Enhancing Cross-Linking Efficiency in Gelatin-Based Hydrogels via Incorporation of Tannic Acid, Pluronic F-127, and Phytic Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Hydrogel Formation
2.3. Characterisation
2.3.1. FTIR Spectroscopy of Reagents and Xerogel
2.3.2. Swelling Capacity of Xerogels
2.3.3. Scanning Electron Microscopy Investigation of Lyophilised Hydrogels
2.3.4. Thermal Analysis of Xerogels and Lyophilised Hydrogels
3. Results and Discussion
3.1. FTIR Spectroscopy Analysis
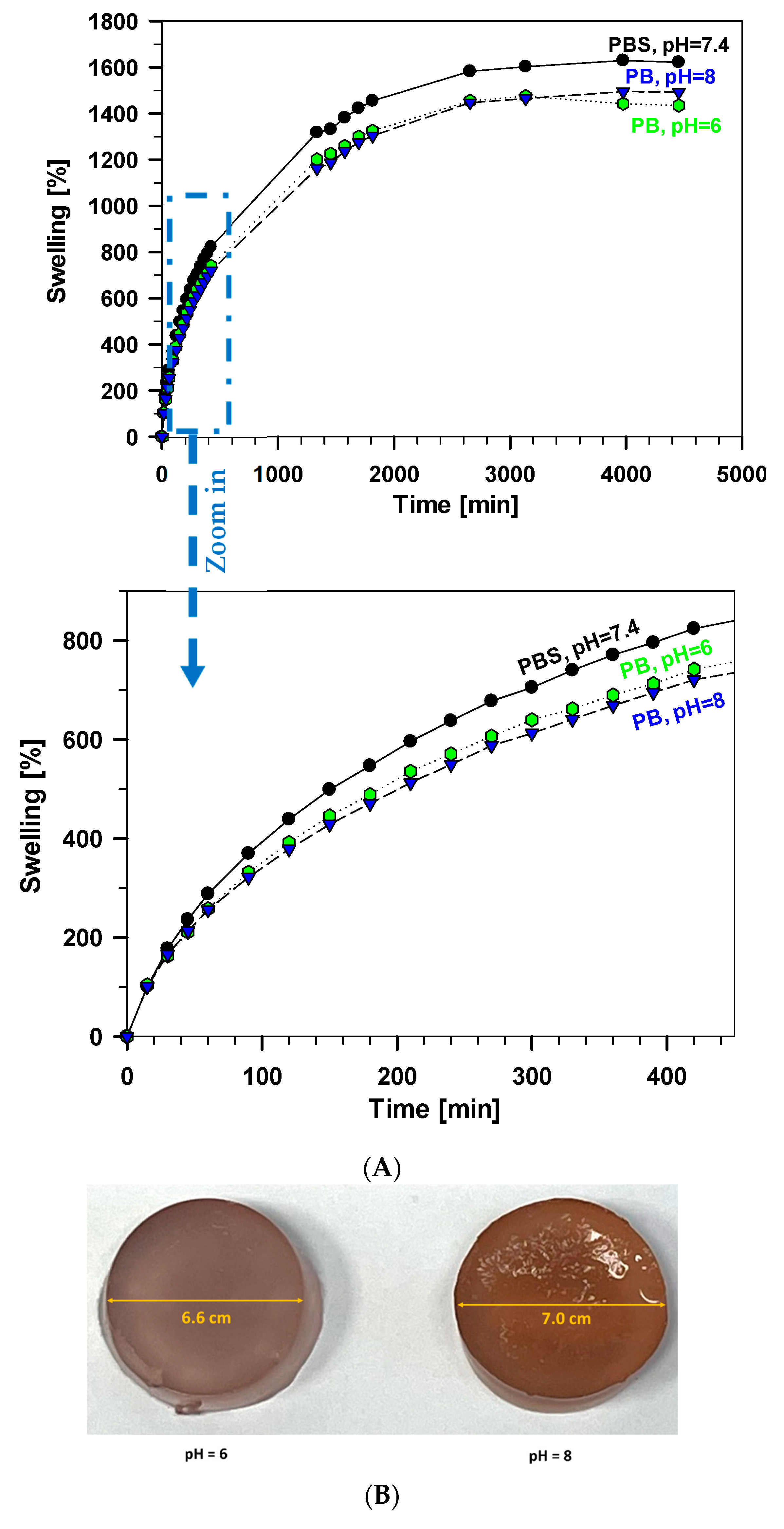
3.2. Swelling Test of Xerogel Samples
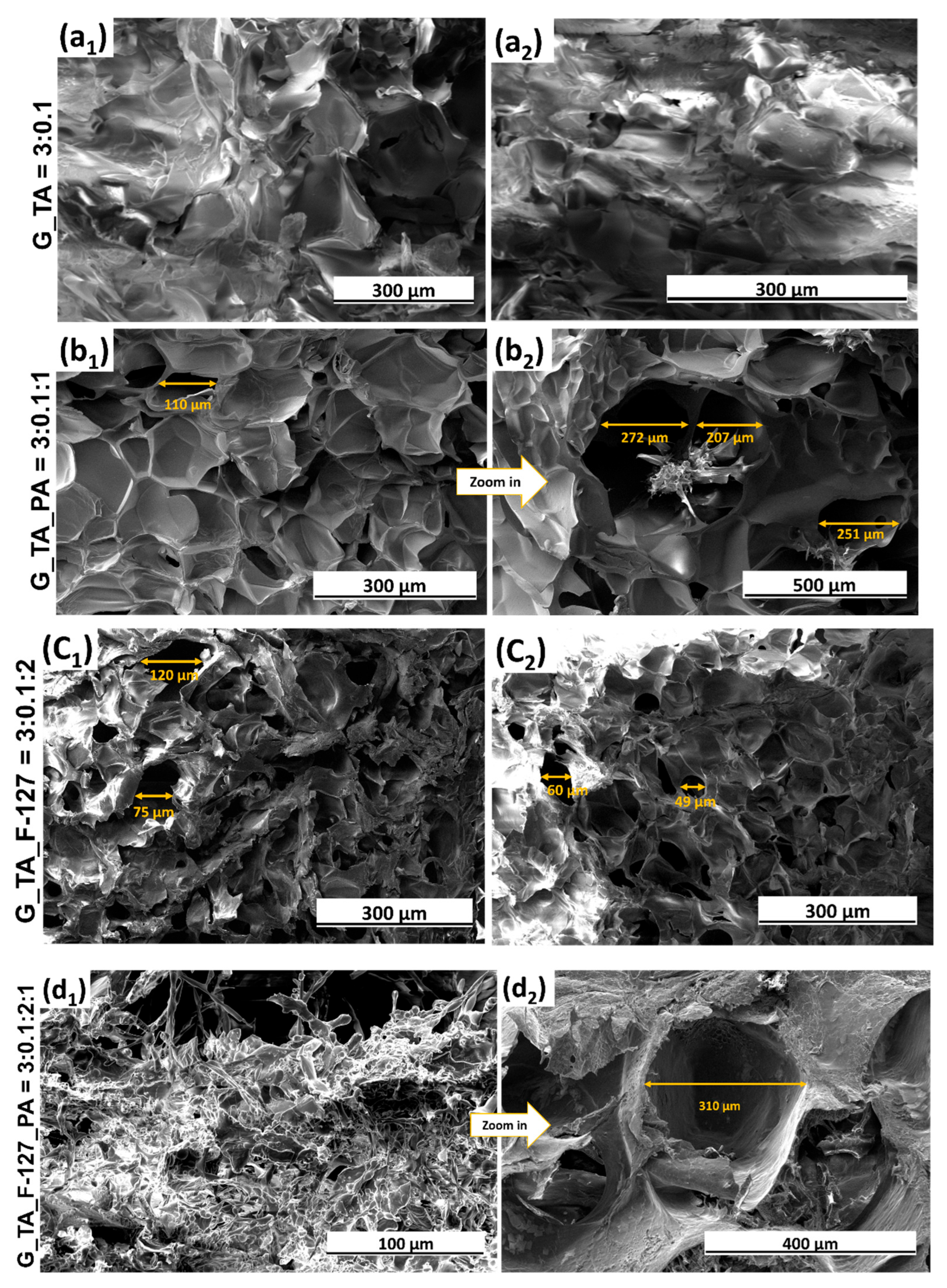
3.3. Structure Investigation Using Scanning Electron Microscopy
3.4. Thermal Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in Biology and Medicine: From Molecular Principles to Bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- Andreazza, R.; Morales, A.; Pieniz, S.; Labidi, J. Gelatin-Based Hydrogels: Potential Biomaterials for Remediation. Polymers 2023, 15, 1026. [Google Scholar] [CrossRef]
- Kasai, R.D.; Radhika, D.; Archana, S.; Shanavaz, H.; Koutavarapu, R.; Lee, D.-Y.; Shim, J. A review on hydrogels classification and recent developments in biomedical applications. Int. J. Polym. Mater. 2023, 72, 1059–1069. [Google Scholar] [CrossRef]
- Jia, B.; Li, G.; Cao, E.; Luo, J.; Zhao, X.; Huang, H. Recent progress of antibacterial hydrogels in wound dressings. Mater. Today Bio 2023, 19, 100582. [Google Scholar] [CrossRef]
- Thang, N.H.; Chien, T.B.; Cuong, D.X. Polymer-Based Hydrogels Applied in Drug Delivery: An Overview. Gels 2023, 9, 523. [Google Scholar] [CrossRef] [PubMed]
- Kapusta, O.; Jarosz, A.; Stadnik, K.; Giannakoudakis, D.A.; Barczyński, B.; Barczak, M. Antimicrobial Natural Hydrogels in Biomedicine: Properties, Applications, and Challenges—A Concise Review. Int. J. Mol. Sci. 2023, 24, 2191. [Google Scholar] [CrossRef]
- Petros, S.; Tesfaye, T.; Ayele, M. A Review on Gelatin Based Hydrogels for Medical Textile Applications. J. Eng. 2020, 2020, 8866582. [Google Scholar] [CrossRef]
- Nam, H.G.; Nam, M.G.; Yoo, P.J.; Kim, J.-H. Hydrogen bonding-based strongly adhesive coacervate hydrogels synthesized using poly(N-vinylpyrrolidone) and tannic acid. Soft Matter 2019, 15, 785–791. [Google Scholar] [CrossRef]
- Ulubayram, K.; Aksu, E.; Gurhan, S.I.D.; Serbetci, K.; Hasirci, N. Cytotoxicity evaluation of gelatin sponges prepared with different cross-linking agents. J. Biomater. Sci. Polym. Ed. 2002, 13, 1203–1219. [Google Scholar] [CrossRef]
- Ribeiro, M.; Simões, M.; Vitorino, C.; Mascarenhas-Melo, F. Hydrogels in Cutaneous Wound Healing: Insights into Characterization, Properties, Formulation and Therapeutic Potential. Gels 2024, 10, 188. [Google Scholar] [CrossRef]
- Mehdi-Sefiani, H.; Chicardi, E.; Romero, A.; Perez-Puyana, V.M. Unveiling the Impact of Gelation Temperature on the Rheological and Microstructural Properties of Type A Gelatin Hydrogels. Polymers 2024, 16, 1842. [Google Scholar] [CrossRef] [PubMed]
- Jaipan, P.; Nguyen, A.; Narayan, R.J. Gelatin-based hydrogels for biomedical applications. MRS Commun. 2017, 7, 416–426. [Google Scholar] [CrossRef]
- Mengyuan, H.; Changlin, W.; Tong, X.; Ping, D.; Xiaojun, Y.; Huaying, S.; Congying, L.; Peng, G.; Zhufeng, C. Modification and preparation of four natural hydrogels and their application in biopharmaceutical delivery. Polym. Bull. 2023, 80, 7101–7144. [Google Scholar] [CrossRef]
- Voorhaar, L.; Hoogenboom, R. Supramolecular polymer networks: Hydrogels and bulk materials. Chem. Soc. Rev. 2016, 45, 4013–4031. [Google Scholar] [CrossRef]
- Berger, J.; Reist, M.; Mayer, J.M.; Felt, O.; Gurny, R. Structure and interactions in chitosan hydrogels formed by complexation or aggregation for biomedical applications. Eur. J. Pharm. Biopharm. 2004, 57, 35–52. [Google Scholar] [CrossRef]
- Chung, K.T.; Wong, T.Y.; Wei, C.I.; Huang, Y.W.; Lin, Y. Tannins and Human Health: A Review. Crit. Rev. Food Sci. Nutr. 1998, 38, 421–464. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Xie, W.; Lu, J.; Guo, X.; Xu, J.; Xu, W.; Chi, Y.; Takuya, N.; Wu, H.; Zhao, L. Tannic acid-based metal phenolic networks for bio-applications: A review. J. Mater. Chem. B 2021, 9, 4098–4110. [Google Scholar] [CrossRef]
- Jing, W.; Xiaolan, C.; Yu, C.; Feng, Q.; Haifeng, Y. Pharmacological effects and mechanisms of tannic acid. Biomed. Pharmacother. 2022, 154, 113561. [Google Scholar] [CrossRef]
- Chen, C.; Yang, H.; Yang, X.; Ma, Q. Tannic acid: A crosslinker leading to versatile functional polymeric networks: A review. RSC Adv. 2022, 12, 7689–7711. [Google Scholar] [CrossRef]
- Kaczmarek, B. Tannic Acid with Antiviral and Antibacterial Activity as A Promising Component of Biomaterials—A Minireview. Materials 2020, 13, 3224. [Google Scholar] [CrossRef]
- Zhao, Q.; Mu, S.; Long, Y.; Zhou, J.; Chen, W.; Astruc, D.; Gaidau, C.; Gu, H. Tannin-Tethered Gelatin Hydrogels with Considerable Self-Healing and Adhesive Performances. Macromol. Mater. Eng. 2019, 304, 1800664. [Google Scholar] [CrossRef]
- Oliveira, G.B.d.; Zamataro, I.S.; Oliveira, M.S.d.; Gomes, A.S.; Barros, M.G.A.; Oliveira, A.G.; Zanella, K.; Gonçalves, C.C.S. Development of Cross-Linked Gelatin Hydrogel Films Using Tannic Acid as Anti-Aging Active with Skin Care Potential. J. Braz. Chem. Soc. 2024, 35, e-20240046. [Google Scholar] [CrossRef]
- Zhou, C.; Chen, L.; Zhou, W.; Wang, L.; Zhang, R.; Yang, C. Antimicrobial polyacrylic acid/tannic acid hydrogel wound dressing facilitating full-thickness skin healing. J. Biomater. Sci. Polym. Ed. 2024, 35, 501–518. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.; Villa, C. Poloxamer Hydrogels for Biomedical Applications. Pharmaceutics 2019, 11, 671. [Google Scholar] [CrossRef]
- Jaquilin, P.J.R.; Oluwafemi, O.S.; Thomas, S.; Oyedeji, A.O. Recent advances in drug delivery nanocarriers incorporated in temperature-sensitive Pluronic F-127–A critical review. J. Drug Deliv. Sci. Technol. 2022, 72, 103390. [Google Scholar] [CrossRef]
- Li, S.; Cheng, Y.; Junqiang, L.; Chao, Z.; Liaoliao, Z.; Yang, S.; Yongdong, G.; Ronglin, W.; Dongxue, G.; Jingjie, S.; et al. Progress in Pluronic F127 Derivatives for Application in Wound Healing and Repair. Int. J. Nanomed. 2023, 18, 4485–4505. [Google Scholar] [CrossRef] [PubMed]
- Yeh, M.-Y.; Zhao, J.-Y.; Hsieh, Y.-R.; Lin, J.-H.; Chen, F.-Y.; Chakravarthy, R.D.; Chung, P.-C.; Lin, H.-C.; Hung, S.-C. Reverse thermo-responsive hydrogels prepared from Pluronic F127 and gelatin composite materials. RSC Adv. 2017, 7, 21252–21257. [Google Scholar] [CrossRef]
- Lin, H.-R.; Sung, K.C.; Vong, W.-J. In Situ Gelling of Alginate/Pluronic Solutions for Ophthalmic Delivery of Pilocarpine. Biomacromolecules 2004, 5, 2358–2365. [Google Scholar] [CrossRef]
- Das, R.P.; Gandhi, V.V.; Verma, G.; Ajish, J.K.; Singh, B.G.; Kunwar, A. Gelatin-lecithin-F127 gel mediated self-assembly of curcumin vesicles for enhanced wound healing. Int. J. Biol. Macromol. 2022, 210, 403–414. [Google Scholar] [CrossRef]
- Dewan, M.; Sarkar, G.; Bhowmik, M.; Das, B.; Chattoapadhyay, A.K.; Rana, D.; Chattopadhyay, D. Effect of gellan gum on the thermogelation property and drug release profile of Poloxamer 407 based ophthalmic formulation. Int. J. Biol. Macromol. 2017, 102, 258–265. [Google Scholar] [CrossRef]
- Nassar, M.; Nassar, R.; Maki, H.; Al-Yagoob, A.; Hachim, M.; Senok, A.; Williams, D.; Hiraishi, N. Phytic Acid: Properties and Potential Applications in Dentistry. Front. Mater. 2021, 8, 638909. [Google Scholar] [CrossRef]
- Wang, R.; Guo, S. Phytic acid and its interactions: Contributions to protein functionality, food processing, and safety. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2081–2105. [Google Scholar] [CrossRef] [PubMed]
- Bloot, A.P.M.; Lahis, K.D.; Soares, A.J.A.; José, B.I.; Canan, C. A Review of Phytic Acid Sources, Obtention, and Applications. Food Rev. Int. 2023, 39, 73–92. [Google Scholar] [CrossRef]
- Ghilan, A.; Nita, L.E.; Pamfil, D.; Simionescu, N.; Tudorachi, N.; Rusu, D.; Rusu, A.G.; Bercea, M.; Rosca, I.; Ciolacu, D.E.; et al. One-Step Preparation of Carboxymethyl Cellulose—Phytic Acid Hydrogels with Potential for Biomedical Applications. Gels 2022, 8, 647. [Google Scholar] [CrossRef]
- Bui, H.L.; Huang, C.-J. Tough Polyelectrolyte Hydrogels with Antimicrobial Property via Incorporation of Natural Multivalent Phytic Acid. Polymers 2019, 11, 1721. [Google Scholar] [CrossRef]
- Liu, G.; Liu, Z.; Li, J.; Zeng, M.; Li, Z.; He, L.; Li, F. Chitosan/phytic acid hydrogel as a platform for facile synthesis of heteroatom-doped porous carbon frameworks for electrocatalytic oxygen reduction. Carbon 2018, 137, 68–77. [Google Scholar] [CrossRef]
- Pan, L.; Yu, G.; Zhai, D.; Lee, H.R.; Zhao, W.; Liu, N.; Wang, H.; Tee, B.C.-K.; Shi, Y.; Cui, Y.; et al. Hierarchical nanostructured conducting polymer hydrogel with high electrochemical activity. Proc. Natl. Acad. Sci. USA 2012, 109, 9287–9292. [Google Scholar] [CrossRef]
- Shuai, Z.; Zhang, Y.; Li, B.; Zhang, P.; Kan, L.; Wang, G.; Wei, H.; Zhang, X.; Ma, N. One-step preparation of highly stretchable, conductive and transparent polyvinyl alcohol-phytic acid hydrogel for casual writing circuits. ACS Appl. Mater. Interfaces 2019, 11, 32441–32448. [Google Scholar] [CrossRef]
- Nita, L.E.; Chiriac, A.P.; Ghilan, A.; Rusu, A.G.; Tudorachi, N.; Timpu, D. Alginate enriched with phytic acid for hydrogels preparation. Int. J. Biol. Macromol. 2021, 181, 561–571. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, X.; Zhang, J.; Duan, L.; Gao, G. A highly conductive hydrogel driven by phytic acid towards a wearable sensor with freezing and dehydration resistance. J. Mater. Chem. A 2021, 9, 22615–22625. [Google Scholar] [CrossRef]
- Ajvazi, N.; Milošev, I.; Cerc Korošec, R.; Rodič, P.; Božić, B. Development and Characterization of Gelatin-Based Hydrogels Containing Triblock Copolymer and Phytic Acid. Gels 2024, 10, 294. [Google Scholar] [CrossRef] [PubMed]

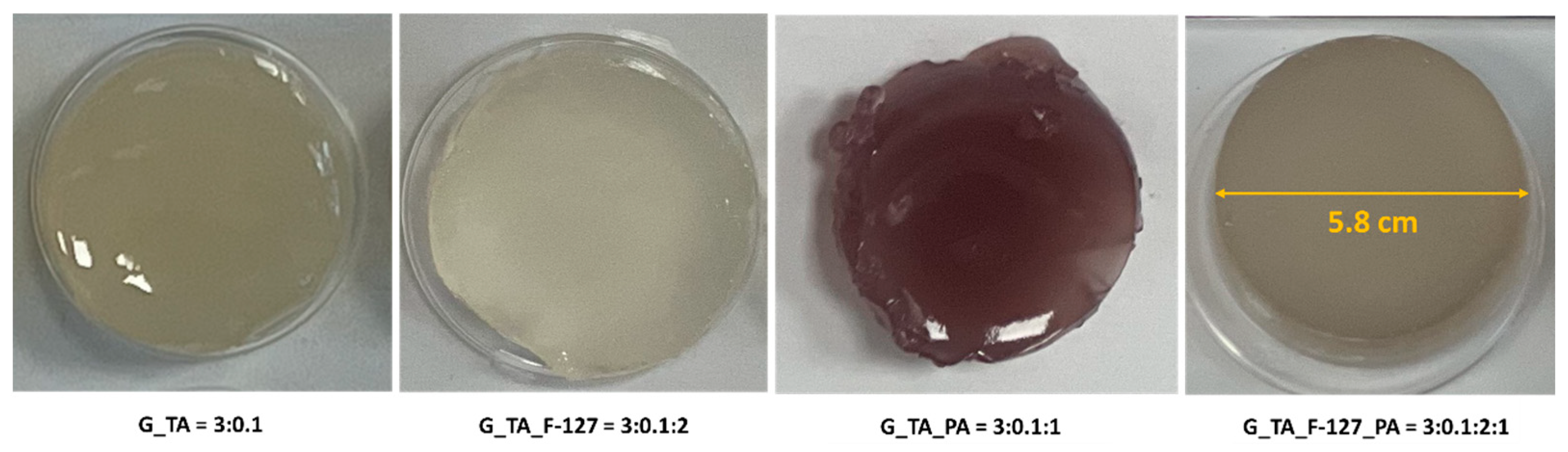

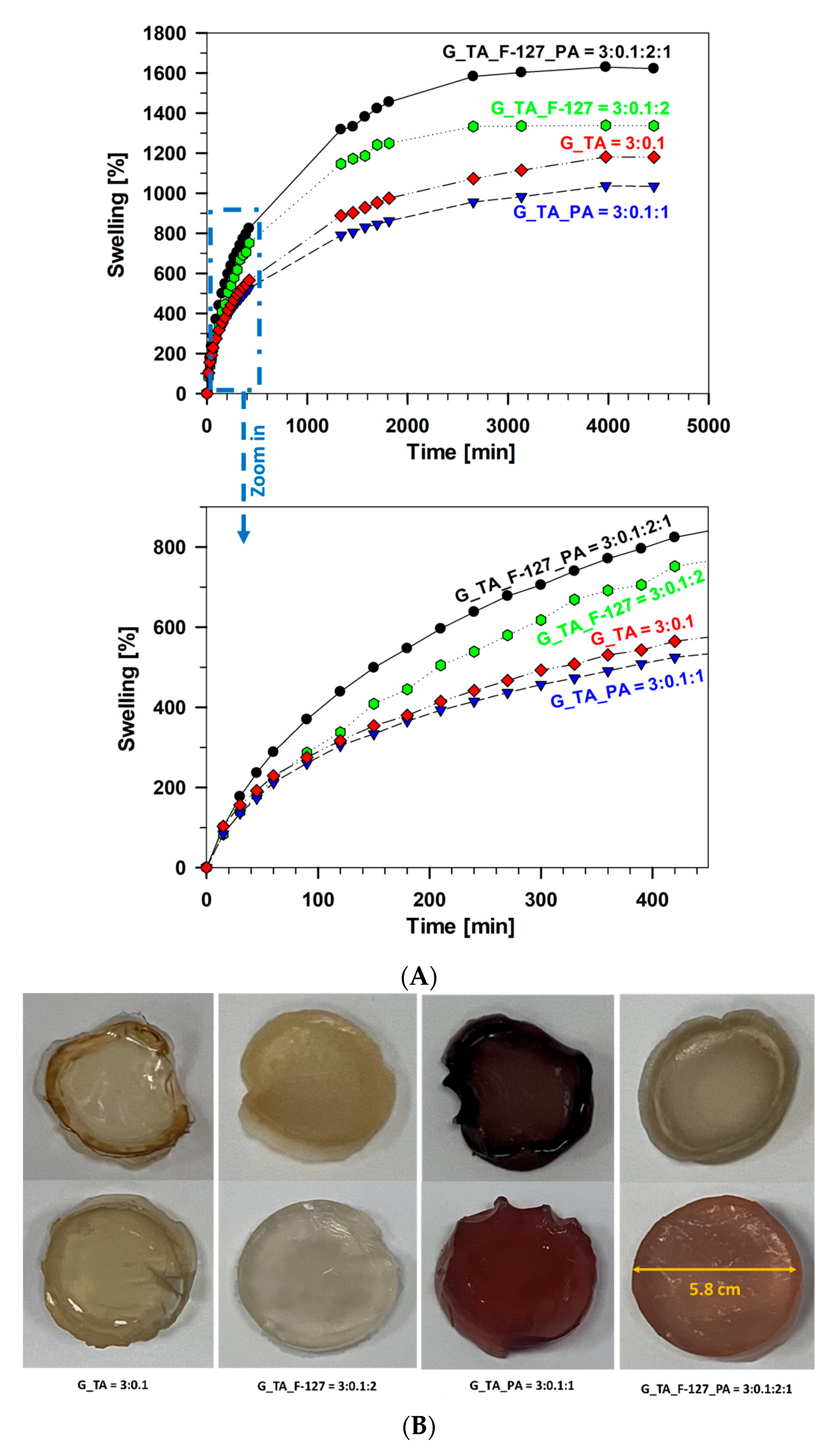


| Gelatin (g) | Tannic Acid (g) | Pluronic F-127 (g) | Phytic Acid (g) | Sample Abbreviations and Component Ratios | Short Abbreviation |
|---|---|---|---|---|---|
| 0.3 | 0.01 | / | / | G_TA = 3:0.1 | G_TA |
| 0.3 | 0.01 | 0.2 | / | G_TA_F-127 = 3:0.1:2 | G_TA_F-127 |
| 0.3 | 0.01 | / | 0.1 | G_TA_PA = 3:0.1:1 | G_TA_PA |
| 0.3 | 0.01 | 0.2 | 0.1 | G_TA_F-127_PA = 3:0.1:2:1 | G_TA_F-127_PA |
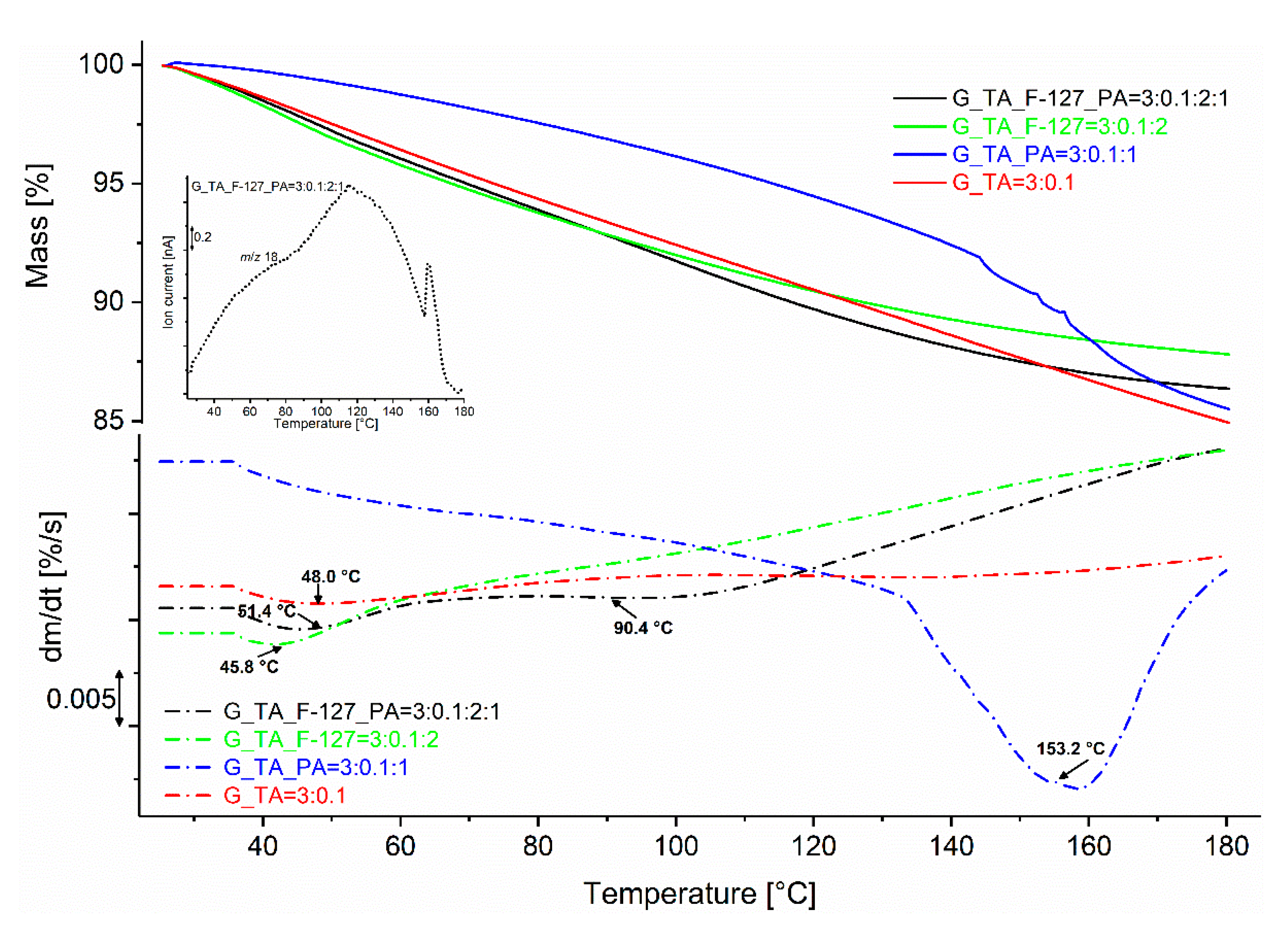
| Sample Designation and Component Ratios | Mass Loss % | TInflect. Pt. (°C) |
|---|---|---|
| G_TA = 3:0.1 | 15.3 | 48.0 |
| G_TA_F-127 = 3:0.1:2 | 12.2 | 45.8 |
| G_TA_PA = 3:0.1:1 | 14.8 | 153.2 |
| G_TA_F-127_PA = 3:0.1:2:1 | 13.7 | 51.4 and 90.4 |
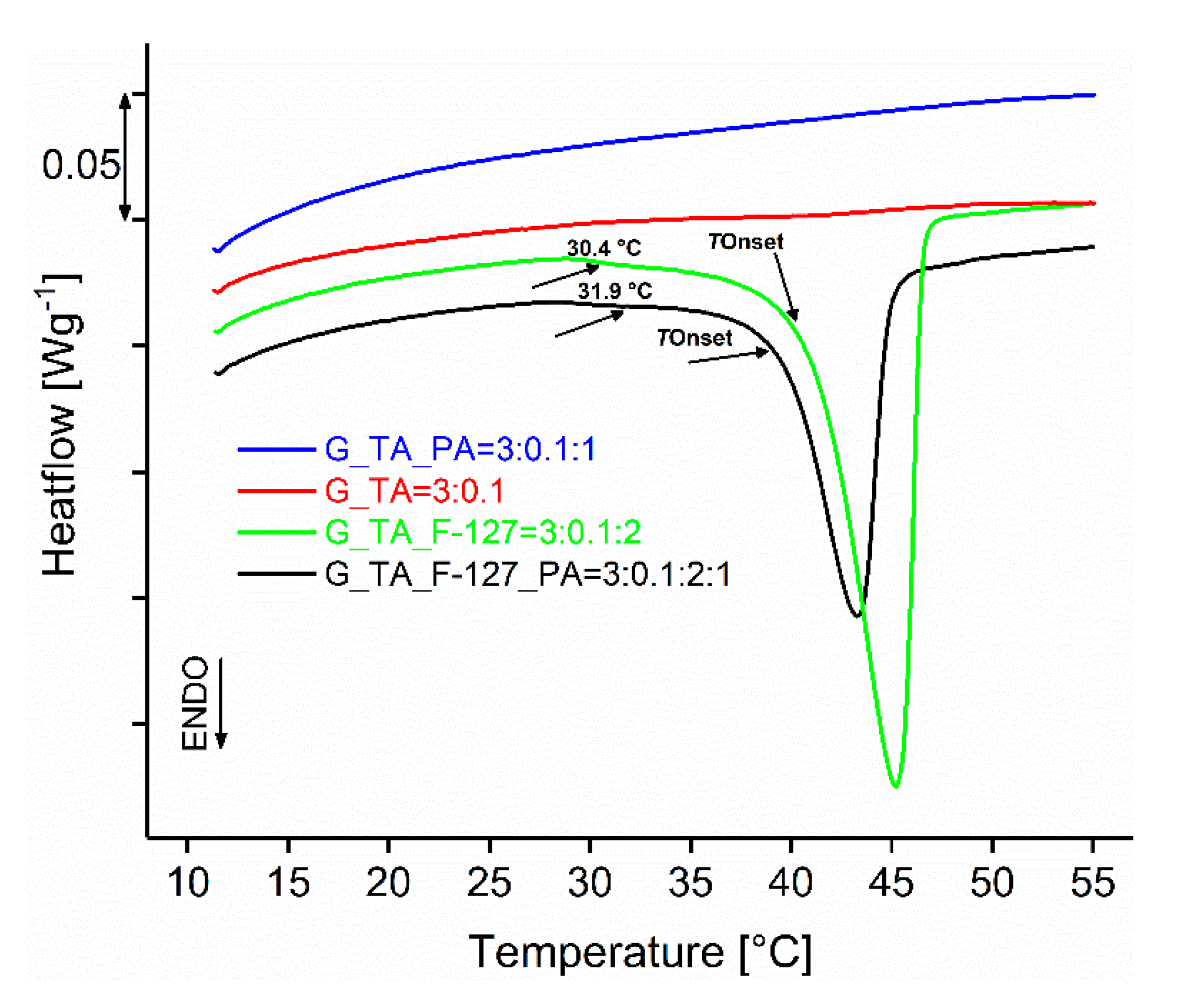
| Sample Designation and Component Ratios | TBegin (Melting) (°C) | TOnset (Melting) (°C) | ΔH (Jg−1) |
|---|---|---|---|
| G_TA = 3:0.1 | / | / | / |
| G_TA_F-127 = 3:0.1:2 | 30.4 | 40.9 | 25.3 |
| G_TA_PA = 3:0.1:1 | / | / | / |
| G_TA_F-127_PA = 3:0.1:2:1 | 31.9 | 39.4 | 15.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ajvazi, N.; Milošev, I.; Čelan Korošin, N.; Rodič, P.; Božić, B. Enhancing Cross-Linking Efficiency in Gelatin-Based Hydrogels via Incorporation of Tannic Acid, Pluronic F-127, and Phytic Acid. Polymers 2025, 17, 1372. https://doi.org/10.3390/polym17101372
Ajvazi N, Milošev I, Čelan Korošin N, Rodič P, Božić B. Enhancing Cross-Linking Efficiency in Gelatin-Based Hydrogels via Incorporation of Tannic Acid, Pluronic F-127, and Phytic Acid. Polymers. 2025; 17(10):1372. https://doi.org/10.3390/polym17101372
Chicago/Turabian StyleAjvazi, Njomza, Ingrid Milošev, Nataša Čelan Korošin, Peter Rodič, and Bojan Božić. 2025. "Enhancing Cross-Linking Efficiency in Gelatin-Based Hydrogels via Incorporation of Tannic Acid, Pluronic F-127, and Phytic Acid" Polymers 17, no. 10: 1372. https://doi.org/10.3390/polym17101372
APA StyleAjvazi, N., Milošev, I., Čelan Korošin, N., Rodič, P., & Božić, B. (2025). Enhancing Cross-Linking Efficiency in Gelatin-Based Hydrogels via Incorporation of Tannic Acid, Pluronic F-127, and Phytic Acid. Polymers, 17(10), 1372. https://doi.org/10.3390/polym17101372










