Advanced Optimization of Optical Carbon Dioxide Sensor Through Sensitivity Enhancement in Anodic Aluminum Oxide Substrate
Abstract
1. Introduction
2. Experimental
2.1. Materials
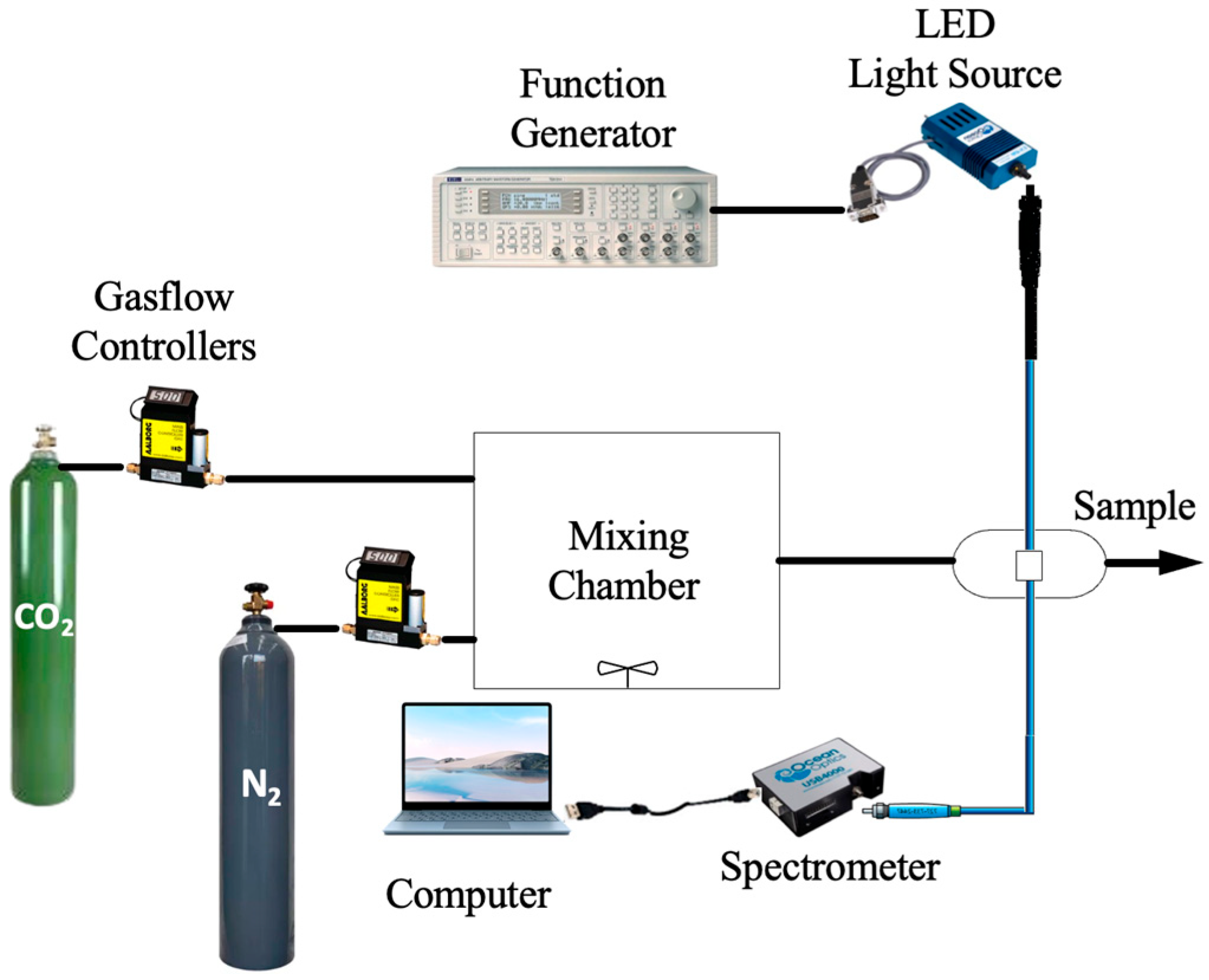
2.2. Instrumentation
3. Results and Discussion
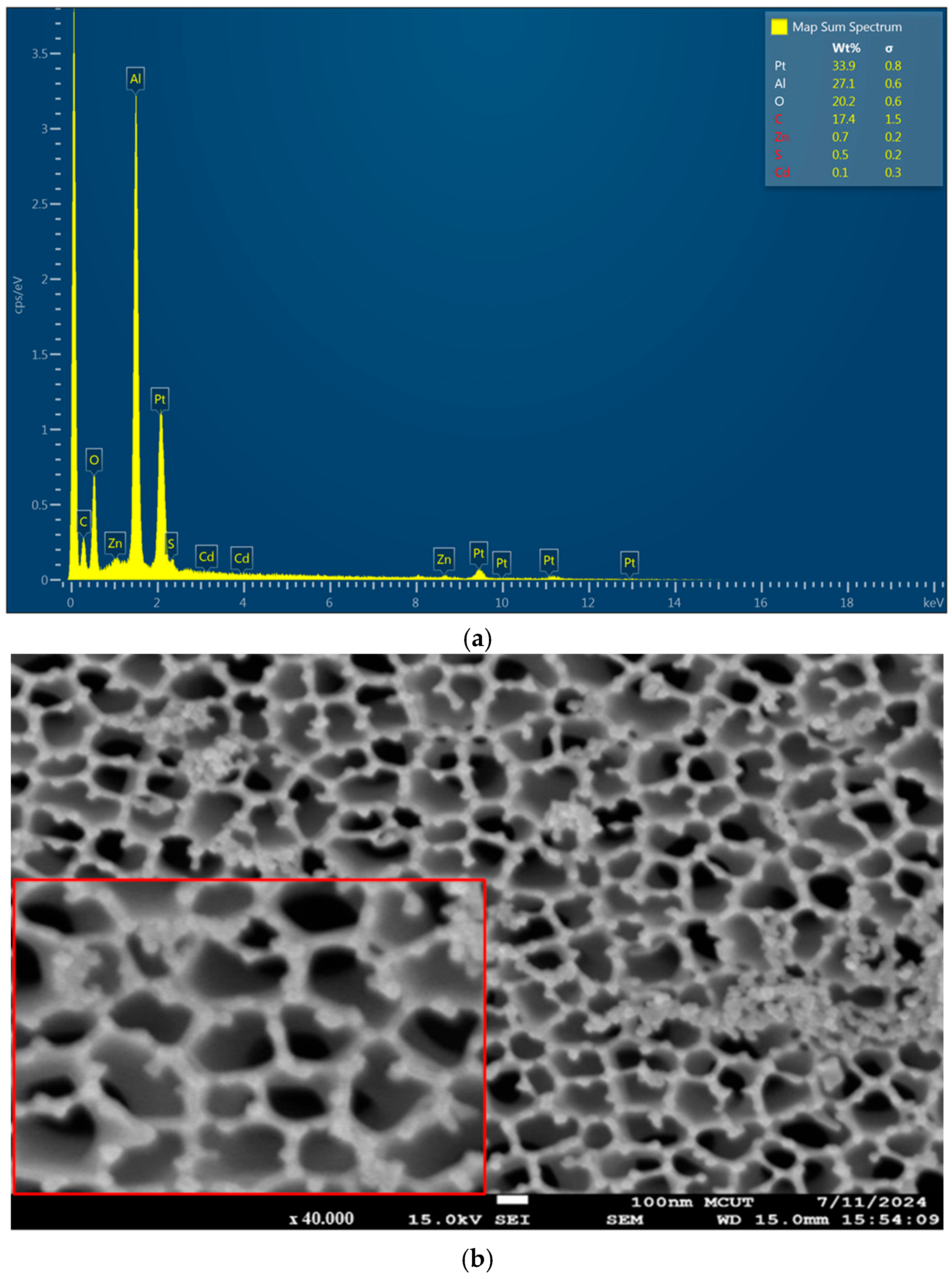
3.1. Morphological Characterization of CdSe/ZnS Quantum Dots via Scanning Electron Microscopy (SEM)
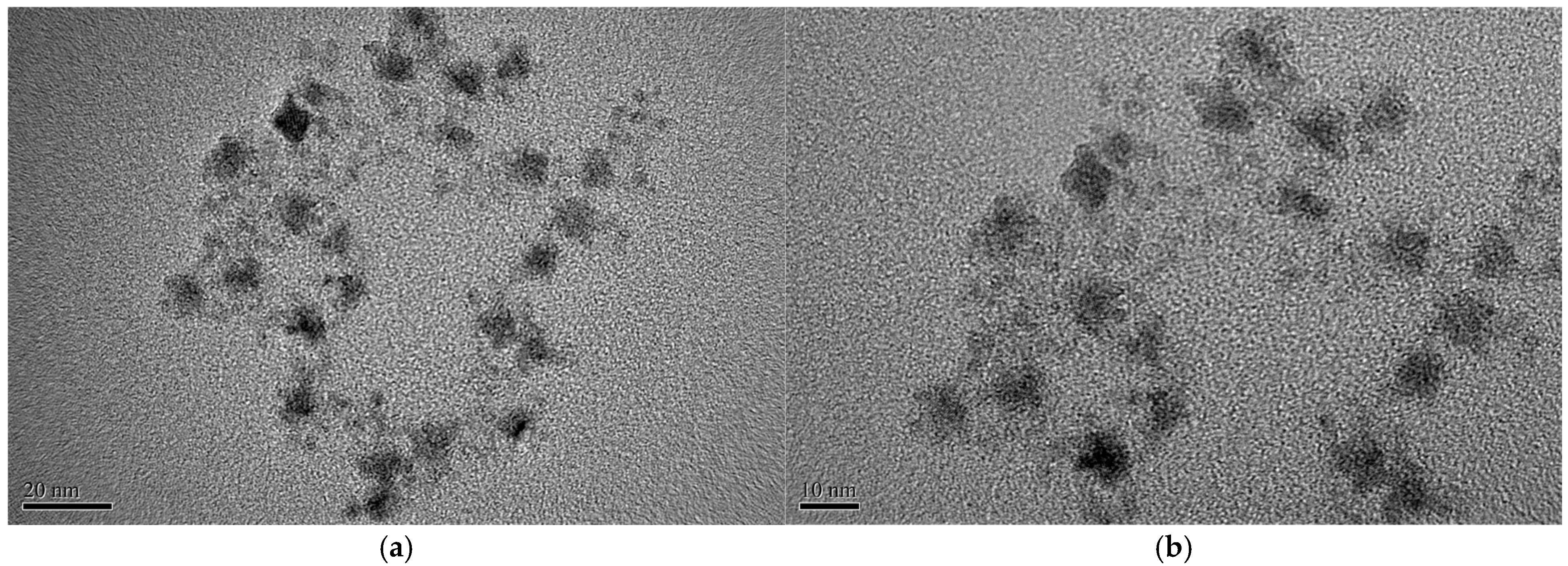
3.2. Fabrication of CO2 Sensing Material on AAO Substrate Characterized by CdSe/ZnS Quantum Dots via Transmission Electron Microscopy (TEM)
3.3. The Absorption and Emission Characteristics of CdSe/ZnS QDs
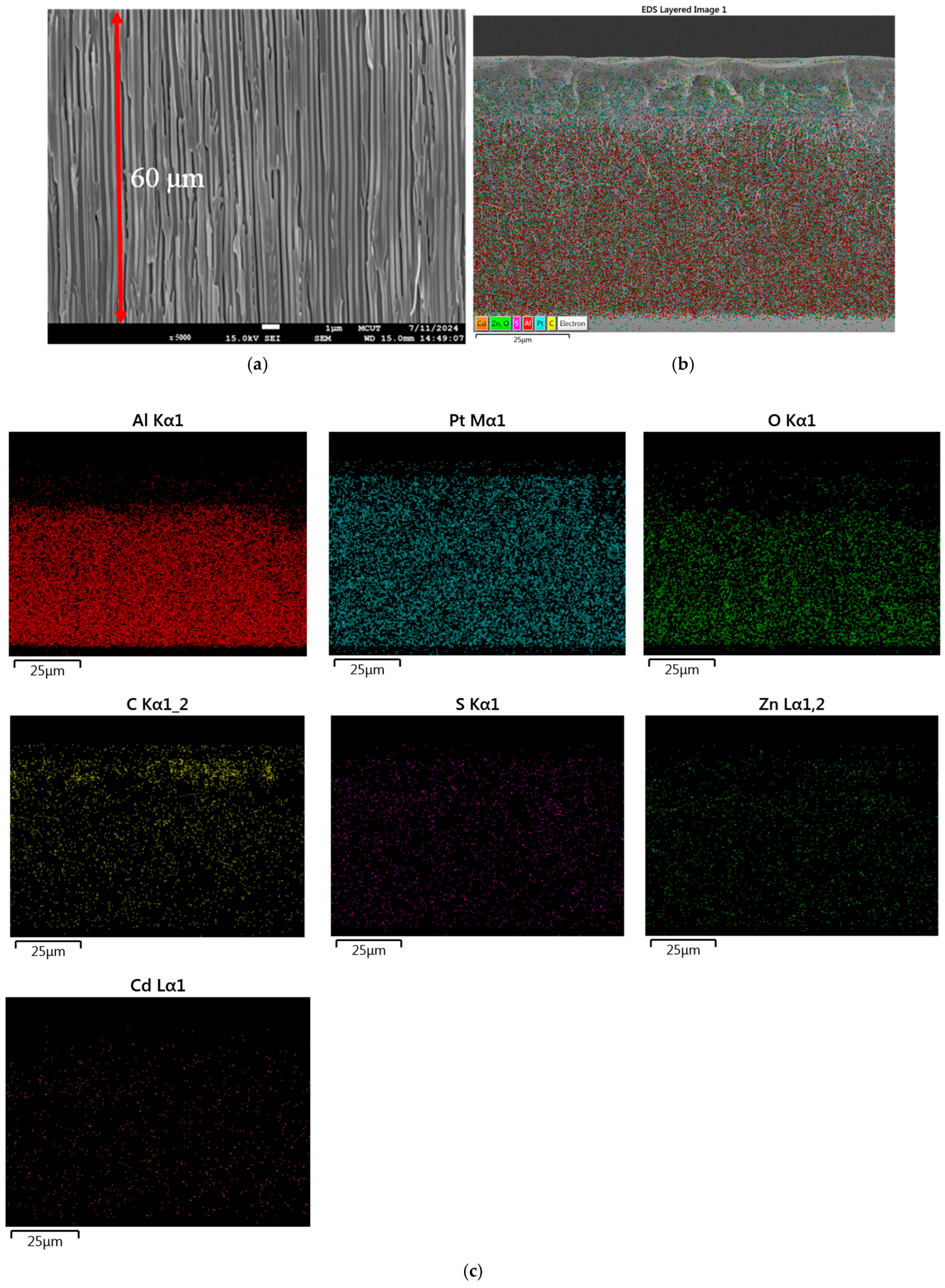
3.4. Cross-Section and Elemental Analysis of Anodic Aluminum Oxide Membrane
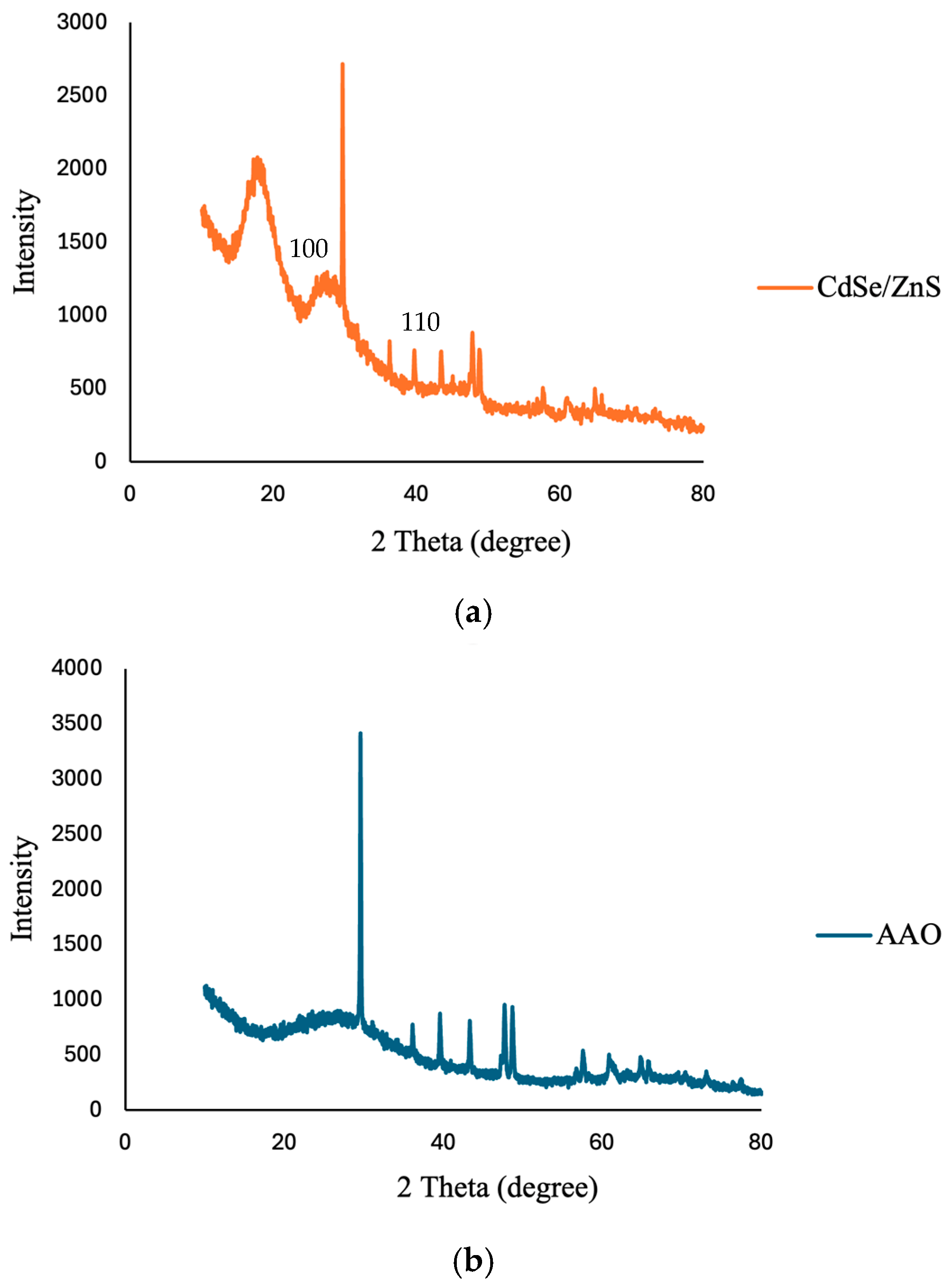
3.5. X-Ray Diffraction (XRD) Analysis of CdSe/ZnS Quantum Dots and AAO Substrate
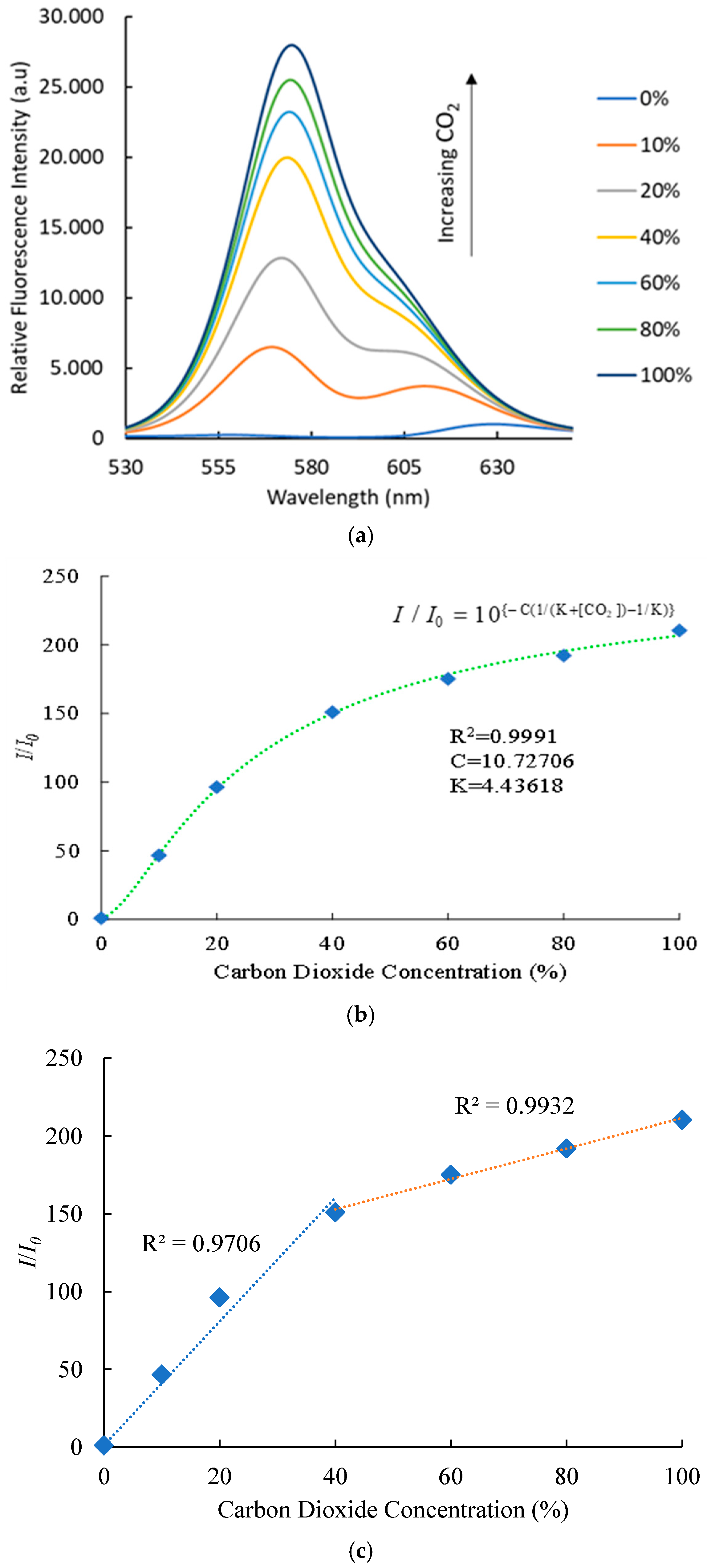
3.6. Optical Carbon Dioxide Sensor Sensing Properties
3.7. Wavelength Shift of Optical Carbon Dioxide Sensor
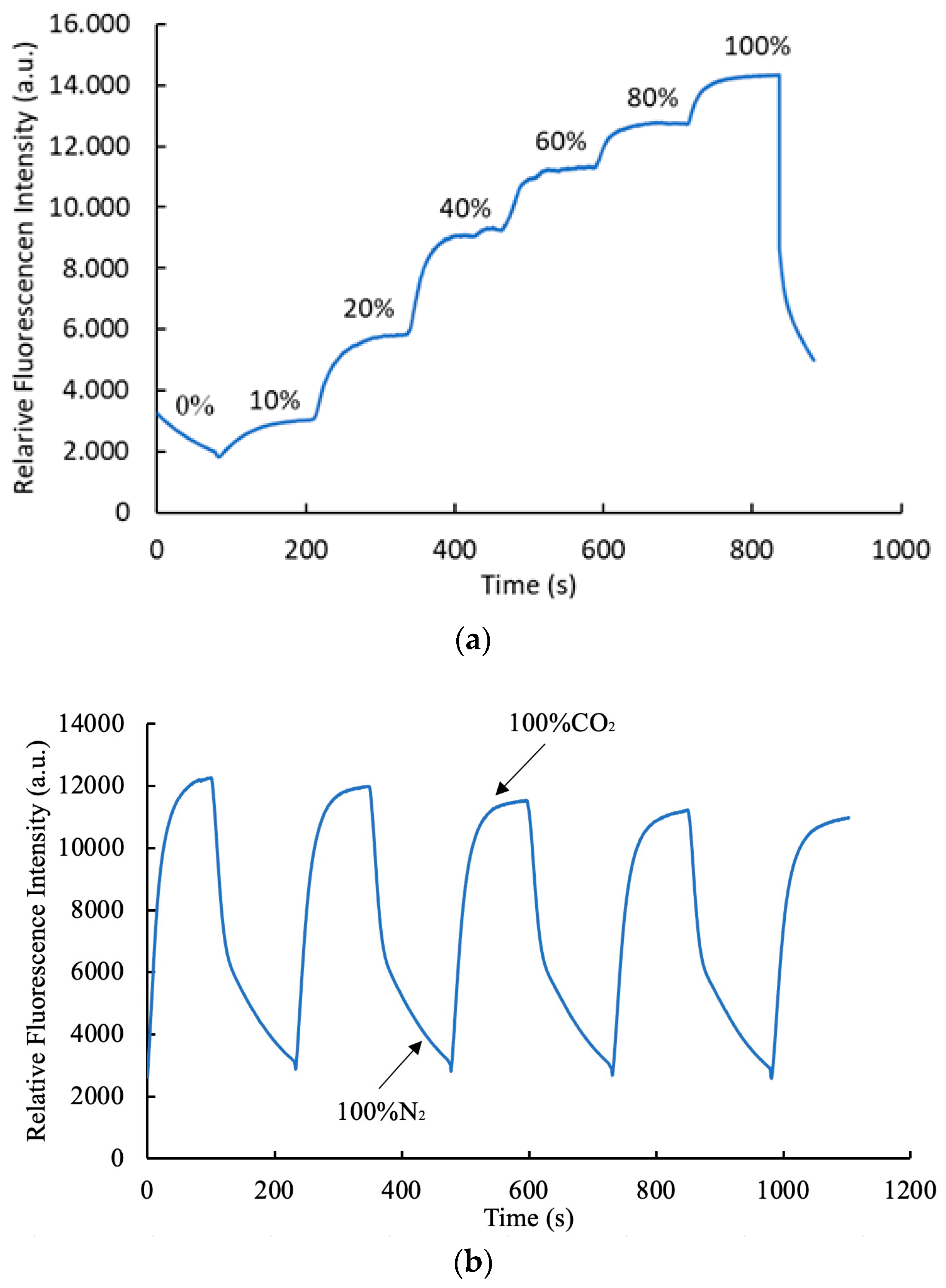
3.8. Response Time and Dynamic Characteristics of Optical Carbon Dioxide Sensor
3.9. The Analytical Selectivity Response of Optical Carbon Dioxide Sensor
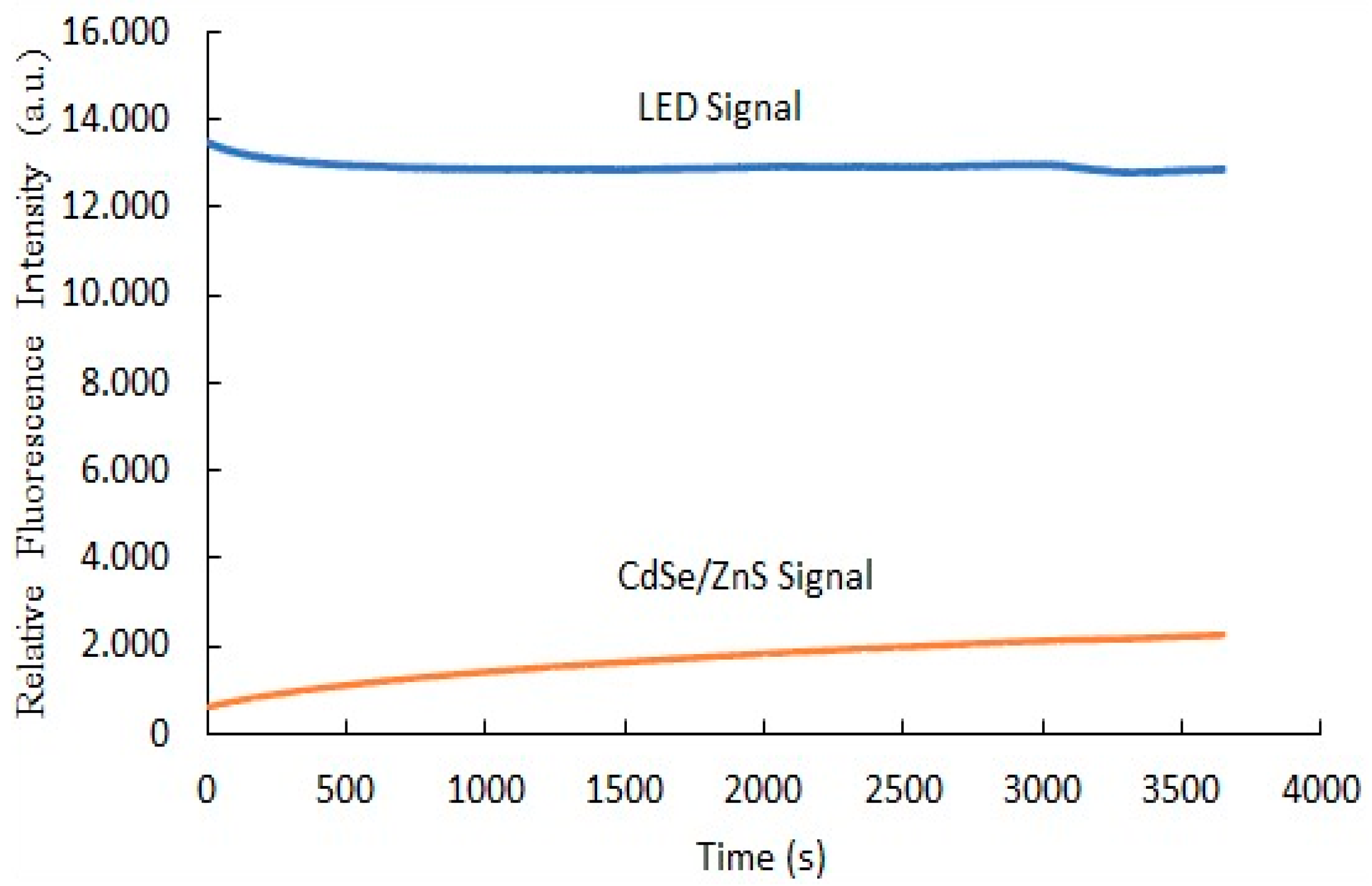
3.10. The Photostability of Optical Carbon Dioxide Sensor
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Benton, E.N.; Perera, N.A.K.R.; Nesterov, V.N.; Perera, W.; Omary, M.A.; Marpu, S.B. A europium-based optical sensor for the detection of carbon dioxide and its application for a fermentation reaction. Chemosensors 2023, 11, 5. [Google Scholar] [CrossRef]
- Zhang, X.; Mu, H.; Li, H.; Zhang, Y.; An, M.; Zhang, X.; Yoon, J.; Yu, H. Dual-channel sensing of CO2: Reversible solution-gel transition and gelatin-induced fluorescence enhancement. Sens. Actuators B Chem. 2018, 255, 2764–2778. [Google Scholar] [CrossRef]
- Sun, J.; Ye, B.; Xia, G.; Zhao, X.; Wang, H. A colorimetric and fluorescent chemosensor for the highly sensitive detection of CO2 gas: Experiment and DFT calculation. Sens. Actuators B Chem. 2016, 233, 76–82. [Google Scholar] [CrossRef]
- Gutierrez, P.K.C.; Rodriguez, S.M.; Castillo, A.L.M.; Sanchez, J.F.F.; Gutierrez, A.F. A new highly sensitive and versatile optical sensing film for controlling CO2 in gaseous and aqueous media. Sens. Actuators B Chem. 2013, 184, 281–287. [Google Scholar] [CrossRef]
- Tsukada, K.; Sakai, S.; Hase, K.; Minamitani, H. Development of catheter-type optical oxygen sensor and applications to bioinstrumentation. Biosens. Bioelectron. 2003, 18, 1439–1445. [Google Scholar] [CrossRef]
- Chu, C.S.; Lo, Y.L. Highly sensitive and linear optical fiber carbon dioxide sensor based on sol-gel matrix doped with silica particles and HPTS. Sens. Actuators B Chem. 2009, 143, 205–210. [Google Scholar] [CrossRef]
- Lakowicz, J.R.; Ray, K.; Chowdhury, M.; Szmacinski, H.; Fu, Y.; Zhang, J.; Nowaczyk, K. Plasmon-controlled fluorescence: A new paradigm in fluorescence spectroscopy. Analyst 2008, 133, 1308–1346. [Google Scholar] [CrossRef]
- Nakamura, N.; Amao, Y. Optical sensor for carbon dioxide combining colorimetric change of a pH indicator and a reference luminescent dye. Anal. Bioanal. Chem. 2003, 376, 642–646. [Google Scholar] [CrossRef]
- Amao, Y.; Komori, T. Optical CO2 sensor of the combination of colorimetric change of α-naphtholphthalein in poly(isobutyl methacrylate) and fluorescent porphyrin in polystyrene. Talanta 2005, 66, 976–981. [Google Scholar] [CrossRef]
- Amao, Y.; Komori, T.; Nishide, H. Rapid responsible optical CO2 sensor of the combination of colorimetric change of alpha-naphtholphthalein in poly(trimethylsiliylpropyne) layer and internal reference fluorescent porphyrin in polystyrene layer. React. Funct. Polym. 2005, 63, 35–41. [Google Scholar] [CrossRef]
- Chu, C.S.; Su, C.J. Fluorescence ratiometric optical broad range pH sensor based on CdSe/ZnS quantum dots and O170 embedded in ethyl cellulose matrix. J. Ligthtwave Tech. 2018, 36, 857–862. [Google Scholar] [CrossRef]
- Amao, Y.; Nakamura, N. Optical CO2 sensor with the combination of colorimetric change of α-naphtholphthalein and internal reference fluorescent porphyrin dye. Sens. Actuators B Chem. 2004, 100, 347–351. [Google Scholar] [CrossRef]
- Baldeck, M.I.P.; Astilean, S. Plasmon-enhanced fluorescence of dye molecules. Nucl. Instru. Meth. B 2009, 267, 403–405. [Google Scholar]
- Eltzov, E.; Prilutsky, D.; Kushmaro, A.; Marks, R.S.; Geddes, C.D. Metal-enhanced bioluminescence: An approach for monitoring biological luminescent processes. Appl. Phys. Lett. 2009, 94, 083901. [Google Scholar] [CrossRef]
- Lee, W.; Park, S.J. Porous Anodic Aluminum Oxide: Anodization and Templated Synthesis of Functional Nanostructures. Chem. Rev. 2014, 114, 7487–7556. [Google Scholar] [CrossRef]
- Masuda, H.; Fukuda, K. Ordered Metal Nanohole Arrays Made by a Two-Step Replication of Honeycomb Structures of Anodic Alumina. Science 1994, 268, 1466–1468. [Google Scholar] [CrossRef]
- Li, A.P.; Müller, F.; Birner, A.; Nielsch, K.; Gösele, U. Hexagonal pore arrays with a 50–420 nm interpore distance formed by self-organization in anodic alumina. J. Appl. Phys. 1998, 84, 6023–6026. [Google Scholar] [CrossRef]
- Liu, C.Y.; Biring, S. Nanoplatform based on ideally ordered arrays of short straight and long beer bottle-shaped nanochannels. Mesoporous Mater. 2019, 287, 71–76. [Google Scholar] [CrossRef]
- Biring, S.; Kolaru, R.B. Achieving high response of poly (3-hexylthiophene-2,5-diyl) molecules to gaseous ammonia using anodic aluminum oxide nanoporous substrate operated under 1 V. Sens. Actuators B Chem. 2022, 373, 132712. [Google Scholar] [CrossRef]
- Mesin, R.; Chu, C.S.; Tseng, Z.L. Ratiometric optical oxygen sensor based on perovskite quantum dots and Rh110 embedded in an ethyl cellulose matrix. Opt. Mater. Express 2023, 13, 945–955. [Google Scholar] [CrossRef]
- Ertekin, K.; Klimant, I.; Neurauter, G.; Wolfbeis, O.S. Characterization of a reservoir-type capillary optical microsensor for pCO2 measurements. Talanta 2003, 59, 261–267. [Google Scholar] [CrossRef]



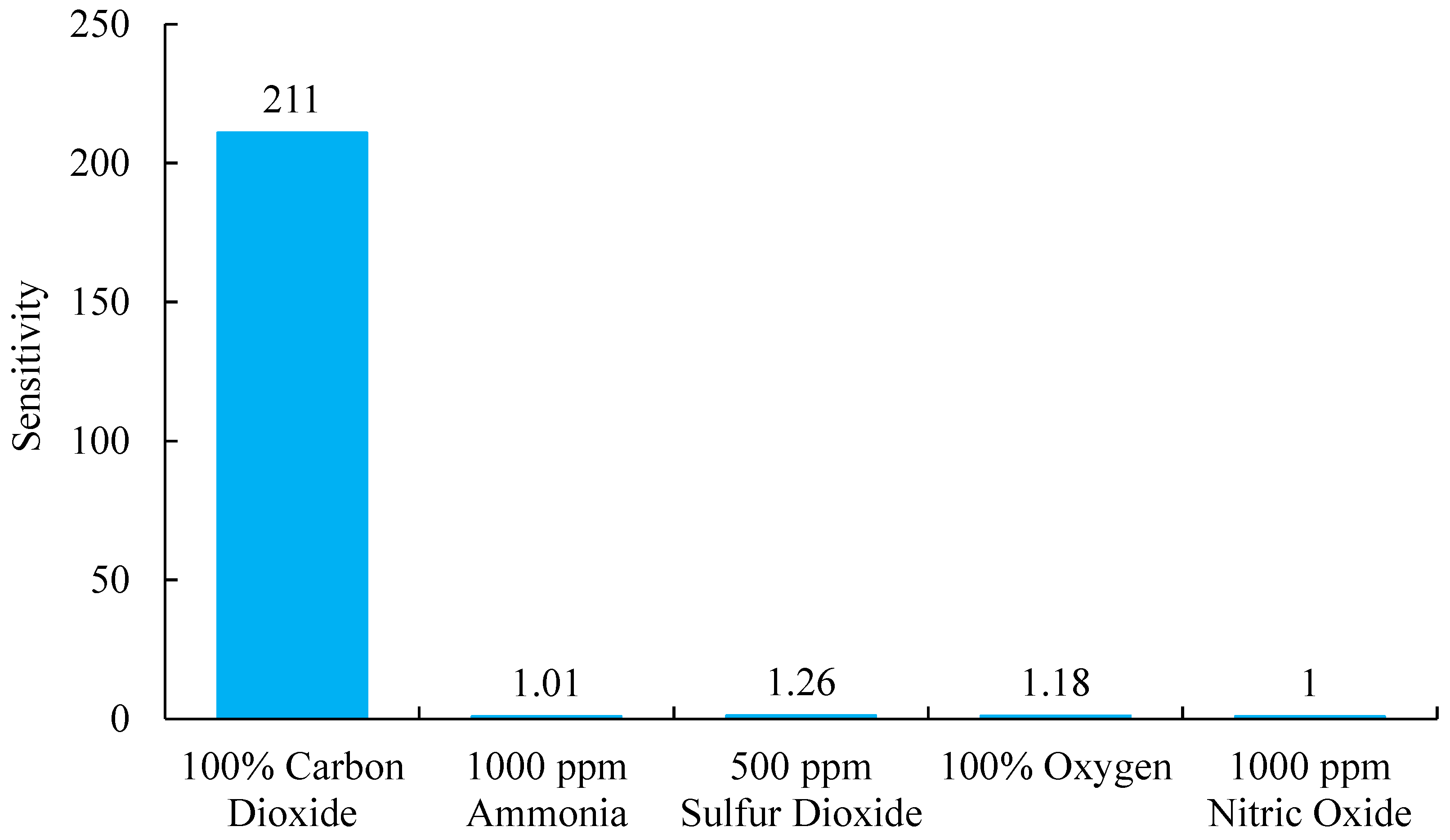
| pH-Sensitive Dye | Internal Reference Dye | Sensor Layer | Sensitivity | Sensing Signal | Ref. |
|---|---|---|---|---|---|
| Cresol Red in ethyl cellulose | Eu(tta)3 in polystyrene | Double layers | 7.1 | Intensity | [8] |
| Phenol Red in ethyl cellulose | Eu(tta)3 in polystyrene | Double layers | 9.9 | Intensity | [8] |
| Thymol blue in ethyl cellulose | Eu(tta)3 in polystyrene | Double layers | 15.6 | Intensity | [8] |
| α- naphtholphthalein in polyIBM | Tetraphenylporphyrin in polystyrene | Double layers | 192 | Intensity | [9] |
| α- naphtholphthalein in poly(TMSP) | Tetraphenylporphyrin in polystyrene | Double Layers | 10.3 | Intensity | [10] |
| α- naphtholphthalein in ethyl cellulose | Tetraphenylporphyrin in polystyrene | Double Layers | 53.9 | Intensity | [12] |
| Phenol Red in polyIBM | CdSe/ZnS QDs in polyIBM | Single layer | 211 | Wavelength shift/Intensity | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simanjuntak, M.S.; Rispandi; Chu, C.-S. Advanced Optimization of Optical Carbon Dioxide Sensor Through Sensitivity Enhancement in Anodic Aluminum Oxide Substrate. Polymers 2025, 17, 1460. https://doi.org/10.3390/polym17111460
Simanjuntak MS, Rispandi, Chu C-S. Advanced Optimization of Optical Carbon Dioxide Sensor Through Sensitivity Enhancement in Anodic Aluminum Oxide Substrate. Polymers. 2025; 17(11):1460. https://doi.org/10.3390/polym17111460
Chicago/Turabian StyleSimanjuntak, Manna Septriani, Rispandi, and Cheng-Shane Chu. 2025. "Advanced Optimization of Optical Carbon Dioxide Sensor Through Sensitivity Enhancement in Anodic Aluminum Oxide Substrate" Polymers 17, no. 11: 1460. https://doi.org/10.3390/polym17111460
APA StyleSimanjuntak, M. S., Rispandi, & Chu, C.-S. (2025). Advanced Optimization of Optical Carbon Dioxide Sensor Through Sensitivity Enhancement in Anodic Aluminum Oxide Substrate. Polymers, 17(11), 1460. https://doi.org/10.3390/polym17111460







