Architecting Porosity Through Monomer Engineering: Hypercrosslinked Polymers for Highly Selective CO2 Capture from CH4 or N2
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Instrumentation
2.3. Expertmental Methods
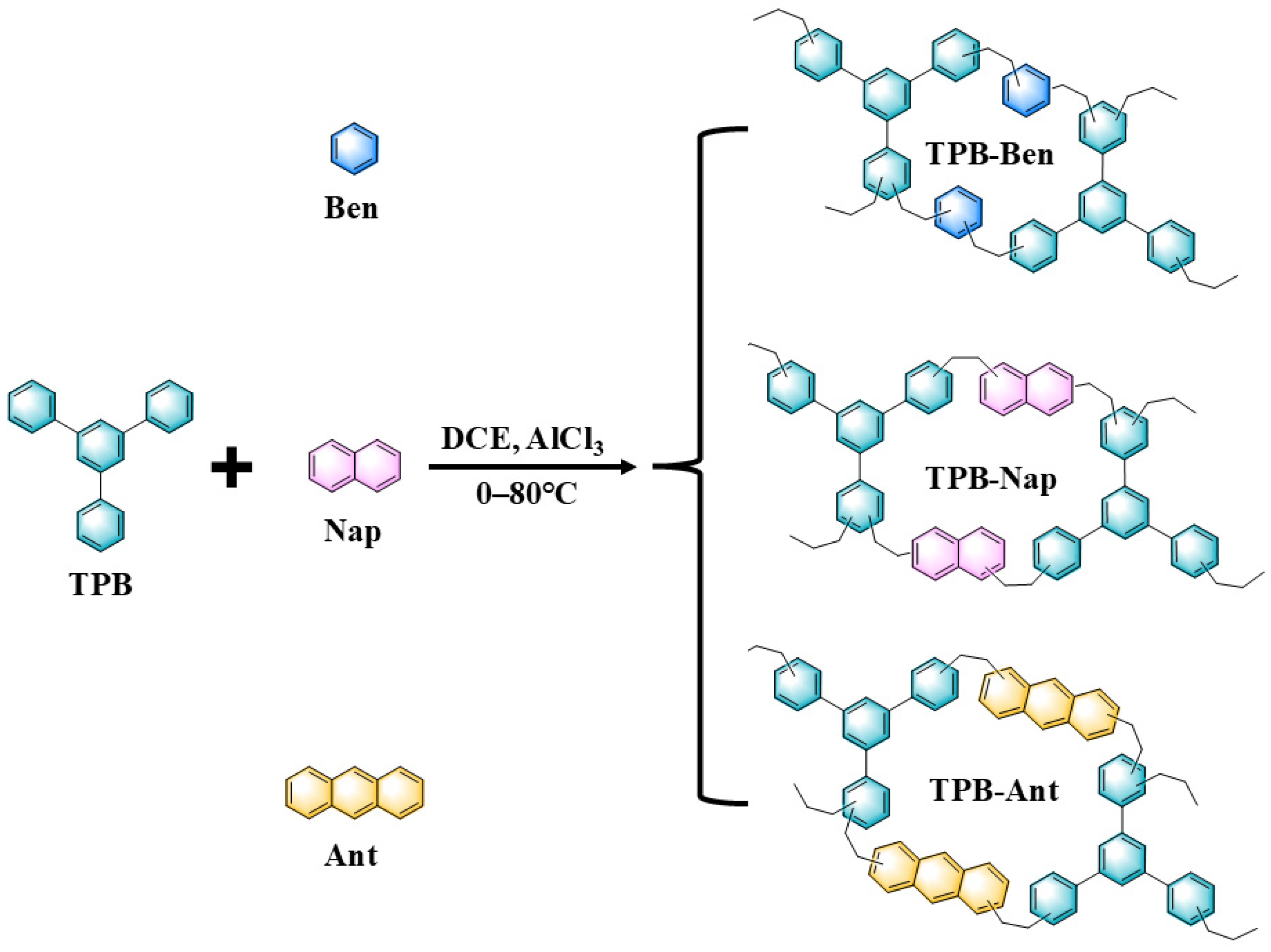
2.4. Synthesis Methods
3. Results and Discussion
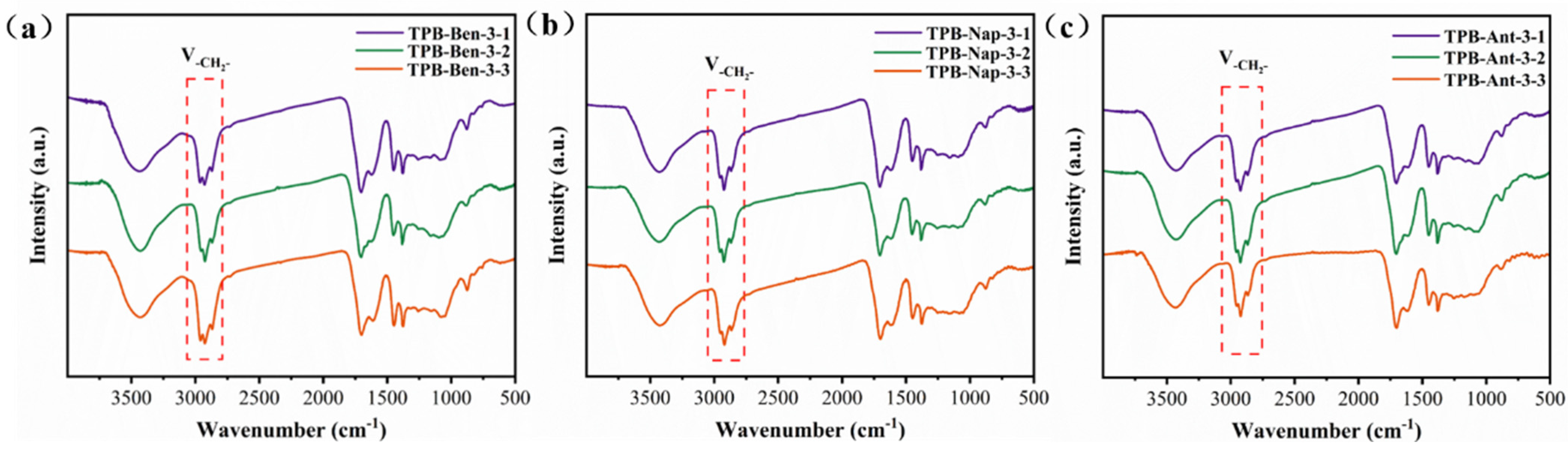
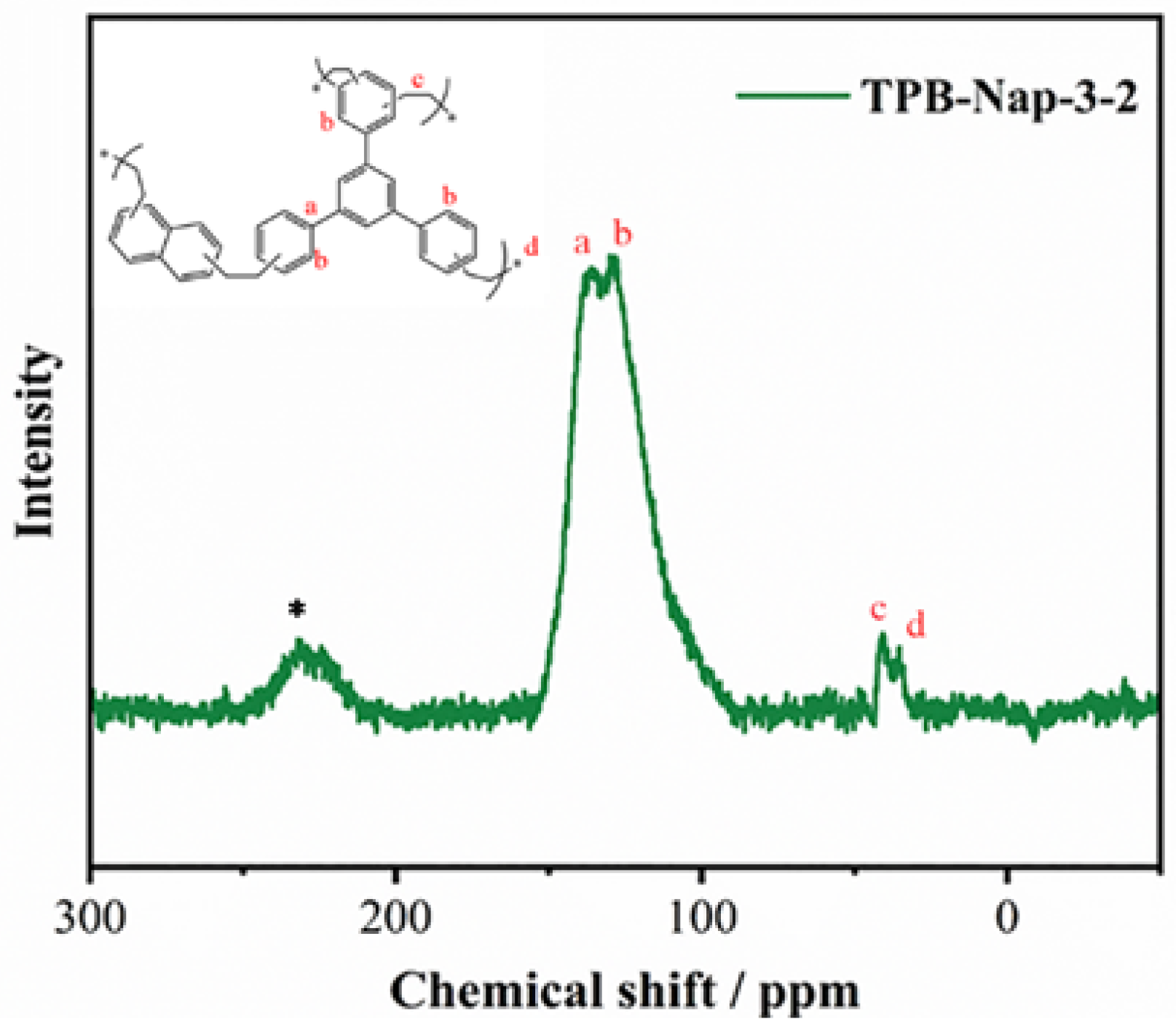
3.1. Structural Characterization
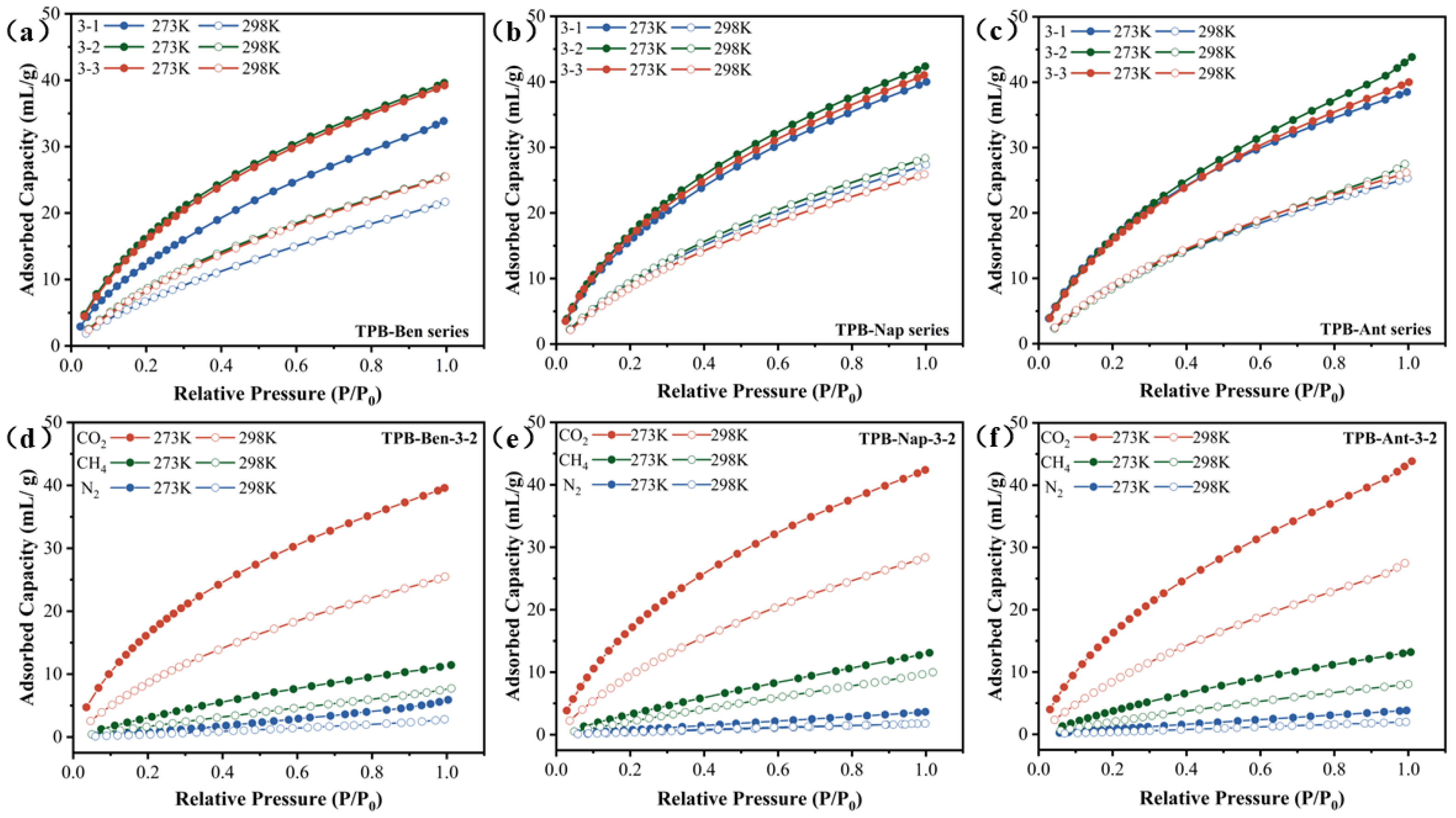
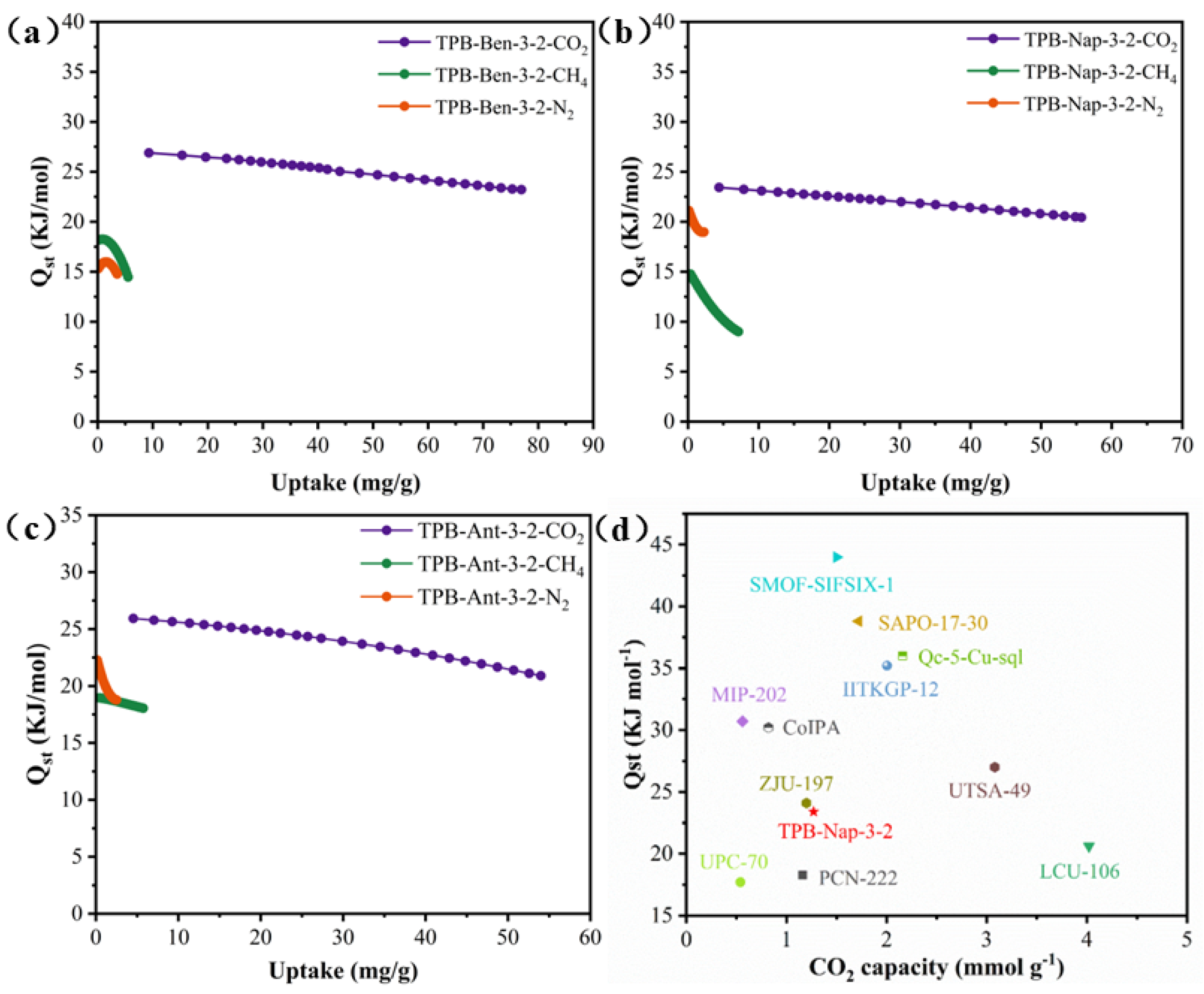
3.2. Single-Component Gas Adsorption Tests
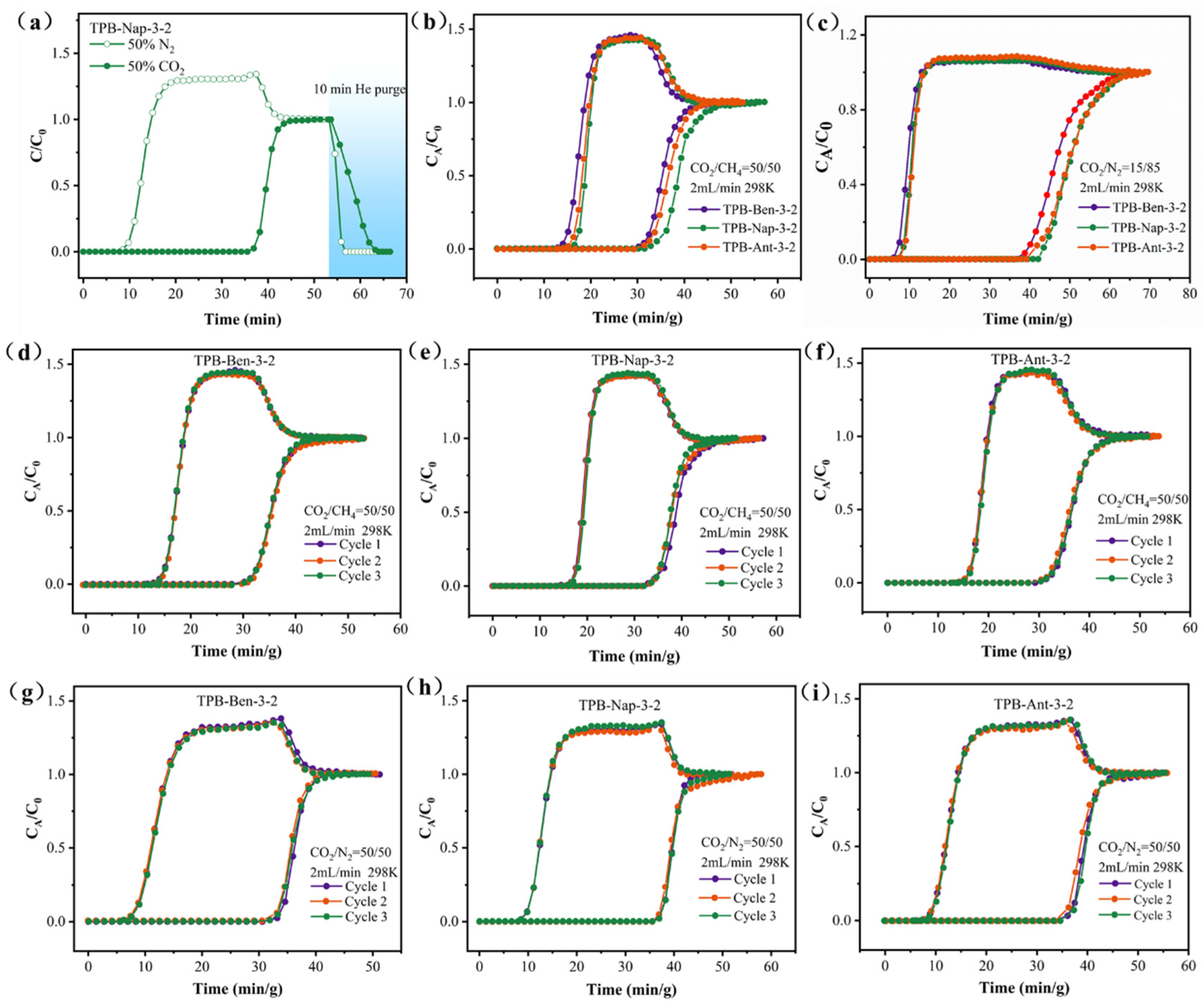
3.3. Dynamic Breakthrough Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rode, A.; Carleton, T.; Delgado, M.; Greenstone, M.; Houser, T.; Hsiang, S.; Hultgren, A.; Jina, A.; Kopp, R.E.; McCusker, K.E.; et al. Estimating a social cost of carbon for global energy consumption. Nature 2021, 598, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Nugent, P.; Belmabkhout, Y.; Burd, S.D.; Cairns, A.J.; Luebke, R.; Forrest, K.; Pham, T.; Ma, S.; Space, B.; Wojtas, L.; et al. Porous materials with optimal adsorption thermodynamics and kinetics for CO2 separation. Nature 2013, 495, 80–84. [Google Scholar] [CrossRef]
- Ning, H.; Ge, X.; Sheng, L.; Zhang, Z.; Shi, M.; Lan, H.; Jie, K.-C.; Zhang, X.; Hu, X.; Wu, Y. Mechanochemical synthesis of type III porous liquids from solid precursors for the removal and conversion of waste CO2 from CH4. Adv. Mater. 2025, 37, 2417106. [Google Scholar] [CrossRef]
- Xu, S.; Li, G.-B.; Yu, R.; Wang, P.; Ji, Y.-L. High-performance carbon capture with fluorine-tailored carbon molecular sieve membranes. Adv. Mater. 2025, 37, 2420477. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.-J.; Zhang, Y.-J.; Cheng, Y.-J.; Qin, Z.-H.; Wang, G.-D.; Bai, Y.; Lin, Y.; Wang, H.; Sui, Y.; Hou, L.; et al. Capture of CO2 from N2 and CH4 over a wide temperature range on a robust MOF with brønsted acidic and lewis basic dual functional sites. J. Mater. Chem. A 2025, 13, 10581–10589. [Google Scholar] [CrossRef]
- Samanta, A.; Zhao, A.; Shimizu, G.K.H.; Sarkar, P.; Gupta, R. Post-combustion CO2 capture using solid sorbents: A review. Ind. Eng. Chem. Res. 2011, 51, 1438–1463. [Google Scholar] [CrossRef]
- Wang, Q.; Luo, J.; Zhong, Z.; Borgna, A. CO2 capture by solid adsorbents and their applications: Current status and new trends. Energy Environ. Sci. 2011, 4, 42–55. [Google Scholar] [CrossRef]
- Radosz, M.; Hu, X.; Krutkramelis, K.; Shen, Y. Flue-gas carbon capture on carbonaceous sorbents: toward a low-cost multifunctional carbon filter for “Green” Energy Producers. Ind. Eng. Chem. Res. 2008, 47, 3783–3794. [Google Scholar] [CrossRef]
- Wang, J.; Huang, L.; Yang, R.; Zhang, Z.; Wu, J.; Gao, Y.; Wang, Q.; O’Hare, D.; Zhong, Z. Recent advances in solid sorbents for CO2 capture and new development trends. Energy Environ. Sci. 2014, 7, 3478–3518. [Google Scholar] [CrossRef]
- Duan, J.; Higuchi, M.; Horike, S.; Foo, M.L.; Rao, K.P.; Inubushi, Y.; Fukushima, T.; Kitagawa, S. High CO2/CH4 and C2 hydrocarbons/CH4 selectivity in a chemically robust porous coordination polymer. Adv. Funct. Mater. 2013, 23, 3525–3530. [Google Scholar] [CrossRef]
- Raza, S.; Nazeer, S.; Abid, A.; Kanwal, A. Recent research progress in the synthesis, characterization and applications of hyper cross-linked polymer. J. Polym. Res. 2023, 30, 414. [Google Scholar] [CrossRef]
- Long, C.; Li, Y.; Yu, W.; Li, A. Adsorption characteristics of water vapor on the hypercrosslinked polymeric adsorbent. Chem. Eng. J. 2012, 180, 106–112. [Google Scholar] [CrossRef]
- Anito, D.A.; Wang, T.-X.; Liu, Z.-W.; Ding, X.; Han, B.-H. Iminodiacetic acid-functionalized porous polymer for removal of toxic metal ions from water. J. Hazard. Mater. 2020, 400, 123188. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, C.; Shu, Y.; Jiang, S.; Xia, Q.; Chen, L.; Jin, S.; Hussain, I.; Cooper, A.I.; Tan, B. Layered microporous polymers by solvent knitting method. Sci. Adv. 2017, 3, e1602610. [Google Scholar] [CrossRef]
- Wang, J.; Wang, W.-Q.; Hao, Z.; Wang, G.; Li, Y.; Chen, J.-G.; Li, M.; Cheng, J.; Liu, Z.-T. A superhydrophobic hyper-cross-linked polymer synthesized at room temperature used as an efficient adsorbent for volatile organic compounds. RSC Adv. 2016, 6, 97048–97054. [Google Scholar] [CrossRef]
- Hu, J.; Yang, S.; Wang, X.; Zhang, D.; Tan, B. High pore volume hyper-cross-linked polymers via mixed-solvent knitting: A route to superior hierarchical porosity for methane storage and delivery. Macromolecules 2024, 57, 5507–5519. [Google Scholar] [CrossRef]
- Li, B.; Gong, R.; Wang, W.; Huang, X.; Zhang, W.; Li, H.; Hu, C.; Tan, B. A New Strategy to Microporous Polymers: Knitting Rigid Aromatic Building Blocks by External Cross-Linker. Macromolecules 2011, 44, 2410–2414. [Google Scholar] [CrossRef]
- Germain, J.; Fréchet, J.M.J.; Svec, F. Hypercrosslinked polyanilines with nanoporous structure and high surface area: Potential adsorbents for hydrogen storage. J. Mater. Chem. 2007, 17, 4989–4997. [Google Scholar] [CrossRef]
- Huang, J.; Turner, S.R. Hypercrosslinked polymers: A review. Poly. Rev. 2017, 58, 1–41. [Google Scholar] [CrossRef]
- Tan, L.; Tan, B. Hypercrosslinked porous polymer materials: Design, synthesis, and applications. Chem. Soc. Rev. 2017, 46, 3322–3356. [Google Scholar] [CrossRef]
- Hou, S.; Tan, B. Naphthyl substitution-induced fine tuning of porosity and gas uptake capacity in microporous hyper-cross-Linked amine polymers. Macromolecules 2018, 51, 2923–2931. [Google Scholar] [CrossRef]
- Hou, S.; Hu, J.; Liang, X.; Zhang, D.; Tan, B. Carbonate-based hyper-cross-linked polymers with pendant versatile electron-withdrawing functional groups for CO2 adsorption and separation. J. Mater. Chem. A 2022, 10, 15062–15073. [Google Scholar] [CrossRef]
- Cheng, Y.; Razzaque, S.; Zhan, Z.; Tan, B. Construction and gas uptake performance of cyano-functional hypercrosslinked polymers via knitting strategy. Chem. Eng. J. 2021, 426, 130731. [Google Scholar] [CrossRef]
- Lin, R.-B.; Wu, H.; Li, L.; Tang, X.-L.; Li, Z.; Gao, J.; Cui, H.; Zhou, W.; Chen, B. Boosting ethane/ethylene separation within isoreticular ultramicroporous Metal–Organic Frameworks. J. Am. Chem. Soc. 2018, 140, 12940–12946. [Google Scholar] [CrossRef]
- Mukesh, C.; Khokarale, S.G.; Virtanen, P.; Mikkola, J.-P. Rapid desorption of CO2 from deep eutectic solvents based on polyamines at lower temperatures: An alternative technology with industrial potential. Sustain. Energy Fuels 2019, 3, 2125–2134. [Google Scholar] [CrossRef]
- Shan, B.; Yu, J.; Armstrong, M.R.; Wang, D.; Mu, B.; Cheng, Z.; Liu, J. A cobalt metal-organic framework with small pore size for adsorptive separation of CO2 over N2 and CH4. AIChe J. 2017, 63, 4532–4540. [Google Scholar] [CrossRef]
- Pal, A.; Chand, S.; Madden, D.G.; Franz, D.; Ritter, L.; Space, B.; Curtin, T.; Chand Pal, S.; Das, M.C. Immobilization of a polar sulfone moiety onto the pore surface of a humid-stable MOF for highly efficient CO2 separation under dry and wet environments through direct CO2–sulfone interactions. ACS Appl. Mater. Interfaces 2020, 12, 41177–41184. [Google Scholar] [CrossRef]
- Ma, H.-Y.; Zhang, Y.-Z.; Yan, H.; Zhang, W.-J.; Li, Y.-W.; Wang, S.-N.; Li, D.-C.; Dou, J.-M.; Li, J.-R. Two microporous CoII-MOFs with dual active sites for highly selective adsorption of CO2/CH4 and CO2/N2. Dalton Trans. 2019, 48, 13541–13545. [Google Scholar] [CrossRef] [PubMed]
- Lv, D.; Chen, J.; Yang, K.; Wu, H.; Chen, Y.; Duan, C.; Wu, Y.; Xiao, J.; Xi, H.; Li, Z.; et al. Ultrahigh CO2/CH4 and CO2/N2 adsorption selectivities on a cost-effectively L-aspartic acid based metal-organic framework. Chem. Eng. J. 2019, 375, 122074. [Google Scholar] [CrossRef]
- Feng, D.; Gu, Z.Y.; Li, J.R.; Jiang, H.L.; Wei, Z.; Zhou, H.C. Zirconium-Metalloporphyrin PCN-222: Mesoporous Metal–Organic Frameworks with Ultrahigh Stability as Biomimetic Catalysts. Angew. Chem. Int. Ed. 2012, 51, 10307–10310. [Google Scholar] [CrossRef]
- He, T.; Xiao, Y.; Zhao, Q.; Zhou, M.; He, G. Ultramicroporous Metal–Organic-Framework Qc-5-Cu for highly selective adsorption of CO2 from C2H4 Stream. Ind. Eng. Chem. Res. 2020, 59, 3153–3161. [Google Scholar] [CrossRef]
- Wang, C.; Li, X.; Liu, S.; Wang, T.; Liu, P.; Song, X.; Liang, Z. Ultrafast synthesis of SAPO-17 zeolites with excellent CO2/N2 and CO2/CH4 separation performance. Inorg. Chem. Front. 2023, 10, 4519–4525. [Google Scholar] [CrossRef]
- Dai, J.; Xie, D.; Liu, Y.; Zhang, Z.; Yang, Y.; Yang, Q.; Ren, Q.; Bao, Z. Supramolecular Metal–Organic-Framework for CO2/CH4 and CO2/N2 separation. Ind. Eng. Chem. Res. 2020, 59, 7866–7874. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Lu, K.; Jiang, W.; Dai, F. A 3D Ba-MOF for selective adsorption of CO2/CH4 and CO2/N2. Chin. Chem. Lett. 2021, 32, 1169–1172. [Google Scholar] [CrossRef]
- Xiong, S.; Gong, Y.; Wang, H.; Wang, H.; Liu, Q.; Gu, M.; Wang, X.; Chen, B.; Wang, Z. A new tetrazolate zeolite-like framework for highly selective CO2/CH4 and CO2/N2 separation. Chem. Commun. 2014, 50, 12101–12104. [Google Scholar] [CrossRef]
- Xu, H.; Li, J.; Liu, L.; Liang, F.S.; Han, Z.B. Pore Space Partitioning MIL-88(Co): Developing robust adsorbents for CO2/CH4 separation featured with high CO2 adsorption and rapid desorption. Inorg. Chem. 2023, 62, 13530–13536. [Google Scholar] [CrossRef]
- Xiang, S.; He, Y.; Zhang, Z.; Wu, H.; Zhou, W.; Krishna, R.; Chen, B. Microporous metal-organic framework with potential for carbon dioxide capture at ambient conditions. Nat. Commun. 2012, 3, 954. [Google Scholar] [CrossRef]
- Li, C.-N.; Wang, S.-M.; Tao, Z.-P.; Liu, L.; Xu, W.-G.; Gu, X.-J.; Han, Z.-B. Green synthesis of MOF-801(Zr/Ce/Hf) for CO2/N2 and CO2/CH4 separation. Inorg. Chem. 2023, 62, 7853–7860. [Google Scholar] [CrossRef]
- Park, H.; Kang, M.; Kang, D.W.; Hong, C.S. A robust ethane-selective hypercrosslinked porous organic adsorbent with high ethane capacity. J. Mater. Chem. A 2022, 10, 3579–3584. [Google Scholar] [CrossRef]
- Pan, H.; Suo, X.; Yang, Z.; Yang, L.; Cui, X.; Xing, H. Engineering the pore structure and functionality of ionic porous polymers for separating acetylene over carbon dioxide. Adv. Funct. Mater. 2023, 33, 202214887. [Google Scholar] [CrossRef]
- Gómez-Gualdrón, D.A.; Moghadam, P.Z.; Hupp, J.T.; Farha, O.K.; Snurr, R.Q. Application of consistency criteria to calculate BET areas of micro- and mesoporous Metal–Organic Frameworks. J. Am. Chem. Soc. 2015, 138, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Amankwah, K.A.G.; Schwarz, J.A. A modified approach for estimating pseudo-vapor pressures in the application of the Dubinin-Astakhov equation. Carbon 1995, 33, 1313–1319. [Google Scholar] [CrossRef]
- Chen, K.-J.; Madden, D.G.; Mukherjee, S.; Pham, T.; Forrest, K.A.; Kumar, A.; Space, B.; Kong, J.; Zhang, Q.-Y.; Zaworotko, M.J. Synergistic sorbent separation for one-step ethylene purification from a four-component mixture. Science. 2019, 366, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Myers, A.L.; Prausnitz, J.M. Thermodynamics of mixed-gas adsorption. AIChe J. 1965, 11, 121–127. [Google Scholar] [CrossRef]
- Yoon, J.W.; Chang, H.; Lee, S.-J.; Hwang, Y.K.; Hong, D.-Y.; Lee, S.-K.; Lee, J.S.; Jang, S.; Yoon, T.-U.; Kwac, K.; et al. Selective nitrogen capture by porous hybrid materials containing accessible transition metal ion sites. Nat. Mater. 2016, 16, 526–531. [Google Scholar] [CrossRef]

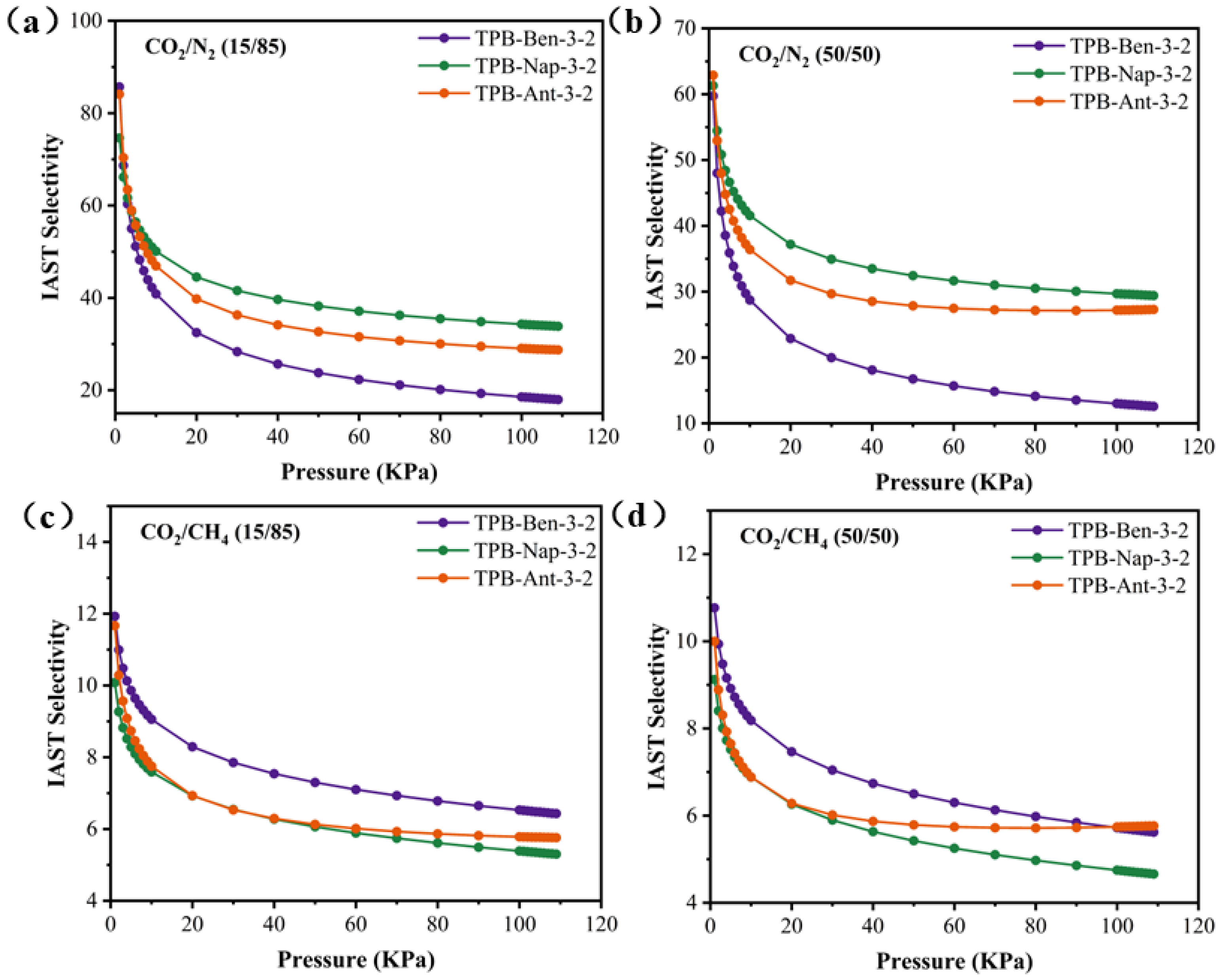
| Adsorbent | IAST Selectivity (298 K, 1 atm) | |||
|---|---|---|---|---|
| CO2/CH4 (15/85) | CO2/CH4 (50/50) | CO2/N2 (15/85) | CO2/N2 (50/50) | |
| TPB-Ben-3-2 | 11.93 | 10.77 | 85.68 | 59.72 |
| TPB-Nap-3-2 | 10.08 | 9.12 | 74.64 | 61.31 |
| TPB-Ant-3-2 | 11.67 | 10.00 | 84.10 | 62.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Zhang, Q.; Leng, X.; Song, R.; Han, Z.-B. Architecting Porosity Through Monomer Engineering: Hypercrosslinked Polymers for Highly Selective CO2 Capture from CH4 or N2. Polymers 2025, 17, 1592. https://doi.org/10.3390/polym17121592
Liu L, Zhang Q, Leng X, Song R, Han Z-B. Architecting Porosity Through Monomer Engineering: Hypercrosslinked Polymers for Highly Selective CO2 Capture from CH4 or N2. Polymers. 2025; 17(12):1592. https://doi.org/10.3390/polym17121592
Chicago/Turabian StyleLiu, Lin, Qi Zhang, Xue Leng, Rui Song, and Zheng-Bo Han. 2025. "Architecting Porosity Through Monomer Engineering: Hypercrosslinked Polymers for Highly Selective CO2 Capture from CH4 or N2" Polymers 17, no. 12: 1592. https://doi.org/10.3390/polym17121592
APA StyleLiu, L., Zhang, Q., Leng, X., Song, R., & Han, Z.-B. (2025). Architecting Porosity Through Monomer Engineering: Hypercrosslinked Polymers for Highly Selective CO2 Capture from CH4 or N2. Polymers, 17(12), 1592. https://doi.org/10.3390/polym17121592







