Synthesis of Acidic Phosphonic Chitosan and the Complexation of La(III) in Acidic Aqueous Solution
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Membrane
2.2. Methods
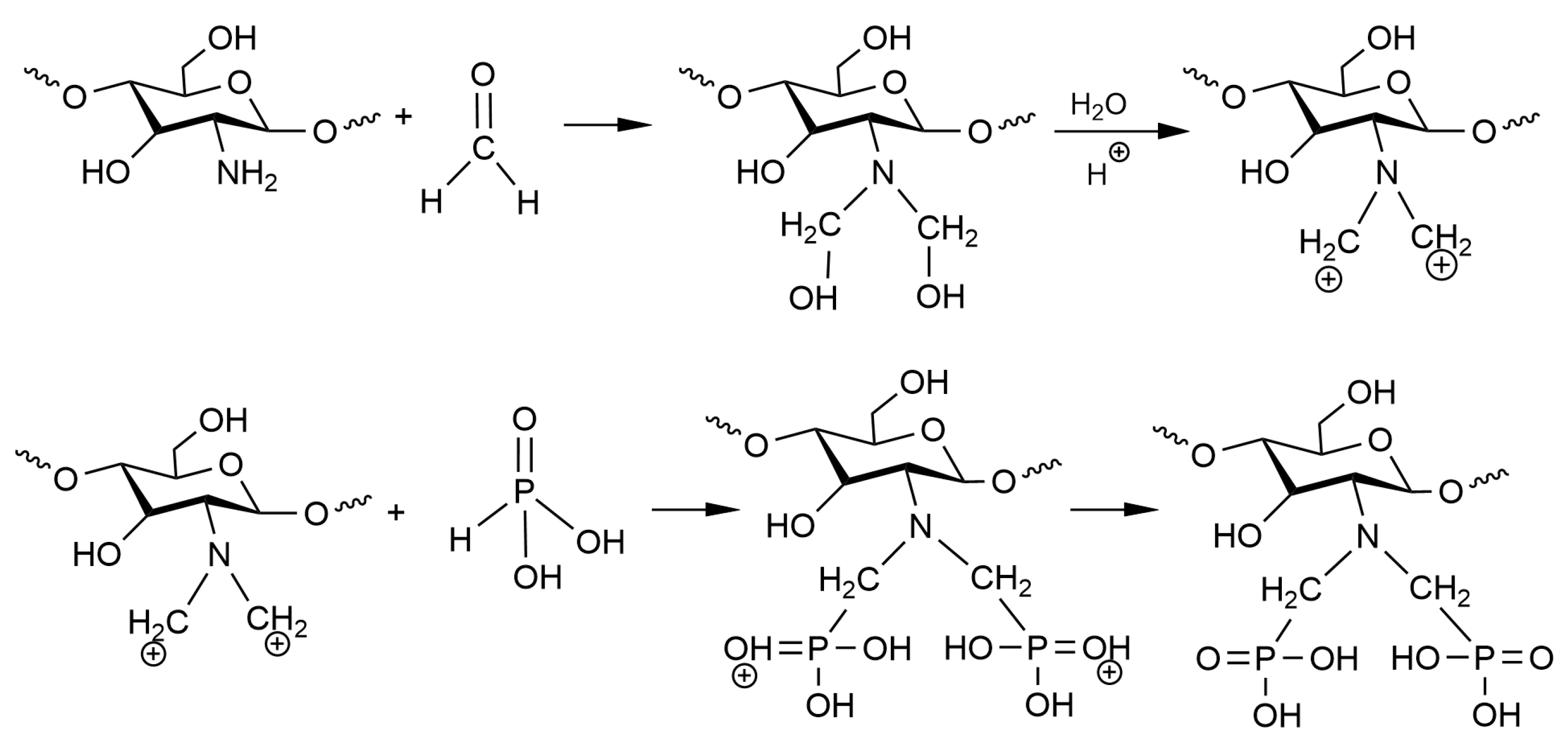
2.2.1. Synthesis of the aPCS
2.2.2. Procedure of C–UF
2.3. Characterization
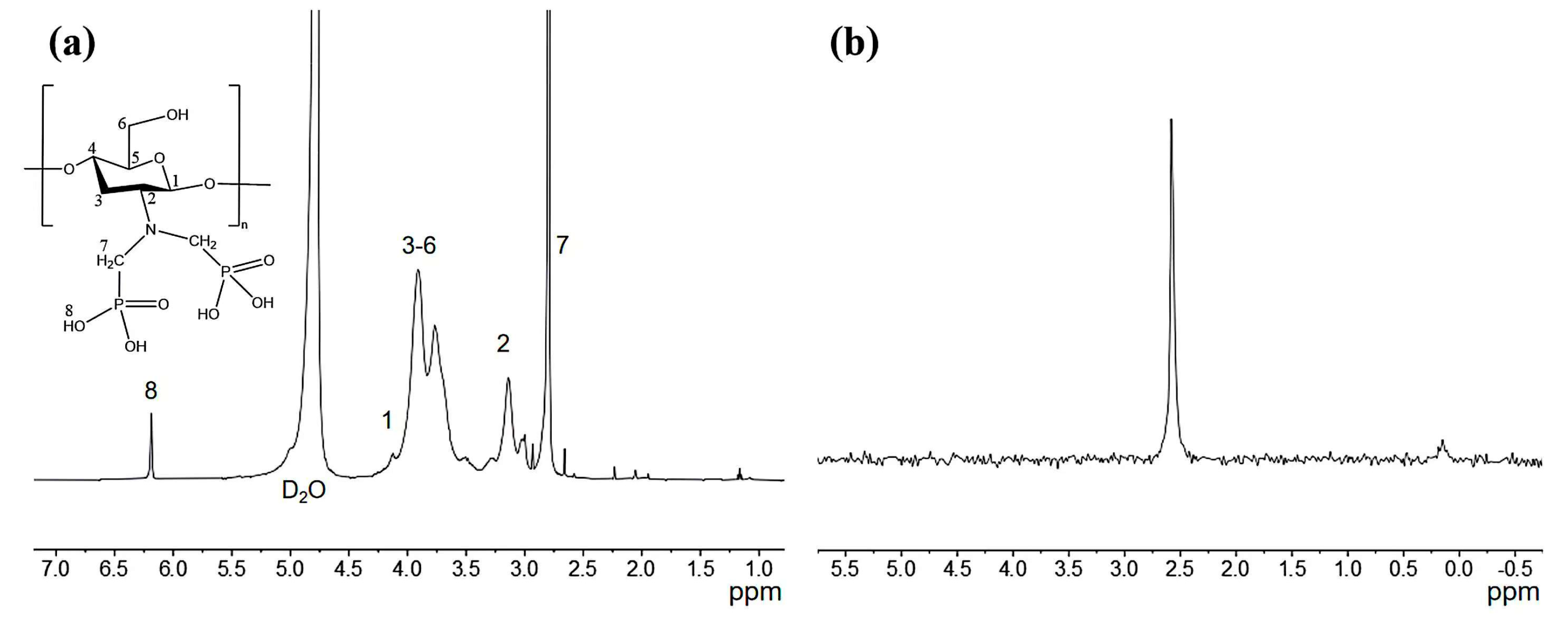
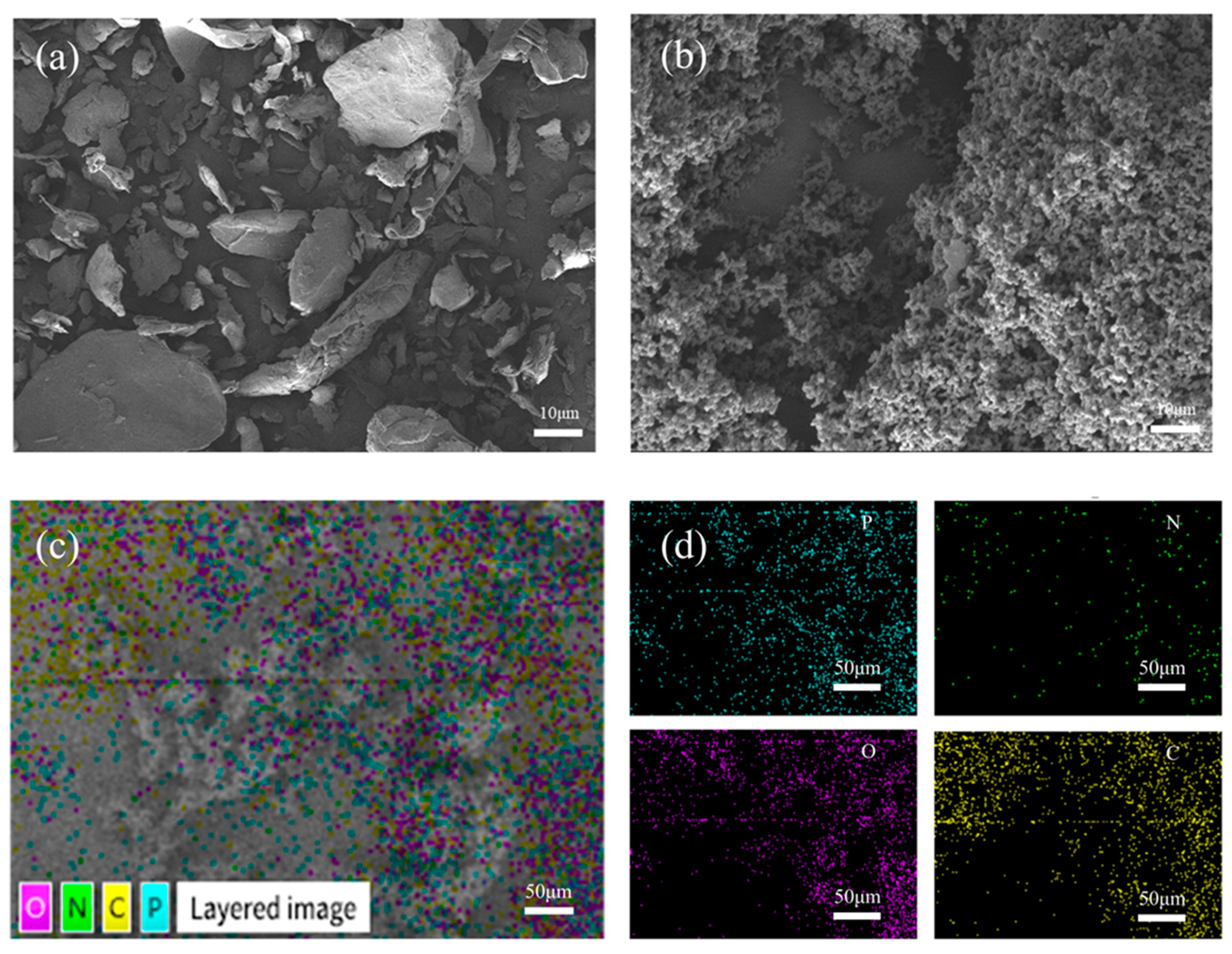
2.4. DFT Caculation
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Z.; Li, Z.; Chen, J.; Kallem, P.; Banat, F.; Qiu, H. Recent advances in selective separation technologies of rare earth elements: A review. J. Environ. Chem. Eng. 2022, 10, 107104. [Google Scholar] [CrossRef]
- Liu, T.; Chen, J. Extraction and separation of heavy rare earth elements: A review. Sep. Purif. Technol. 2021, 276, 119263. [Google Scholar] [CrossRef]
- Xu, L.; Pu, N.; Li, Y.; Wei, P.; Sun, T.; Xiao, C.; Chen, J.; Xu, C. Selective Separation and Complexation of Trivalent Actinide and Lanthanide by a Tetradentate Soft–Hard Donor Ligand: Solvent Extraction, Spectroscopy, and DFT Calculations. Inorg. Chem. 2019, 58, 4420–4430. [Google Scholar] [CrossRef]
- Xiao, Y.; Feng, Z.; Huang, X.; Huang, L.; Chen, Y.; Liu, X.; Wang, L.; Long, Z. Recovery of rare earth from the ion-adsorption type rare earths ore: II. Compound leaching. Hydrometallurgy 2016, 163, 83–90. [Google Scholar] [CrossRef]
- Traore, M.; Gong, A.; Wang, Y.; Qiu, L.; Bai, Y.; Zhao, W.; Liu, Y.; Chen, Y.; Liu, Y.; Wu, H.; et al. Research progress of rare earth separation methods and technologies. J. Rare Earths 2023, 41, 182–189. [Google Scholar] [CrossRef]
- Kahloul, M.; Mahfoudhi, S.; Ounifi, I.; Elabed, B.; Amor, T.B.; Hafiane, A. Green complexation for heavy metals removal from wastewater by Keggin-polyoxometalates enhanced ultrafiltration. Water Sci. Technol. 2022, 86, 1510–1526. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wen, Y.; Chu, Y.; Liu, W.; Liu, J.; Wang, R.; Xu, Z. Separation of Rare Earth and Aluminum by Selective Complexation. Minerals 2022, 12, 743. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Y.; Luo, J.; Wan, Y.; Song, W. Membrane pre-concentration as an efficient strategy to enhance the enrichment of rare earth by MgO precipitation. Chem. Eng. J. 2024, 491, 152083. [Google Scholar] [CrossRef]
- Kuang, S.; Zhang, Z.; Li, Y.; Wei, H.; Liao, W. Extraction and separation of heavy rare earths from chloride medium by α-aminophosphonic acid HEHAPP. J. Rare Earths 2018, 36, 304–310. [Google Scholar] [CrossRef]
- Zhou, H.; Dong, Y.; Wang, Y.; Zhao, Z.; Xiao, Y.; Sun, X. Recovery of Th(IV) from leaching solutions of rare earth residues using a synergistic solvent extraction system consisting of Cyanex 572 and n-octyl diphenyl phosphate (ODP). Hydrometallurgy 2019, 183, 186–192. [Google Scholar] [CrossRef]
- Pandit, A.H.; Nisar, S.; Imtiyaz, K.; Nadeem, M.; Mazumdar, N.; Rizvi, M.M.A.; Ahmad, S. Injectable, Self-Healing, and Biocompatible N,O-Carboxymethyl Chitosan/Multialdehyde Guar Gum Hydrogels for Sustained Anticancer Drug Delivery. Biomacromolecules 2021, 22, 3731–3745. [Google Scholar] [CrossRef]
- Welchoff, M.A.; Wittenberg, A.T.; Jimenez, J.C.; Kamireddi, D.; Snelling, E.K.; Street, R.M.; Schauer, C.L. Post-modification of electrospun chitosan fibers. Polym. Eng. Sci. 2023, 63, 1921–1931. [Google Scholar] [CrossRef]
- Zhang, Y.; Haris, M.; Zhang, L.; Zhang, C.; Wei, T.; Li, X.; Niu, Y.; Li, Y.; Guo, J.; Li, X. Amino-modified chitosan/gold tailings composite for selective and highly efficient removal of lead and cadmium from wastewater. Chemosphere 2022, 308, 136086. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Zhang, Y.; Wang, H.; Li, C. Research progress in the removal of heavy metals by modified chitosan. Tenside Surfactants Deterg. 2022, 59, 281–293. [Google Scholar] [CrossRef]
- Yin, Z.; Qiu, D.; Zhang, M. Molecular level study of cadmium adsorption on dithiocarbamate modified chitosan. Environ. Pollut. 2021, 271, 116322. [Google Scholar] [CrossRef]
- Li, L.; Xu, Y.; Xu, Z.; Wu, C.; Chen, Q.; Xu, K.; Shi, Z. Synthesis, characterization and antifungal properties of dehydroabietic acid modified chitosan. Int. J. Biol. Macromol. 2024, 255, 128056. [Google Scholar] [CrossRef]
- Tan, F.; Hao, N. Synthesis and Surface Property Characterization of N-Phthaloyl Hydroxypropyl Chitosan. J. Wenshan Univ. 2016, 29, 24–27. [Google Scholar]
- Romal, J.R.A.; Ong, S.K. Opportunity for a greener recovery of dysprosium(III) from secondary sources by a novel Mannich reaction-modified phosphorylated chitosan hydrogel. Int. J. Biol. Macromol. 2024, 267, 131449. [Google Scholar] [CrossRef]
- Hamza, M.F.; Abdellah, W.M.; Zaki, D.I.; Wei, Y.; Althumayri, K.; Brostow, W.; Hamad, N.A. A Phosphonic Functionalized Biopolymer for the Sorption of Lanthanum (III) and Application in the Recovery of Rare Earth Elements. Sustainability 2023, 15, 2843. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Y.; Lu, D.; Qiu, Y. Synthesis of a Novel Water-Soluble Polymer Complexant Phosphorylated Chitosan for Rare Earth Complexation. Polymers 2022, 14, 419. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Baboul, A.G.; Curtiss, L.A.; Redfern, P.C.; Raghavachari, K. Gaussian-3 theory using density functional geometries and zero-point energies. J. Chem. Phys. 1999, 110, 7650–7657. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Schäfer, A.; Horn, H.; Ahlrichs, R. Fully optimized contracted Gaussian basis sets for atoms Li to Kr. J. Chem. Phys. 1992, 97, 2571–2577. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. C.01; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Zidan, T.A.; Abdelhamid, A.E.; Zaki, E.G. N-Aminorhodanine modified chitosan hydrogel for antibacterial and copper ions removal from aqueous solutions. Int. J. Biol. Macromol. 2020, 158, 32–42. [Google Scholar] [CrossRef]
- Liu, T.; Gou, S.; He, Y.; Fang, S.; Zhou, L.; Gou, G.; Liu, L. N-methylene phosphonic chitosan aerogels for efficient capture of Cu2+ and Pb2+ from aqueous environment. Carbohydr. Polym. 2021, 269, 118355. [Google Scholar] [CrossRef]
- Sashiwa, H.; Kawasaki, N.; Nakayama, A.; Muraki, E.; Yamamoto, N.; Aiba, S.-i. Chemical Modification of Chitosan. 14: Synthesis of Water-Soluble Chitosan Derivatives by Simple Acetylation. Biomacromolecules 2002, 3, 1126–1128. [Google Scholar] [CrossRef]
- Luo, X.; Zhao, L.; Khan, I.M.; Yue, L.; Zhang, Y.; Wang, Z. Chitosan-curcumin conjugate prepared by one-step free radical grafting: Characterization, and functional evaluation. Carbohydr. Res. 2024, 545, 109297. [Google Scholar] [CrossRef]
- Wu, B.; Huang, J.; Tan, G.; Hao, Z.; Hu, G.; Luo, J.; Cui, C. DFT Study on the Structure and Properties of Hydantoin and Its Derivatives in the Alkaline Aqueous Solution. Chemistry 2021, 84, 610–619. [Google Scholar]
- Li, W.; Zhou, M.; Fan, L.; Dai, Z.; Qiu, Y. Selective separation of Dy(III), Yb(III) and Y(III) by complexation and orderly decomplexation coupling with ultrafiltration based on rotating disk membrane and a DFT study. Sep. Purif. Technol. 2024, 341, 126665. [Google Scholar] [CrossRef]
- Francos, J.; Elorriaga, D.; Crochet, P.; Cadierno, V. The chemistry of Group 8 metal complexes with phosphinous acids and related POH ligands. Coord. Chem. Rev. 2019, 387, 199–234. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, M.; Liu, Z.; Lu, D.; Wang, J.; Chen, Z.; Qiu, Y. Synthesis of Acidic Phosphonic Chitosan and the Complexation of La(III) in Acidic Aqueous Solution. Polymers 2025, 17, 1341. https://doi.org/10.3390/polym17101341
Zhou M, Liu Z, Lu D, Wang J, Chen Z, Qiu Y. Synthesis of Acidic Phosphonic Chitosan and the Complexation of La(III) in Acidic Aqueous Solution. Polymers. 2025; 17(10):1341. https://doi.org/10.3390/polym17101341
Chicago/Turabian StyleZhou, Min, Zhenglin Liu, Dandan Lu, Jiajun Wang, Zili Chen, and Yunren Qiu. 2025. "Synthesis of Acidic Phosphonic Chitosan and the Complexation of La(III) in Acidic Aqueous Solution" Polymers 17, no. 10: 1341. https://doi.org/10.3390/polym17101341
APA StyleZhou, M., Liu, Z., Lu, D., Wang, J., Chen, Z., & Qiu, Y. (2025). Synthesis of Acidic Phosphonic Chitosan and the Complexation of La(III) in Acidic Aqueous Solution. Polymers, 17(10), 1341. https://doi.org/10.3390/polym17101341





