Fabrication of PVDF Membranes with a PVA Layer for the Effective Removal of Volatile Organic Compounds in Semiconductor Wastewater
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
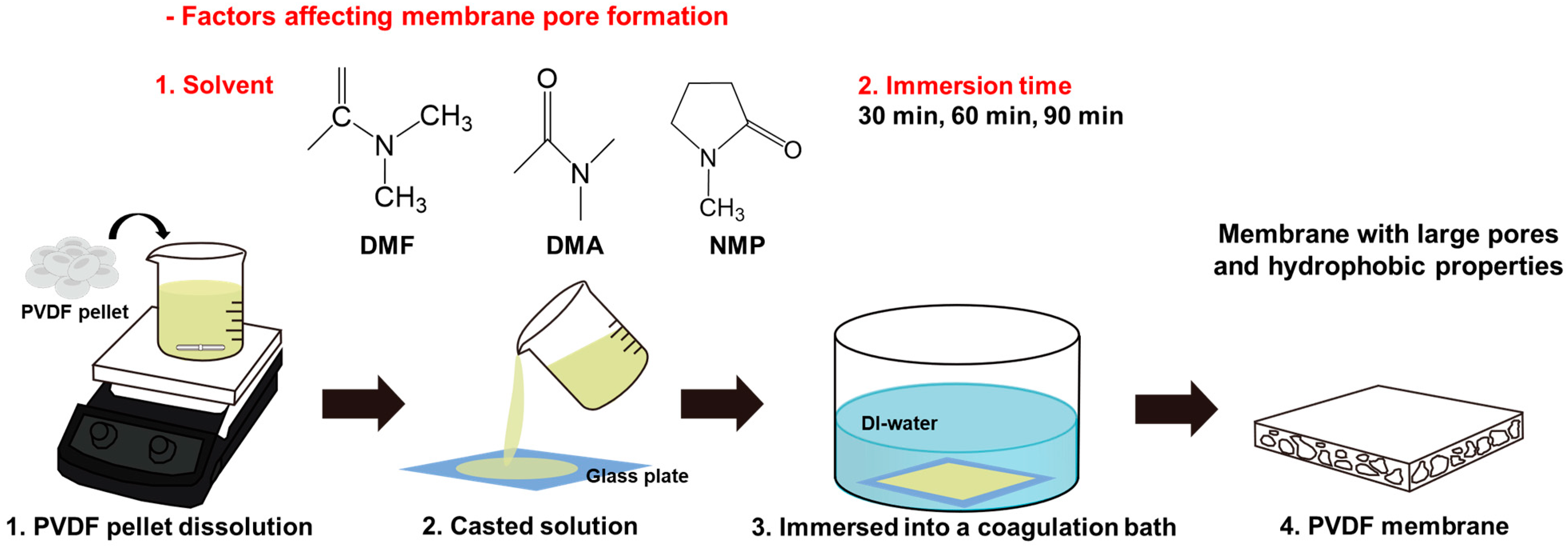
2.2. Preparation of a Pristine PVDF Membrane
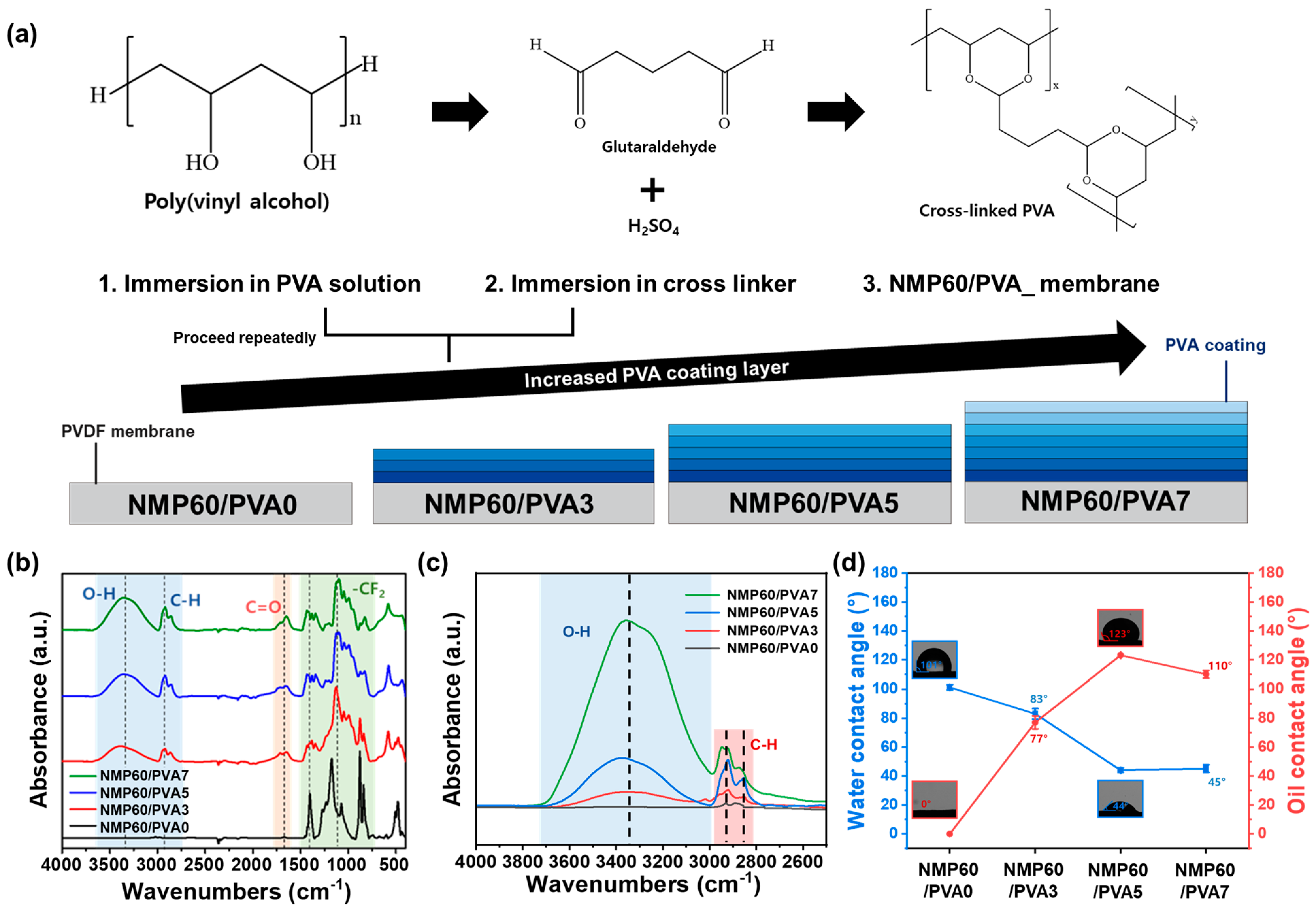
2.3. Preparation of the NMP60/PVA Membrane
2.4. Characterizations
2.5. Pure-Water and Emulsion Flux of the Membrane
2.6. Flux Decline Rate of the Membranes
2.7. Chemical Stability of the Membranes
2.8. Oil Rejection by the Membranes
2.9. Antifouling Performance of the Membranes
3. Results and Discussion
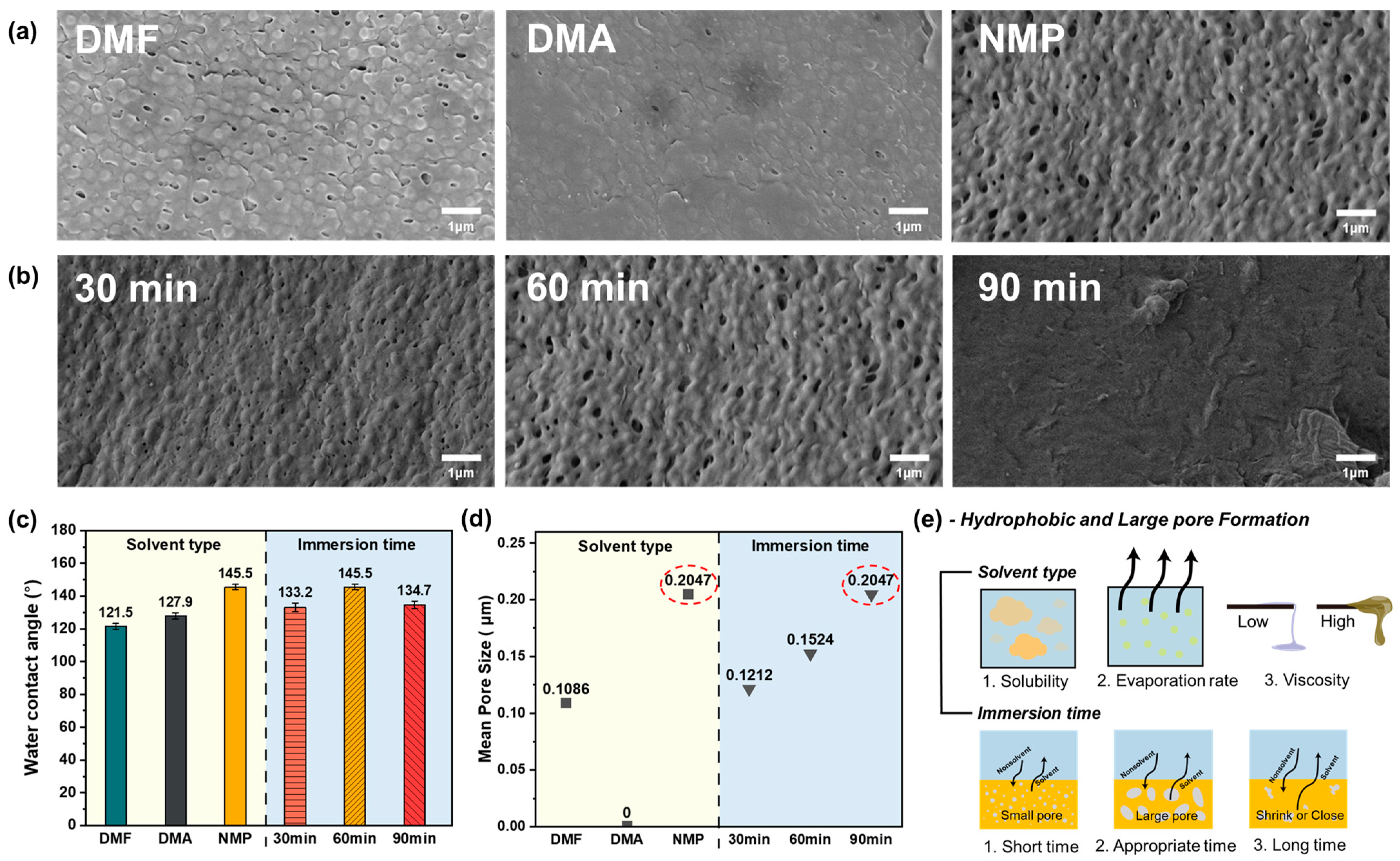
3.1. Membrane Fabrication
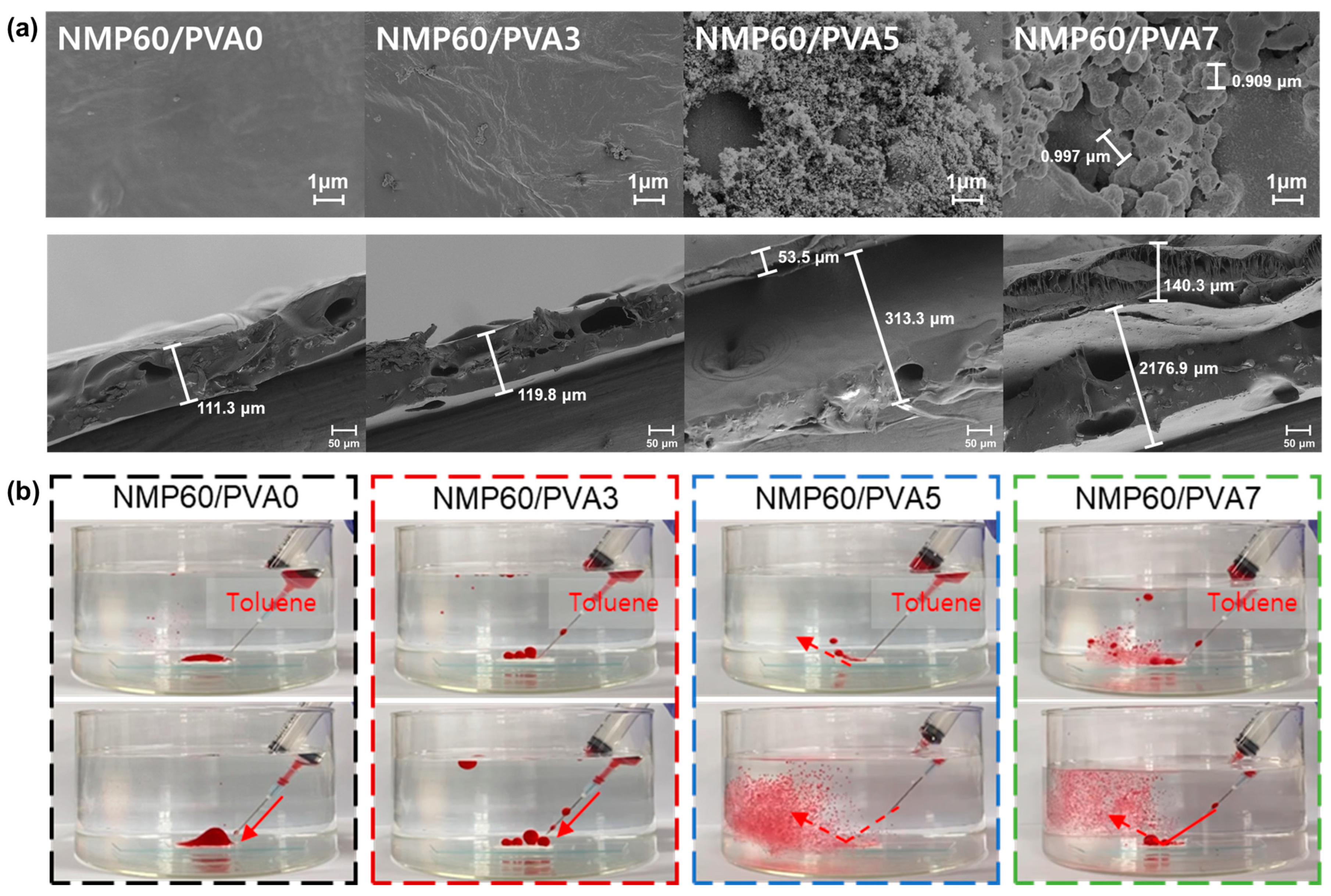
3.2. Membrane Morphology
3.3. Membrane Modification
3.4. Membrane Surface Properties
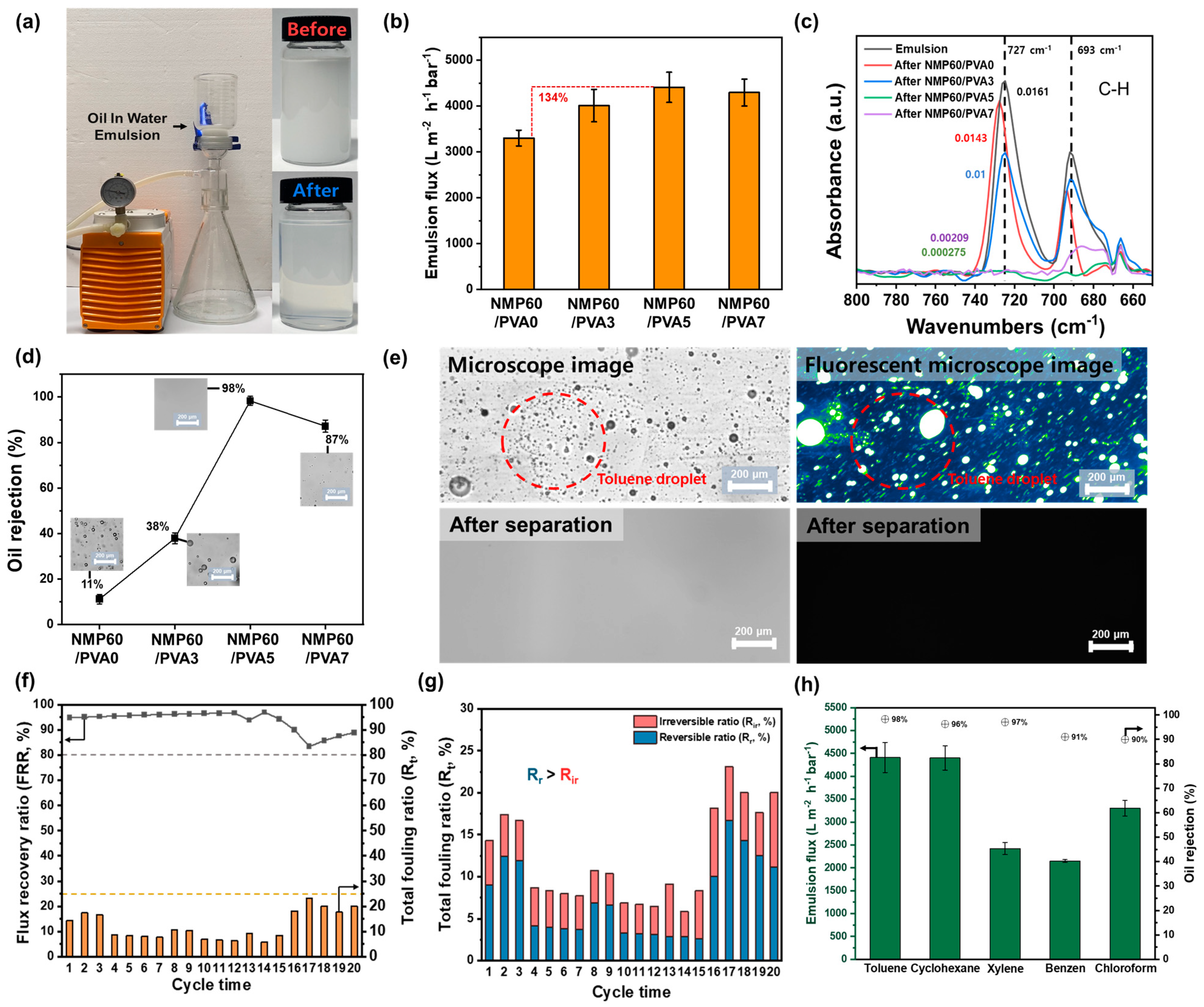
3.5. Membrane Performance
3.6. Oil/Water Separation by the Membrane
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Sim, J.; Lee, J.; Rho, H.; Park, K.-D.; Choi, Y.; Kim, D.; Kim, H.; Woo, Y.C. A review of semiconductor wastewater treatment processes: Current status, challenges, and future trends. J. Clean. Prod. 2023, 429, 139570. [Google Scholar] [CrossRef]
- Lin, S.H.; Kiang, C.D. Combined physical, chemical and biological treatments of wastewater containing organics from a semiconductor plant. J. Hazard. Mater. 2003, 97, 159–171. [Google Scholar] [CrossRef]
- Choi, J.; Chung, J. Reply to “Comments on ‘Reuse of Semiconductor Wastewater Using Reverse Osmosis and Metal-Immobilized Catalyst-Based Advanced Oxidation Process’”. Ind. Eng. Chem. Res. 2014, 53, 18587–18588. [Google Scholar] [CrossRef]
- Huang, H.; Liu, J.; Zhang, P.; Zhang, D.; Gao, F. Investigation on the simultaneous removal of fluoride, ammonia nitrogen and phosphate from semiconductor wastewater using chemical precipitation. Chem. Eng. J. 2017, 307, 696–706. [Google Scholar] [CrossRef]
- Chuang, T.-C.; Huang, C.J.; Liu, J.C. Treatment of Semiconductor Wastewater by Dissolved Air Flotation. J. Environ. Eng. 2002, 128, 974–980. [Google Scholar] [CrossRef]
- Rueda-Márquez, J.; Sillanpää, M.; Pocostales, P.; Acevedo, A.; Manzano, M. Post-treatment of biologically treated wastewater containing organic contaminants using a sequence of H2O2 based advanced oxidation processes: Photolysis and catalytic wet oxidation. Water Res. 2015, 71, 85–96. [Google Scholar] [CrossRef]
- Bhargava, S.K.; Tardio, J.; Prasad, J.; Föger, K.; Akolekar, D.B.; Grocott, S.C. Wet Oxidation and Catalytic Wet Oxidation. Ind. Eng. Chem. Res. 2006, 45, 1221–1258. [Google Scholar] [CrossRef]
- Radha, K.V.; Sridevi, V.; Kalaivani, K. Electrochemical oxidation for the treatment of textile industry wastewater. Bioresour. Technol. 2009, 100, 987–990. [Google Scholar] [CrossRef]
- Martínez-Huitle, C.A.; Panizza, M. Electrochemical oxidation of organic pollutants for wastewater treatment. Curr. Opin. Electrochem. 2018, 11, 62–71. [Google Scholar] [CrossRef]
- Sanz, J.L.; Köchling, T. Molecular biology techniques used in wastewater treatment: An overview. Process Biochem. 2007, 42, 119–133. [Google Scholar] [CrossRef]
- Ji, Y.; Wang, Y.; Sun, J.; Yan, T.; Li, J.; Zhao, T.; Yin, X.; Sun, C. Enhancement of biological treatment of wastewater by magnetic field. Bioresour. Technol. 2010, 101, 8535–8540. [Google Scholar] [CrossRef]
- Ma, X.; Wang, A.; Miao, J.; Fan, T. 2D lamellar membrane with MXene hetero-intercalated small sized graphene oxide for harsh environmental wastewater treatment. Sep. Purif. Technol. 2023, 311, 123248. [Google Scholar] [CrossRef]
- Kusworo, T.D.; Kumoro, A.C.; Aryanti, N.; Hasbullah, H.; Chaesarifa, D.R.S.; Fauzan, M.D.; Dalanta, F. Developing a robust photocatalytic and antifouling performance of PVDF membrane using spinel NiFe2O4/GO photocatalyst for efficient industrial dye wastewater treatment. J. Environ. Chem. Eng. 2023, 11, 109449. [Google Scholar] [CrossRef]
- Park, S.; Kim, Y.; Lee, W.; Nam, C. Superhydrophobic polypropylene sorbent derived from discarded face masks: A highly efficient adsorbent for oil spill sorbent. Chemosphere 2022, 303, 135186. [Google Scholar] [CrossRef]
- Kang, J.; Kim, H.; Nam, C. Ultrafast and on-demand oil/water separation with vertically aligned cellulosic smart sponge. J. Hazard. Mater. 2023, 445, 130559. [Google Scholar] [CrossRef]
- Le, T.M.H.; Singto, S.; Sajomsang, W.; Mongkolnavin, R.; Nuisin, R.; Painmanakul, P.; Sairiam, S. Hydrophobic PVDF hollow fiber membrane modified with pulse inductively coupling plasma activation and chloroalkylsilanes for efficient dye wastewater treatment by ozonation membrane contactor. J. Memb. Sci. 2021, 635, 119443. [Google Scholar] [CrossRef]
- Tyszler, D.; Zytner, R.G.; Batsch, A.; Brügger, A.; Geissler, S.; Zhou, H.; Klee, D.; Melin, T. Reduced fouling tendencies of ultrafiltration membranes in wastewater treatment by plasma modification. Desalination 2006, 189, 119–129. [Google Scholar] [CrossRef]
- Varin, K.J.; Lin, N.H.; Cohen, Y. Biofouling and cleaning effectiveness of surface nanostructured reverse osmosis membranes. J. Memb. Sci. 2013, 446, 472–481. [Google Scholar] [CrossRef]
- Sheikh, M.; Pazirofteh, M.; Dehghani, M.; Asghari, M.; Rezakazemi, M.; Valderrama, C.; Cortina, J.-L. Application of ZnO nanostructures in ceramic and polymeric membranes for water and wastewater technologies: A review. Chem. Eng. J. 2020, 391, 123475. [Google Scholar] [CrossRef]
- Enemuo, N.; Richards, H.; Daramola, M.O. Preparation of hydrophilic PVDF membrane via blending with Fe3O4 nanoparticles and PVA for improved membrane performance in BTEX removal from wastewater. J. Environ. Chem. Eng. 2025, 13, 116135. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, C.; Yang, F. TiO2 and polyvinyl alcohol (PVA) coated polyester filter in bioreactor for wastewater treatment. Water Res. 2012, 46, 1969–1978. [Google Scholar] [CrossRef]
- Nayak, K.; Tripathi, B.P. Molecularly grafted PVDF membranes with in-air superamphiphilicity and underwater superoleophobicity for oil/water separation. Sep. Purif. Technol. 2021, 259, 118068. [Google Scholar] [CrossRef]
- Guillen, G.R.; Pan, Y.; Li, M.; Hoek, E.M.V. Preparation and Characterization of Membranes Formed by Nonsolvent Induced Phase Separation: A Review. Ind. Eng. Chem. Res. 2011, 50, 3798–3817. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Ideris, N.; Ooi, B.S.; Low, S.C.; Ismail, A. Synthesis of polyvinylidene fluoride (PVDF) membranes for protein binding: Effect of casting thickness. J. Appl. Polym. Sci. 2013, 128, 3438–3445. [Google Scholar] [CrossRef]
- Prihatiningtyas, I.; Li, Y.; Hartanto, Y.; Vananroye, A.; Coenen, N.; Van der Bruggen, B. Effect of solvent on the morphology and performance of cellulose triacetate membrane/cellulose nanocrystal nanocomposite pervaporation desalination membranes. Chem. Eng. J. 2020, 388, 124216. [Google Scholar] [CrossRef]
- Karimi, A.; Khataee, A.; Vatanpour, V.; Safarpour, M. The effect of different solvents on the morphology and performance of the ZIF-8 modified PVDF ultrafiltration membranes. Sep. Purif. Technol. 2020, 253, 117548. [Google Scholar] [CrossRef]
- Mallick, Z.; Sarkar, R.; Kundu, T.K.; Mandal, D. Molecular dipole regulated surface potential and ferroelectric characteristics in nanoconfined P(VDF-TrFE) architectures. Appl. Surf. Sci. 2024, 646, 158925. [Google Scholar] [CrossRef]
- Sridhar, C.; Neha; Seo, Y.-S.; Rabani, I.; Turpu, G.; Tigga, S.; Padmaja, G. Electrochemical, Photocatalytic and Photoluminescence properties of BaWO4 and rGO-BaWO4 nano-composites: A Comparative study. Curr. Appl. Phys. 2024, 58, 79–90. [Google Scholar] [CrossRef]
- Yao, M.; Ren, J.; Akther, N.; Woo, Y.C.; Tijing, L.D.; Kim, S.-H.; Shon, H.K. Improving membrane distillation performance: Morphology optimization of hollow fiber membranes with selected non-solvent in dope solution. Chemosphere 2019, 230, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Lalia, B.S.; Kochkodan, V.; Hashaikeh, R.; Hilal, N. A review on membrane fabrication: Structure, properties and performance relationship. Desalination 2013, 326, 77–95. [Google Scholar] [CrossRef]
- Jung, J.T.; Kim, J.F.; Wang, H.H.; di Nicolo, E.; Drioli, E.; Lee, Y.M. Understanding the non-solvent induced phase separation (NIPS) effect during the fabrication of microporous PVDF membranes via thermally induced phase separation (TIPS). J. Memb. Sci. 2016, 514, 250–263. [Google Scholar] [CrossRef]
- Kolb, H.; Schmitt, R.; Dittler, A.; Kasper, G. On the accuracy of capillary flow porometry for fibrous filter media. Sep. Purif. Technol. 2018, 199, 198–205. [Google Scholar] [CrossRef]
- Tan, K.; Obendorf, S.K. Surface modification of microporous polyurethane membrane with poly(ethylene glycol) to develop a novel membrane. J. Memb. Sci. 2006, 274, 150–158. [Google Scholar] [CrossRef]
- Ley, A.; Altschuh, P.; Thom, V.; Selzer, M.; Nestler, B.; Vana, P. Characterization of a macro porous polymer membrane at micron-scale by Confocal-Laser-Scanning Microscopy and 3D image analysis. J. Memb. Sci. 2018, 564, 543–551. [Google Scholar] [CrossRef]
- Xiao, T.; Wang, P.; Yang, X.; Cai, X.; Lu, J. Fabrication and characterization of novel asymmetric polyvinylidene fluoride (PVDF) membranes by the nonsolvent thermally induced phase separation (NTIPS) method for membrane distillation applications. J. Memb. Sci. 2015, 489, 160–174. [Google Scholar] [CrossRef]
- Ashtiani, S.; Khoshnamvand, M.; Číhal, P.; Dendisová, M.; Randová, A.; Bouša, D.; Shaliutina-Kolešová, A.; Sofer, Z.; Friess, K. Fabrication of a PVDF membrane with tailored morphology and properties via exploring and computing its ternary phase diagram for wastewater treatment and gas separation applications. RSC Adv. 2020, 10, 40373–40383. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.; Wang, B.; Li, K. High-performance PVDF membranes prepared by the combined crystallisation and diffusion (CCD) method using a dual-casting technique: A breakthrough for water treatment applications. Energy Environ. Sci. 2021, 14, 5491–5500. [Google Scholar] [CrossRef]
- Thomas, R.; Guillen-Burrieza, E.; Arafat, H.A. Pore structure control of PVDF membranes using a 2-stage coagulation bath phase inversion process for application in membrane distillation (MD). J. Memb. Sci. 2014, 452, 470–480. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Q.; Miao, W.; Lu, P.; You, C.; Wang, Z. Hydrophilic PVDF membrane with versatile surface functions fabricated via cellulose molecular coating. J. Memb. Sci. 2021, 640, 119817. [Google Scholar] [CrossRef]
- He, T.; Mulder, M.; Strathmann, H.; Wessling, M. Preparation of composite hollow fiber membranes: Co-extrusion of hydrophilic coatings onto porous hydrophobic support structures. J. Memb. Sci. 2002, 207, 143–156. [Google Scholar] [CrossRef]
- Eom, J.; Kwak, Y.; Nam, C. Electrospinning fabrication of magnetic nanoparticles-embedded polycaprolactone (PCL) sorbent with enhanced sorption capacity and recovery speed for spilled oil removal. Chemosphere 2022, 303, 135063. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, H.S.; Han, X.; Nowinski, A.K.; Ella-Menye, J.-R.; Wimbish, C.; Marek, P.; Senecal, K.; Jiang, S. One-Step Dip Coating of Zwitterionic Sulfobetaine Polymers on Hydrophobic and Hydrophilic Surfaces. ACS Appl. Mater. Interfaces 2014, 6, 6664–6671. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, L.; Deng, F.; Zhao, D.; Zhang, C.; Zhang, C. Hydrophilic modification of PVDF porous membrane via a simple dip-coating method in plant tannin solution. RSC Adv. 2016, 6, 71287–71294. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, Q.; Li, S.; Zhang, S. Fabrication of thin film composite nanofiltration membranes by coating water soluble disulfonated poly(arylene ether sulfone) and in situ crosslinking. Desalination 2016, 387, 25–34. [Google Scholar] [CrossRef]
- Sakarkar, S.; Muthukumaran, S.; Jegatheesan, V. Evaluation of polyvinyl alcohol (PVA) loading in the PVA/titanium dioxide (TiO2) thin film coating on polyvinylidene fluoride (PVDF) membrane for the removal of textile dyes. Chemosphere 2020, 257, 127144. [Google Scholar] [CrossRef]
- Yuan, H.-K.; Ren, J.; Ma, X.-H.; Xu, Z.-L. Dehydration of ethyl acetate aqueous solution by pervaporation using PVA/PAN hollow fiber composite membrane. Desalination 2011, 280, 252–258. [Google Scholar] [CrossRef]
- Nam, C.; Zimudzi, T.J.; Wiencek, R.A.; Chung, T.M.; Hickner, M.A. Improved ATR-FTIR detection of hydrocarbons in water with semi-crystalline polyolefin coatings on ATR elements. Analyst 2018, 143, 5589–5596. [Google Scholar] [CrossRef]
- Shalaby, M.S.; Sołowski, G.; Abbas, W. Recent Aspects in Membrane Separation for Oil/Water Emulsion. Adv. Mater. Interfaces 2021, 8, 2100448. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, G.; Bai, R.; Shen, S.; Zhou, X.; Wyman, I. Fabrication of superhydrophilic and underwater superoleophobic membranes via an in situ crosslinking blend strategy for highly efficient oil/water emulsion separation. J. Memb. Sci. 2019, 569, 60–70. [Google Scholar] [CrossRef]
- Carballo, G.; Yang, H.-L.; Hsu, Y.-X.; Leron, R.; Tsai, H.-A.; Lee, K.-R. Incorporation of zwitterionic carbon quantum dots in cellulose acetate tubular membrane for oil/water separation. Sep. Purif. Technol. 2024, 337, 126301. [Google Scholar] [CrossRef]
- Gu, Y.; Zhang, B.; Fu, Z.; Li, J.; Yu, M.; Li, L.; Li, J. Poly (vinyl alcohol) modification of poly(vinylidene fluoride) microfiltration membranes for oil/water emulsion separation via an unconventional radiation method. J. Memb. Sci. 2021, 619, 118792. [Google Scholar] [CrossRef]
- Wu, J.; Hou, Z.; Yu, Z.; Lang, J.; Cui, J.; Yang, J.; Dai, J.; Li, C.; Yan, Y.; Xie, A. Facile preparation of metal-polyphenol coordination complex coated PVDF membrane for oil/water emulsion separation. Sep. Purif. Technol. 2021, 258, 118022. [Google Scholar] [CrossRef]
- Tian, S.; Zhang, Y.; Sha, Q.; Zhang, X.; Yang, T.; Yan, X.; Han, N. PPS/TA-PEI/β-FeOOH membranes with powerful photo-Fenton self-cleaning capability for efficient oil–water emulsion separation. Chem. Eng. J. 2024, 485, 150069. [Google Scholar] [CrossRef]

| Membrane | Pure Water Flux (L m−2 h−1 bar−1) | Emulsion Type | Emulsion Flux (L m−2 h−1 bar−1) | Oil Rejection (%) | Ref. |
|---|---|---|---|---|---|
| M40 | - | 1 wt% hexadecane | 90.0 | 98.0 | [49] |
| ZQDs/CA | 581.3 | 1 wt% diesel | 350.5 | 98.0 | [50] |
| m-PVDF-2.5 | ~3700 | 1 wt% n-dodecane | 690 | 99.5 | [51] |
| TA-Ti-5@PVDF | 7643.5 | 1 wt% toluene | 802.5 | 98.5 | [52] |
| PPS/TA-PEI/β-FeOOH-15 | ~6200 | 1 wt% hexane | 3770.9 | 98.1 | [53] |
| NMP60/PVA5 | 5515.6 | 1 wt% toluene | 4412.16 | 98.3 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, Y.; Nam, C. Fabrication of PVDF Membranes with a PVA Layer for the Effective Removal of Volatile Organic Compounds in Semiconductor Wastewater. Polymers 2025, 17, 1332. https://doi.org/10.3390/polym17101332
Choi Y, Nam C. Fabrication of PVDF Membranes with a PVA Layer for the Effective Removal of Volatile Organic Compounds in Semiconductor Wastewater. Polymers. 2025; 17(10):1332. https://doi.org/10.3390/polym17101332
Chicago/Turabian StyleChoi, Youngmin, and Changwoo Nam. 2025. "Fabrication of PVDF Membranes with a PVA Layer for the Effective Removal of Volatile Organic Compounds in Semiconductor Wastewater" Polymers 17, no. 10: 1332. https://doi.org/10.3390/polym17101332
APA StyleChoi, Y., & Nam, C. (2025). Fabrication of PVDF Membranes with a PVA Layer for the Effective Removal of Volatile Organic Compounds in Semiconductor Wastewater. Polymers, 17(10), 1332. https://doi.org/10.3390/polym17101332






