1. Introduction
Porphyrinic complexes are an important class due to their versatile properties and features [
1,
2]. Structurally, porphyrin is composed of four pyrrolic units linked in a coplanar fashion by four methane bridges that give a planar macrocyclic structure. It has an extended conjugated 18 π-electron system, which is responsible for its aromatic behavior. Indeed, the free-base porphyrin reacts with metal cations to form complexes with a variety of conformations, depending especially on the size of metal cations, the axial ligands, the size of peripheral substituents, etc. [
3,
4]. The properties of metalloporphyrins can be controlled by the fine-tuning of the out-of-plane distance in the porphyrin cavity. Owing to these intriguing features, porphyrins and metalloporphyrins have been widely used in several interesting fields, including theragnostic [
5,
6,
7,
8,
9,
10,
11,
12], opto-electronics [
13,
14,
15], photocatalysis [
16], and other important applications [
17,
18]. In particular, there is a considerable increase in the usage of metalloporphyrin-based materials for the removal of several harmful contaminants from water in order to limit toxic substance use and manufacture. In previous work, we and other researchers showed that the characteristics, and hence the application, of a metalloporphyrin complex may be altered by modifying the metal [
19,
20,
21,
22], the axial ligand(s) coupled to the metal [
23,
24], and the porphyrin perimeter [
25,
26,
27]. Recently, some investigations have concentrated on the construction of new porphyrins modified with polymers formed predominantly through intermolecular coordination and hydrogen-bonding interactions [
28,
29].
Polyethyleneimine, which is an organic polyamine polymer, is considered one of the most important examples of cationic polymers. It is rich in amine groups. These amine groups could rapidly interact with several organic and inorganic pollutants. In the literature, some works have reported on the chemical modification of a series of compounds using polyethyleneimine. For instance, Teng et al. [
30] used polyethyleneimine to modify polyvinylidene fluoride membrane. The modified membranes exhibited good ability to adsorb organic dyes and heavy metal ions from water and reject them via filtration. Lang et al. [
31] fabricated a polyethyleneimine/nanocellulose/MXene/loofah composite aerogel. The aerogel was able to efficiently adsorb heavy crude oil and can selectively adsorb methylene blue, malachite green, Congo red, and methyl orange from mixed dye systems. Yan et al. [
32] synthesized a composite aerogel via the lyophilization of cellulose modified with polyethyleneimine and cadmium sulfide. This composite demonstrated exceptional efficacy in degrading methyl orange and methylene blue. In our previous work, we reported the extraction and chemical functionalization of cellulose from
Echinops bannaticus leaves. The extracted cellulose was functionalized with Poly (diallyldimethylammonium chloride) and branched polyethyleneimine. The modified samples demonstrated good adsorption capacities of acid blue 25, an anionic dye [
33].
Herein, we report, first, the synthesis and the characterization of the following complexes (1–3); meso-tetrakis(2,4,6-trimethylphenyl) porphyrinato)zinc(II): ([Zn(TMP)] (1), meso-tetrakis-(tetraphenyl)porphyrin iron(III))chloride): [Fe(TPP)Cl] (2), meso-tetrakis(phenyl)porphyrin manganese(III) chloride): [Mn(TPP)Cl] (3). Then, the prepared porpyrinic complexes (1–3) were functionalized with branched polyethyleneimine in order to impart reactive amino groups and improve their adsorption capacities toward anionic species. All prepared materials were thoroughly analyzed using several analytical techniques, including 1H NMR, FT-IR, UV-vis, EDX, XRF, SEM, TGA-DTA, and XRD. Materials were further used for the adsorption of Naphthol blue black B from water. Factors influencing the adsorption mechanism, such as pH, time, initial Naphthol blue black B concentration, and temperature, were assessed. The adsorption results were fitted to theoretical kinetic and isotherm models in order to understand the adsorption phenomenon. The thermodynamic parameters were also calculated.
2. Experimental
2.1. Reagents, Materials, and Apparatus
Branched polyethyleneimine solution (50 wt.% in H2O, average Mw ~750,000) was purchased from Sigma Aldrich, S. Louis and Burlington, MA, USA and used for the chemical modification of the porphyrinic compounds. Naphthol blue black B (M.W = 616.5 g/mol, purity = 85%), an anionic dye, was used as the adsorbate during adsorption experiments. The pH values of the solutions were adjusted using sodium hydroxide and sulfuric acid solutions. All other used solvents or reagents were laboratory grade. An Ultrashield spectrometer, Quassim, Saudi Arabia (CDCl3 is used as solvent, and trimethylsilane is the reference) was used to record (1H)-NMR spectra. A Perkin Elmer Spectrum FT-IR (Monastir, Tunis) was used to provide FT-IR spectra. The absorption measurements were collected using a diluted sample solution in dichloromethane (Sigma Aldrich, USA, ST) The morphological characteristics of the porphyrinic compounds under study were described using a JEOL JSM-5400 SEM (Quassim, Saudi Arabia). An X-ray diffraction (XRD) pattern was obtained using PANalytical X’Pert PRO MPD equipment (Quassim, Saudi Arabia). The samples were evaluated within a 2ϴ range (10 to 90 degrees). Thermogravimetric data (TGA/DTA) were collected in an air flow with a heating rate of 10 °C per minute. Thermal events were investigated using a platinum crucible and the NE-TZSCH STA 449F3 instrument (Quassim, Saudi Arabia).
2.2. Synthesis of Complexes 1–3
The meso-tetrakis(2,4,6-trimethylphenyl) porphyrinato)zinc(II): complex
(1), meso-tetrakis-(tetraphenyl) porphyrin iron(III)chloride: complex
(2) and meso-tetrakis-(tetraphenyl) porphyrin manganese(III)chloride: complex
(3) were synthesized following a method reported in our previous studies [
34,
35,
36].
2.3. Synthesis of Complex (1)-PEI: Meso-Tetrakis(2,4,6-trimethylphenyl) Porphyrinato)zinc(II) Functionalized with Branched Polyethyleneimine: [Zn(TMP)]-PEI
[Zn(TMP)] (500 mg, 0.591 mmol) and excess branched PEI polymer solution were mixed in dichloromethane (10 mL) overnight at 25 °C. The hue of the solution shifted from pink to purple. The complex [Zn(TMP)]-branched polyethyleneimine was obtained as a pink powder. UV/Vis [CH2Cl2, λmax in nm (log Ɛ)]: 424 (4.85), 554(3.68), 603(3.25).
2.4. Synthesis of Complex (2)-PEI: Meso-Tetrakis-(tetraphenyl) Porphyrin Iron(III)chloride Functionalized with Branched Polyethylenemine: [Fe(TPP)Cl]-PEI
[Fe(TPP)Cl] (500 mg, 0.747 mmol) and an excess of branched PEI polymer solution were mixed in dichloromethane (10 mL) at 25 °C overnight. The color of the solution turned purple. The complex [Fe(TPP)Cl]- branched PEI was obtained as a purple powder. UV/Vis [CH2Cl2, λmax in nm (log Ɛ)]: 425 (4.98), 515(4.02).
2.5. Synthesis of the Complex (3)-PEI: Meso-Tetrakis-(tetraphenyl) Porphyrin Manganese(III)chloride Functionalized with Branched Polyethyleneimine: [Mn(TPP)Cl]-PEI
[Mn(TPP)Cl] (500 mg, 0.749 mmol) and an excess of branched PEI polymer solution were mixed for 12 h at 25 °C in dichloromethane (10 mL). The solution changed color from green to purple. The complex [Mn(TPP)Cl]-PEI was produced as a purple powder. UV/Vis [CH2Cl2, λmax in nm (log Ɛ)]: 375 (3.97), 411 (4.74), 580(4.97), and 619(5.63).
2.6. Adsorption Experiments
An Erlenmeyer flask containing a mixture of 0.001 g of non-modified or functionalized porphyrinic complexes and 10 mL of Naphthol blue black B solution was continuously stirred (125 rpm) for a period of 2 h. Then, the contents of the flasks were filtered using Whatman filter paper, Riyadh, Saudi Arabia. The absorbance of the resulting solutions was measured using a UV-Vis spectrophotometer (Riyadh, Saudi Arabia) at the maximum wavelength absorption (λmax = 610 nm). The adsorption capacities were further calculated. The studies were designed to test the effects of various contact periods (ranging from 0 to 120 min), Naphthol blue black B concentrations (ranging from 0 mg/L to 600 mg/L), and temperatures (22 °C to 53 °C).
3. Results
3.1. Sample Characterizations
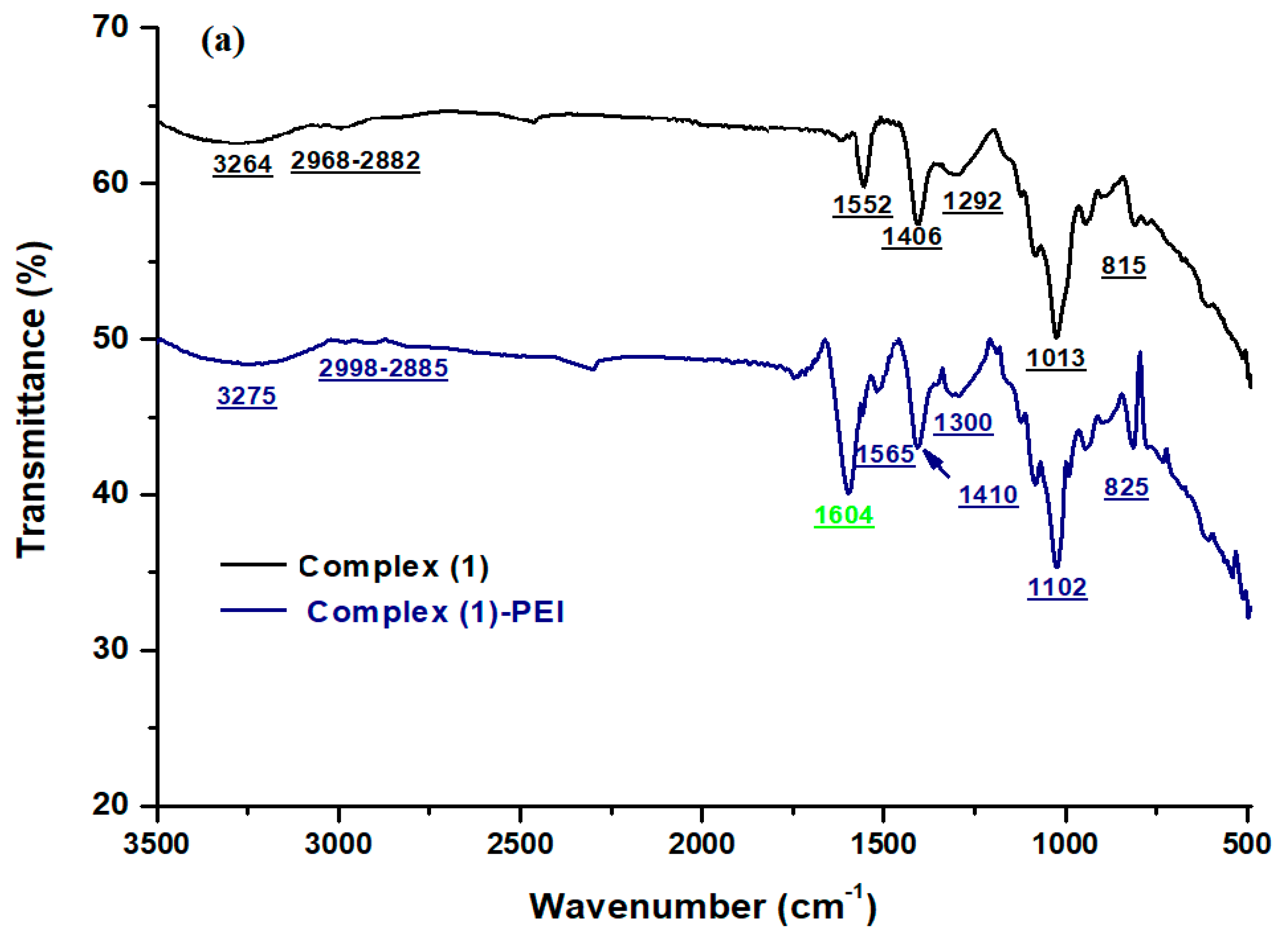
3.1.1. FT-IR Spectroscopy
The FT–IR spectra of complexes
(1–
3) and the modified complexes are given in
Figure 1. For complexes
(1–
3), the spectra show the presence of the main peaks of metalloporphyrins. The C-H group of the porphyrinic core is detected around 3264–3380 cm
−1. The ν (C-N) peak is detected between 1430 and 1500 cm
−1. The ν(C-C) peak is seen at 1590–1552 cm
−1. The absorption peak for the vibration v (C-C) is around 1102–1230 cm
−1. The vibration mode δ (CCH) of the porphyrin core is located at around 1008 cm
−1. The absorption peak at 810 cm
−1 corresponds to the v (C-C) phenyl group [
37,
38]. After chemical functionalization of the porphyrinic complexes
(1–
3) with branched polyethyleneimine, new absorption peaks appear at 1604, 1608, and 1607 cm
−1 for complex
(1)-PEI, complex
(2)-PEI, and complex
(3)-PEI, respectively. These absorption peaks can be assigned to the amine groups of polyethyleneimine [
39]. This result confirms that the prepared porphyrinic complexes chemically interact with the polymer through amine groups.
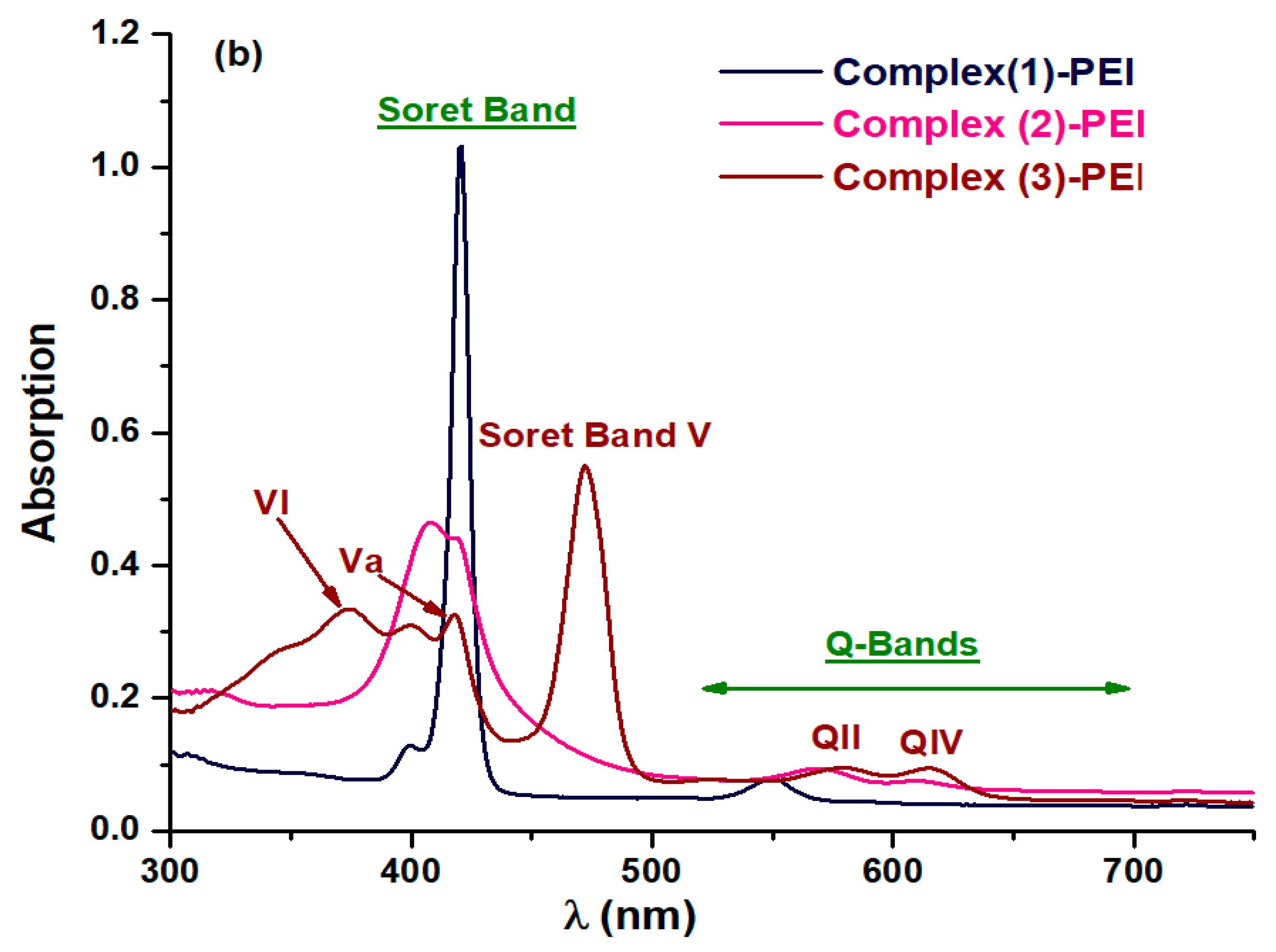
3.1.2. Optical Spectroscopy
The UV-visible spectra of the complexes
(1–
3) and the modified complexes were studied in dichloromethane at a concentration of ca 10
−6 M.
Figure 2 elucidates the spectroscopic data of complex
(1–
3) and the modified ones. As observed, the change in the spectral profile is due to the presence of Zn, Fe, and Mn metals. We noticed that the spectrum of complex
(1) exhibits a very intense band known as the Soret band, allowing the transition of the electrons from the ground state S0 to the second excited state S2 at 422 nm. In addition, two less intense absorption bands are observed at 550 nm and 596 nm, which are known as Q-Bands corresponding to the transition of electrons from the ground state to the first excited state S1 [
30,
31,
34]. Regarding complex
(2), the UV-vis spectrum reveals the presence of a Soret band at 421 nm and one Q-band at 510 nm [
35]. We observe significant changes for complex
(3) compared to complex
(1) and
(2). This is related to the manganese metal [
36]. Complex
(3) contains a hypertype electronic spectrum that includes a half-vacant metal orbital with symmetry, such as [dπ:dxz and dyz] [
36]. The Soret band V, or the strongest band, is identified at 471 nm. This band results from the transfer of the porphyrin’s a1u(π) and a2u(π) orbitals to manganese orbitals, such as [dπ: dxz and dyz]. The two less intense bands than the Soret band, the VI and Va bands, are detected at 372 nm and 408 nm, respectively. In addition, there are two Q-bands, the QII and QIV bands, observed at 577 nm and 615 nm, respectively. After chemical modification of the complexes
(1–
3) with polyethyleneimine, we observe a slight bathochromic shift in the values of λ
max of the bands. This suggests the interaction of complexes
(1–
3) with the polyethyleneimine polymer.
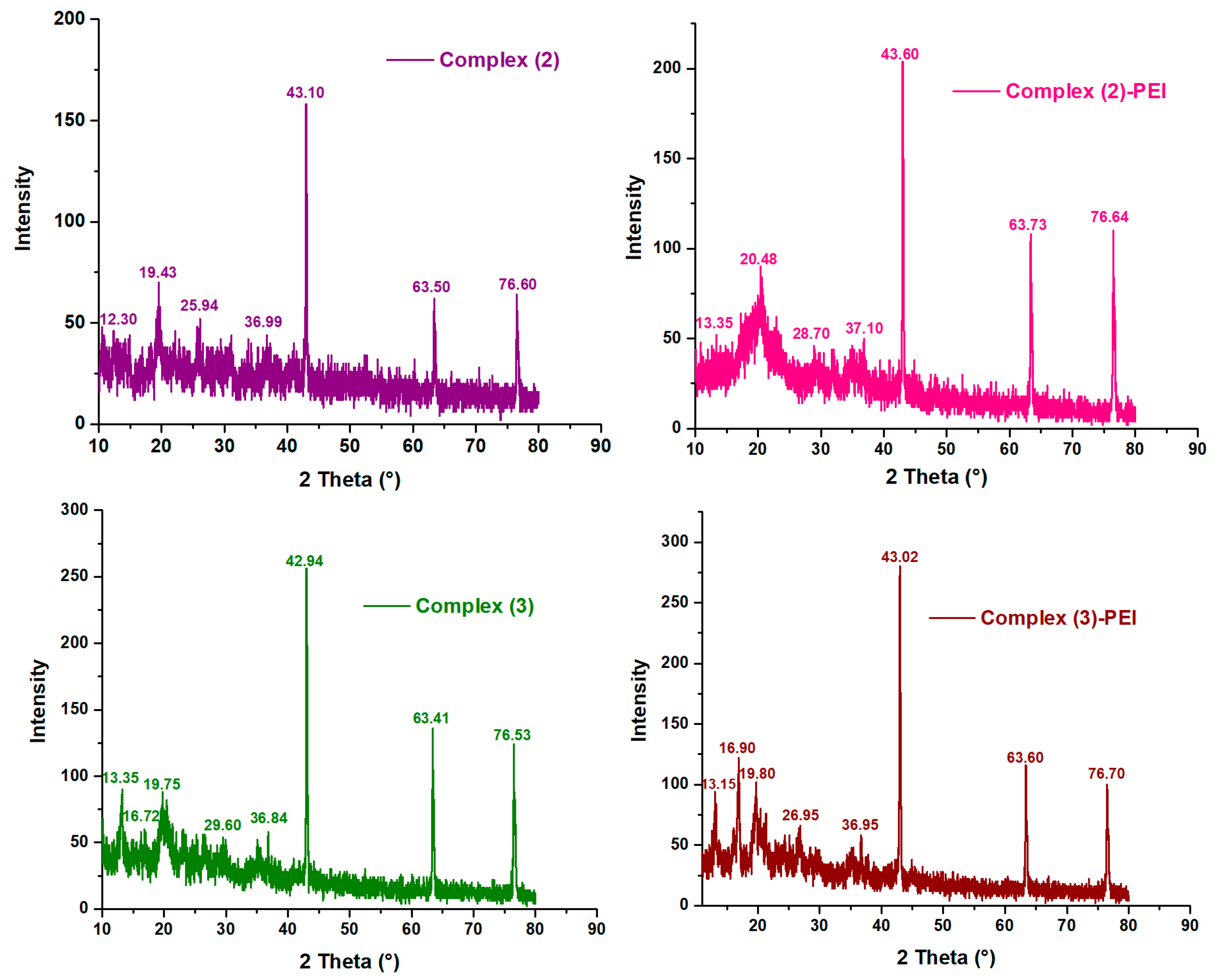
3.1.3. XRD Analysis
Figure 3 shows the XRD patterns of complex
(1–
3) and modified complexes. As shown, the complex
1 reveals sharp peaks observed at 2θ = 12.29°, 20.05°, 26.60°, 36.75°, 43.05°, 63.40°, and 76.52°. However, the complex
2 exhibits a series of peaks observed at 2θ = 12.30°, 19.43°, 25.94°, 36.99°, 43.10°, 63.50°, and 76.60°. The complex
3 displays a series of peaks observed at 2θ = 13.35°, 16.72°, 19.75°, 29.60°, 36.84°, 42.94°, 63.41°, and 76.53°. These peaks point to the crystalline character of the porphyrinic complexes [
40,
41]. In addition, we find a slight displacement of the position of the principal peaks for the modified complexes
(1–
3) compared to the unmodified ones. This shifting suggests again the chemical interaction between the complexes
(1–
3) and polyethyleneimine.
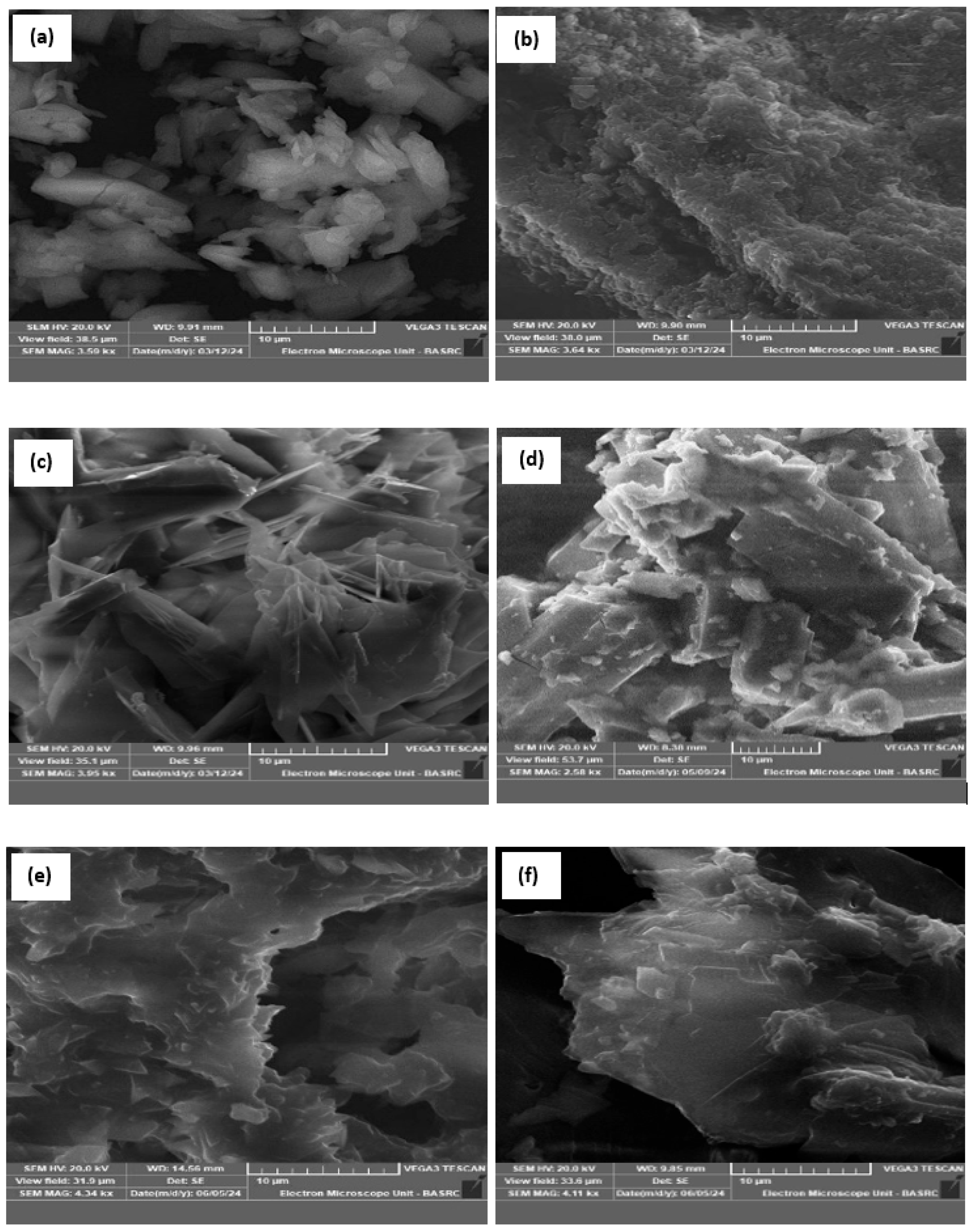
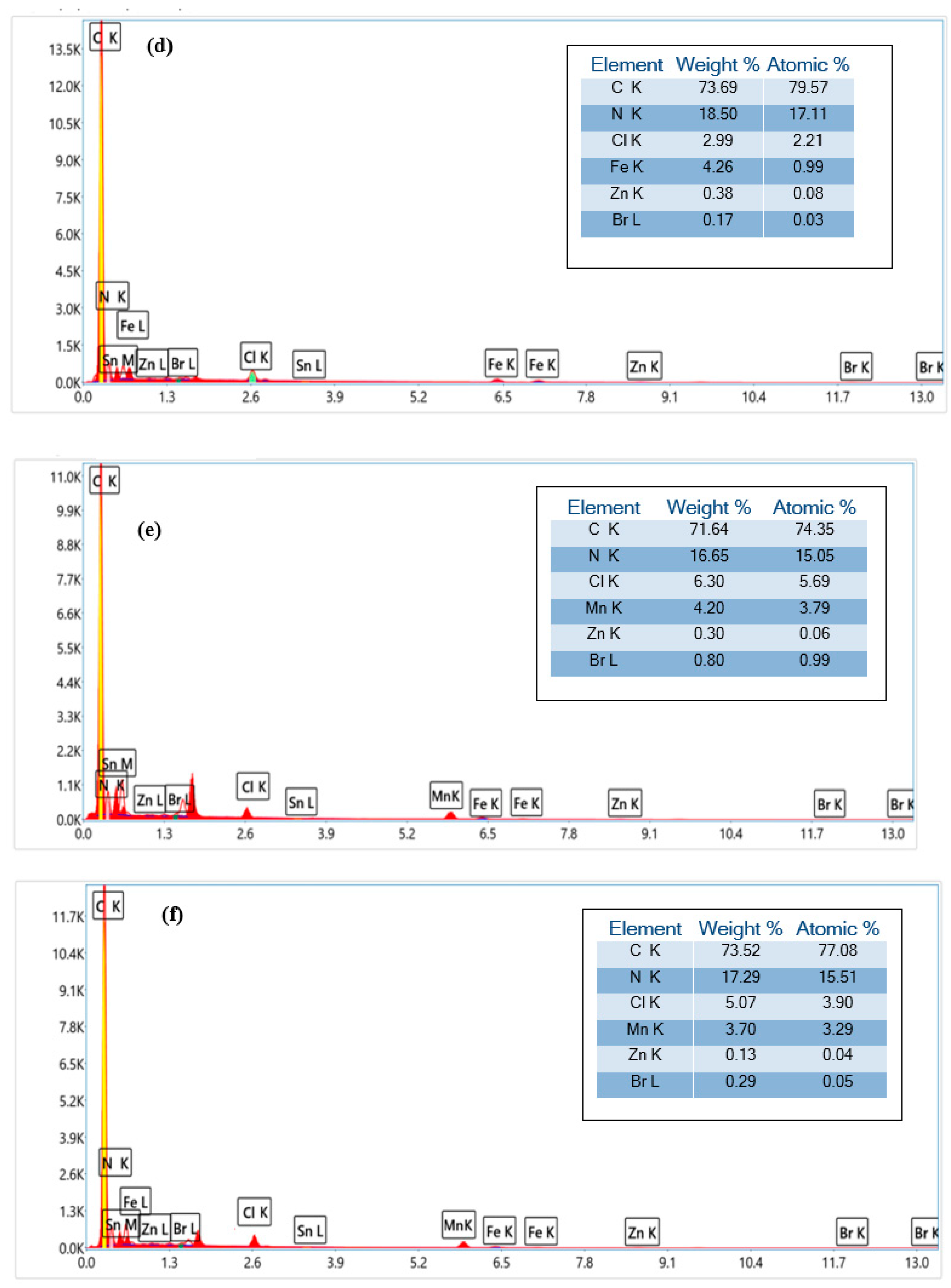
3.1.4. SEM and EDX Analysis
Figure 4 gives the morphological characteristics of the complexes
(1–
3) and modified complexes. As observed, the chemical modification of the prepared complexes clearly affects the morphological properties of the porphyrinic complexes. In particular, the particles become more agglomerated due to the chemical interaction between polyethyleneimine and the porphyrinic complexes.
The EDX data support the complexation of zinc, iron, and manganese metals with the porphyrinic core (
Figure 5). As expected, an increase in carbon and nitrogen contents after the addition of PEI polymer to the complexes
(1–
3) is visible. This confirms the interaction between the porphyrinic complexes and polyethyleneimine.
3.1.5. X-Ray Fluorescence Analysis
The X-ray fluorescence (XRF) data of complexes
(1–
3) and modified complexes, showing the relative abundance of elements, are summarized in
Table 1. The results show that the metals Zn, Fe, and Mn are present in the complexes
(1–
3) with relative amounts of 20.63%, 52.60%, and 70.59%, respectively. Other elements, such as Cl, Na, Al, Ca, K, Ti, Nb, Mo, In, Hf, Sx, and V are also found in the prepared complexes. As observed, the relative abundance of these elements is slightly changed after chemical modification with polyethyleneimine. This result can be explained by the electron transfer pathway between the metals and the nitrogen atom in polyethyleneimine polymer [
42,
43].
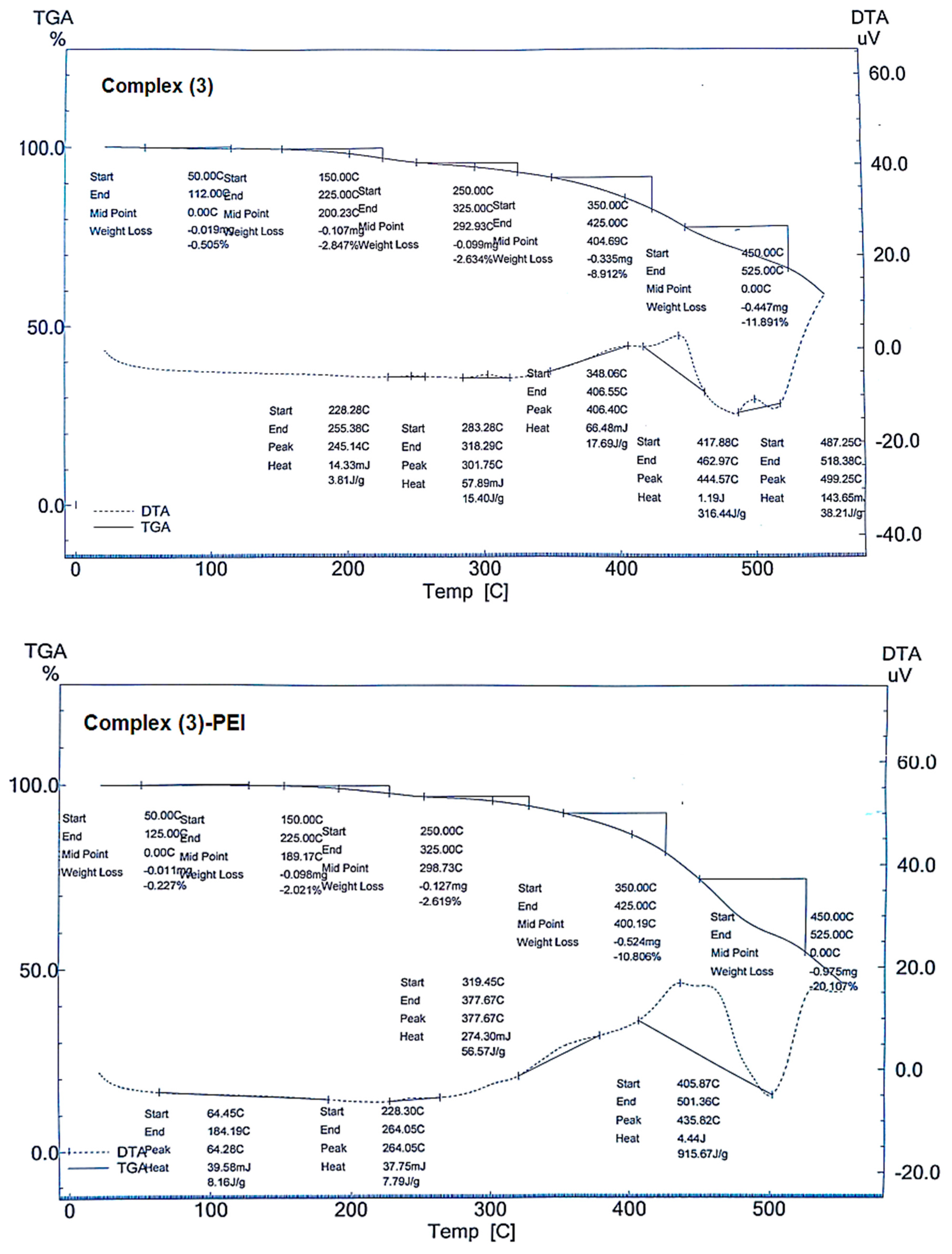
3.1.6. Thermal Analysis
The thermal analysis of complexes
(1–
3) and modified complexes is shown in
Figure 6. All the obtained complexes show thermal processes at temperatures below 100 °C, indicating the release of adsorbed water [
44]. The total mass reduction is 72.97%, 66.77%, and 26.78% for complexes
1,
2, and
3, respectively. For the modified complexes, five occurrences are noticed in the range [50–450 °C], with total mass loss of 83.12% (complex
1-PEI), 81.88% (complex
2-PEI), and 35.78% (complex
3-PEI). The increase in the mass loss for the modified complexes confirms the chemical interaction of the porphyrinic complexes modified with polyethyleneimine. This trend also justifies the existence and degradation of the polymeric material. The thermal events indicate that the non-modified compounds are more thermostable than the functionalized ones.
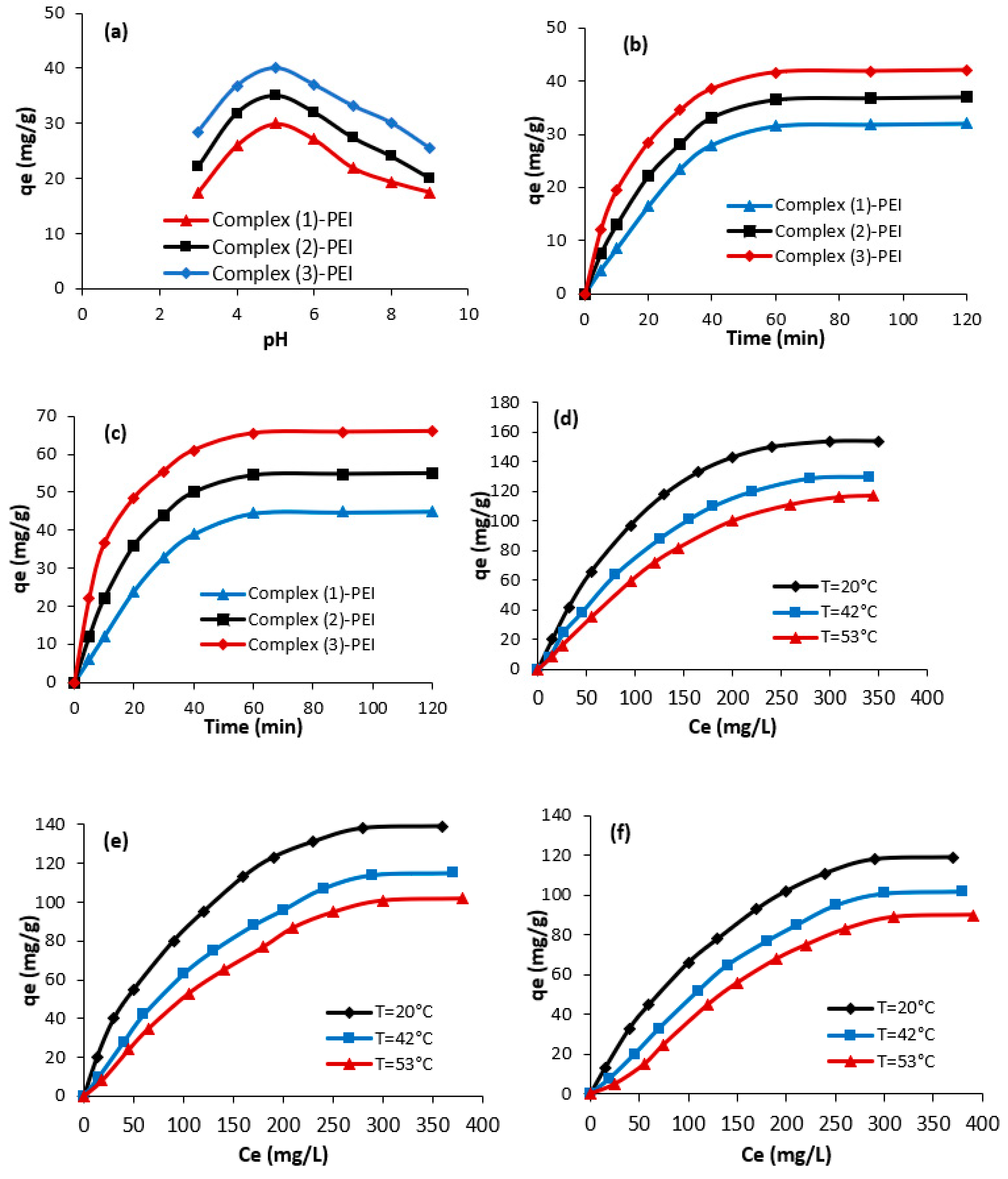
3.2. Application of the Prepared Complexes in Naphthol Blue Black B Adsorption
3.2.1. Effect of the Experimental Parameters on the Adsorption Mechanism
The prepared complexes
(1–
3) and the modified complexes were studied for the adsorption of Naphthol blue black B from water. The effects of changing pH, dye concentration, duration, and temperature on adsorption capabilities were examined. First, it is noticed that the adsorption capacities of Naphthol blue black B using the prepared complexes
(1–
3) are poor. However, complex
(1)-PEI, complex
(2)-PEI, and complex
(3)-PEI showed high adsorption capacities. The next sections cover the adsorption of Naphthol blue black B utilizing three adsorbents: complex
(1)-PEI, complex
(2)-PEI, and complex
(3)-PEI.
Figure 7a shows the change in the adsorbed amounts of Naphthol blue black B against pH using complex
(1)-PEI, complex
(2)-PEI, and complex
(3)-PEI. As observed, the adsorption capacities of Naphthol blue black B depend significantly on the pH value and it increase from 3 to 5.
At pH = 5, the three studied adsorbents reached their highest adsorption capabilities. At this pH, the negative charges of Naphthol blue black B molecules form strong electrostatic contacts with the positive charges on the surfaces of complexes (1)-PEI, (2)-PEI, and (3)-PEI. In acidic conditions, high concentrations of hydronium ions facilitate anionic dye adsorption. However, high concentrations of hydroxide ions in alkaline environments limit the adsorption capacity of anionic color molecules.
Figure 7b–d describe the change in the adsorption amounts of Naphthol blue black B using complex
(1)-PEI, complex
(2)-PEI, and complex
(3)-PEI. As shown, the adsorption of Naphthol blue black B increases promptly with the reaction time (from 0 to 30 min). In fact, during this stage of adsorption, the number of adsorption sites on the surface of the studied adsorbents results in high adsorption capacity. After 60 min of reaction, the adsorption level tends to stabilize due to saturation of the available adsorption sites on the surfaces of complexes
(1)-PEI,
(2)-PEI, and
(3)-PEI.
Figure 7d–f show the effect of the change in Naphthol blue black B concentration on the adsorption capacities. At low Naphthol blue black B concentrations, dye adsorption capabilities are readily achieved. This is owing to the accessibility of multiple adsorption sites at this phase, as well as the quick dispersion of dye from the original dye solution to the surface of complexes
(1)-PEI,
(2)-PEI, and
(3)-PEI. At optimum adsorption conditions (T = 20 °C, time = 60 min, pH = 5), the highest adsorption capacities achieve 154 mg/g, 139 mg/g, and 119 mg/g for complex
(3)-PEI, complex
(2)-PEI, and complex
(1)-PEI, respectively. The obtained results confirm the use of the prepared compounds as efficient adsorbents of Naphthol blue black B from water. In some cases, the obtained adsorption capacities are higher compared to many adsorbents studied in the literature, and in other cases, they are comparable to some adsorbents (
Table 2). In fact, the chemical modification of the metalloporphyrinic complexes with polyethyleneimine greatly improves the adsorption of Naphthol blue black B due to the existence of reactive amine groups. As also observed in
Figure 7d–f, the adsorption capacities vary as a function of temperature. The adsorption abilities were reduced with rising temperatures, indicating an exothermic phenomenon. The decrease in adsorption capabilities with temperature can be attributed to the partial breakdown of the association between Naphthol blue black B molecules and the modified metalloporphyrinic complex.
3.2.2. Kinetic Investigation
The adsorption of Naphthol blue black B was evaluated using first order, second order, intra-particular dispersion, and Elovich kinetic models [
48] for complexes
(1)-PEI,
(2)-PEI, and (3)-PEI. The computed variables are listed in
Table 3. The high regression coefficients (R
2 > 0.98) and correlation of measured adsorbed amounts of Naphthol blue black B with theoretically calculated amounts in the pseudo second order model imply a chemical adsorption mechanism [
49,
50]. The deviation from the origin in the intra-particular diffusion plots indicates that the adsorption of Naphthol blue black B is driven by both the intra-particular diffusion trend and other kinetic mechanisms [
51].
3.2.3. Determination of Thermodynamic Parameters
The three isotherms of Langmuir, Freundlich, and Temkin were evaluated in attempts to analyze the adsorption process of Naphthol blue black B employing the three adsorbents complex
(1)-PEI, complex
(2)-PEI, and complex
(3)-PEI.
Table 3 provides the fitting parameters and regression coefficients (R
2). The high regression coefficients (R
2 ≥ 0.97) indicate that the adsorption mechanism is consistent with the Freundlich and Temkin equations. According to these predictions, active adsorption sites may be unevenly distributed on the surfaces of complex
(1)-PEI, complex
(2)-PEI, and complex
(3)-PEI [
52].
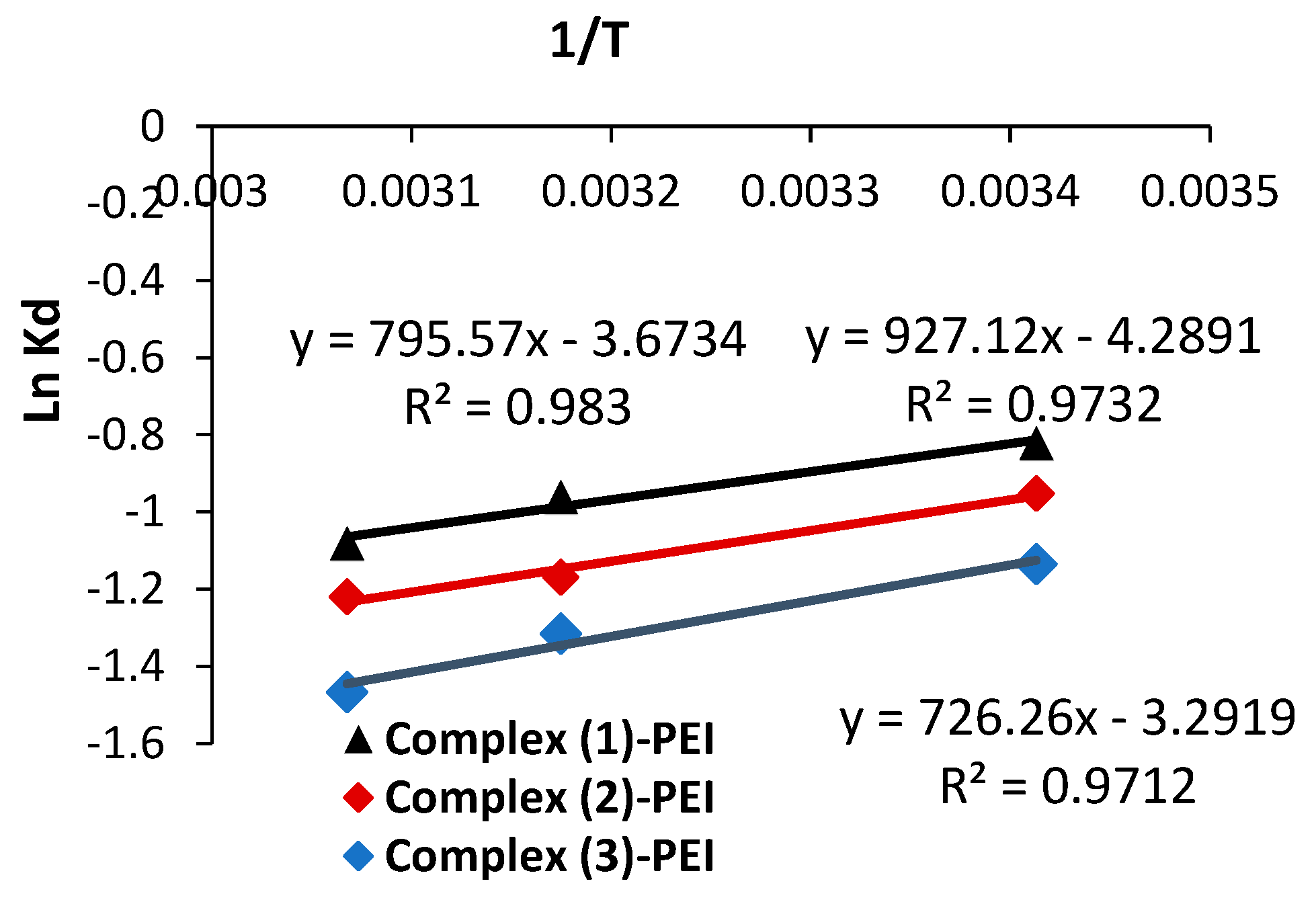
Figure 8 shows graphs of Ln (K
d) vs. 1/T to derive the thermodynamic parameters (entropy and enthalpy).
Table 3 shows the calculated values. The computed negative enthalpy values confirm that the adsorbent complexes
(1)-PEI,
(2)-PEI, and
(3)-PEI underwent an exothermic reaction. As the temperature rises from 20 °C to 53 °C, the amount of adsorbed Naphthol blue black B molecules decreases, supporting this finding. The entropy values are negative, indicating that the disorder in the examined adsorption system has decreased, as well as the presence of many structural alterations [
53]. The positive free energy values obtained during Naphthol blue black B adsorption show that the process is not spontaneous.
4. Conclusions
In this work, meso-tetrakis(2,4,6-trimethylphenyl) porphyrinato)zinc(II): ([Zn(TMP)] (1), meso-tetrakis-(tetraphenyl)porphyrin iron(III))chloride): [Fe(TPP)Cl] (2), and meso-tetrakis(phenyl)porphyrin manganese(III) chloride): [Mn(TPP)Cl] (3) were prepared. Then, the three prepared porphyrinic complexes (1–3) were chemically modified with branched polyethyleneimine (PEI). The synthesized compounds were examined using 1H NMR, FT-IR, UV-vis, XRD, XRF, TGA-DTA, SEM, and EDX. The presence of sharp peaks at 2θ between 10° and 80°, in XRD analysis, for all studied compounds suggested the crystalline nature of the porphyrinic complexes. The morphological properties of the porphyrinic complexes were significantly affected by the chemical modification with polyethyleneimine. EDX result confirmed the complexation of zinc, iron, and manganese metals with the porphyrinic core. The increase in carbon and nitrogen contents after the addition of polyethyleneimine to the complexes 1–3 was noticeable. After thermal decomposition, the total mass losses were equal to 92.97%, 66.77%, and 26.78% for complexes 1, 2, and 3, respectively. However, for the complex (1)-PEI, complex (2)-PEI, and complex (3)-PEI, the total mass losses were 83.12%, 81.88%, and 35.78%, respectively. The synthesized compounds were then utilized to adsorb Naphthol Blue Black B from water. At optimum adsorption conditions (T = 20 °C, time = 60 min, pH = 5), the highest adsorption capacities were 154 mg/g, 139 mg/g, and 119 mg/g for complex (3)-PEI, complex (2)-PEI, and complex (1)-PEI, respectively. The adsorption mechanism followed the pseudo second order, Freundlich, and Temkin models. The process was exothermic and non-spontaneous. Future studies will be extended for the design of other composites based porphyrinic complexes for the adsorption of other pollutants from contaminated water.
Author Contributions
Conceptualization, R.S.; Methodology, A.A.A. and M.J.; Software, S.Y.R., R.S., and M.J.; Validation, S.Y.R. and A.A.A.; Formal analysis, A.A.A.; Investigation, A.A.A. and R.S.; Resources, S.Y.R., R.S., and M.J.; Writing—review and editing, M.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research has been funded by the Scientific Research Deanship at the University of Ha’il, Saudi Arabia, through project number BA-24 020.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Acknowledgments
This research has been funded by the Scientific Research Deanship at the University of Ha’il, Saudi Arabia, through project number BA-24 020.
Conflicts of Interest
The authors declare no conflict of interest.
References
- O’Neill, J.S.; Kearney, L.; Brandon, M.P.; Pryce, M.T. Design components of porphyrin-based photocatalytic hydrogen evolution systems: A review. Coord. Chem. Rev. 2022, 467, 214599. [Google Scholar] [CrossRef]
- Das, R.; Verma, P.K.; Nagaraja, C.M. Design of porphyrin-based frameworks for artificial photosynthesis and environmental remediation: Recent progress and future prospects. Coord. Chem. Rev. 2024, 514, 215944. [Google Scholar] [CrossRef]
- Vaz, B.; Pérez-Lorenzo, M. Unraveling structure–performance relationships in porphyrin-sensitized TiO2 photocatalysts. Nanomaterials 2023, 13, 1097. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Ramzan, M.; Qureshi, A.K.; Khan, M.A.; Tariq, M. Emerging applications of porphyrins and metalloporphyrins in biomedicine and diagnostic magnetic resonance imaging. Biosensors 2018, 8, 95. [Google Scholar] [CrossRef]
- Ptaszyńska, A.A.; Trytek, M.; Borsuk, G.; Buczek, K.; Rybicka-Jasińska, K.; Gryko, D. Porphyrins inactivate Nosema spp. microsporidia. Sci. Rep. 2018, 8, 5523. [Google Scholar] [CrossRef]
- Varchi, G.; Foglietta, F.; Canaparo, R.; Ballestri, M.; Arena, F.; Sotgiu, G.; Fanti, S. Engineered porphyrin loaded core-shell nanoparticles for selective sonodynamic anticancer treatment. Nanomedicine 2015, 10, 3483–3494. [Google Scholar] [CrossRef]
- Tsolekile, N.; Nelana, S.; Oluwafemi, O.S. Porphyrin as diagnostic and therapeutic agent. Molecules 2019, 24, 2669. [Google Scholar] [CrossRef]
- Hammerer, F.; Garcia, G.; Chen, S.; Poyer, F.; Achelle, S.; Fiorini-Debuisschert, C.; Maillard, P. Synthesis and characterization of glycoconjugated porphyrin triphenylamine hybrids for targeted two-photon photodynamic therapy. J. Org. Chem. 2014, 79, 1406–1417. [Google Scholar] [CrossRef]
- Dong, X.; Chen, H.; Qin, J.; Wei, C.; Liang, J.; Liu, T.; Lv, F. Thermosensitive porphyrin-incorporated hydrogel with four-arm PEG-PCL copolymer(II): Doxorubicin loaded hydrogel as a dual fluorescent drug delivery system for simultaneous imaging tracking in vivo. Drug Deliv. 2017, 24, 641–650. [Google Scholar] [CrossRef]
- Zhang, W.; Taheri-Ledari, R.; Ganjali, F.; Mirmohammadi, S.S.; Qazi, F.S.; Saeidirad, M.; KashtiArayb, A.; Zarei-Shokatb, S.; Tianc, Y.; Maleki, A. Effects of morphology and size of nanoscale drug carriers on cellular uptake and internalization process: A review. RSC Adv. 2023, 13, 80–114. [Google Scholar] [CrossRef]
- Jenkins, S.V.; Srivatsan, A.; Reynolds, K.Y.; Gao, F.; Zhang, Y.; Heyes, C.D.; Chen, J. Understanding the interactions between porphyrin-containing photosensitizers and polymer-coated nanoparticles in model biological environments. J. Colloid Interface Sci. 2016, 461, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Song, W.; Rieffel, J.; Lovell, J.F. Emerging applications of porphyrins in photomedicine. Front. Phys. 2015, 3, 23. [Google Scholar] [CrossRef]
- Dini, D.; Calvete, M.J.; Hanack, M. Nonlinear optical materials for the smart filtering of optical radiation. Chem. Rev. 2016, 116, 13043–13233. [Google Scholar] [CrossRef]
- Charisiadis, A.; Nikolaou, V.; Nikoloudakis, E.; Ladomenou, K.; Charalambidis, G.; Coutsolelos, A.G. Metalloporphyrins in bio-inspired photocatalytic conversions. Chem. Commun. 2025, 61, 4630–4646. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Zhu, W.H.; Xie, Y. Development of ion chemosensors based on porphyrin analogues. Chem. Rev. 2017, 117, 2203–2256. [Google Scholar] [CrossRef]
- Leng, F.; Liu, H.; Ding, M.; Lin, Q.P.; Jiang, H.L. Boosting Photocatalytic Hydrogen Production of Porphyrinic MOFs: The Metal Location in Metalloporphyrin Matters. ACS Catal. 2018, 8, 4583–4590. [Google Scholar] [CrossRef]
- Zucca, P.; Neves, C.; Simões, M.M.; Neves, M.D.G.P.; Cocco, G.; Sanjust, E. Immobilized lignin peroxidase-like metalloporphyrins as reusable catalysts in oxidative bleaching of industrial dyes. Molecules 2016, 21, 964. [Google Scholar] [CrossRef] [PubMed]
- Rabiee, N.; Yaraki, M.T.; Garakani, S.M.; Ahmadi, S.; Lajevardi, A.; Bagherzadeh, M.; Rabiee, M.; Tayebi, L.; Tahriri, M.; Hamblin, M.R. Recent advances in porphyrin-based nanocomposites for effective targeted imaging and therapy. Biomaterials 2020, 232, 119707. [Google Scholar] [CrossRef]
- Soury, R.; Alenezi, K.M.; Jabli, M.; Haque, A.; Al Otaibi, A.; El Moll, H.; Philouze, C. Synthesis and characterization of axially modified Zn (II) porphyrin complexes for methylene blue dye oxidative degradation. J. Mol. Struct. 2021, 1243, 130791. [Google Scholar] [CrossRef]
- Soury, R.; Jabli, M.; Saleh, T.A.; Kechich, A.; Loiseau, F.; Saint-Aman, E.; Nasri, H. Degradation of Calmagite by dichloride (5, 10, 15, 20tetraphenylporphyrinato) antimony hexachloridoantimonate: [Sb (TPP) Cl2] SbCl6. Inorg. Chem. Commun. 2019, 104, 54–60. [Google Scholar] [CrossRef]
- Nikoloudakis, E.; López-Duarte, I.; Charalambidis, G.; Ladomenou, K.; Ince, M.; Coutsolelos, A.G. Porphyrins and phthalocyanines as biomimetic tools for photocatalytic H2 production and CO2 reduction. Chem. Soc. Rev. 2022, 51, 6965–7045. [Google Scholar] [CrossRef] [PubMed]
- Grössl, D.M.; Hafner, A.V.; Fischer, R.C.; Saf, R.; Torvisco, A.; Uhlig, F. Bis (chlorido) tin (IV) meso-substituted Porphyrins-Characterization and Solubility. Eur. J. Inorg. Chem. 2023, 26, e202300286. [Google Scholar] [CrossRef]
- Lismont, M.; Dreesen, L.; Wuttke, S. Metal-organic framework nanoparticles in photodynamic therapy: Current status and perspectives. Adv. Funct. Mater. 2017, 27, 1606314. [Google Scholar] [CrossRef]
- Liang, Z.; Wang, H.Y.; Zheng, H.; Zhang, W.; Cao, R. Porphyrin-based frameworks for oxygen electrocatalysis and catalytic reduction of carbon dioxide. Chem. Soc. Rev. 2021, 50, 2540–2581. [Google Scholar] [CrossRef]
- Soury, R.; Chaabene, M.; Haque, A.; Jabli, M.; Alenezi, K.M.; Latif, S.; Abdulaziz, F.; Bchetnia, A.; Philouze, C. Two novel pyrazine Zn (II)-porphyrins complexes: Synthesis, photophysical properties, structure study, DFT-Calculation and assessment of an azo dye removal from aqueous solution. J. Solid State Chem. 2022, 310, 123048. [Google Scholar] [CrossRef]
- Soury, R.; Jabli, M.; Saleh, T.A.; Abdul-Hassan, W.S.; Saint-Aman, E.; Loiseau, F.; Philouze, C.; Nasri, H. (ethyl-4 (4-butyryl) oxyphenyl) porphyrinato zinc complexes with 4, 4′-bpyridin: Synthesis, characterization, and its catalytic degradation of Calmagite. RSC Adv. 2018, 8, 20143–20156. [Google Scholar] [CrossRef]
- Soury, R.; Chaabene, M.; Jabli, M.; Saleh, T.A.; Chaabane, R.B.; Saint-Aman, E.; Loiseau, F.; Philouze, C.; Allouche, A.-R.; Nasri, H. Meso-tetrakis (3, 4, 5-trimethoxyphenyl) porphyrin derivatives: Synthesis, spectroscopic characterizations and adsorption of NO2. Chem. Eng. J. 2019, 375, 122005. [Google Scholar] [CrossRef]
- Soury, R.; Alhar, M.S.; Jabli, M. Synthesis, Characterization, and Application of Dichloride (5,10,15,20-Tetraphenylporphyrinato) Antimony Functionalized Pectin Biopolymer to Methylene Blue Adsorption. Polymers 2023, 15, 1030. [Google Scholar] [CrossRef]
- Soury, R.; Jabli, M.; Latif, S.; Alenezi, K.M.; El Oudi, M.; Abdulaziz, F.; Teka, S.; El Moll, H.; Haque, A. Synthesis and characterization of a new meso-tetrakis (2,4,6-trimethylphenyl) porphyrinto) zinc(II) supported sodium alginate gel beads for improved adsorption of methylene blue dye. Int. J. Biol. Macromol. 2022, 202, 161–176. [Google Scholar] [CrossRef]
- Teng, L.; Yue, C.; Zhang, G. Epoxied SiO2 nanoparticles and polyethyleneimine (PEI) coated polyvinylidene fluoride (PVDF) membrane for improved oil water separation, anti-fouling, dye and heavy metal ions removal capabilities. J. Colloid Interface Sci. 2023, 630 Pt A, 416–429. [Google Scholar] [CrossRef]
- Lang, J.Q.; Li, C.; Chen, L.; Mai, T.; Guo, Z.H.; Ma, M.G. A polyethyleneimine-functionalized cellulose nanofiber/MXene composite aerogel: Towards highly efficient adsorption of crude oil, organic solvents, and dyes. J. Water Process Eng. 2025, 74, 107808. [Google Scholar] [CrossRef]
- Yan, B.; Dai, Y.; Li, Y.; Xin, L.; Li, M.; Long, H.; Gao, X. Preparation of polyethyleneimine modified cellulose/nano-CdS composite aerogel and its photocatalytic properties for organic dyes under visible light. Int. J. Biol. Macromol. 2025, 306 Pt 4, 141748. [Google Scholar] [CrossRef]
- Soury, R.; Jabli, M.; Al Otaibi, A. Rapid removal of anionic dyes from water, using poly (diallyldimethylammonium chloride) and branched polyethyleneimine functionalized cellulose extracted from Echinops bannaticus leaves. Results Chem. 2025, 15, 102209. [Google Scholar] [CrossRef]
- Soury, R.; Jabli, M.; Alenezi, K.M.; Haque, A.; Moll, H.E.; Rein, R.; Solladié, N.; Azzam, E.M.S.; Nasri, H. A novel meso-tetrakis(2,4,6-trimethylphenyl) porphyrinato ([Zn(TMP)(4,4’-bpy)]) complex: Synthesis, characterization, and its performance for oxidative degradation of calmagite. Inorg. Chem. Commun. 2021, 130, 108716. [Google Scholar] [CrossRef]
- Alzabny, M.H.; Soury, R.; Alenezi, K.M. Mn(III) and Fe(III) Porphyrin Complexes as Electrocatalysts for Hydrogen Evolution Reaction: A comparative study. Int. J. Electrochem. Sci. 2021, 16, 210718. [Google Scholar] [CrossRef]
- Soury, R.; Elamri, A.; El Oudi, M.; Alenezi, K.M.; Jabli, M.; Al Otaibi, A.; Alanazi, A.A.; Albadri, A.E.A.E. Design of a New Catalyst, Manganese(III) Complex, for the Oxidative Degradation of Azo Dye Molecules in Water Using Hydrogen Peroxide. Molecules 2024, 29, 5217. [Google Scholar] [CrossRef]
- Kurochkin, I.Y.; Olshevskaya, V.A.; Zaitsev, A.; Girichevac, N.; Girichev, G. Vibrational Spectra of 5,10,15,20-Tetraphenylporphyrin (H2TPP) and Platinum(II) 5,10,15,20 Tetra(phenyl/pentafluorophenyl)porphyrins (PtTPP and PtTF5PP). Macroheterocycles 2021, 14, 334–341. [Google Scholar] [CrossRef]
- Soury, R.; Jabli, M.; Oudi, M.E.; Alenezi, K.M.; Otaibi, A.A.; Abdulaziz, F.; Al Ghamdi, H.A.; Bchetnia, A. New complexes of [5,10,15,20-(tetraphenylporphyrin)] and dichloride (5,10,15,20-tetraphenylporphyrinato) antimony(V) hexachloridoantimonate(V) functionalized with polyethyleneimine: Synthesis, characterization, and application in Eriochrome Black T adsorption from water. Polyhedron 2025, 266, 117305. [Google Scholar]
- Jabli, M.; Sebeia, N.; El-Ghoul, Y.; Soury, R.; Al-Ghamdi, Y.O.; Saleh, T.A. Chemical modification of microcrystalline cellulose with polyethyleneimine and hydrazine: Characterization and evaluation of its adsorption power toward anionic dyes. Int. J. Biol. Macromol. 2023, 229, 210–223. [Google Scholar] [CrossRef]
- Gao, W.Y.; Chrzanowski, M.; Ma, S. Metal–metalloporphyrin frameworks: A resurging class of functional materials. Chem. Soc. Rev. 2014, 43, 5841–5866. [Google Scholar] [CrossRef]
- Gamelas, S.R.; Tomé, J.P.; Tomé, A.C.; Lourenço, L.M. Porphyrin-containing materials for photodegradation of organic pollutants in wastewaters: A review. Catal. Sci. Technol. 2024, 14, 2352–2389. [Google Scholar] [CrossRef]
- Bao, Y. Polymerization-Mediated Through-Space Charge Transfer: An Emerging Strategy for Light-Emitting Materials. Langmuir 2024, 40, 3275–3282. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Li, H.; Liu, C.; Liu, J.; Feng, Y.; Wee, A.G.; Zhang, B. Porphyrin-and porphyrinoid-based covalent organic frameworks (COFs): From design, synthesis to applications. Coord. Chem. Rev. 2021, 435, 213778. [Google Scholar] [CrossRef]
- Trache, D.; Donnot, A.; Khimeche, K.; Benelmir, R.; Brosse, N. Physicochemical properties and thermal stability of microcrystalline cellulose isolated from Alfa fibres. Carbohydr. Polym. 2014, 104, 223–230. [Google Scholar] [CrossRef]
- Manal Alkhabbas Alaa, M. Al-Ma’abreh, Gada Edris, Tasneem Saleh, Heba Alhmood. Adsorption of Anionic and Cationic Dyes on Activated Carbon Prepared from Oak Cupules: Kinetics and Thermodynamics Studies. Int. J. Environ. Res. Public Health 2023, 20, 3280. [Google Scholar] [CrossRef] [PubMed]
- Fathurrahmi, F.; Robbani, F. Adsorption of Naphtol Blue Black (NBB) Dye over Immobilized Chitosan from Shrimp Shells onto Glass Plate. J. Pharm. Sci. 2022, 5, 21–32. [Google Scholar]
- Benammar, H.S.; Guergazi, S.; Youcef, S.; Youcef, L. Removal of Congo red and Naphthol blue black dyes from aqueous solution by adsorption on activated carbon. Characterization, kinetic and equilibrium in nonlinear models studies. Desalin. Water Treat. 2021, 221, 396–405. [Google Scholar] [CrossRef]
- Costescu, A.; Pasuk, I.; Ungureanu, F.; Dinischiotu, A.; Costache, M.; Huneau, F.; Galaup, S.; Le Coustumer, P.; Predoi, D. Physico-chemical properties of nano-sized hexagonal hydroxyapatite powder synthesized by sol-gel. Dig. J. Nanomater. Biostruct. 2010, 5, 989–1000. [Google Scholar]
- Bulina, N.V.; Makarova, S.V.; Baev, S.G.; Matvienko, A.A.; Gerasimov, K.B.; Logutenko, O.A.; Bystrov, V.S. A Study of Thermal Stability of Hydroxyapatite. Minerals 2021, 11, 1310. [Google Scholar] [CrossRef]
- Einhorn-Stoll, U.; Kunzek, H.; Dongowski, G. Thermal analysis of chemically and mechanically modified pectins. Food Hydrocoll. 2007, 21, 1101–1112. [Google Scholar] [CrossRef]
- Wei, W.; Yang, L.; Zhong, W.H.; Li, S.Y.; Cui, J.; Wei, Z.G. Fast removal of methylene blue from aqueous solution by adsorption onto poorly crystalline hydroxyapatite nanoparticles. Dig. J. Nanomater. Biostruct. 2015, 10, 1343–1363. [Google Scholar]
- Zhang, J.; Ping, Q.; Niu, M.; Shi, H.; Li, N. Kinetics and equilibrium studies from the methylene blue adsorption on diatomite treated with sodium hydroxide. Appl. Clay Sci. 2013, 83–84, 12–16. [Google Scholar] [CrossRef]
- Nasuha, N.; Hameed, B.H.; Din, A.T. Rejected tea as a potential low-cost adsorbent for the removal of methylene blue. J. Hazard. Mater. 2010, 175, 126–132. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
FT-IR spectra of (a) complex (1) and complex (1)-PEI, (b) complex (2) and complex (2)-PEI, (c) complex (3) and complex (3)-PEI.
Figure 1.
FT-IR spectra of (a) complex (1) and complex (1)-PEI, (b) complex (2) and complex (2)-PEI, (c) complex (3) and complex (3)-PEI.
Figure 2.
Spectroscopic data of: (a); complexes (1–3) and (b); complex (1)-PEI, complex (2)-PEI, and complex (3)-PEI in dichloromethane.
Figure 2.
Spectroscopic data of: (a); complexes (1–3) and (b); complex (1)-PEI, complex (2)-PEI, and complex (3)-PEI in dichloromethane.
Figure 3.
XRD patterns of complexes (1–3), complex (1)-PEI, complex (2)-PEI, and complex (3)-PEI.
Figure 3.
XRD patterns of complexes (1–3), complex (1)-PEI, complex (2)-PEI, and complex (3)-PEI.
Figure 4.
SEM images of: (a) complex (1), (b) complex (2), (c) complex (3), (d) complex (1)-PEI, (e) complex (2)-PEI, and (f) complex (3)-PEI.
Figure 4.
SEM images of: (a) complex (1), (b) complex (2), (c) complex (3), (d) complex (1)-PEI, (e) complex (2)-PEI, and (f) complex (3)-PEI.
Figure 5.
EDX result of: (a) complex (1), (c) complex (2), (e) complex (3), (b) complex (1)-PEI, (d) complex (2)-PEI, and (f) complex (3)-PEI.
Figure 5.
EDX result of: (a) complex (1), (c) complex (2), (e) complex (3), (b) complex (1)-PEI, (d) complex (2)-PEI, and (f) complex (3)-PEI.
Figure 6.
TGA/DTA curves of complexes (1–3) and modified complexes.
Figure 6.
TGA/DTA curves of complexes (1–3) and modified complexes.
Figure 7.
Influence of some experimental conditions on the adsorption of Naphthol blue black B using complex (1)-PEI, complex (2)-PEI, and complex (3)-PEI: (a) pH (C0 = 50 mg/L, T = 19 °C, time = 100 min), (b,c) time (C0 = 50 mg/L, C0 = 100 mg/L, pH = 5, T = 19 °C), and (d–f) temperature (data is replicated 3 times).
Figure 7.
Influence of some experimental conditions on the adsorption of Naphthol blue black B using complex (1)-PEI, complex (2)-PEI, and complex (3)-PEI: (a) pH (C0 = 50 mg/L, T = 19 °C, time = 100 min), (b,c) time (C0 = 50 mg/L, C0 = 100 mg/L, pH = 5, T = 19 °C), and (d–f) temperature (data is replicated 3 times).
Figure 8.
Plots of Ln Kd versus 1/T for Naphthol blue black B adsorption utilizing complexes (1)-PEI, (2)-PEI, and (3)-PEI (results replicated three times).
Figure 8.
Plots of Ln Kd versus 1/T for Naphthol blue black B adsorption utilizing complexes (1)-PEI, (2)-PEI, and (3)-PEI (results replicated three times).
Table 1.
Relative abundance of elements present in complexes 1–3 and modified complexes.
Table 1.
Relative abundance of elements present in complexes 1–3 and modified complexes.
| m/m (%) |
|---|
| Elements | Complex (1) | Complex (1)-PEI | Complex (2) | Complex (2)-PEI | Complex (3) | Complex (3)-PEI |
|---|
| Na | 51.52 | 48.69 | - | - | - | - |
| K | 05.14 | 08.96 | - | - | - | - |
| Zn | 20.63 | 19.75 | - | - | - | - |
| Al | 19.20 | 18.59 | - | - | - | - |
| Ca | 01.70 | 01.59 | - | - | - | - |
| Hf | 01.14 | 01.05 | - | - | - | - |
| Ti | 00.67 | 01.37 | - | - | - | - |
| Fe | - | - | 52.60 | 51.93 | - | - |
| Cl | - | - | 40.17 | 39.58 | - | - |
| Sx | - | - | 06.02 | 07.01 | - | - |
| V | - | - | 00.69 | 00.71 | - | - |
| Ni | - | - | 00.44 | 00.70 | - | - |
| Nb | - | - | 00.02 | 00.05 | - | - |
| Mo | - | - | 00.10 | 00.02 | - | - |
| Mn | - | - | - | - | 70.59 | 68.70 |
| Cl | - | - | - | - | 28.60 | 27.25 |
| Co | - | - | - | - | 0.706 | 01.14 |
| Nb | - | - | - | - | 0.044 | 01.05 |
| Mo | - | - | - | - | 0.035 | 01.04 |
| In | - | - | - | - | 0.016 | 00.07 |
Table 2.
Comparison of the adsorption capacities of Naphthol blue black B using some studied adsorbents.
Table 2.
Comparison of the adsorption capacities of Naphthol blue black B using some studied adsorbents.
| | Q Max (mg/g) | Reference |
|---|
| Complex (3)-PEI | 154 | This study |
| Complex (2)-PEI | 139 | This study |
| Complex (1)-PEI | 119 | This study |
| H3PO4 activated carbon produced from oak cupules | 208 | [45] |
| Immobilized Chitosan from Shrimp Shells onto Glass Plate | 45.5 | [46] |
| Commercial powdered activated carbon | 70.6 | [47] |
Table 3.
Kinetics, isotherms, and thermodynamic parameters computed during the adsorption of Naphthol blue black B employing complexes (1)-PEI, (2)-PEI, and (3)-PEI.
Table 3.
Kinetics, isotherms, and thermodynamic parameters computed during the adsorption of Naphthol blue black B employing complexes (1)-PEI, (2)-PEI, and (3)-PEI.
| | Constants | Naphthol Blue Black B (mg/L) | Isotherms | Parameters | Temperature (°C) |
|---|
| Complex (1)-PEI |
| First order | | 50 | 100 | | | 20 | 42 | 53 |
| K1 (min−1) | 0.029 | 0.0306 | qm (mg·g−1) | 222.22 | 250 | 243.90 |
| q (mg·g−1) | 45.74 | 66.99 | Langmuir | KL (L·g−1) | 0.0078 | 0.0046 | 0.39 |
| R2 | 0.98 | 0.98 | R2 | 0.98 | 0.85 | 0.90 |
| Second order | K2 | 0.0017 | 0.0005 | Thermodynamic parameters | ΔH° (KJ mol−1) | −6.038 |
| q | 47.39 | 60.97 | ΔS° (J mol−1) | −27.35 |
| R2 | 0.99 | 0.99 | ΔG° (KJ mol−1) | 8.014 | 8.62 | 8.89 |
| Freundlich | KF (L·g−1) | 28.84 | 2.40 | 1.38 |
| Elovich | α (mg·g−1·min−1) | 32.06 | 45.37 | n | 1.54 | 1.24 | 1.21 |
| β (mg·g−1·min−1) | 0.102 | 0.072 | R2 | 0.97 | 0.97 | 0.98 |
| R2 | 0.95 | 0.95 | Temkin | bT (J·mol−1) | 51.17 | 64.36 | 71.76 |
| Intra-particular-Diffusion | K (mg·g1·min1/2) | 3.36 | 4.73 | A (L·g−1) | 11.50 | 13.34 | 15.99 |
| R2 | 0.88 | 0.88 | R2 | 0.98 | 0.97 | 0.97 |
| Complex (2)-PEI |
| First order | K1 (min−1) | 0.029 | 0.031 | | qm (mg·g−1) | 188.67 | 204.08 | 227.27 |
| q (mg·g−1) | 47.86 | 68.23 | Langmuir | KL (L·g−1) | 0.0088 | 0.0042 | 0.392 |
| R2 | 0.98 | 0.98 | R2 | 0.99 | 0.95 | 0.82 |
| Second order | K2 | 0.0011 | 0.0009 | Thermodynamic parameters | ΔH° (KJ mol−1) | −6.61 |
| q | 44.84 | 64.51 | ΔS° (J mol−1) | −30.51 |
| R2 | 0.99 | 0.99 | ΔG° (KJ mol−1) | 8.94 | 8.94 | 8.94 |
| Freundlich | KF (L·g−1) | 40.74 | 2.75 | 1.24 |
| Elovich | α (mg·g−1·min−1) | 23.49 | 27.43 | n | 1.67 | 1.30 | 1.19 |
| β (mg·g−1·min−1) | 0.096 | 0.068 | R2 | 0.97 | 0.97 | 0.97 |
| R2 | 0.95 | 0.94 | Temkin | bT (J·mol−1) | 60.86 | 55.02 | 78.04 |
| Intra-particular-Diffusion | K (mg·g1·min1/2) | 3.75 | 5.47 | A (L·g−1) | 10.086 | 8.24 | 19.21 |
| R2 | 0.89 | 0.87 | R2 | 0.97 | 0.97 | 0.97 |
| Complex (3)-PEI |
| First order | K1 (min−1) | 0.0294 | 0.032 | | qm (mg·g−1) | 212.76 | 285.71 | 217.39 |
| q (mg·g−1) | 45.92 | 68.55 | Langmuir | KL (L·g−1) | 0.0038 | 0.0018 | 0.37 |
| R2 | 0.98 | 0.98 | R2 | 0.95 | 0.68 | 0.73 |
| Second order | K2 | 0.0017 | 0.015 | Thermodynamic parameters | ΔH° (KJ mol−1) | −7.71 |
| q | 47.39 | 72.46 | ΔS° (J mol−1) | −35.66 |
| R2 | 0.99 | 0.99 | ΔG° (KJ mol−1) | 10.45 | 11.24 | 11.59 |
| Freundlich | KF (L·g−1) | 6.92 | 2.51 | 39.81 |
| Elovich | α (mg·g−1·min−1) | 23.43 | 27.20 | n | 1.43 | 1.12 | 1.01 |
| β (mg·g−1·min−1) | 0.098 | 0.070 | R2 | 0.97 | 0.97 | 0.97 |
| R2 | 0.94 | 0.94 | Temkin | bT (J·mol−1) | 65.73 | 71.82 | 75.66 |
| Intra-particular-Diffusion | K (mg·g1·min1/2) | 4.007 | 6.02 | A (L·g−1) | 14.10 | 21.98 | 29.57 |
| R2 | 0.87 | 0.84 | R2 | 0.97 | 0.97 | 0.97 |
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).