Thermal Insulation Performance of Epoxy-Based Intumescent Coatings: Influence of Temperature-Induced Porosity Evolution on Heat Transfer Resistance
Abstract
1. Introduction
1.1. Intumescent Coatings and Fire Protection: Motivation and Research Gaps
1.2. Numerical and Experimental Analysis: Need for Integrated Studies
1.3. Heat Transfer Mechanisms in Intumescent Coatings
1.4. Influence of Porosity on Thermal Insulation
1.5. Research Significance and Objectives
2. Experimental Methods
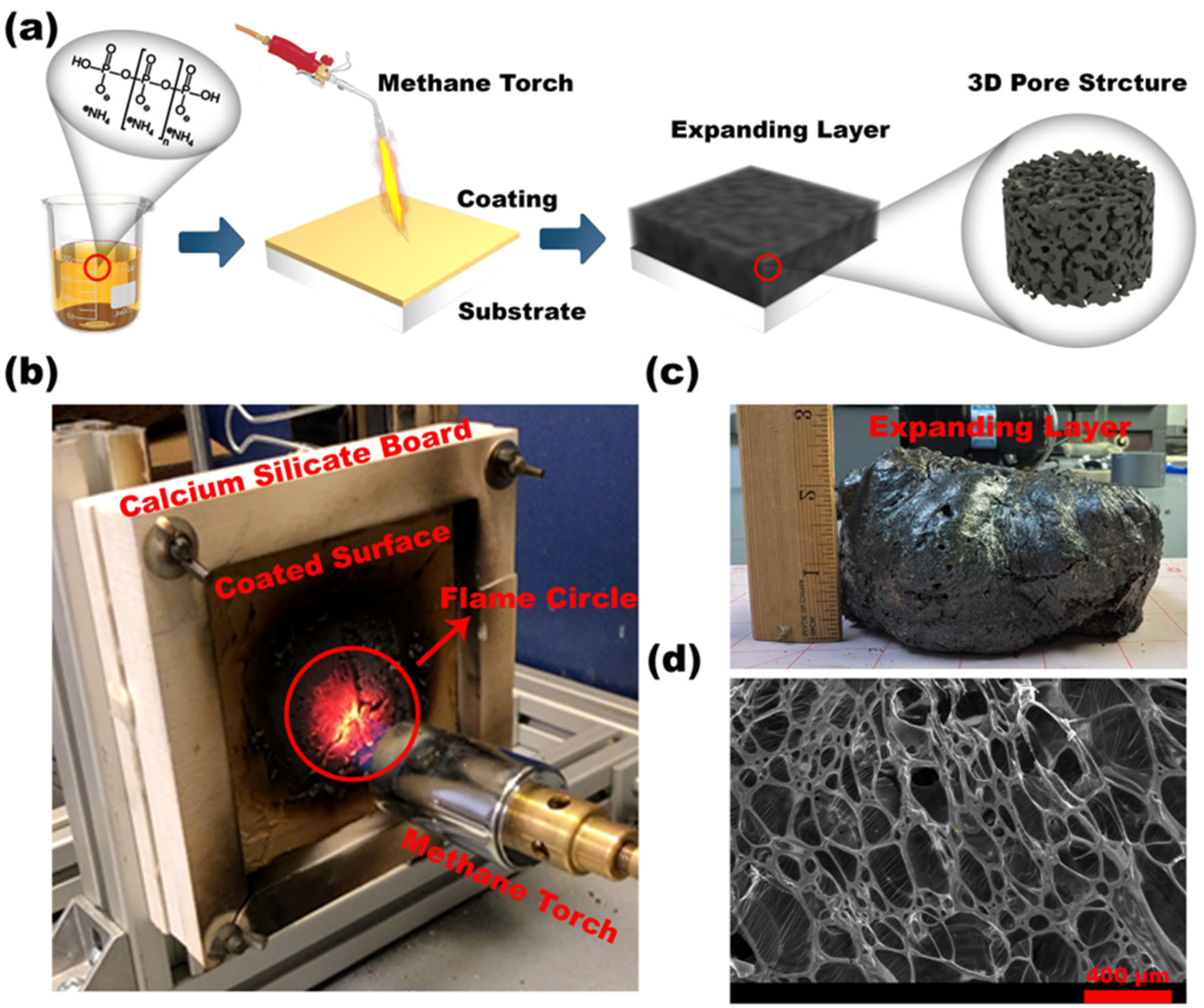
2.1. Sample Preparation
RSI Coating Preparation Method for Fire Testing and Specific Concentration
2.2. Fire Testing and Thermal Behavior Assessment
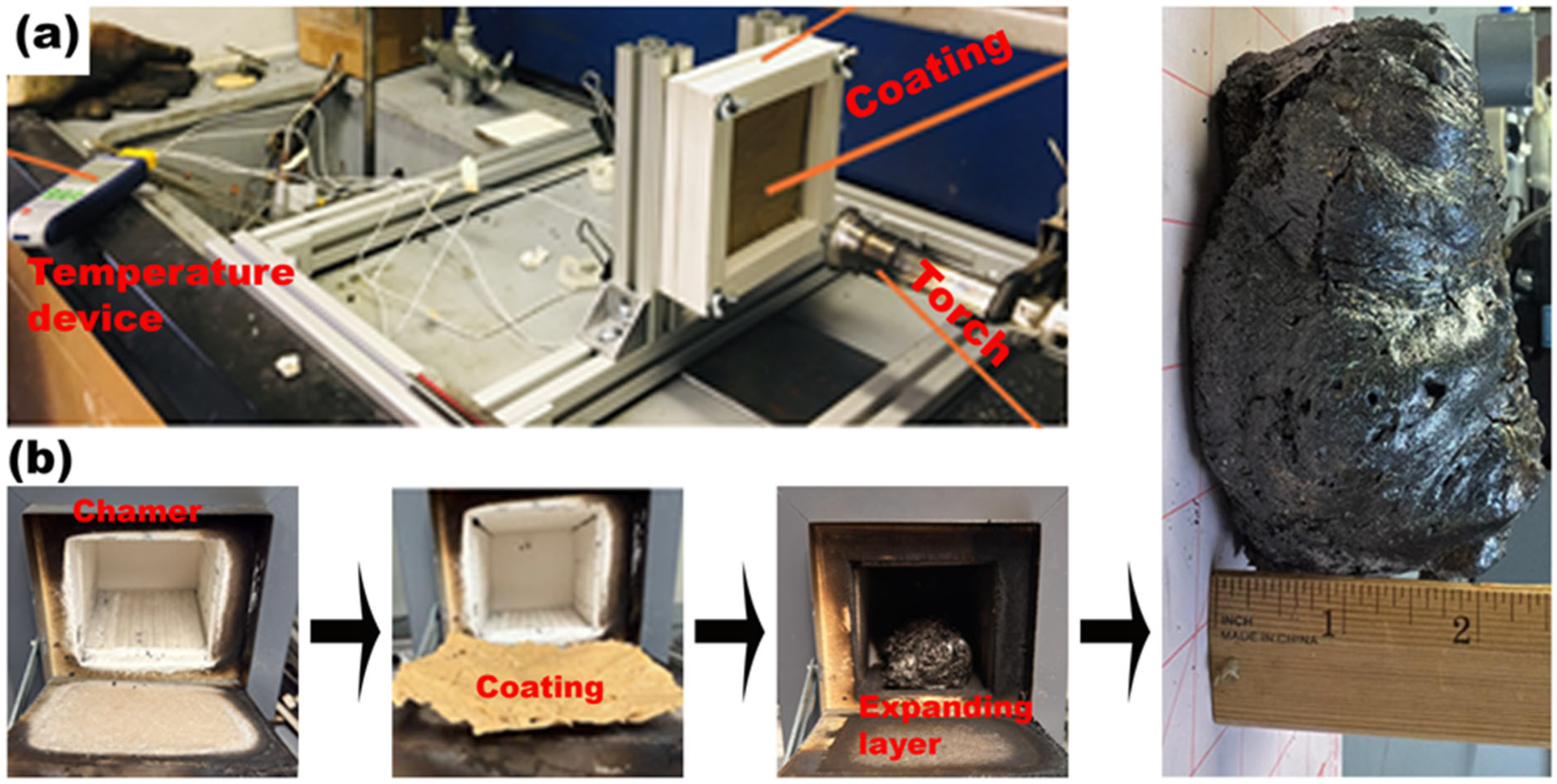
2.2.1. Test 1: Diffusive Methane Flame Exposure (Fire Testing and Thermal Behavior Assessment)
2.2.2. Test 2: Oven Controlled Heating Experiment (Thermal Heat Chamber)
2.3. Characterization of RSI Coatings: Thermal, Microstructural, and Expansion Analysis
2.3.1. Bench Scale Pyrolysis
2.3.2. Thermal Conductivity Measurement
2.3.3. Thermal Decomposition and Char Formation
2.4. SEM and MATLAB Analysis of Char Morphology and Porosity
3. Results and Discussion
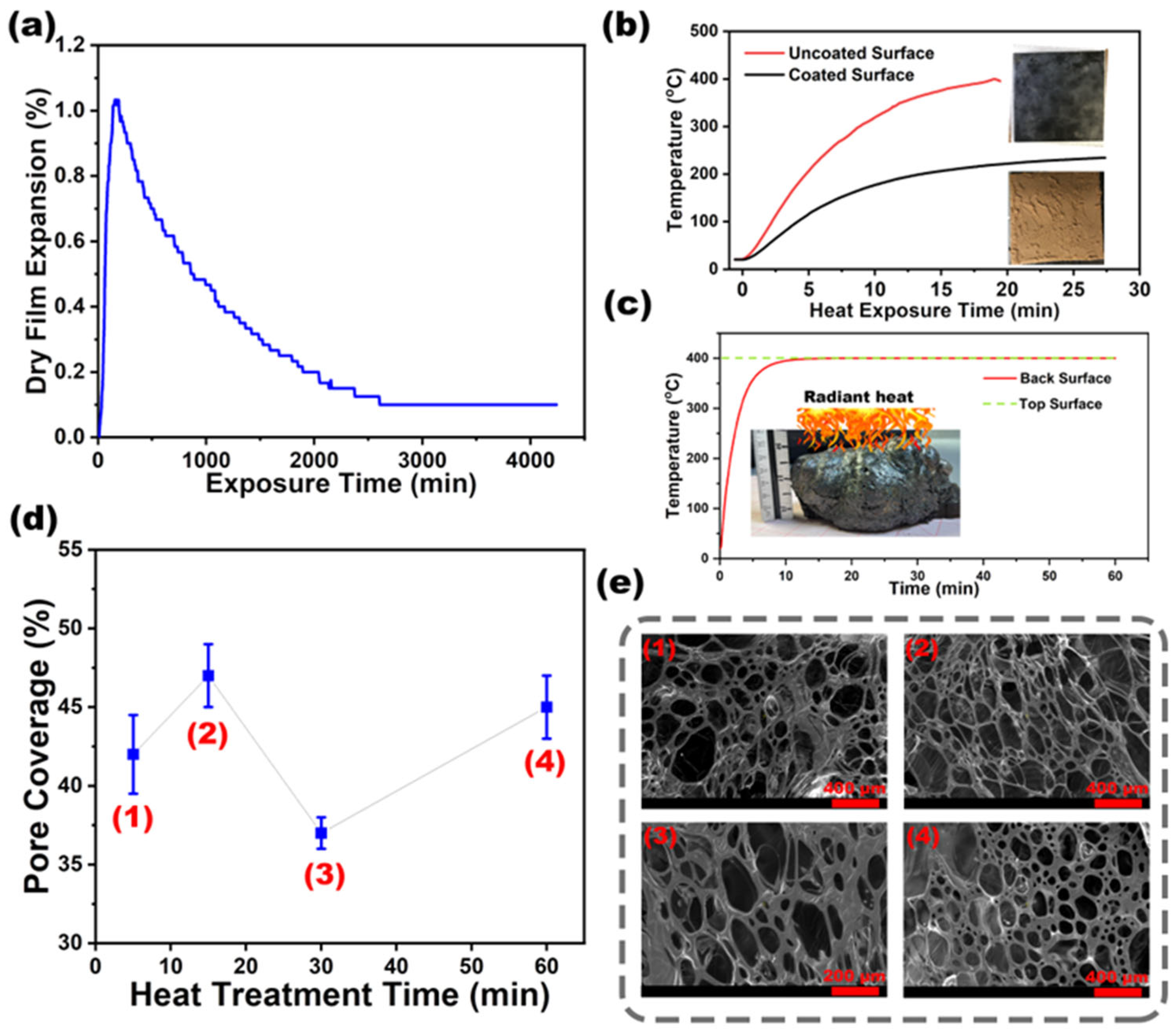
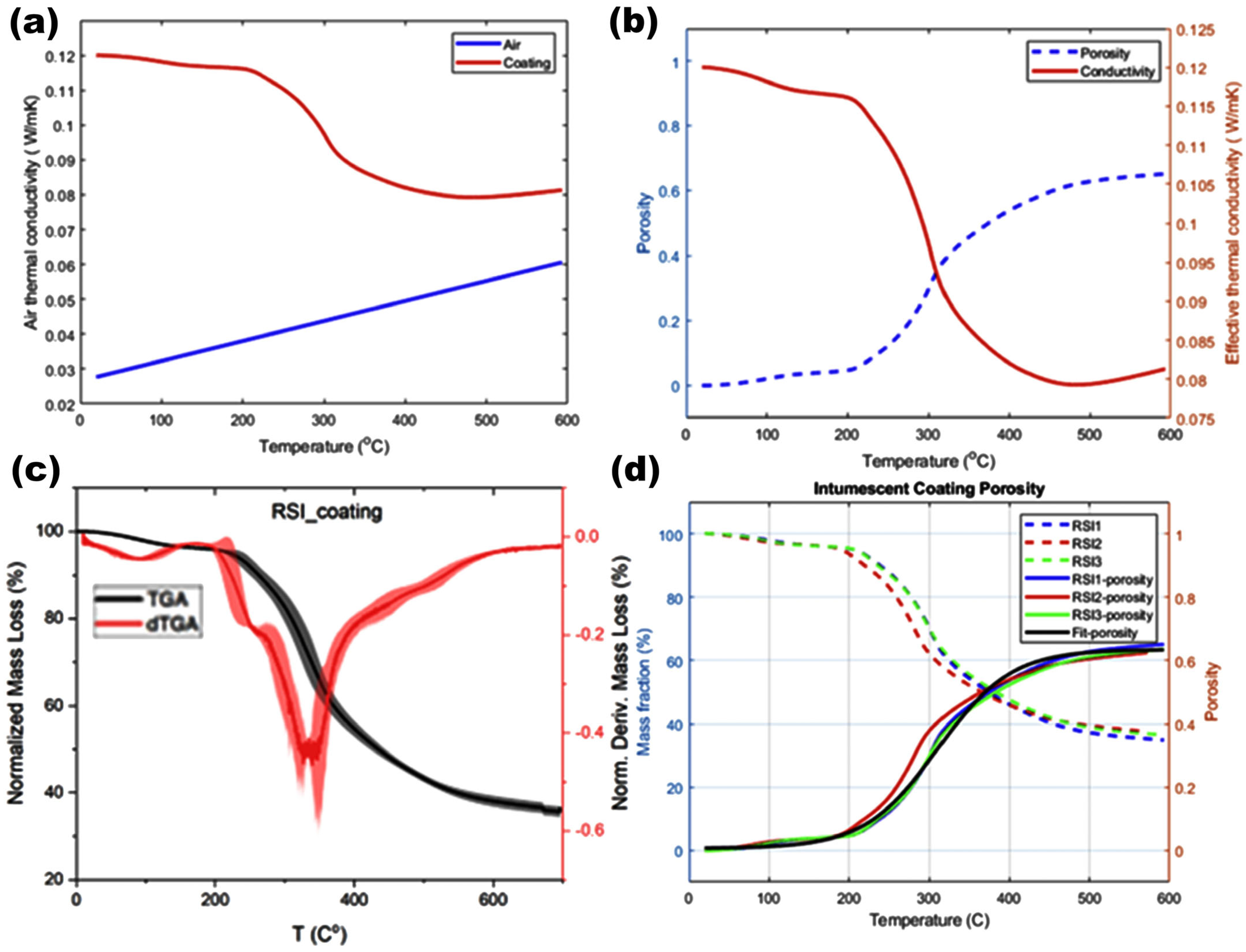
3.1. Thermal Decomposition and Char Formation
3.2. Temperature-Dependent Porosity
- represents the temperature shift (onset of significant porosity change),
- controls the spread/steepness of the transition or the curve, dictating how quickly porosity changes with temperature,
- is the amplitude (maximum change in porosity), and
- is the offset (porosity at very high temperatures).
3.3. Thermal Conductivity
3.4. Numerical Prediction of Thermal Performance in RSI Coatings with COMSOL Multiphysics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fang, Q.; Zhan, Y.; Chen, X.; Wu, R.; Zhang, W.; Wang, Y.; Wu, X.; He, Y.; Zhou, J.; Yuan, B. A bio-based intumescent flame retardant with biomolecules functionalized ammonium polyphosphate enables polylactic acid with excellent flame retardancy. Eur. Polym. J. 2022, 177, 111479. [Google Scholar] [CrossRef]
- Narayanan, D.K.; Ravoof, A.A.; Jayapriya, J.; Revathi, G.; Murugan, M. 4—Hazards in oil, gas, and petrochemical industries. In Crises in Oil, Gas and Petrochemical Industries; Rahimpour, M.R., Omidvar, B., Shirazi, N.A., Makarem, M.A., Eds.; Elsevier: London, UK, 2023; pp. 71–99. [Google Scholar] [CrossRef]
- Zuccaro, G. A case of choice of passive fire protection (PFP) in an Oil & Gas EPC project. Chem. Eng. Trans. 2012, 26, 315–320. [Google Scholar]
- Brameld, M.; Baensch, T. Development of a novel Passive Fire Protection system–Humidur Char. APPEA J. 2023, 63, S155–S158. [Google Scholar] [CrossRef]
- Tian, N.; Delichatsios, M.A.; Zhang, J.; Fateh, T. A methodology and a simple engineering fire performance model for Intumescent Fire Retardant coatings. Fire Saf. J. 2018, 98, 120–129. [Google Scholar] [CrossRef]
- Hu, X.; Zhu, X.; Sun, Z. Development of an SEM image analysis method to characterize intumescent fire retardant char layer. Prog. Org. Coat. 2020, 139, 105461. [Google Scholar] [CrossRef]
- Wang, F.; Liu, H.; Yan, L. Comparative Study of Fire Resistance and Char Formation of Intumescent Fire-Retardant Coatings Reinforced with Three Types of Shell Bio-Fillers. Polymers 2021, 13, 4333. [Google Scholar] [CrossRef]
- Price, E.J.; Covello, J.; Tuchler, A.; Wnek, G.E. Intumescent, Epoxy-Based Flame-Retardant Coatings Based on Poly(acrylic acid) Compositions. ACS Appl. Mater. Interfaces 2020, 12, 18997–19005. [Google Scholar] [CrossRef] [PubMed]
- Zverev, V.G.; Zinchenko, V.I.; Tsimbalyuk, A.F. Physical and mechanical properties and thermal protection efficiency of intumescent coatings. IOP Conf. Ser. Mater. Sci. Eng. 2016, 124, 012109. [Google Scholar] [CrossRef]
- Deshpande, S.; Kulkarni, A.; Sampath, S.; Herman, H. Application of image analysis for characterization of porosity in thermal spray coatings and correlation with small angle neutron scattering. Surf. Coat. Technol. 2004, 187, 6–16. [Google Scholar] [CrossRef]
- Takahashi, F. Fire blanket and intumescent coating materials for failure resistance. MRS Bull. 2021, 46, 429–434. [Google Scholar] [CrossRef]
- Cirpici, B.K.; Wang, Y.; Rogers, B. Assessment of the thermal conductivity of intumescent coatings in fire. Fire Saf. J. 2016, 81, 74–84. [Google Scholar] [CrossRef]
- Kang, S.; Choi, J.Y.; Choi, S. Mechanism of Heat Transfer through Porous Media of Inorganic Intumescent Coating in Cone Calorimeter Testing. Polymers 2019, 11, 221. [Google Scholar] [CrossRef] [PubMed]
- Maluk, C.; Bisby, L.; Krajcovic, M.; Torero, J.L. A Heat-Transfer Rate Inducing System (H-TRIS) Test Method. Fire Saf. J. 2019, 105, 307–319. [Google Scholar] [CrossRef]
- Takahashi, F.; Abbott, A.; Murray, T.M.; T’ien, J.S.; Olson, S.L. Thermal response characteristics of fire blanket materials: Characteristics of fire blanket materials. Fire Mater. 2014, 38, 609–638. [Google Scholar] [CrossRef]
- Hostler, S.R.; Abramson, A.R.; Gawryla, M.D.; Bandi, S.A.; Schiraldi, D.A. Thermal conductivity of a clay-based aerogel. Int. J. Heat Mass Transf. 2009, 52, 665–669. [Google Scholar] [CrossRef]
- Mesquita, L.M.R.; Piloto, P.A.G.; Vaz, M.A.P.; Pinto, T.M.G. Decomposition of Intumescent Coatings: Comparison between a Numerical Method and Experimental Results. Acta Polytech. 2009, 49, 60–65. [Google Scholar] [CrossRef]
- Fateh, T.; Guillaume, E.; Joseph, P. An experimental study of the thermal performance of a novel intumescent fire protection coating. Fire Saf. J. 2017, 92, 132–141. [Google Scholar] [CrossRef]
- Solis-Pomar, F.; Díaz-Gómez, A.; Berrío, M.E.; Ramírez, J.; Jaramillo, A.F.; Fernández, K.; Rojas, D.; Melendrez, M.F.; Pérez-Tijerina, E. A Dual Active-Passive Coating with Intumescent and Fire-Retardant Properties Based on High Molecular Weight Tannins. Coatings 2021, 11, 460. [Google Scholar] [CrossRef]
- Evtushenko, Y.M.; Grigoriev, Y.A.; Rudakova, T.A.; Ozerin, A.N. Effect of aluminum hydroxide on the fireproofing properties of ammonium polyphosphate–pentaerythritol-based intumescent coating. J. Coat. Technol. Res. 2019, 16, 1389–1398. [Google Scholar] [CrossRef]
- Zhu, J.; Frerich, T.; Herrmann, A.S. CFD modeling and validation of heat transfer inside an autoclave based on a mesh independency study. J. Compos. Mater. 2021, 55, 2469–2487. [Google Scholar] [CrossRef]
- Qin, D.; Gao, P.; Aslam, F.; Sufian, M.; Alabduljabbar, H. A comprehensive review on fire damage assessment of reinforced concrete structures. Case Stud. Constr. Mater. 2022, 16, e00843. [Google Scholar] [CrossRef]
- Yi, L.; Feng, S.; Wang, Z.; Ding, Y.; Chu, T.; Zhuang, Y. A comprehensive model to predict the fire performance of intumescent fire-retardant coating on steel substrate. J. Build. Eng. 2024, 95, 110127. [Google Scholar] [CrossRef]
- Zhou, X.; Li, R.; Fu, F.; Shen, M.; Li, Q.; Liu, H.; Xu, X.; Song, Z. A novel efficient flame-retardant curing agent for epoxy resin based on P-N synergistic effect: Bio-based benzoxazine phosphate ester. Chem. Eng. J. 2024, 499, 156441. [Google Scholar] [CrossRef]
- Carson, J.K.; Lovatt, S.J.; Tanner, D.J.; Cleland, A.C. Thermal conductivity bounds for isotropic, porous materials. Int. J. Heat Mass Transf. 2005, 48, 2150–2158. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Bailey, C.; Taylor, A. Global modelling of fire protection performance of intumescent coating under different cone calorimeter heating conditions. Fire Saf. J. 2012, 50, 51–62. [Google Scholar] [CrossRef]
- Griffin, G. The Modeling of Heat Transfer across Intumescent Polymer Coatings. J. Fire Sci. 2009, 28, 249–277. [Google Scholar] [CrossRef]
- de Silva, D.; Bilotta, A.; Nigro, E. Experimental investigation on steel elements protected with intumescent coating. Constr. Build. Mater. 2019, 205, 232–244. [Google Scholar] [CrossRef]
- Lucherini, A.; Hidalgo, J.P.; Torero, J.L.; Maluk, C. Numerical heat transfer model for swelling intumescent coatings during heating. Int. J. Therm. Sci. 2023, 184, 107922. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, X.; Yu, J.; Cheng, Y.; Zhang, F. A simplified heat transfer model for intumescent coatings. J. Therm. Anal. Calorim. 2024, 149, 1343–1350. [Google Scholar] [CrossRef]
- Okyay, G.; Naik, A.D.; Samyn, F.; Jimenez, M.; Bourbigot, S. Fractal conceptualization of intumescent fire barriers, toward simulations of virtual morphologies. Sci. Rep. 2019, 9, 1872. [Google Scholar] [CrossRef]
- Rabbani, A.; Jamshidi, S.; Salehi, S. An automated simple algorithm for realistic pore network extraction from micro-tomography images. J. Pet. Sci. Eng. 2014, 123, 164–171. [Google Scholar] [CrossRef]
- Jimenez, M.; Duquesne, S.; Bourbigot, S. Intumescent fire protective coating: Toward a better understanding of their mechanism of action. Thermochim. Acta 2006, 449, 16–26. [Google Scholar] [CrossRef]
- Yew, M.C.; Saw, L.H.; Ng, T.C.; Durairaj, R.; Beh, J.H. Influences of nano bio-filler on the fire-resistive and mechanical properties of water-based intumescent coatings. Prog. Org. Coat. 2018, 124, 33–40. [Google Scholar] [CrossRef]
- Hafiz, T.; Covello, J.; Wnek, G.; Liao, Y.-T.; Gassama, E.; Melaiye, A.K. Experimental and Numerical Approaches to Optimize Heat Blocking Efficiency in Intumescent Coatings. Eng. Model. Anal. Simul. 2025, 2. [Google Scholar] [CrossRef]
- Kang, J.; Takahashi, F.; T’ien, J.S. In situ thermal-conductivity measurements and morphological characterization of intumescent coatings for fire protection. J. Fire Sci. 2018, 36, 419–437. [Google Scholar] [CrossRef]
- Francl, J.; Kingery, W.D. Thermal Conductivity: IX, Experimental Investigation of Effect of Porosity on Thermal Conductivity. J. Am. Ceram. Soc. 1954, 37, 99–107. [Google Scholar] [CrossRef]
- Gravit, M.; Korolchenko, D.; Nedviga, E.; Portnov, F.; Diachenko, S. Impact of Jet Fires on Steel Structures: Application of Passive Fire Protection Materials. Fire 2024, 7, 281. [Google Scholar] [CrossRef]
- Oterkus, E.; Jo, S. Thermal and Structural Behaviour of Offshore Structures with Passive Fire Protection. Sustain. Mar. Struct. 2022, 4, 16–28. [Google Scholar] [CrossRef]
- Yazici, C.; Özkal, F.M.; Orhan, S.N.; Cirpici, B.K. Reformative Effects of Intumescent Coating on the Structural Characteristics of Cold-Formed Steel. ACS Omega 2022, 7, 42560–42569. [Google Scholar] [CrossRef]
- Wang, Z.; Gong, G.; Gao, L.; Cui, W.; Wang, Y. Preparation and performance of intumescent water-based coatings with both thermal insulation and flame retardant functions. Chem. Eng. J. 2024, 480, 148165. [Google Scholar] [CrossRef]
- Tavman, I. Effective thermal conductivity of granular porous materials. Int. Commun. Heat Mass Transf. 1996, 23, 169–176. [Google Scholar] [CrossRef]

| System | Component | Content (%) |
|---|---|---|
| RSI | TA | 28 |
| APP | 6.8 | |
| EPOXY | 65 | |
| Polyamine | 21.2 |
| Material Property | Value | Comments |
|---|---|---|
| Thermal Conductivity of K_matrix | 0.12 W/km | In this work |
| Specific Heat Matrix CP_Matrix | 1500 [J/kg/K] | In this work |
| Specific Heat | = 971, = 0.06, = 1.66 × 10−4, = −6.79 × 10−8 | |
| Air thermal conductivity K_air | ||
| Thermal expansion | Alpha = 1.0 × 10−2 (mm/s/k) p1 = the value from Equation (4) | |
| Porosity () | From Equation (4) | From porosity values |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hafiz, T.; Covello, J.; Wnek, G.E.; Hostler, S.; Gassama, E.; Wei, Y.; Ji, J. Thermal Insulation Performance of Epoxy-Based Intumescent Coatings: Influence of Temperature-Induced Porosity Evolution on Heat Transfer Resistance. Polymers 2025, 17, 1426. https://doi.org/10.3390/polym17111426
Hafiz T, Covello J, Wnek GE, Hostler S, Gassama E, Wei Y, Ji J. Thermal Insulation Performance of Epoxy-Based Intumescent Coatings: Influence of Temperature-Induced Porosity Evolution on Heat Transfer Resistance. Polymers. 2025; 17(11):1426. https://doi.org/10.3390/polym17111426
Chicago/Turabian StyleHafiz, Taher, James Covello, Gary E. Wnek, Stephen Hostler, Edrissa Gassama, Yen Wei, and Jiujiang Ji. 2025. "Thermal Insulation Performance of Epoxy-Based Intumescent Coatings: Influence of Temperature-Induced Porosity Evolution on Heat Transfer Resistance" Polymers 17, no. 11: 1426. https://doi.org/10.3390/polym17111426
APA StyleHafiz, T., Covello, J., Wnek, G. E., Hostler, S., Gassama, E., Wei, Y., & Ji, J. (2025). Thermal Insulation Performance of Epoxy-Based Intumescent Coatings: Influence of Temperature-Induced Porosity Evolution on Heat Transfer Resistance. Polymers, 17(11), 1426. https://doi.org/10.3390/polym17111426








