High Performance and Recyclable Polypropylene/Styrene–Ethylene–Butylene–Styrene Blends for Next Generation Cable Insulation with Enhanced Breakdown Strength Through Controlling Crystallinity
Abstract
1. Introduction
2. Experiment
2.1. Materials and Sample Preparation
2.2. Characteristics
3. Results and Discussion
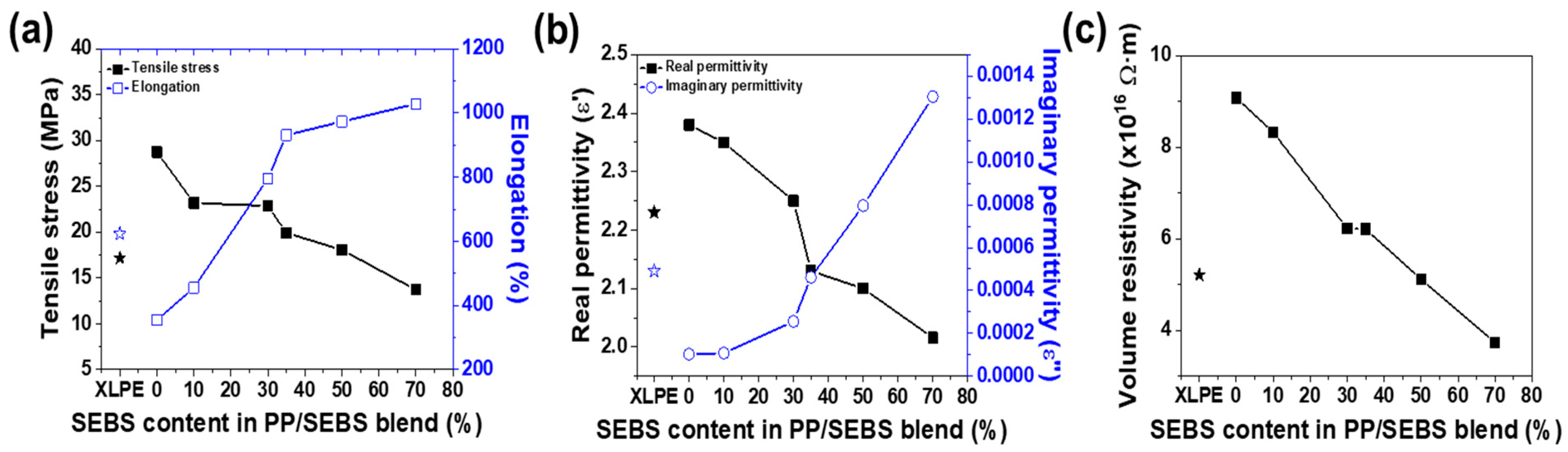
3.1. Mechanical and Electrical Properties
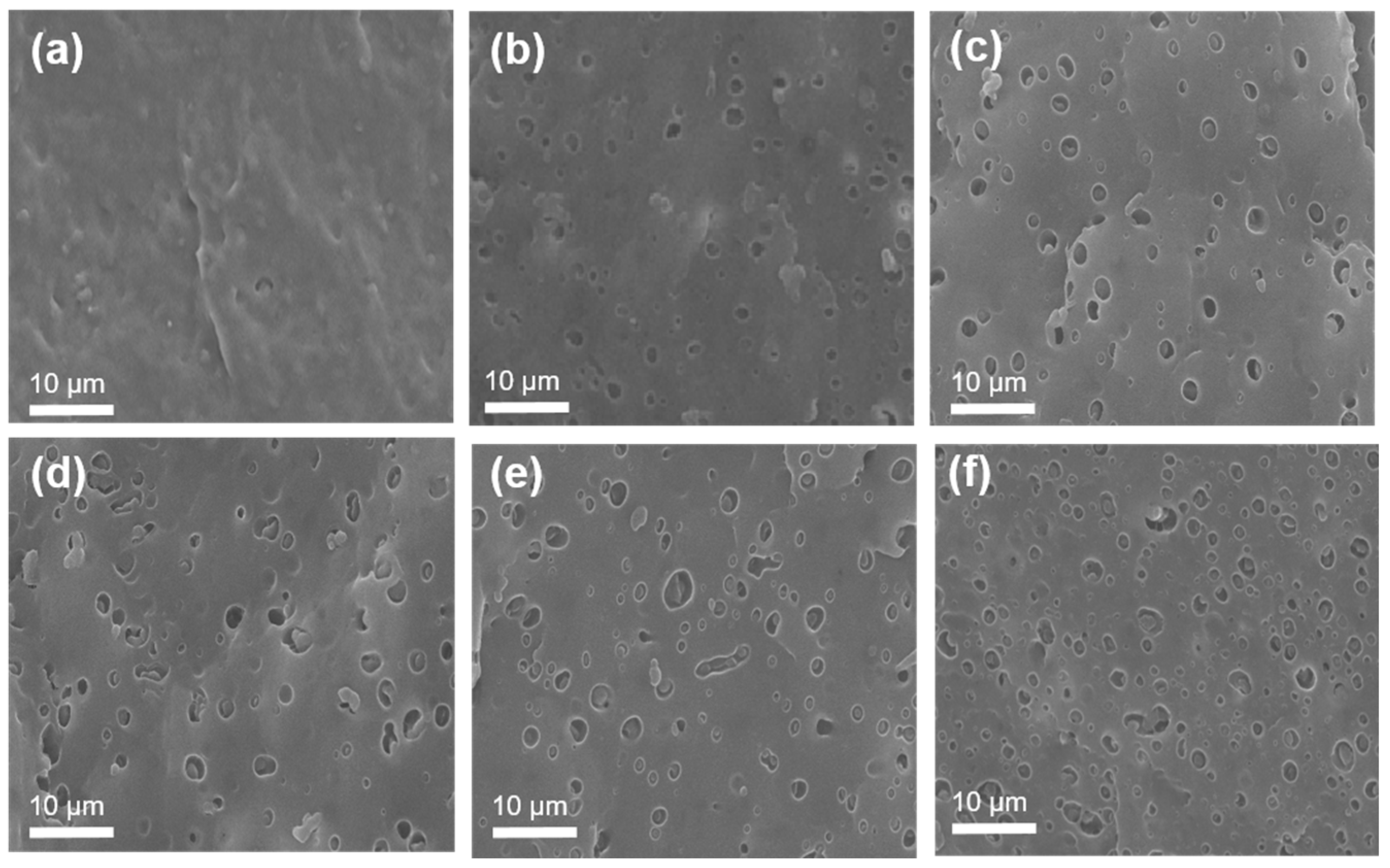
3.2. Morphology
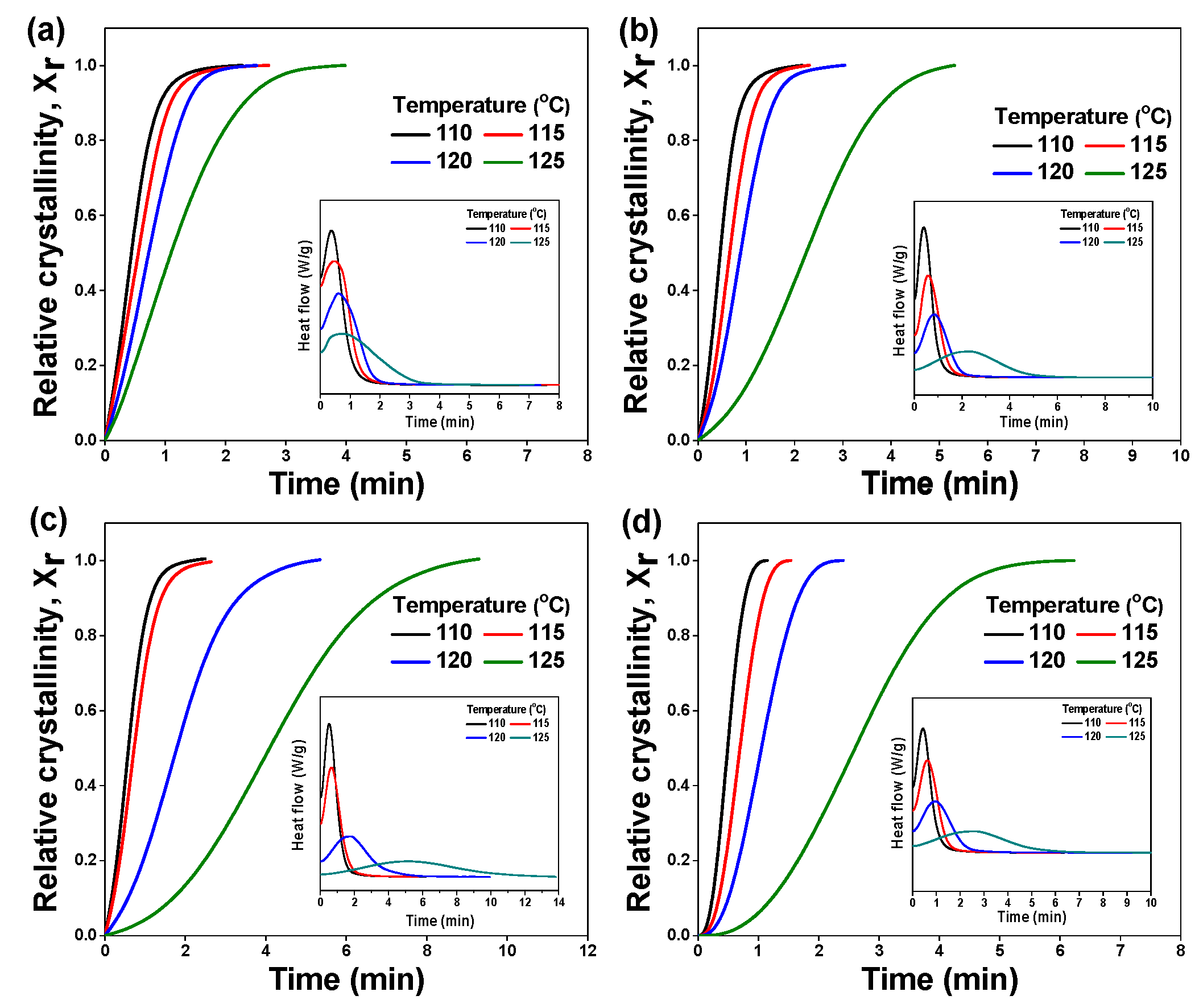
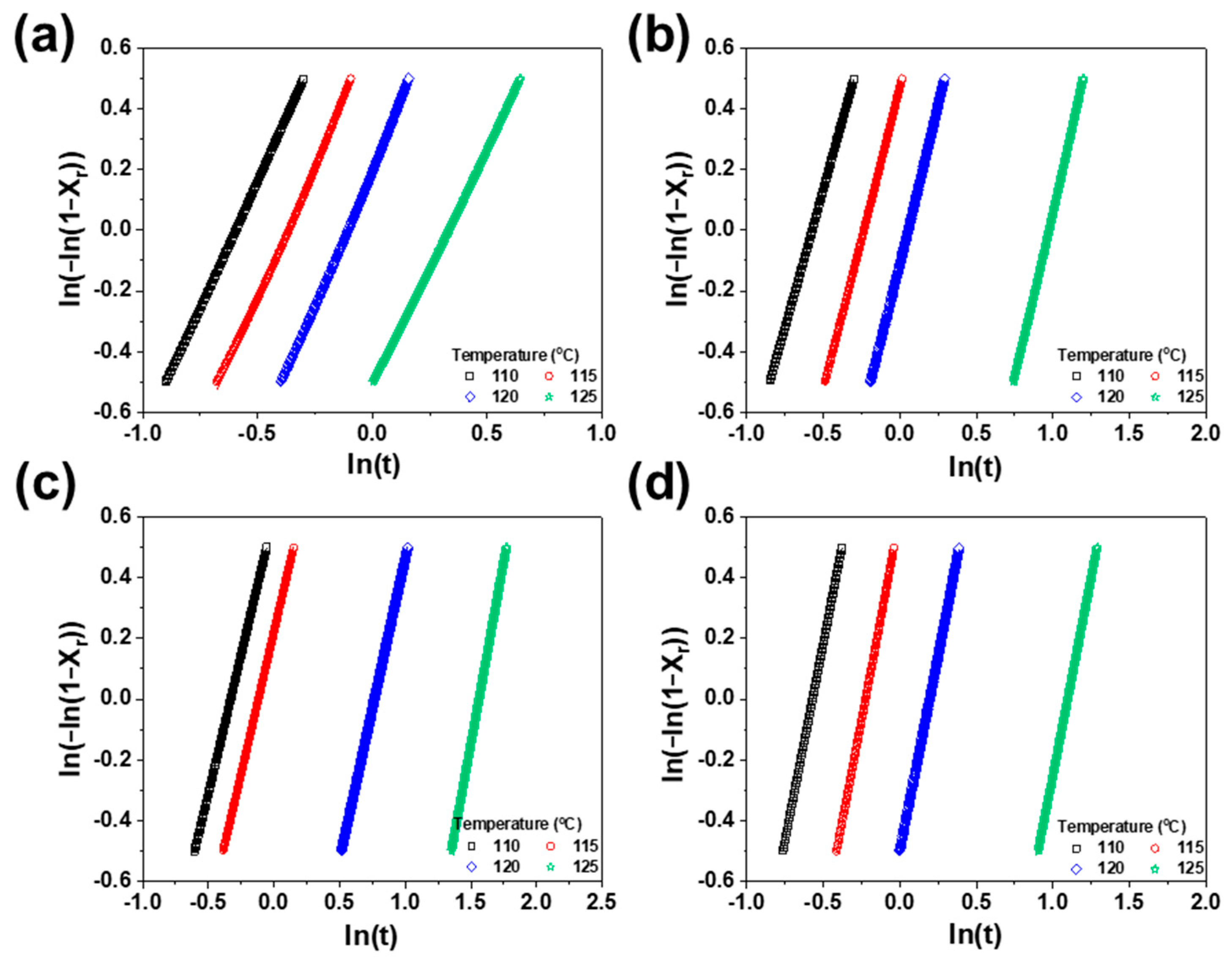
3.3. Isothermal and Non-Isothermal Crystallization
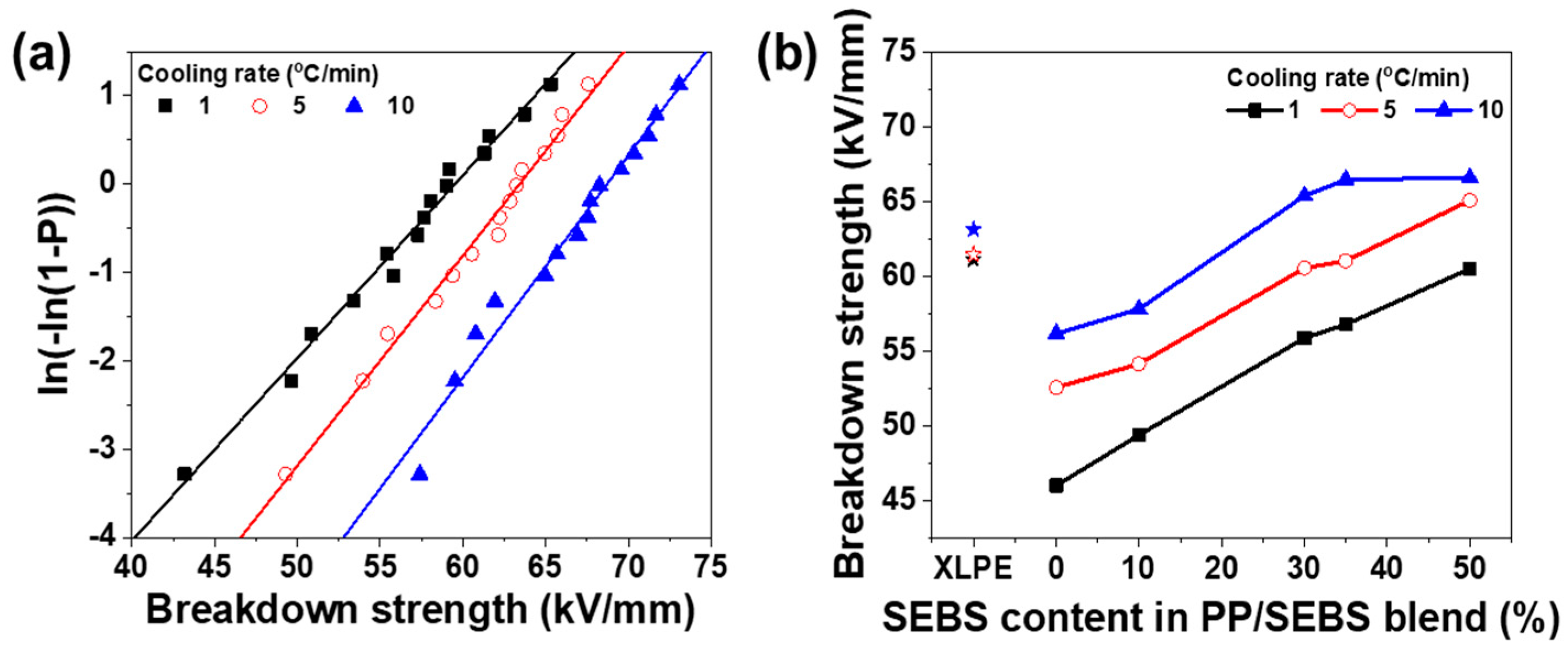
3.4. Breakdown Strength
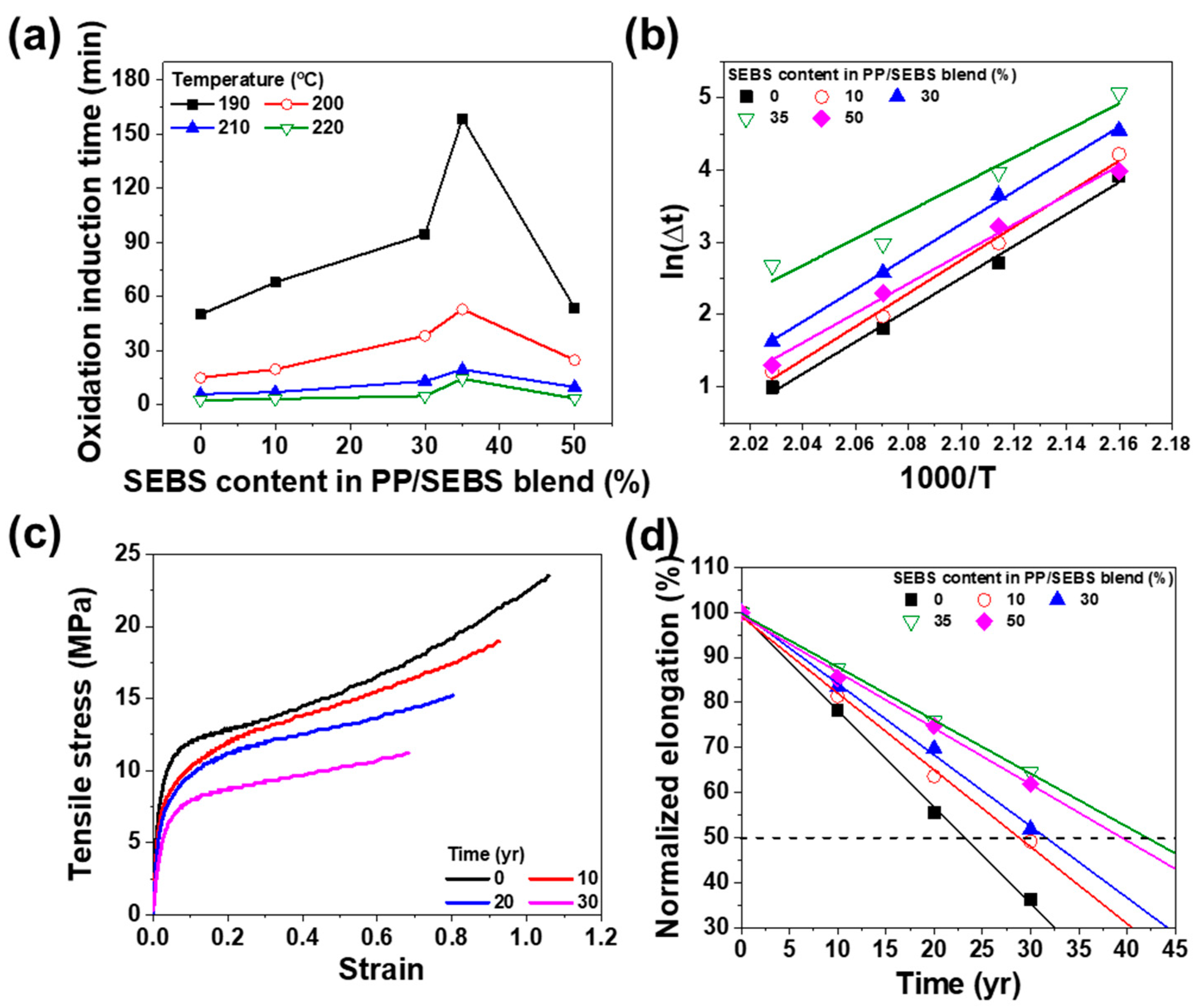
3.5. Accelerated Lifetime Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reed, C.W. An Assessment of Material Selection for High Voltage DC Extruded Polymer Cables. IEEE Electr. Insul. Mag. 2017, 17, 22–26. [Google Scholar] [CrossRef]
- Meng, F.; Chen, X.; Dai, C.; Zhang, M.; Paramane, A.; Zheng, L.; Tanaka, Y. Effect of Thermal Ageing on Physico-Chemical and Electrical Properties of EHVDC XLPE Cable Insulation. IEEE Trans. Dielectr. Electr. Insul. 2021, 28, 1012–1019. [Google Scholar] [CrossRef]
- Zhu, X.; Yin, Y.; Wu, J. Study on Aging Characteristics of XLPE Cable Insulation Based on Quantum Chemical Calculation. IEEE Trans. Dielectr. Electr. Insul. 2020, 27, 1942–1950. [Google Scholar] [CrossRef]
- Zhou, Y.; Dang, B.; Wang, H.; Liu, J.; Li, Q.; Hu, J.; He, J. Polypropylene-based ternary nanocomposites for recyclable high-voltage direct-current cable insulation. Compos. Sci. Technol. 2018, 165, 168–174. [Google Scholar] [CrossRef]
- Hosier, I.L.; Vaughan, A.S.; Swingler, S.G. An investigation of the potential of ethylene vinyl acetate/polyethylene blends for use in recyclable high voltage cable insulation systems. J. Mater. Sci. 2010, 45, 2747–2759. [Google Scholar] [CrossRef]
- Andrews, T.; Hampton, R.N.; Smedberg, A.; Wald, D.; Waschk, V.; Weissenberg, W. The role of Degassing in XLPE Power Cable Manufacture. IEEE Electr. Insul. Mag. 2006, 22, 5–16. [Google Scholar] [CrossRef]
- Suh, K.S.; Kim, J.Y.; Lee, C.R. Charge Distribution in Polyethylene/Ethylene Vinylacetate Laminates and Blends. IEEE Trans. Dielectr. Electr. Insul. 1996, 3, 1996. [Google Scholar] [CrossRef]
- Li, J.; Yang, K.; Wu, K.; Jing, Z.; Dong, J.Y. Eco-friendly polypropylene power cable insulation: Present status and perspective. IET Nanodielectrics 2023, 6, 130–146. [Google Scholar] [CrossRef]
- Shirvanimoghaddam, K.; Balaji, K.V.; Yadav, R.; Zabihi, O.; Ahmadi, M.; Adetunji, P.; Naebe, M. Balancing the toughness and strength in polypropylene composites. Compos. Part B Eng. 2021, 223, 109121. [Google Scholar] [CrossRef]
- Zhan, Y.; Yang, X.; Yang, J.; Hou, S.; Fu, M. Improved electrical properties of organic modified thermoplastic insulation material for direct current cable application. Polymers 2024, 16, 46. [Google Scholar] [CrossRef]
- Xu, C.; Zheng, Z.; Wu, W.; Wang, Z.; Fu, L. Dynamically vulcanized PP/EPDM blends with balanced stiffness and toughness via in-situ compatibilization of MAA and excess ZnO nanoparticles: Preparation, structure and properties. Compos. Part B Eng. 2019, 160, 147–157. [Google Scholar] [CrossRef]
- Panaitescu, D.M.; Vuluga, Z.; Sanporean, C.G.; Nicolae, C.A.; Gabor, A.R.; Trusca, R. High flow polypropylene/SEBS composites reinforced with differently treated hemp fibers for injection molded parts. Compos. Part B Eng. 2019, 174, 107062. [Google Scholar] [CrossRef]
- Song, H.S.; Kim, H.S.; Jung, J.H.; Lee, B.W. Comparison for Accelerated Degradation of New and Old 6.6 kV AC XLPE Cables. J. Electr. Eng. Technol. 2024, 19, 5407–5417. [Google Scholar] [CrossRef]
- Zhang, Z.; Assala, P.D.S.; Wu, L. Residual life assessment of 110 kV XLPE cable. Electr. Power Syst. Res. 2018, 163, 572–580. [Google Scholar] [CrossRef]
- Peinado, C.; Corrales, T.; Catalina, F.; Pedron, S.; Quiteria, V.R.S.; Parellada, M.D.; Barrio, J.A.; Olmos, D.; Gonzalez-Benito, J. Effects of ozone in surface modification and thermal stability of SEBS block copolymers. Polym. Degrad. Stab. 2010, 95, 975–986. [Google Scholar] [CrossRef]
- Yazdani-Pedram, M.; Quijada, R.; Lopez-Manchado, M.A. Use of Monomethyl Itaconate Grafted Poly(propylene)(PP) and Ethylene Propylene Rubber (EPR) as Compatibilizers for PP/EPR Blends. Macromol. Mater. Eng. 2003, 288, 875–885. [Google Scholar] [CrossRef]
- Hosier, I.L.; Vaughan, A.S.; Pye, A.; Stevens, G.C. High performance polymer blend systems for HVDC applications. IEEE Trans. Dielectr. Electr. Insul. 2019, 26, 1197–1203. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Z.; Wang, H.; Zheng, Z.; Du, B. Enhanced Dielectric Breakdown Property of Polypropylene Based on Mesoscopic Structure Modulation by Crystal Phase Transformation for High Voltage Power Cable Insulation. ACS Appl. Polym. Mater. 2024, 6, 3031–3041. [Google Scholar] [CrossRef]
- Li, Y.; Han, Y.; Pang, J.; Jin, D.; Sun, Y.; Li, Z. Electric Field Assist on Enhancing the Electrical Breakdown Strength of Cross-Linked Polyethylene for Power Cable Insulation. Macromolecules 2024, 57, 5497–5506. [Google Scholar] [CrossRef]
- Lou, C.W.; Huang, C.L.; Pan, Y.J.; Lin, Z.L.; Song, X.M.; Lin, J.H. Crystallization, mechanical, and electromagnetic properties of conductive polypropylene/SEBS composites. J. Polym. Res. 2016, 23, 84. [Google Scholar] [CrossRef]
- Nazrin, A.; Kuan, T.M.; Mansour, D.E.A.; Farade, R.A.; Ariffin, A.M.; Rahman, M.S.A.; Wahab, N.I.B.A. Innovative approaches for augmenting dielectric properties in cross-linked polyethylene (XLPE): A review. Heliyon 2024, 10, e34737. [Google Scholar] [CrossRef] [PubMed]
- Das, A.K.; Chatterjee, S.; Pradhan, A.K.; Chatterjee, B.; Dalai, S. Estimation of moisture content in XLPE cable insulation using electric modulus. IEEE Trans. Dielectr. Electr. Insul. 2022, 29, 1030–1037. [Google Scholar] [CrossRef]
- Banerjee, S.S.; Burbine, S.; Shivaprakash, N.K.; Mead, J. 3D-Printable PP/SEBS Thermoplastic Elastomeric Blends: Preparation and Properties. Polymers 2019, 11, 347. [Google Scholar] [CrossRef]
- Li, Z.; Wu, Y.; Du, B. Effect of Crystalline Morphology on Electrical Tree Morphology and Growth Characteristics of PP Insulation: From Mesoscopic to Macroscopic. IEEE Trans. Dielectr. Electr. Insul. 2023, 30, 989–996. [Google Scholar] [CrossRef]
- Li, S.; Cheng, P.; Liu, X.; Li, G.; Ma, Y. Fabrication and toughening mechanism of high toughness PP/SEBS/HDPE blends with core-shell particles. J. Polym. Sci. 2023, 61, 2389–2402. [Google Scholar] [CrossRef]
- Uthaipan, N.; Jarnthong, M.; Peng, Z.; Junhasavasdikul, B.; Nakason, C.; Thitithammawong, A. Effects of cooling rates on crystallization behavior and melting characteristics of isotactic polypropylene as neat and in the TPVs EPDM/PP and EOC/PP. Polym. Test. 2015, 44, 101–111. [Google Scholar] [CrossRef]
- Seo, Y.; Kim, J.; Kim, K.U.; Kim, Y.C. Study of the crystallization behaviors of polypropylene and maleic anhydride grafted polypropylene. Polymer 2000, 41, 2639–2646. [Google Scholar] [CrossRef]
- Zhang, Z.; Yu, F.; Zhang, H. Isothermal and Non-Isothermal Crystallization Studies of Long Chain Branched Polypropylene Containing Poly(ethylene-co-octene) under Quiescent and Shear Conditions. Polymers 2017, 236, 236. [Google Scholar] [CrossRef]
- Abbasi, A.; Abadi, A.N.; Hemmati, F.; Mohammadi-Roshandeh, J.; Farizeh, T. Structure-properties correlations in compatibilized polyamide/thermoplastic elastomer/nanoclay mixtures: Interrelationship among non-isothermal crystallization kinetics, morphology and viscoelastic responses. J. Therm. Anal. Calorim. 2023, 148, 3373–3394. [Google Scholar] [CrossRef]
- Patra, P.K.; Jaisingh, A.; Goel, V.; Kapur, G.S.; Nebhani, L. Crystallization kinetics of compatibilized blends of polypropylene and polyethylenimine. J. Therm. Anal. Calorim. 2022, 147, 6689–6699. [Google Scholar] [CrossRef]
- 930-2004; IEEE Guide for the Statistical Analysis of Electrical Insulation Breakdown Data. IEEE: New York, NY, USA, 2004.
- Guo, Q.; Li, X.; Li, W.; Yao, Z. The Balanced Insulating Performance and Mechanical Property of PP by Introducing PP-g-PS Graft Copolymer and SEBS Elastomer. Ind. Eng. Chem. Res. 2018, 57, 6696–6704. [Google Scholar] [CrossRef]
- Fan, M.; Zhou, S.; Li, Z.; Du, B.; Yu, F.; Yan, H. Effect of Crystalline Morphology on DC-Prestressed Breakdown Characteristics of PP-based Cable Insulation. In Proceedings of the 3rd IEEE International Conference on Electrical Materials and Power Equipment, Chongqing, China, 11–15 April 2021; pp. 1–4. [Google Scholar] [CrossRef]
- Dai, X.; Hao, J.; Liao, R.; Zheng, X.; Gao, Z.; Peng, W. Multi-dimensional Analysis and Correlation Mechanism of Thermal Degradation Characteristics of XLPE Insulation for Extra High Voltage Submarine Cable. IEEE Trans. Dielectr. Electr. Insul. 2021, 28, 1488–1496. [Google Scholar] [CrossRef]
- Shan, B.; Du, C.; Cheng, J.; Wang, W.; Li, C. Residual life prediction of XLPE distribution cables based on time-temperature superposition principle by non-destructive BIS measuring on site. Polymers 2022, 14, 5478. [Google Scholar] [CrossRef] [PubMed]
- Zaharescu, T. Insight into the stabilization activity of n-SiO2 powder in SEBS phase. J. Therm. Anal. Calorim. 2025, 150, 1217–1223. [Google Scholar] [CrossRef]
- Shebani, A.; Algoul, S.; Al-Qish, A.; Alaeb, A.; Trish, A. Enhancement of mechanical properties and thermal stability of recycled polyethylene, polypropylene and their blends via incorporation of elastomer. J. Elastomers Plast. 2025, 57, 233–248. [Google Scholar] [CrossRef]
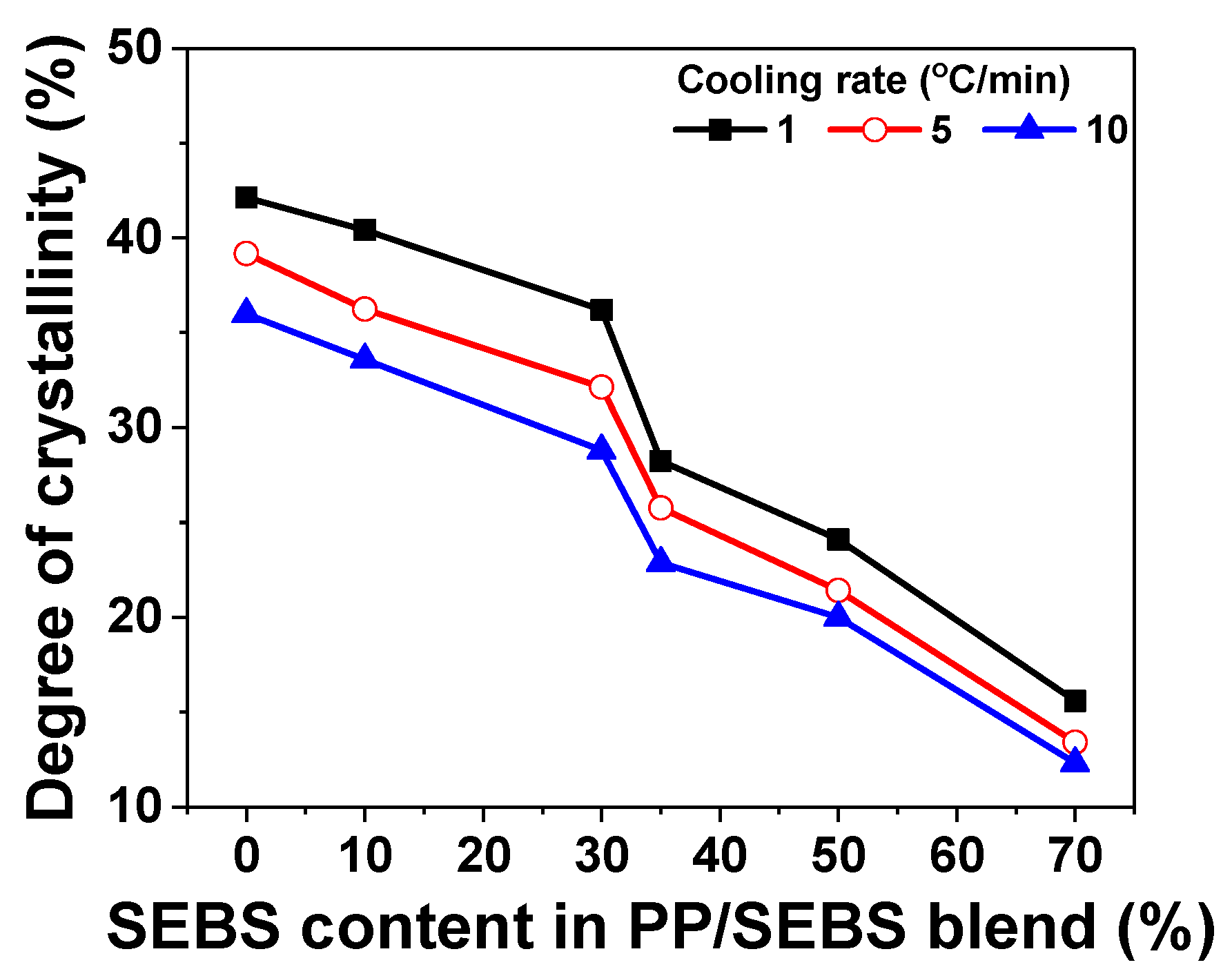


| Cooling Rate | Content of SEBS (%) | Tm (°C) | Tc (°C) | ΔHm (J/g) | Xc (%) |
|---|---|---|---|---|---|
| 1 °C/min | 0 | 171.30 | 122.1 | 88.07 | 42.14 |
| 10 | 170.93 | 120.9 | 76.03 | 40.42 | |
| 30 | 170.32 | 119.5 | 52.97 | 36.21 | |
| 35 | 170.05 | 118.2 | 38.36 | 28.24 | |
| 50 | 167.97 | 116.3 | 25.19 | 24.11 | |
| 5 °C/min | 0 | 170.92 | 114.6 | 81.88 | 39.18 |
| 10 | 170.02 | 113.4 | 68.18 | 36.25 | |
| 30 | 169.84 | 111.6 | 47.03 | 32.14 | |
| 35 | 169.64 | 110.4 | 34.99 | 25.76 | |
| 50 | 168.72 | 109.0 | 22.37 | 21.41 | |
| 10 °C/min | 0 | 171.23 | 116.9 | 75.25 | 36.00 |
| 10 | 170.87 | 111.8 | 63.17 | 33.58 | |
| 30 | 170.13 | 110.1 | 42.14 | 28.81 | |
| 35 | 170.05 | 108.9 | 31.07 | 22.87 | |
| 50 | 169.05 | 107.7 | 20.88 | 19.98 |
| Isothermal Temperature | Content of SEBS (%) | n | k | |
|---|---|---|---|---|
| 110 | 0 | 0.42 | 1.67 | 2.71 |
| 10 | 0.44 | 1.82 | 2.84 | |
| 35 | 0.56 | 1.83 | 1.82 | |
| 50 | 0.49 | 2.60 | 4.40 | |
| 115 | 0 | 0.51 | 1.72 | 1.90 |
| 10 | 0.63 | 1.99 | 1.60 | |
| 35 | 0.73 | 1.86 | 1.25 | |
| 50 | 0.70 | 2.67 | 1.82 | |
| 120 | 0 | 0.68 | 1.80 | 1.22 |
| 10 | 0.84 | 2.07 | 0.90 | |
| 35 | 1.72 | 1.98 | 0.22 | |
| 50 | 1.05 | 2.57 | 0.61 | |
| 125 | 0 | 1.05 | 1.56 | 0.60 |
| 10 | 2.17 | 2.20 | 0.12 | |
| 35 | 3.79 | 2.39 | 0.02 | |
| 50 | 2.62 | 2.60 | 0.06 |
| Cooling Rate | Content of SEBS (%) | σ (kV/mm) | β |
|---|---|---|---|
| 1 °C/min | 0 | 45.99 | 7.89 |
| 10 | 49.37 | 9.10 | |
| 30 | 55.85 | 11.92 | |
| 35 | 56.76 | 11.15 | |
| 50 | 60.49 | 10.89 | |
| 5 °C/min | 0 | 52.55 | 9.32 |
| 10 | 54.11 | 10.70 | |
| 30 | 60.53 | 13.42 | |
| 35 | 61.02 | 13.83 | |
| 50 | 65.06 | 13.50 | |
| 10 °C/min | 0 | 56.14 | 11.46 |
| 10 | 57.79 | 12.53 | |
| 30 | 65.36 | 14.50 | |
| 35 | 66.44 | 16.38 | |
| 50 | 66.57 | 14.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nam, C.Y.; Lee, J.H.; Kim, M.A.; Yoon, H.G. High Performance and Recyclable Polypropylene/Styrene–Ethylene–Butylene–Styrene Blends for Next Generation Cable Insulation with Enhanced Breakdown Strength Through Controlling Crystallinity. Polymers 2025, 17, 1361. https://doi.org/10.3390/polym17101361
Nam CY, Lee JH, Kim MA, Yoon HG. High Performance and Recyclable Polypropylene/Styrene–Ethylene–Butylene–Styrene Blends for Next Generation Cable Insulation with Enhanced Breakdown Strength Through Controlling Crystallinity. Polymers. 2025; 17(10):1361. https://doi.org/10.3390/polym17101361
Chicago/Turabian StyleNam, Chae Yun, Jun Hyung Lee, Min Ah Kim, and Ho Gyu Yoon. 2025. "High Performance and Recyclable Polypropylene/Styrene–Ethylene–Butylene–Styrene Blends for Next Generation Cable Insulation with Enhanced Breakdown Strength Through Controlling Crystallinity" Polymers 17, no. 10: 1361. https://doi.org/10.3390/polym17101361
APA StyleNam, C. Y., Lee, J. H., Kim, M. A., & Yoon, H. G. (2025). High Performance and Recyclable Polypropylene/Styrene–Ethylene–Butylene–Styrene Blends for Next Generation Cable Insulation with Enhanced Breakdown Strength Through Controlling Crystallinity. Polymers, 17(10), 1361. https://doi.org/10.3390/polym17101361






