carmona, M.L.; Van der Biest-Rodríguez, F.R. Requerimientos máximos de agua de enfriamiento en el área de polimerización de la planta PVC II, El Tablazo; Universidad Rafael Urdaneta: Maracaibo, Venezuela, 2007; Available online: https://documentos.uru.edu/pdf/2101-07-01736.pdf (accessed on 30 April 2025).
- Chan, J.H.; Foo, D.C.Y.; Kumaresan, S.; Abdul Aziz, R.; Abu Hassan, M.A. Process modelling of a PVC production plant. In Proceedings of the 2nd International Conference on Chemical and Bioprocess Engineering in conjunction with the 19th Symposium of Malaysian Chemical Engineers (SOMChE 2005), Kota Kinabalu, Malaysia, 8–10 December 2005; Available online: https://eprints.utm.my/5375/ (accessed on 30 April 2025).
- Wieme, J.; De Roo, T.; Marin, G.B.; Heynderickx, G.J. Simulation of pilot- and industrial-scale vinyl chloride batch suspension polymerization reactors. Ind. Eng. Chem. Res. 2007, 46, 1179–1196. [Google Scholar] [CrossRef]
- Hu, J.; Xiao, S.; Chen, Y. Chapter Eleven—Information security risk-based inherently safer design for intelligent oil and gas pipeline systems. In Methods in Chemical Process Safety; Khan, F.I., Amyotte, P.R., Alauddin, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; Volume 7, pp. 279–309. ISBN 978-0-443-19380-4. [Google Scholar] [CrossRef]
- Meramo-Hurtado, S.I.; Sanchez-Tuiran, E.; Ponce-Ortega, J.M.; El-Halwagi, M.M.; Ojeda-Delgado, K.A. Síntesis y evaluación de la sostenibilidad de una biorrefinería de múltiples materias primas lignocelulósicas considerando indicadores de desempeño técnico. ACS Omega 2020, 5, 9259–9275. [Google Scholar] [CrossRef] [PubMed]
- Abedi, P.; Shahriari, M. Inherent safety evaluation in process plants—A comparison of methodologies. Open Chem. 2005, 3, 756–779. [Google Scholar] [CrossRef]
- Chemical Safety Software. SDS Search. Chemical Safety Software. Available online: https://chemicalsafety.com/sds-search/ (accessed on 16 April 2025).
- National Institute for Occupational Safety and Health (NIOSH). NIOSH Pocket Guide to Chemical Hazards; Centers for Disease Control and Prevention (CDC): Atlanta, GA, USA, 1978. Available online: https://www.cdc.gov/niosh/npg/ (accessed on 16 April 2025).
- World Health Organization. Vinyl Chloride: Environmental Health Criteria 215; WHO: Geneva, Switzerland, 1999; Available online: https://apps.who.int/iris/bitstream/handle/10665/42217/WHO_EHC_215.pdf (accessed on 16 April 2025).
- Estrada-Marroquín, M.A. Automatización del Proceso de Llenado de Hipoclorito de Sodio en Una Planta de Producción de Artículos del Cuidado del Hogar; Universidad de San Carlos de Guatemala: Guatemala City, Guatemala, 2018; Available online: http://www.repositorio.usac.edu.gt/8643/1/Manuel%20Alejandro%20Estrada%20Marroqu%C3%ADn.pdf (accessed on 10 March 2025).
- National Oceanic and Atmospheric Administration (NOAA). CAMEO Chemicals. Available online: https://cameochemicals.noaa.gov/ (accessed on 16 April 2025).
- Salocks, C.; Kaley, K.B. Technical Support Document: Toxicology—Clandestine Drug Labs: Methamphetamine. Volume 1, Number 7: Sodium Hydroxide; California Environmental Protection Agency, Office of Environmental Health Hazard Assessment: Sacramento, CA, USA, 2004; Available online: https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=56ca70fcc699801802833d296cadc9895ee1f54d (accessed on 10 March 2025).
- Straccia-Cepeda, V.G. Caracterización Estructural del PVC y Correlación con su Estabilidad Térmica; Universidad del Zulia, Facultad Experimental de Ciencias, Departamento de Química: Maracaibo, Venezuela, 2017; Available online: https://www.academia.edu/39348046/Caracterizaci%C3%B3n_estructural_del_PVC_y_correlaci%C3%B3n_con_su_estabilidad_t%C3%A9rmica (accessed on 10 March 2025).
- Xu, Z.; Kolapkar, S.S.; Zinchik, S.; Bar-Ziv, E.; McDonald, A.G. Comprehensive kinetic study of thermal degradation of polyvinylchloride (PVC). Polym. Degrad. Stab. 2020, 176, 109148. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services. Toxicological Profile for Vinyl Chloride; Agency for Toxic Substances and Disease Registry, U.S. Department of Health and Human Services: Atlanta, GA, USA, 2023. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp20.pdf (accessed on 15 June 2023).
- Ogle, R.A.; Megerle, M.V.; Morrison, D.R.; Carpenter, A.R. Explosion caused by flashing liquid in a process vessel. J. Hazard. Mater. 2004, 115, 133–140. [Google Scholar] [CrossRef]
- Ministère Chargé de l’Environnement. Vinyl Chloride Leak in a Chemical Plant. 2006. Available online: https://www.aria.developpement-durable.gouv.fr/accident/44911_en/?lang=en (accessed on 26 May 2025).
- Leal-Alanís, S.A. Caracterización de Aceros Inoxidables y Estudio de su Resistencia Mecánica y Conformabilidad. Master’s thesis, Universidad Autónoma de Nuevo León, San Nicolás de los Garza, México. 2011. Available online: http://eprints.uanl.mx/2495/ (accessed on 26 May 2025).
- Gardner, L.; Insausti, A.; Ng, K.T.; Ashraf, M. Elevated temperature material properties of stainless steel alloys. J. Constr. Steel Res. 2010, 66, 634–647. [Google Scholar] [CrossRef]
- Mejía-Meza, R.J.; Ricardo-Ricardo, L.G.R. Automatización y Control de Una extrusora para la Producción de Tuberías de PVC (Polyvinyl Chloride). Proyecto de grado, Universidad Tecnológica de Bolívar, Cartagena de Indias D.T. y C. 2016. Available online: https://repositorio.utb.edu.co/server/api/core/bitstreams/4b609912-86b8-467c-b7d1-27ea95a82126/content (accessed on 26 May 2025).
- Feliu, S., Jr.; Veleva, L.; García-Galvan, F. Effect of Temperature on the Corrosion Behavior of Biodegradable AZ31B Magnesium Alloy in Ringer’s Physiological Solution. Metals 2019, 9, 591. [Google Scholar] [CrossRef]
- Yang, X.; Sun, F.; Li, Q.; Zhu, R.; Liu, Z.; Du, C.; Li, X. Effect of Hydrogen Charging on the Stress Corrosion Cracking Behavior of X70 Steel in Simulated Deep Seawater Environment. Metals 2022, 12, 334. [Google Scholar] [CrossRef]
- Nogara, J.; Zarrouk, S.J. Corrosion in geothermal environment: Part 1: Fluids and their impact. Renew. Sustain. Energy Rev. 2018, 82, 1333–1346. [Google Scholar] [CrossRef]
- Inspenet. Stress Corrosion Cracking: Mechanism, Control and Practical Cases. Inspenet. 2023. Available online: https://inspenet.com/en/articulo/low-stress-corrosion-cracking/ (accessed on 26 May 2025).
- Cole Parmer. Chemical Resistance. Available online: https://www.coleparmer.com/Chemical-Resistance (accessed on 16 April 2025).
- Song, W.; Zhao, X.; Jin, Z.; Fan, L.; Ji, X.; Deng, J.; Duan, J. Poly(vinyl alcohol) for multi-functionalized corrosion protection of metals: A review. J. Clean. Prod. 2023, 394, 136390. [Google Scholar] [CrossRef]
- Carvalho, A.; Gani, R.; Matos, H. Design of sustainable chemical processes: Systematic retrofit analysis generation and evaluation of alternatives. Process. Saf. Environ. Prot. 2008, 86, 328–346. [Google Scholar] [CrossRef]
- Blanco-Jaén, L. Desarrollo y Aplicación de Tratamientos Avanzados de Regeneración de Efluentes en la Producción de PVC. Ph.D. Thesis, Universidad Complutense de Madrid, Madrid, Spain, 2017. Available online: https://hdl.handle.net/20.500.14352/16159 (accessed on 26 May 2025).
- El Sol de Puebla. Primex, la Explosión que Aterró a los Poblanos en 1977. Available online: https://oem.com.mx/elsoldepuebla/cultura/primex-la-explosion-que-aterro-a-los-poblanos-en-1977-los-tiempos-idos-13433104 (accessed on 16 April 2025).
- U.S. Chemical Safety and Hazard Investigation Board. U.S. CSB Investigation Report, Vinyl Chloride Monomer Explosion. 2007. Available online: https://www.csb.gov/assets/1/20/formosa_il_report.pdf (accessed on 16 April 2025).
- La Nueva. White: Se Cumplen 16 Años del Escape de Cloro en el Complejo Petroquímico. Available online: https://www.lanueva.com/nota/2016-8-20-7-23-0-white-se-cumplen-16-anos-del-escape-de-cloro-en-el-complejo-petroquimico (accessed on 16 April 2025).
- Blászquez, A. Diseño de Seguridad Inherente. 2020. Available online: http://oa.upm.es/62862/1/TFG_ALVARO_LOZANO_BLAZQUEZ.pdf (accessed on 26 May 2025).
- Tan, Z.Y.; Huo, J.L.; Yu, Y.M.; Wu, Z.Z. Safety Analysis of VCM Storage Tanks Based on AHP-Fuzzy. Adv. Mater. Res. 2011, 233–235, 2447–2450. [Google Scholar] [CrossRef]
- Energy Cle, S.A.S. Estadísticas Mundiales de Producción y Eliminación de PVC. Available online: https://www.energycle.com/es/estadisticas-mundiales-de-produccion-y-eliminacion-de-pvc/ (accessed on 16 April 2025).
- Schettler, T. El Policloruro de Vinilo en la Atención de la Salud: Fundamentos Para la Elección de Alternativas. Salud Sin Daño, Enero de 2020. Available online: https://lac.saludsindanio.org/sites/default/files/documents-files/6577/Policloruro%20de%20vinilo.pdf (accessed on 26 May 2025).
- González-Delgado, Á.D.; Aguilar-Vásquez, E.A.; Ramos-Olmos, M. Chemical and Process Inherent Safety Analysis of Large-Scale Suspension Poly(Vinyl Chloride) Production. ChemEngineering 2023, 7, 76. [Google Scholar] [CrossRef]
- Mejía-Ochoa, D.I.; Ruiz-Tizón, D.S. Diseño e Implementación de un Sistema de Monitoreo y Control en la Línea de Extrusión de la Empresa Vulcano Plástico. Bachelor’s Thesis, Universidad Politécnica Salesiana, Ecuador, July 2022. Available online: http://dspace.ups.edu.ec/handle/123456789/24153 (accessed on 26 May 2025).
- Catalán-Tobar, F.A. Análisis y Prevención de Riesgos e Implementación de un Sistema de Seguridad Industrial, en una Planta de Extrusión de Tubería PVC, Basado en la Norma OHSAS 18000. Master’s Thesis, Universidad de San Carlos de Guatemala, Ciudad de Guatemala, Guatemala, 2017. Available online: http://www.repositorio.usac.edu.gt/id/eprint/7379 (accessed on 26 May 2025).
- Agency for Toxic Substances and Disease Registry (ATSDR). Policloruro de vinilo (PVC): Hoja Informativa. Available online: https://www.atsdr.cdc.gov/es/toxfaqs/es_tfacts20.html (accessed on 16 April 2025).
- Escudero-Caviedes, A.R.; Pérez-Cuesta, D.C. Condiciones de Trabajo y Salud de los Trabajadores de la Planta de Producción de una Fábrica de Envases Plásticos, Durante el mes de Octubre de 2018. Universidad. 2018. Available online: http://hdl.handle.net/10554/38993 (accessed on 26 May 2025).
- ISO 45001:2018; Occupational Health and Safety Management Systems—Requirements with Guidance for Use; Official Spanish Translation. International Organization for Standardization (ISO): Geneva, Switzerland, 2018. Available online: https://ergosourcing.com.co/wp-content/uploads/2018/05/iso-45001-norma-Internacional.pdf (accessed on 26 May 2025).
- Kiparissides, C.; Achilias, D.S.; Frantzikinakis, C.E. The effect of oxygen on the kinetics and particle size distribution in vinyl chloride emulsion polymerization. Ind. Eng. Chem. Res. 2002, 41, 3097–3109. [Google Scholar] [CrossRef]
- Starnes, W.H. Structural and mechanistic aspects of the thermal degradation of poly(vinyl chloride). Prog. Polym. Sci. 2002, 27, 2133–2170. [Google Scholar] [CrossRef]
- State of California Air Resources Board. Technical Support Document, Part A: Vinyl Chloride; Stationary Source Division: Sacramento, CA, USA, 1990. Available online: https://ww2.arb.ca.gov/sites/default/files/classic/toxics/id/summary/vinyl_a.pdf (accessed on 26 May 2025).
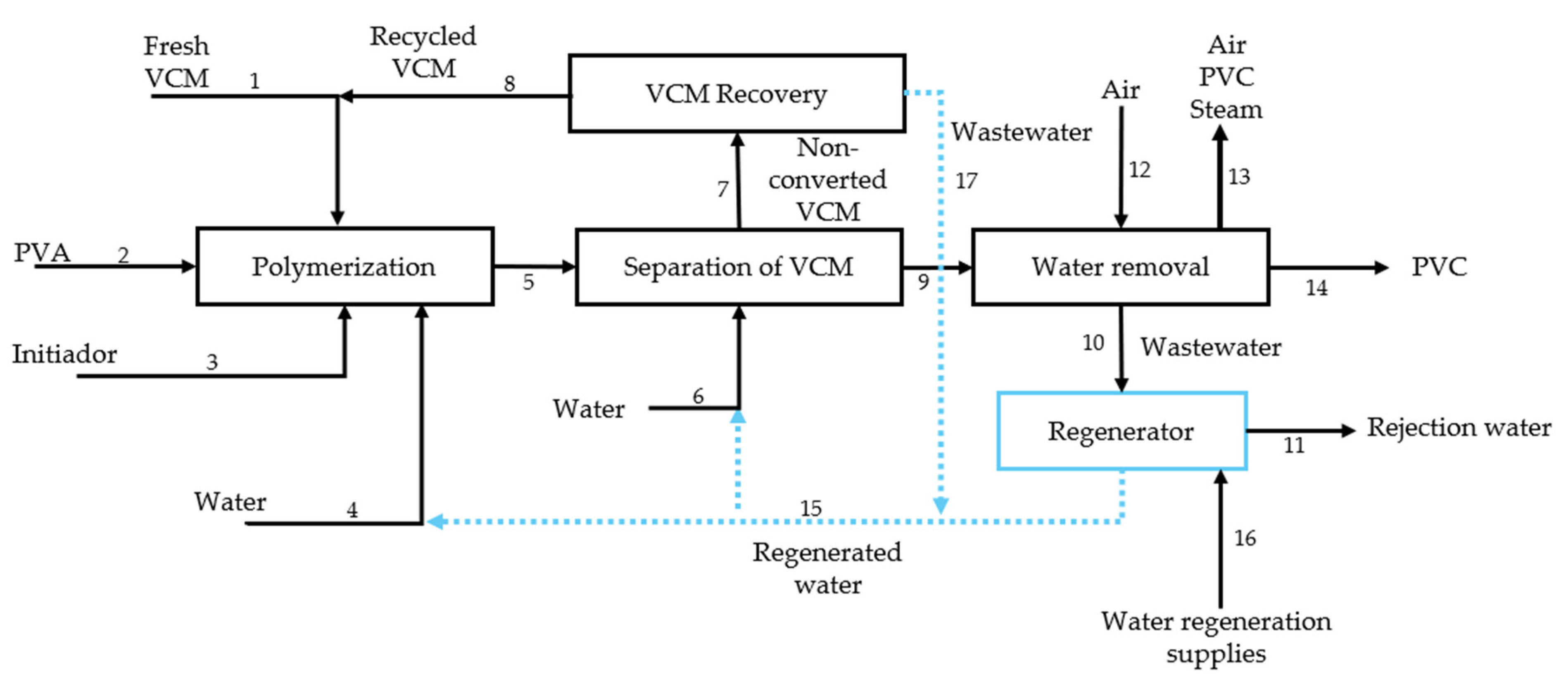
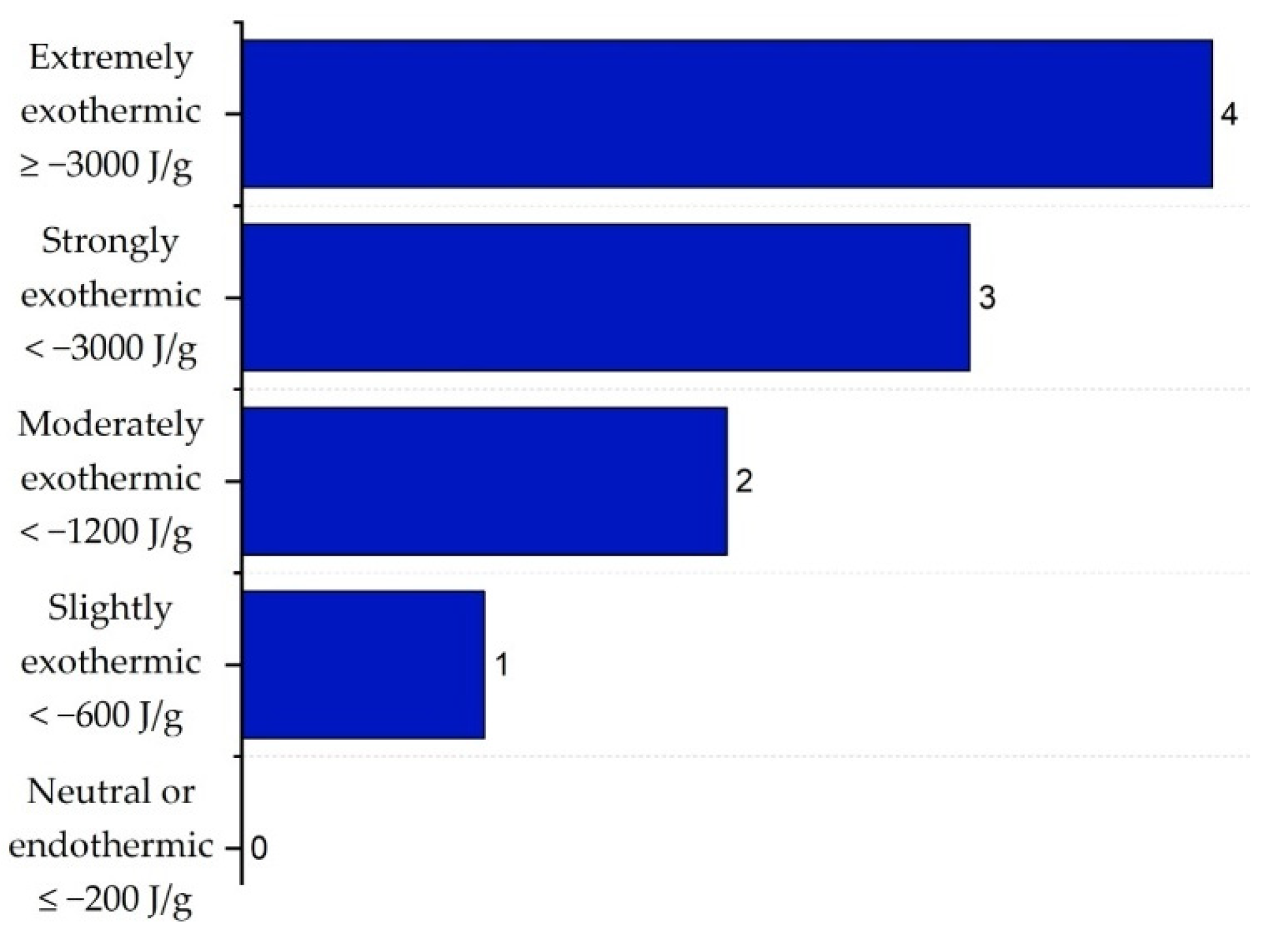


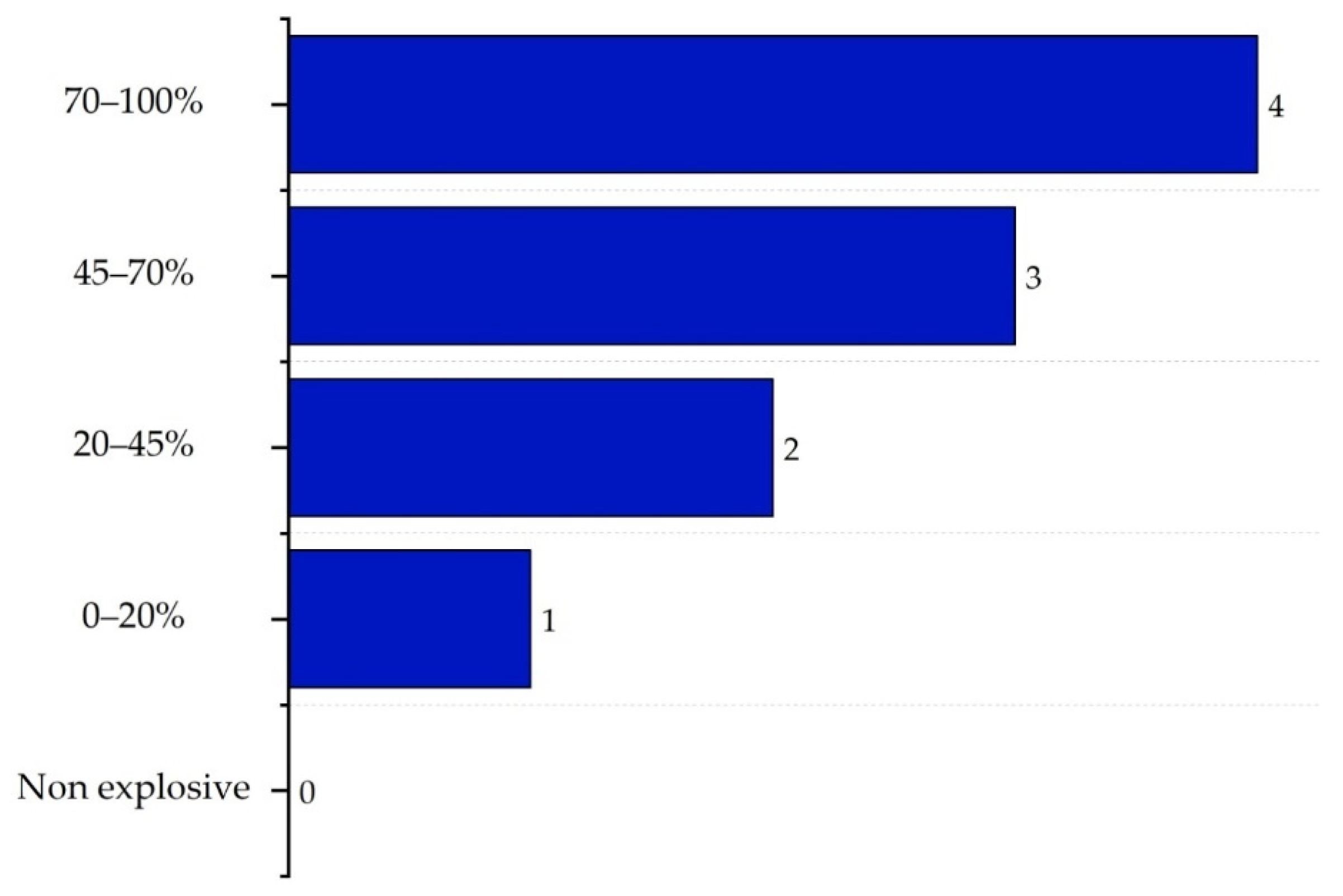
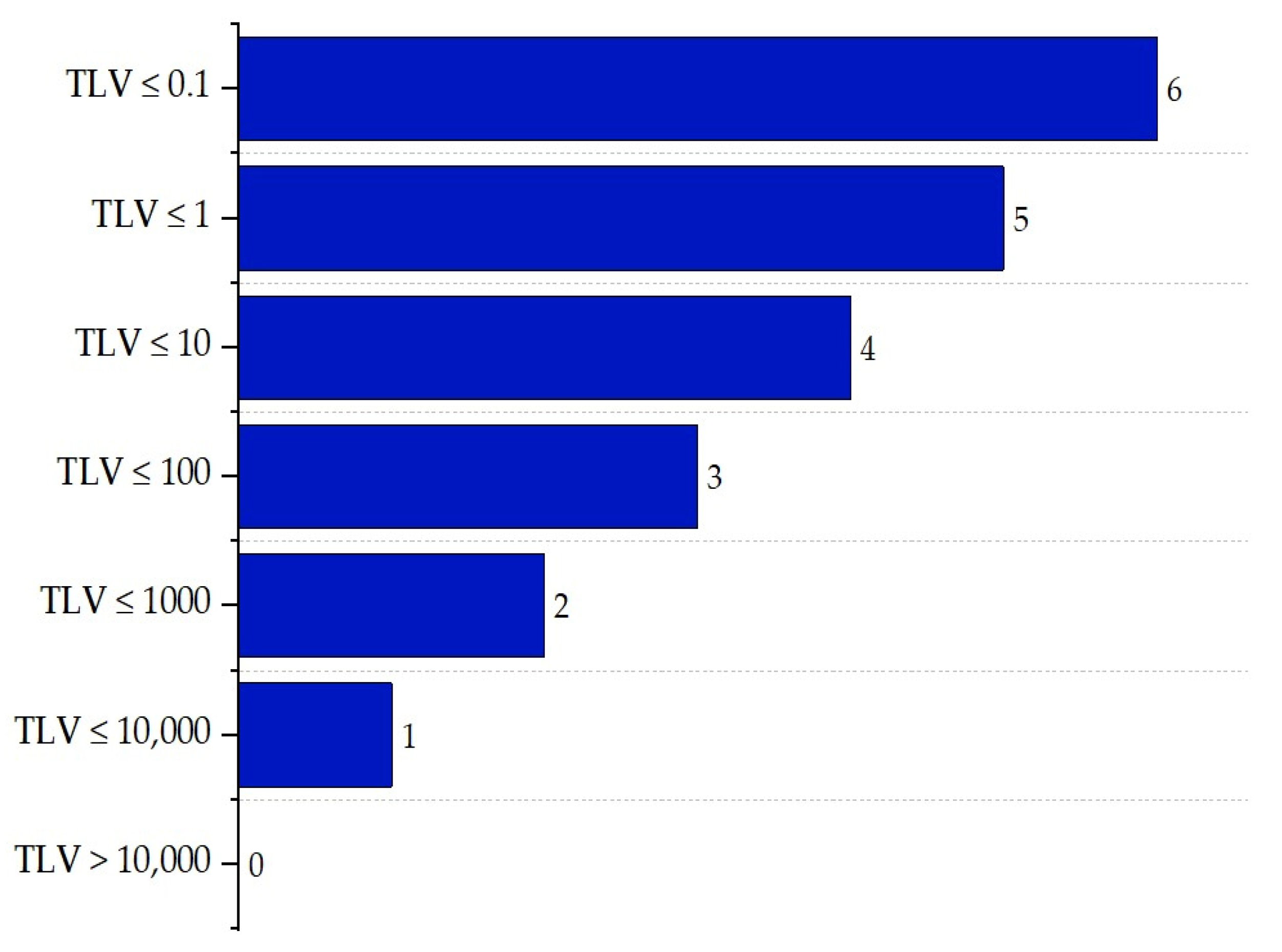


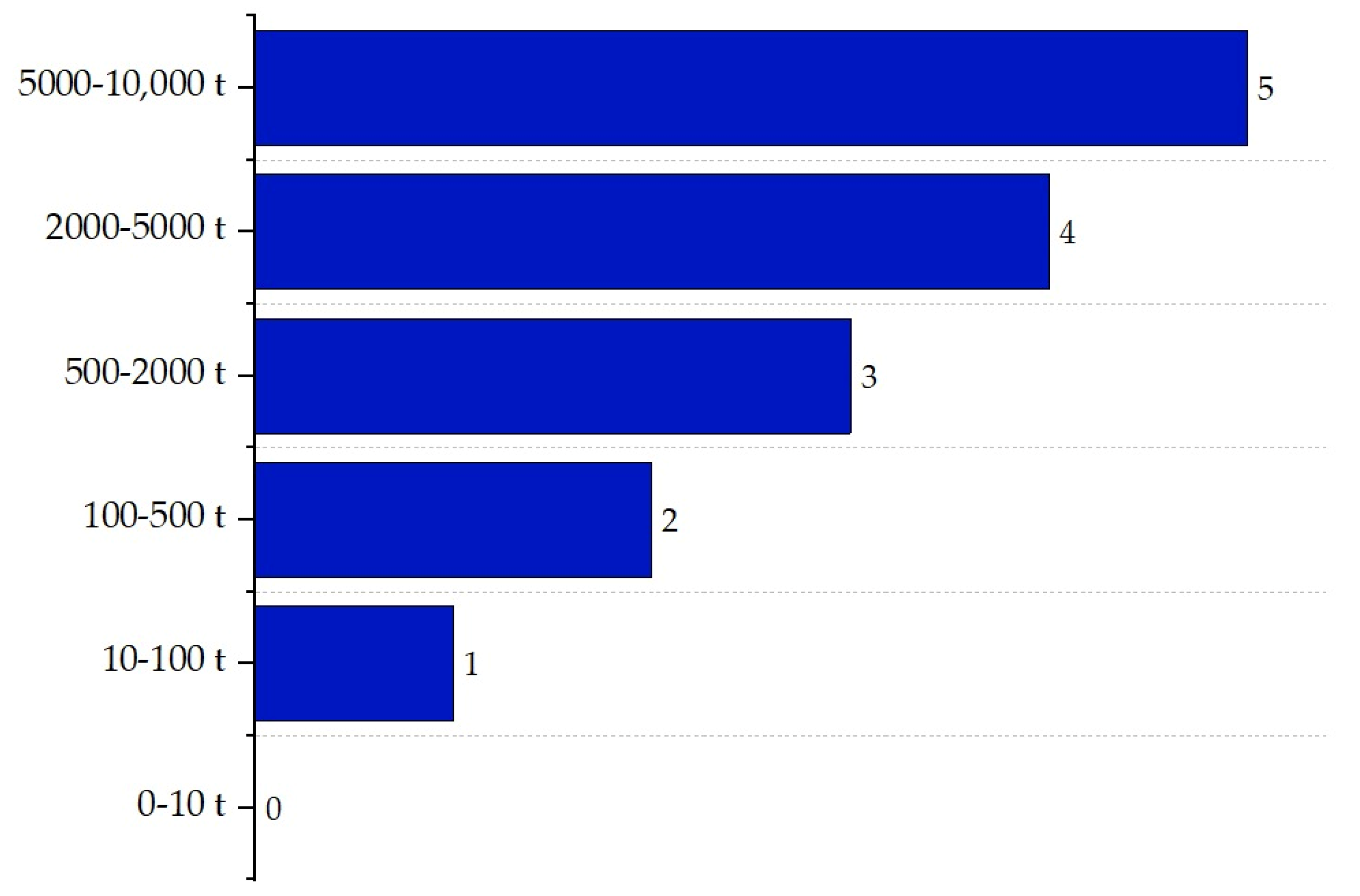
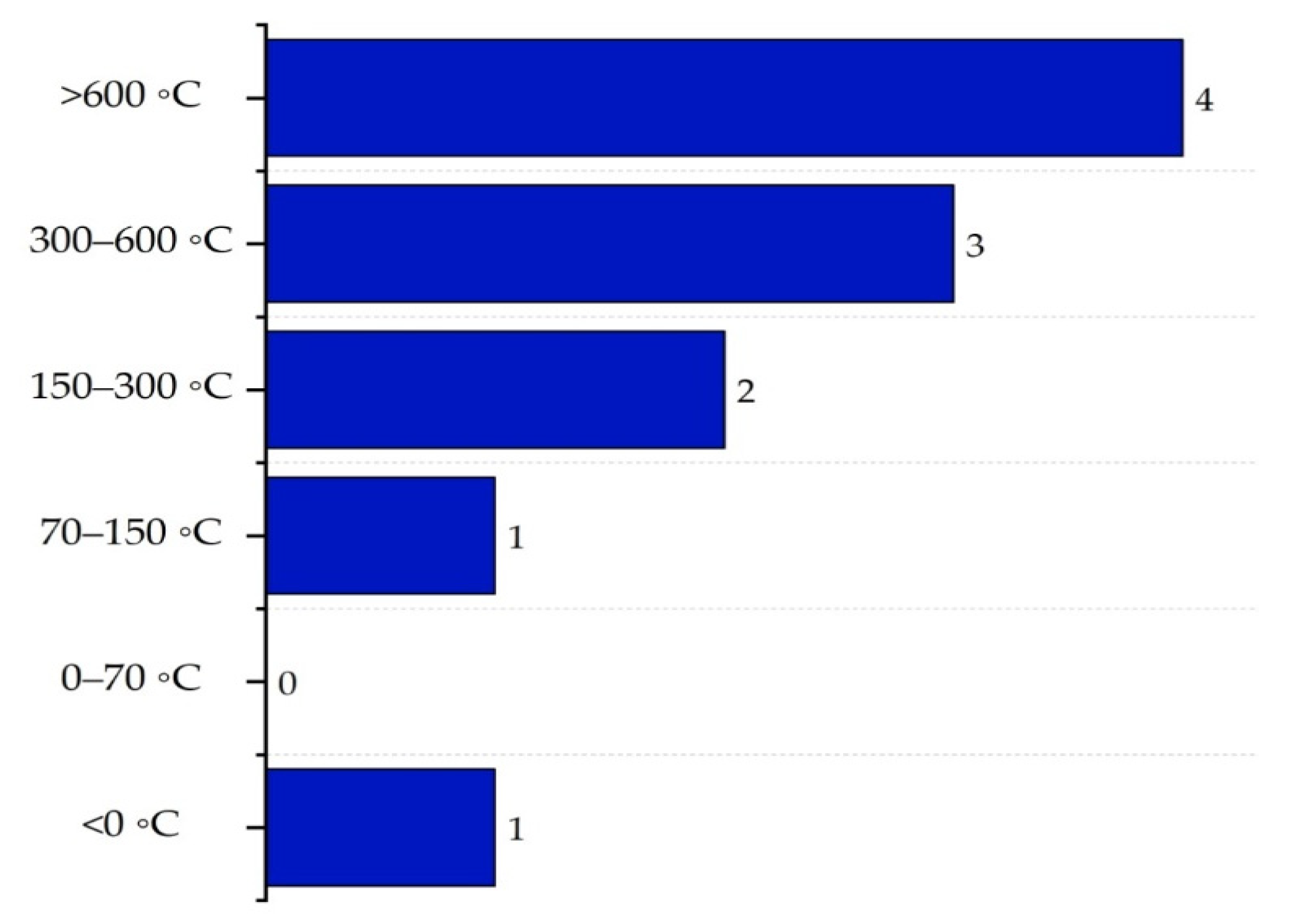

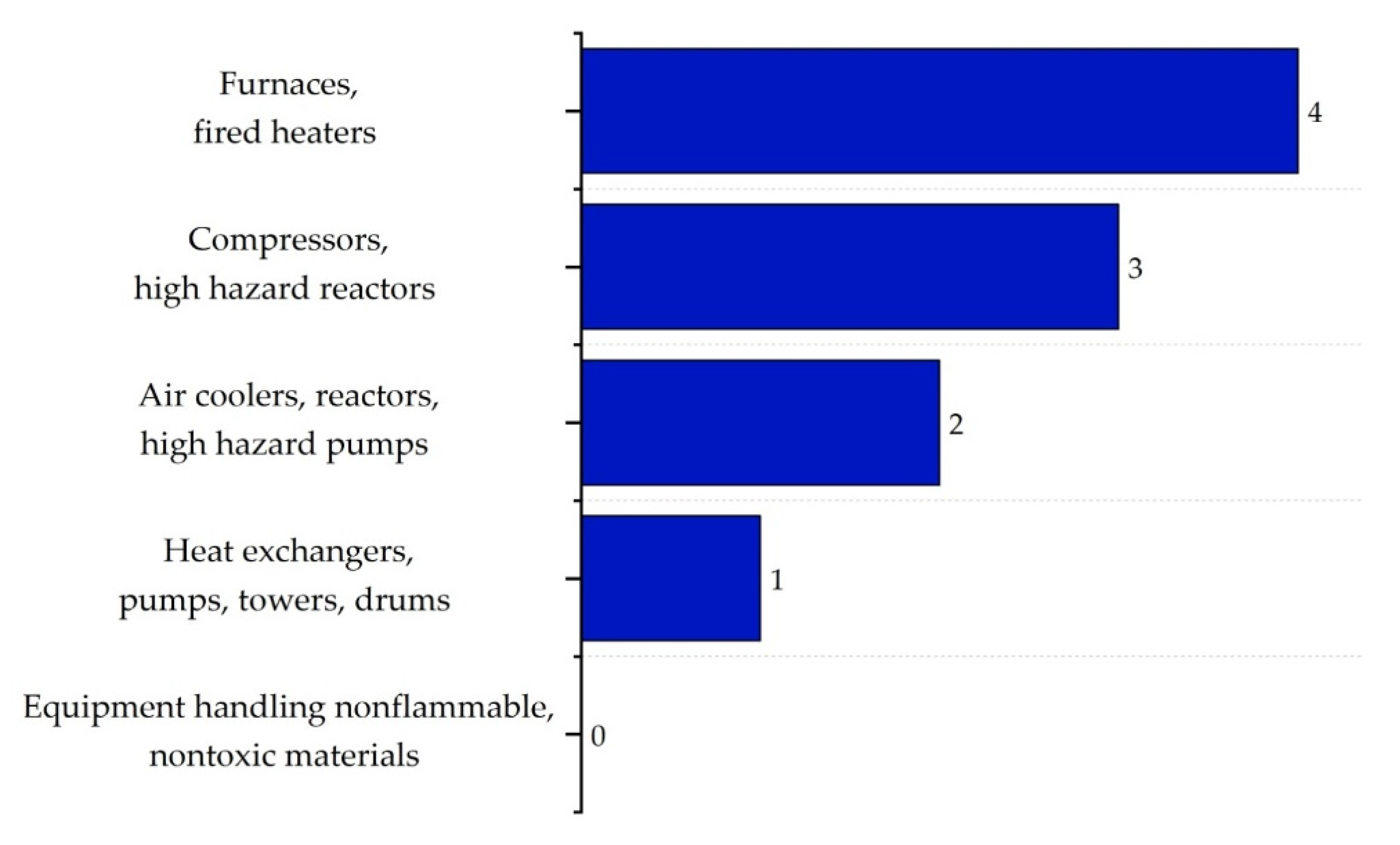
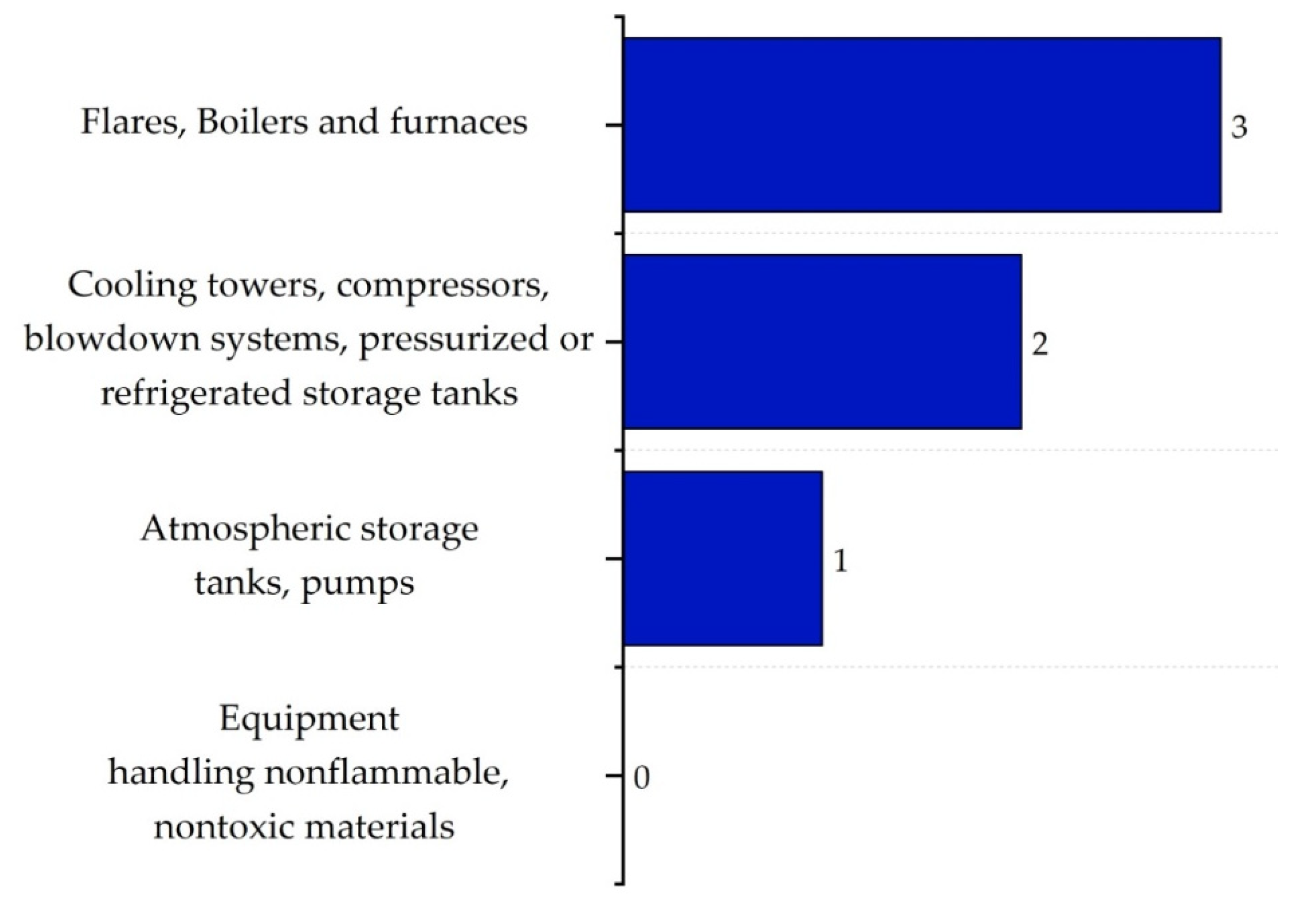
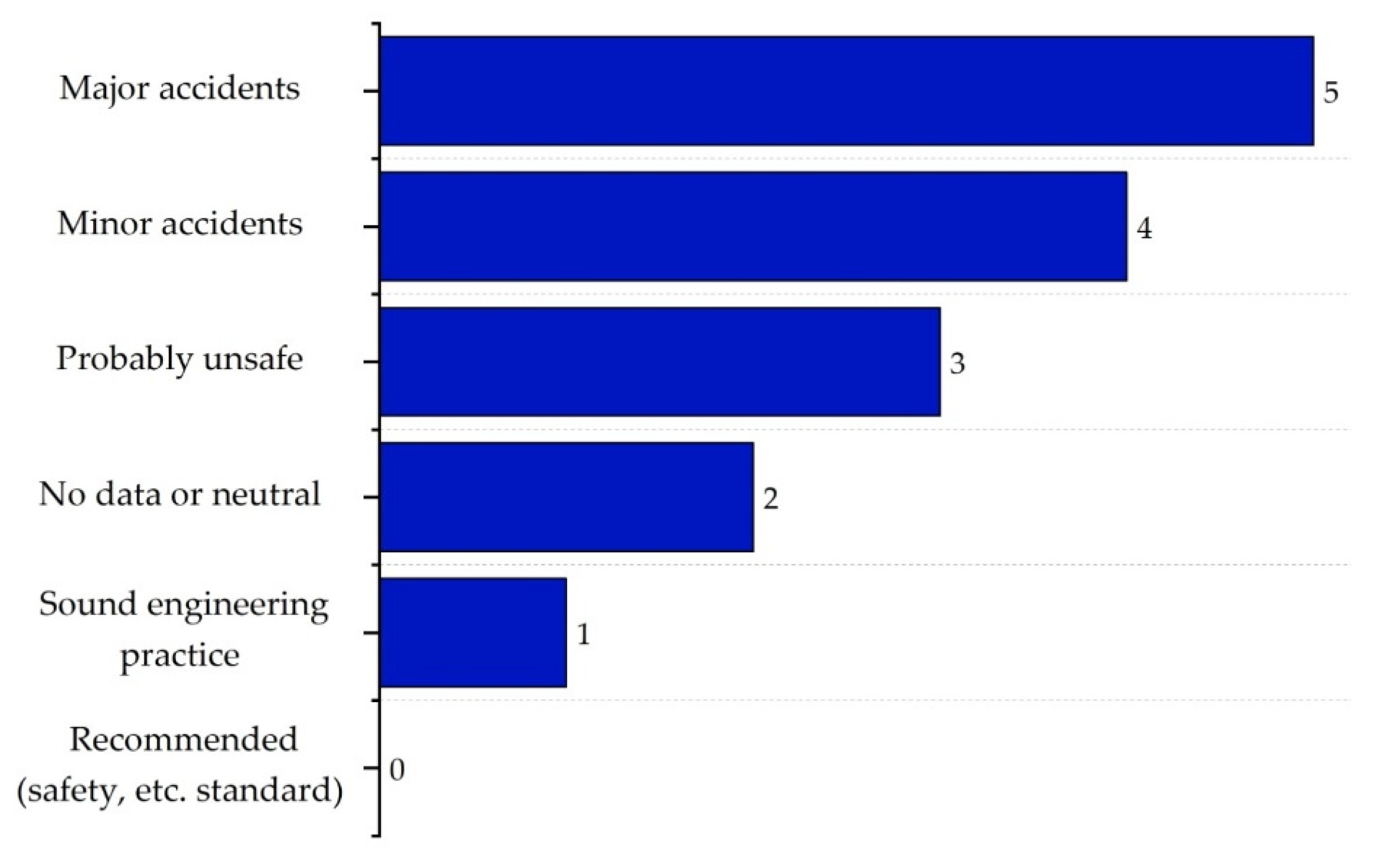
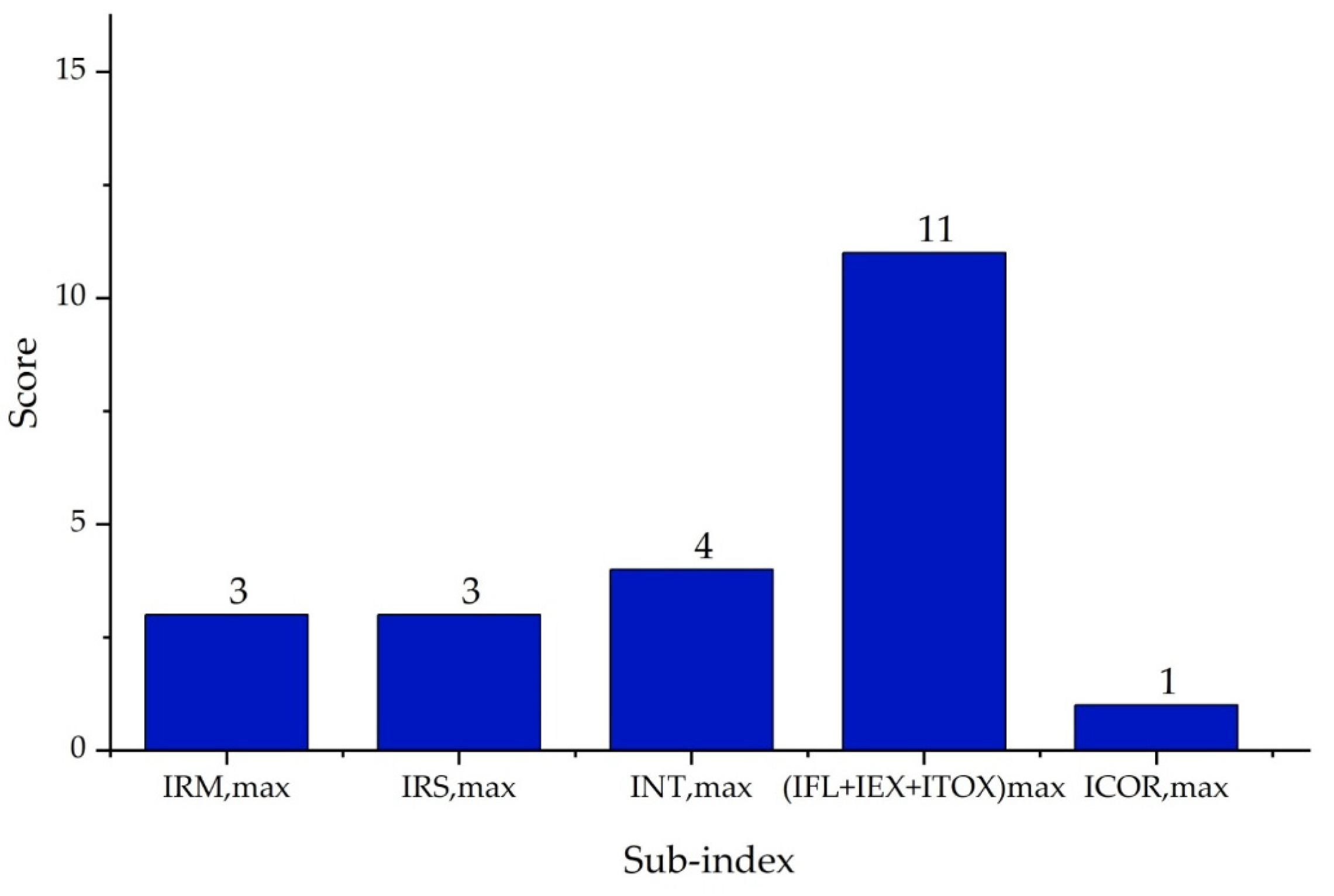
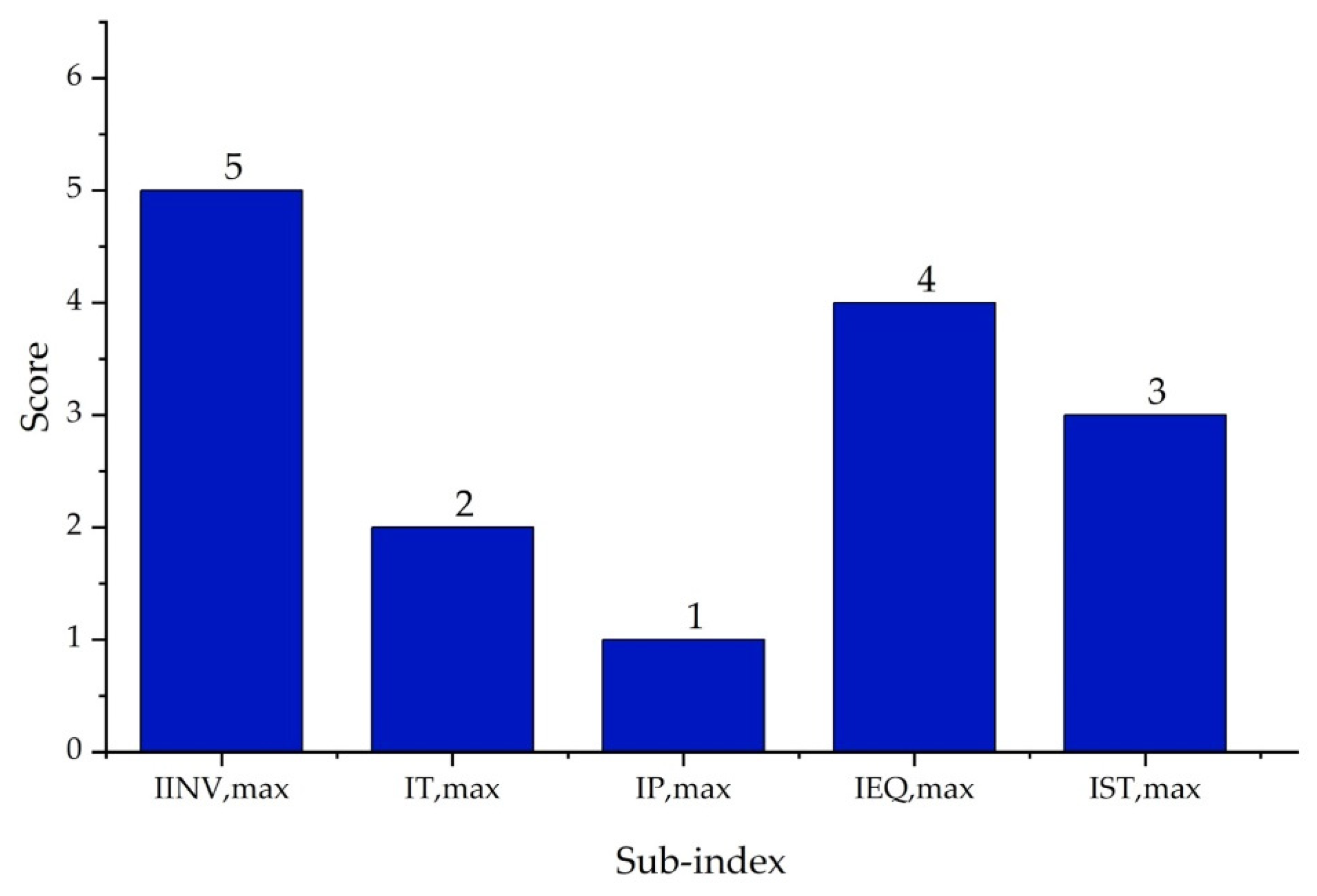
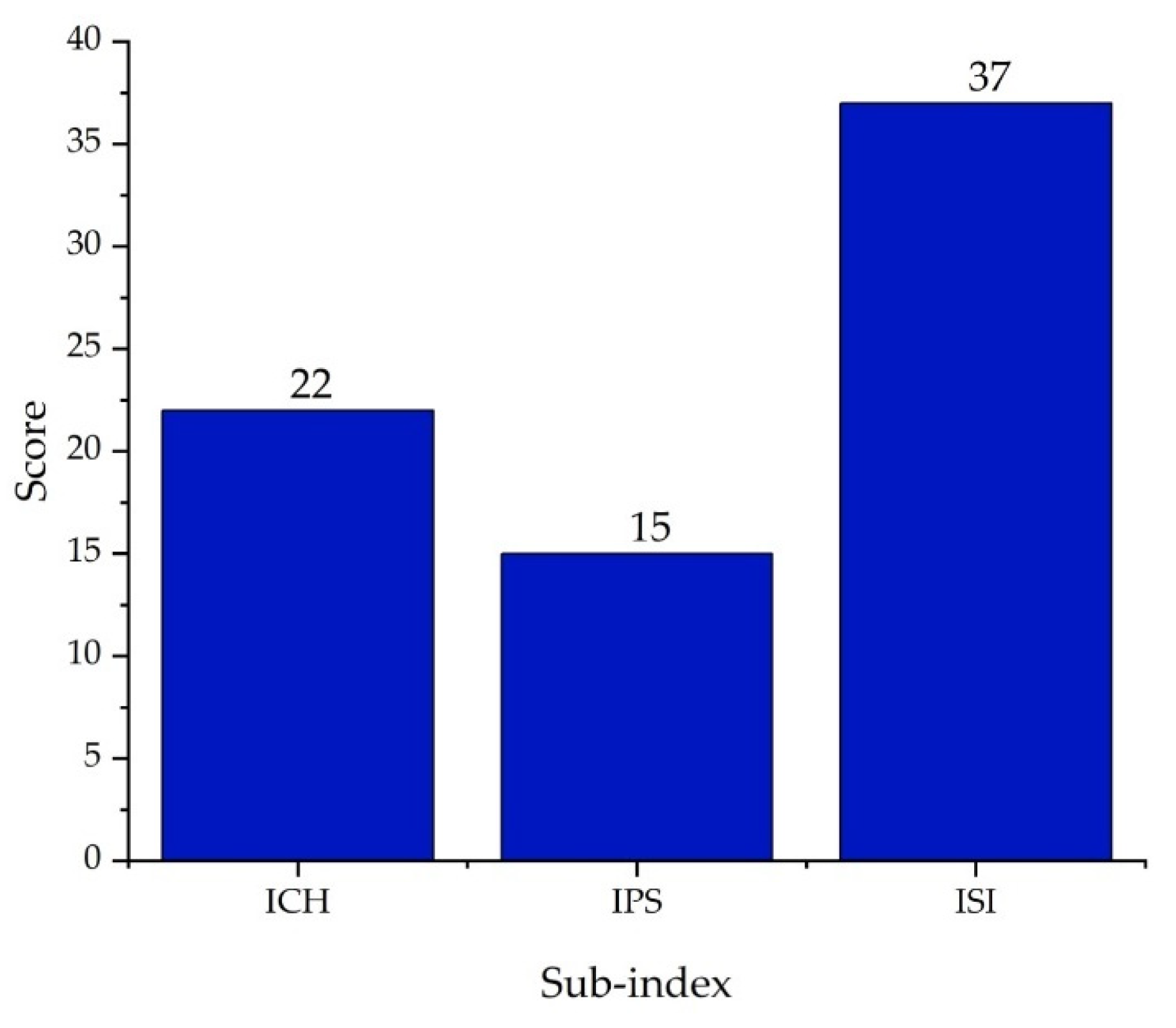
| Substance | Explosivity (UEL%–LEL%) | Flammability | Toxicity TVL (ppm) | Chemical Interaction |
|---|---|---|---|---|
| VCM | 29.40% | Highly flammable | TLV ≤ 1 | Explosion |
| NaOH | 0 | Non-flammable | TLV ≤ 10 | Fire |
| NaOCL | 0 | Non-flammable | TLV ≤ 10 | Explosion |
| PVC | 0 | Combustible | TLV ≤ 1 | Explosion |
| Substance | Explosivity (UEL%–LEL%) | Flammability | Toxicity TVL (ppm) | Chemical Interaction |
|---|---|---|---|---|
| VCM | 2 | 4 | 5 | 4 |
| NaOH | 0 | 0 | 4 | 4 |
| NaOCL | 0 | 0 | 4 | 4 |
| PVC | 0 | 1 | 5 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bustamante-Miranda, A.; Aguilar-Vásquez, E.; Ramos-Olmos, M.; Rojas-Flores, S.; González-Delgado, Á.D. Influence of Water Regeneration on Chemical and Process Indices in an Energy-Integrated PVC Production Process. Polymers 2025, 17, 1639. https://doi.org/10.3390/polym17121639
Bustamante-Miranda A, Aguilar-Vásquez E, Ramos-Olmos M, Rojas-Flores S, González-Delgado ÁD. Influence of Water Regeneration on Chemical and Process Indices in an Energy-Integrated PVC Production Process. Polymers. 2025; 17(12):1639. https://doi.org/10.3390/polym17121639
Chicago/Turabian StyleBustamante-Miranda, Arelmys, Eduardo Aguilar-Vásquez, Miguel Ramos-Olmos, Segundo Rojas-Flores, and Ángel Darío González-Delgado. 2025. "Influence of Water Regeneration on Chemical and Process Indices in an Energy-Integrated PVC Production Process" Polymers 17, no. 12: 1639. https://doi.org/10.3390/polym17121639
APA StyleBustamante-Miranda, A., Aguilar-Vásquez, E., Ramos-Olmos, M., Rojas-Flores, S., & González-Delgado, Á. D. (2025). Influence of Water Regeneration on Chemical and Process Indices in an Energy-Integrated PVC Production Process. Polymers, 17(12), 1639. https://doi.org/10.3390/polym17121639







