Application of Gellan Hydrogel and Kaz-6 in Wheat Seed Coating for Improved Productivity and Environmental Resilience
Abstract
1. Introduction
2. Materials and Methods
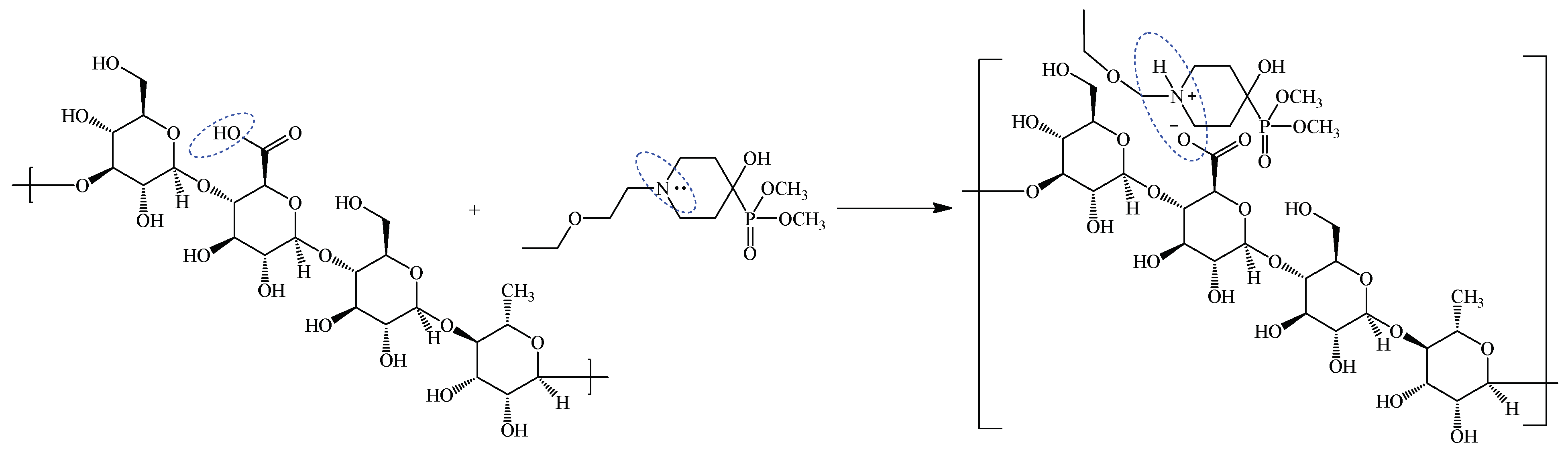
2.1. Preparation of Gellan-Based Hydrogel for Seed Coating
2.2. Measurement of Elastic Modulus of Obtained Hydrogels and Calculation of Polymer Network Structural Parameters
2.3. Seed Coating Process
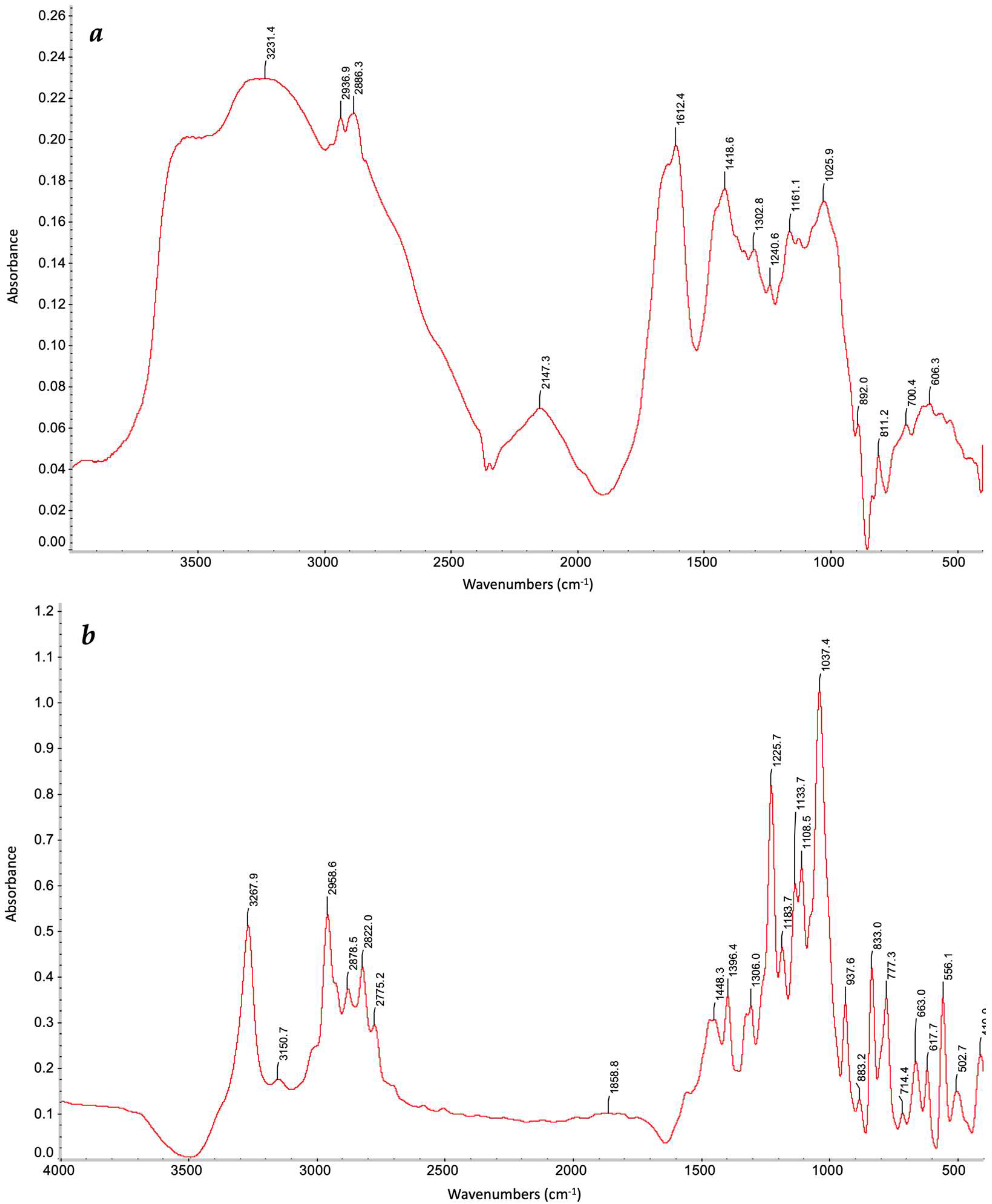
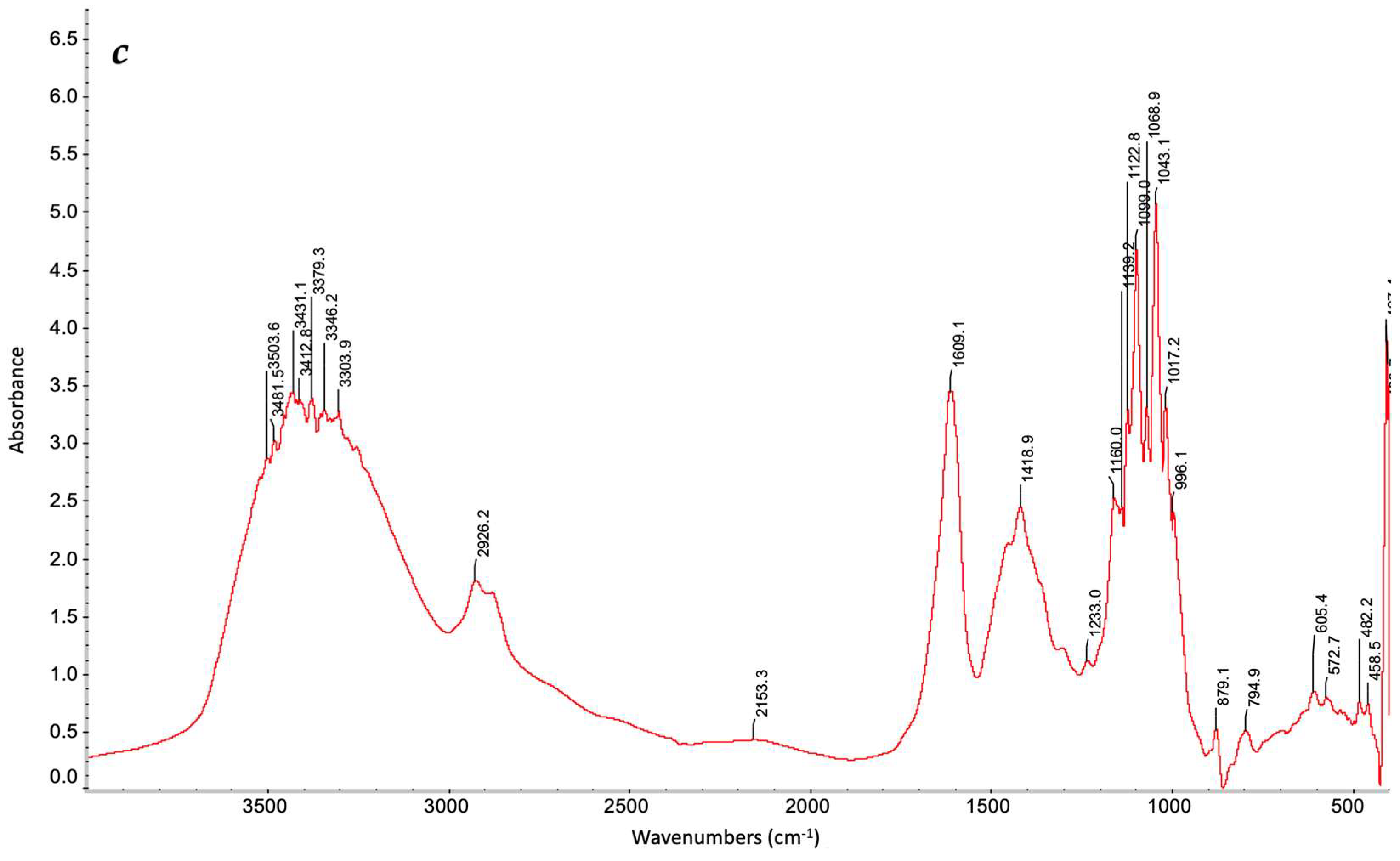
2.4. Characterization of Gel Formation
2.5. Laboratory Germination Tests
2.6. Effect of Hydrogel Layer Thickness
2.7. Drought Resistance Testing
2.8. Greenhouse Trials
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dias, T.A.C.; Lora, E.E.S.; Maya, D.M.Y.; del Olmo, O.A. Global potential assessment of available land for bioenergy projects in 2050 within food security limits. Land Use Policy 2021, 105, 105346. [Google Scholar] [CrossRef]
- Sharma, N.; Bohra, B.; Pragya, N.; Ciannella, R.; Dobie, P.; Lehmann, S. Bioenergy from agroforestry can lead to improved foodsecurity, climate change, soil quality, and rural development. Food Energy Secur. 2016, 5, 165–183. [Google Scholar] [CrossRef]
- Jain, S.; Srivastava, A.; Khadke, L.; Chatterjee, U.; Elbeltagi, A. Global-scale water security and desertification management amidst climate change. Environ. Sci. Pollut. Res. Int. 2024, 31, 58720–58744. [Google Scholar] [CrossRef]
- Lewicka, K.; Szymanek, I.; Rogacz, D.; Wrzalik, M.; Lagiewka, J.; Nowik-Zając, A.; Zawierucha, I.; Coseri, S.; Puiu, I.; Falfushynska, H.; et al. Current Trends of Polymer Materials’ Application in Agriculture. Sustainability 2024, 16, 8439. [Google Scholar] [CrossRef]
- Ehsanfar, S.; Modarres-Sanavy, S.A. Crop protection by seed coating. Commun. Agric. Appl. Biol. Sci. 2005, 70, 225–229. [Google Scholar] [PubMed]
- Su, L.Q.; Li, J.G.; Xue, H.; Wang, X.F. Super absorbent polymer seed coatings promote seed germination and seedling growth of Caragana korshinskii in drought. J. Zhejiang Univ. Sci. B 2017, 18, 696–706. [Google Scholar] [CrossRef]
- Sohail, M.; Pirzada, T.; Opperman, C.H.; Khan, S.K. Recent advances in seed coating technologies: Transitioning toward sustainable agriculture. Green. Chem. 2022, 24, 6052–6085. [Google Scholar] [CrossRef]
- Kumar, P.; Meena; Tanveer, N.; Dhiman, S.; Rajput, S.; Rajput, M.; Rajput, Y.; Pandey, N. A Review on Seed Storage Technology: Recent Trends and Advances in Sustainable Techniques for Global Food Security. AgroEnviron. Sustain. 2024, 2, 34–50. [Google Scholar] [CrossRef]
- Seed Coating Market by Additive Type (Polymers, Colorants, Binders, Minerals/Pumice, Active Ingredients), Crop Type, Process (Film Coating, Pelleting, Encrusting), Coating Type (Bio-Based, Synthetic), Form, and Region–Global Forecast to 2030. Report Code: AGI 2754. Published: April 2025. Available online: https://www.marketsandmarkets.com/Market-Reports/seed-coating-materials-market-149045530.html (accessed on 14 April 2025).
- Ioannou, A.; Gohari, G.; Papaphilippou, P.; Panahirad, S.; Akbari, A.; Dadpour, M.R.; Krasia-Christoforou, T.; Fotopoulos, V. Advanced nanomaterials in agriculture under a changing climate: The way to the future? Environ. Exp. Bot. 2020, 176, 104048. [Google Scholar] [CrossRef]
- Patel, C.; Singh, J.; Karunakaran, A.; Ramakrishna, W. Evolution of Nano-Biofertilizer as a Green Technology for Agriculture. Agriculture 2023, 13, 1865. [Google Scholar] [CrossRef]
- Kaur, P.; Agrawal, R.; Pfeffer, F.M.; Williams, R.; Bohidar, H. Hydrogels in Agriculture: Prospects and Challenges. J. Polym. Environ. 2023, 31, 3701–3718. [Google Scholar] [CrossRef]
- Patra, S.K.; Poddar, R.; Brestic, M.; Acharjee, P.U.; Bhattacharya, P.; Sengupta, S.; Pal, P.; Bam, N.; Biswas, B.; Barek, V.; et al. Prospects of Hydrogels in Agriculture for Enhancing Crop and Water Productivity under Water Deficit Condition. Int. J. Polym. Sci. 2022, 2022, 4914836. [Google Scholar] [CrossRef]
- Rathore, S.S.; Shekhawat, K.; Babu, S.; Singh, V.K. Mitigating moisture stress in Brassica juncea through deficit irrigation scheduling and hydrogel in ustocherpts soils of semi-arid India. Heliyon 2020, 6, e05786. [Google Scholar] [CrossRef]
- Krasnopeeva, E.L.; Panova, G.G.; Yakimansky, A.V. Agricultural Applications of Superabsorbent Polymer Hydrogels. Int. J. Mol. Sci. 2022, 23, 15134. [Google Scholar] [CrossRef]
- Ghobashy, M.M. Chapter 12—The application of natural polymer-based hydrogels for agriculture. In Hydrogels Based on Natural Polymers; Elsevier: Amsterdam, The Netherlands, 2020; pp. 329–356. [Google Scholar] [CrossRef]
- Ali, K.; Asad, Z.; Agbna, G.H.D.; Saud, A.; Khan, A.; Zaidi, S.J. Progress and Innovations in Hydrogels for Sustainable Agriculture. Agronomy 2024, 14, 2815. [Google Scholar] [CrossRef]
- Woodhouse, J.M.; Johnson, M.S. The effect of gel-forming polymers on seed germination and establishment. J. Arid. Environ. 1991, 20, 375–380. [Google Scholar] [CrossRef]
- Amirkhani, M.; Mayton, H.; Loos, M.; Taylor, A. Development of Superabsorbent Polymer (SAP) Seed Coating Technology to Enhance Germination and Stand Establishment in Red Clover Cover Crop. Agronomy 2023, 13, 438. [Google Scholar] [CrossRef]
- Pathak, V.; Kingsly Ambrose, R.P. Starch-based biodegradable hydrogel as seed coating for corn to improve early growth under water shortage. J. Appl. Polym. Sci. 2020, 137, 48523. [Google Scholar] [CrossRef]
- Skrzypczak, D.; Jarzembowski, Ł.; Izydorczyk, G.; Mikula, K.; Hoppe, V.; Mielko, K.A.; Pudełko-Malik, N.; Młynarz, P.; Chojnacka, K.; Witek-Krowiak, A. Hydrogel Alginate Seed Coating as an Innovative Method for Delivering Nutrients at the Early Stages of Plant Growth. Polymers 2021, 13, 4233. [Google Scholar] [CrossRef]
- Morris, E.R.; Nishinari, K.; Rinaudo, M. Gelation of gellan—A review. Food Hydrocoll. 2012, 28, 373–411. [Google Scholar] [CrossRef]
- Morris, E.R.; Gothard, M.G.E.; Hember, M.W.N.; Manning, C.E.; Robinson, G. Conformational and rheological transitions of welan, rhamsan and acylated gellan. Carbohydr. Polym. 1996, 30, 165–175. [Google Scholar] [CrossRef]
- Hu, C.; Yang, X.; Atya, M.E.; Kohinata, Y.; Kimura, M.; Matsukawa, S. Gelation mechanism of gellan in coexisting trivalent with monovalent cations as studied by NMR and particle tracking. Food Hydrocoll. 2025, 167, 111437. [Google Scholar] [CrossRef]
- Yao, H.-H.-Y.; Wang, J.-Q.; Yin, J.-Y.; Nie, S.-P.; Xie, M.-Y. A review of NMR analysis in polysaccharide structure and conformation: Progress, challenge and perspective. Int. Food Res. 2021, 143, 110290. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Si, Y.; Zhou, F.; Hao, W.; Zhang, P.; Jiang, P.; Cha, R. Advances in polysaccharides for probiotic delivery: Properties, methods, and applications. Carbohydr. Polym. 2024, 323, 121414. [Google Scholar] [CrossRef] [PubMed]
- Yu, V.K.; Praliyev, K.D. Prev. Pat. 5011 RK. 1-(2-Ethoxyethyl)-4-(Dimethoxyphosphoryl)-4-Hydroxypiperidine, Which Possesses Plant Growth Stimulating Activity. Available online: https://kz.patents.su/0-pp5011-1-2-etoksietil-4-dimetoksifosforil-4-gidroksipiperidin-obladayushhijj-stimuliruyushhejj-rost-rastenijj-aktivnostyu.html (accessed on 13 February 2025).
- Kystaubayeva, N.U.; Durap, F.; Zharkynbek, T.Y.; Yu, V.K.; Aydemir, M.; Meric, N.; Zazybin, A.G.; Ten, A.Y.; Rafikova, K.S.; Binbay, N.E.; et al. Synthesis, and characterization of palladium (II) and platinum (II) Complexes with α-hydroxy [1-(2-ethoxyethyl) piperidin-4-yl]phosphonate and se of the palladium (II) complex as pre-catalyst in Suzuki-Miyaura cross-coupling reactions. J. Mol. Struct. 2022, 1270, 133912. [Google Scholar] [CrossRef]
- Fasina, A.B.; Stepto, R.F.T. Formation and properties of triol-based polyester networks. Die Makromol. Chem. 1981, 182, 2479–2493. [Google Scholar] [CrossRef]
- Agibayeva, L.E.; Kaldybekov, D.B.; Porfiryeva, N.N.; Garipova, V.R.; Mangazbayeva, R.A.; Moustafine, R.I.; Semina, I.I.; Mun, G.A.; Kudaibergenov, S.E.; Khutoryanskiy, V.V. Gellan gum and its methacrylated derivatives as in situ gelling mucoadhesive formulations of pilocarpine: In vitro and in vivo studies. Int. J. Pharm. 2020, 577, 119093. [Google Scholar] [CrossRef]
- Flores-Huicochea, E.; Rodríguez-Hernández, A.I.; Espinosa-Solares, T.; Tecante, A. Sol-gel transition temperatures of high acyl gellan with monovalent and divalent cations from rheological measurements. Food Hydrocoll. 2013, 31, 299–305. [Google Scholar] [CrossRef]
- Dobre, V.; Velescu, I.; Rosca, R. Research Regarding Barley Germination with the Jacobsen Machine Used for Obtaining Green Malt. Bull. UASVM Agric. 2012, 69, 207–213. [Google Scholar]
- Yang, L.; Paulson, A.T.; Nickerson, M.T. Mechanical and physical properties of calcium-treated gellan films. J. Food Res. Int. 2010, 43, 1439–1443. [Google Scholar] [CrossRef]
- Yagobin, B.A.; Zhukov, Y.P.; Kozbarenko, B.I. Agrochemistry; Yagodin B.A.: Moscow, Russia, 2002; 584p. [Google Scholar]
- Xu, L.; Dong, M.; Gong, H.; Sun, M.; Li, Y. Effects of inorganic cations on the rheology of aqueous welan, xanthan, gellan solutions and their mixtures. Carbohydr. Polym. 2015, 121, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Pandit, J.K. Comparative evaluation of Ca++ and Zn++ cross-linked gellan gum based floating beads. J. Arch. Des. Sci. 2012, 65, 75–84. [Google Scholar]
- Kystaubayeva, N.; Praliyev, K.D.; Iskakova, T.K.; Al-Saed, N.; Seilkhanov, T.M.; Zazybin, A.; Yu, V. Novel α-hydroxy[1-(2-ethoxyethyl)piperidin-4-yl]phosphonate as a candidate for multi-purpose substances. In Proceedings of the 4th International Congress on Technology—Engineering & Science (ICONTES), Kuala Lumpur, Malaysia, 5–6 August 2017; p. 112. Available online: https://www.academia.edu/35386478/PROCEDIA (accessed on 25 January 2025).
- Kystaubayeva, N.; Zharkynbek, T.; Rakhmatulina, R.; Faskhutdinov, M.; Malmakova, A.E.; Zhumakova, S.S.; Praliyev, K.D.; Yu, V.K. Complexes of 1-(2-ethoxyethyl)-4-(dimethoxyphosphoryl)-4-hydroxypiperidine with ions of biogenic metals: Synthesis and properties. Chem. J. Kaz. 2018, 3, 144–154. Available online: https://chemjournal.kz/index.php/journal/article/view/336/303 (accessed on 31 January 2025).



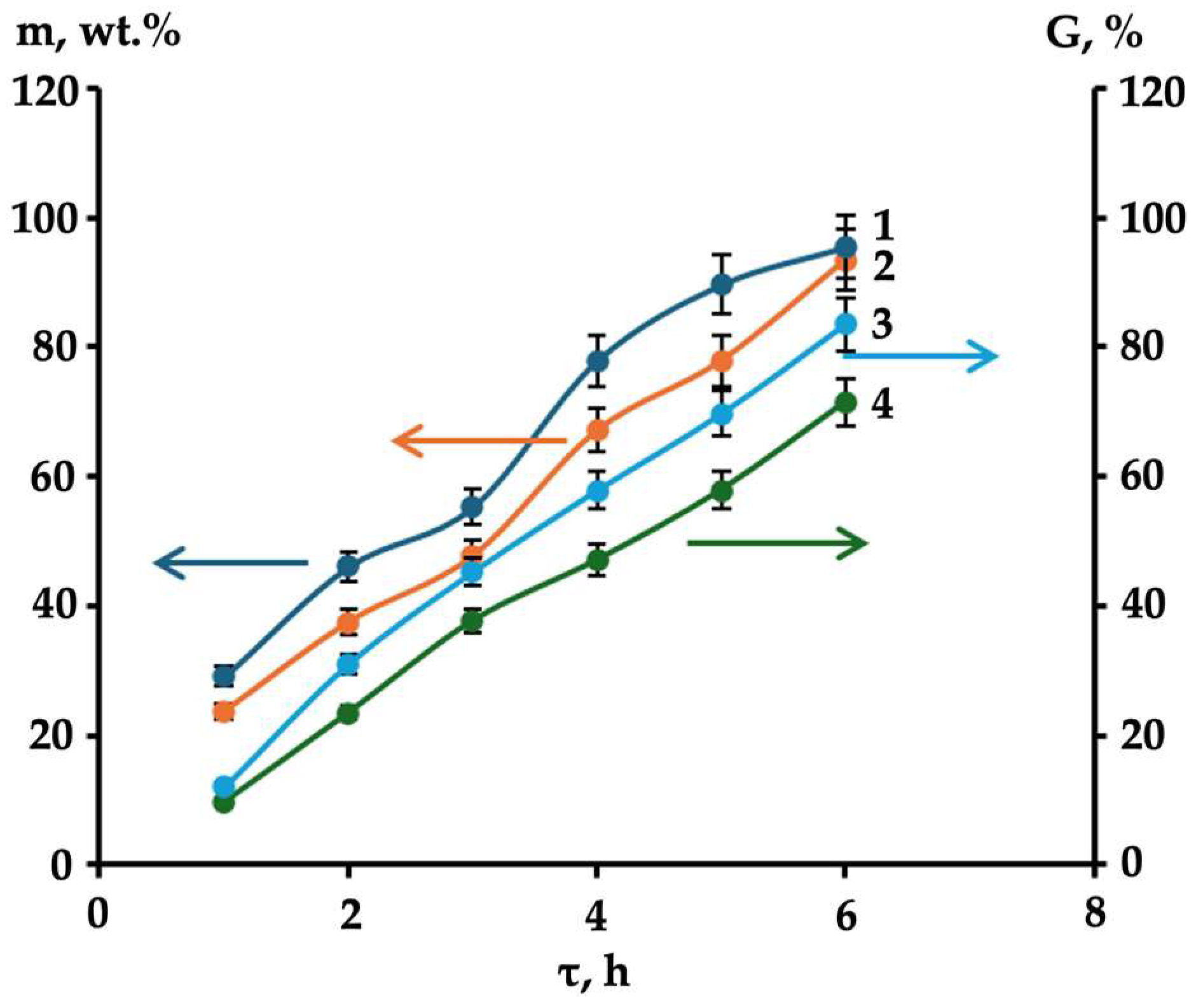


| Gel Formation Time (τ), h | Equilibrium Swelling Degree in Water (α) of the Gellan Hydrogel Formed Upon Salt Addition to the Aqueous Gellan Solution, g/g | |||
|---|---|---|---|---|
| Potassium Chloride Concentration 0.08 mol/L | Potassium Chloride Concentration 0.12 mol/L | Ammonium Sulfate Concentration 0.08 mol/L | Ammonium Sulfate Concentration 0.12 mol/L | |
| 1.0 | 275.7 ± 5.5 | 226.9 ± 5.3 | 209.5 ± 4.3 | 199.1 ± 3.3 |
| 2.0 | 247.4 ± 3.3 | 216.9 ± 3.1 | 197.7 ± 2.9 | 175.3 ± 2.9 |
| 3.0 | 212.5 ± 3.1 | 193.1 ± 2.9 | 167.1 ± 2.9 | 147.9 ± 2.7 |
| 4.0 | 183.9 ± 2.9 | 173.7 ± 2.9 | 137.9 ± 2.7 | 129.7 ± 2.5 |
| 6.0 | 147.4 ± 2.9 | 117.7 ± 2.5 | 107.5 ± 2.6 | 93.5 ± 2.5 |
| Gel Formation Time (τ), h | Elastic Modulus (G) of Gellan Hydrogels Formed Upon Salt Addition to Aqueous Gellan Solution, kPa | |||
|---|---|---|---|---|
| Potassium Chloride Concentration 0.08 mol/L | Potassium Chloride Concentration 0.12 mol/L | Ammonium Sulfate Concentration 0.08 mol/L | Ammonium Sulfate Concentration 0.12 mol/L | |
| 1.0 | 0.75 ± 0.25 | 0.95 ± 0.15 | 1.11 ± 0.25 | 1.31 ± 0.25 |
| 1.0 | 0.91 ± 0.15 | 1.31 ± 0.25 | 1.51 ± 0.21 | 1.71 ± 0.21 |
| 3.0 | 1.71 ± 0.21 | 1.83 ± 0.35 | 2.73 ± 0.35 | 3.93 ± 0.35 |
| Gel Formation Time (τ), h | Values of the Cross-Linking Density Parameter (nc) of Gellan Hydrogel Formed Upon Salt Addition to Aqueous Gellan Solution, mol/m3 | |||
|---|---|---|---|---|
| Potassium Chloride Concentration 0.08 mol/L | Potassium Chloride Concentration 0.12 mol/L | Ammonium Sulfate Concentration 0.08 mol/L | Ammonium Sulfate Concentration 0.12 mol/L | |
| 1.0 | 0.302 ± 0.101 | 0.383 ± 0.061 | 0.448 ± 0.101 | 0.528 ± 0.101 |
| 3.0 | 0.367 ± 0.061 | 0.529 ± 0.101 | 0.609 ± 0.085 | 0.690 ± 0.085 |
| 6.0 | 0.690 ± 0.085 | 0.739 ± 0.141 | 1.102 ± 0.141 | 1.586 ± 0.141 |
| Gel Formation Time (τ), h | Values of the Structural Parameter Mc of Gellan Hydrogel Formed Upon Salt Addition to Aqueous Gellan Solution, g/mol | |||
|---|---|---|---|---|
| Potassium Chloride Concentration 0.08 mol/L | Potassium Chloride Concentration 0.12 mol/L | Ammonium Sulfate Concentration 0.08 mol/L | Ammonium Sulfate Concentration 0.12 mol/L | |
| 1.0 | 2973 ± 991 | 2347 ± 371 | 2008 ± 452 | 1702 ± 325 |
| 3.0 | 2450 ± 404 | 1702 ± 325 | 1477 ± 205 | 1304 ± 160 |
| 6.0 | 1304 ± 160 | 1218 ± 233 | 817 ± 105 | 567 ± 51 |
| Solution | pH | Temperature |
|---|---|---|
| Gellan | 6.38 | 24.3 °C |
| Kaz-6 (10−3 %) | 8.48 | |
| Gellan with Kaz-6 | 7.68 |
| Observation Day | No Coating (Control 1) | No Coating in the Presence of Kaz-6 (Control 2) | Coated with Gellan Without Kaz-6 (Control 3) | Coated with Gellan Containing Kaz-6 | ANOVA Results for Data of Comparison of Wheat Seedling Growth Length Under Different Treatment Conditions Over 10 Days | |
|---|---|---|---|---|---|---|
| F-Value | p-Value | |||||
| 1st | 0.0 ± 0.00 cm | 0.0 ± 0.00 cm | 0.0 ± 0.00 cm | 0.0 ± 0.00 cm | ||
| 2nd | 0.0 ± 0.00 cm | 0.1 ± 0.06 cm * | 0.0 ± 0.00 cm | 0.0 ± 0.00 cm | 8.36 | 0.0075 |
| 3rd | 0.0 ± 0.00 cm | 0.2 ± 0.08 cm | 0.0 ± 0.00 cm | 0.0 ± 0.00 cm | 108.54 | <0.0001 |
| 4th | 0.2 ± 0.09 cm | 0.4 ± 0.07 cm | 0.4 ± 0.18 cm | 2.4 ± 0.21 cm * | 136.73 | <0.0001 |
| 5th | 0.4 ± 0.18 cm | 0.9 ± 0.29 cm | 0.8 ± 0.05 cm | 4.0 ± 0.38 cm * | 234.73 | <0.0001 |
| 6th | 0.6 ± 0.24 cm | 1.6 ± 0.18 cm | 1.5 ± 0.09 cm | 6.0 ± 0.41cm * | 198.54 | <0.0001 |
| 7th | 0.8 ± 0.17 cm | 2.0 ± 0.16 cm | 2.1 ± 0.12 cm | 7.4 ± 0.63 cm * | 156.77 | <0.0001 |
| 8th | 1.2 ± 0.12 cm | 2.5 ± 0.39 cm | 2.6 ± 0.33 cm | 10.2 ± 0.85 cm * | 179.04 | <0.0001 |
| 9th | 1.8 ± 0.16 cm | 4.0 ± 0.12 cm | 4.2 ± 0.45 cm | 14.0 ± 1.12 cm * | 213.26 | <0.0001 |
| 10th | 2.5 ± 0.35 cm | 5.0 ± 0.28 cm | 5.5 ± 0.51 cm | 16.0 ± 0.96 cm * | 8.36 | 0.0075 |
| Number of Gellan Layers | 0 (Control) | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|---|
| Shell Thickness, mm | 0 | 0.15 ± 0.03 | 0.35 ± 0.03 | 0.55 ± 0.03 | 0.71 ± 0.04 | 0.85 ± 0.04 |
| Percentage of germinated pellicle seeds, % | (95–100) | (95–100) | (90–100) | (30–40) | (10–20) | (5–10) |
| Average seedling length of wheat seeds 5 days after planting, cm | 0.2 ± 0.09 | 3.0 ± 0.2 | 4.0 ± 0.2 | 6.0 ± 0.6 | 4.0 ± 0.5 | 2.0 ± 0.4 |
| Average seedling length of wheat seeds 10 days after planting | 2.5 ± 0.35 | 10.0 ± 0.4 | 16.0 ± 0.5 | 18.0 ± 0.6 | 14.0 ± 0.5 | 12.0 ± 0.4 |
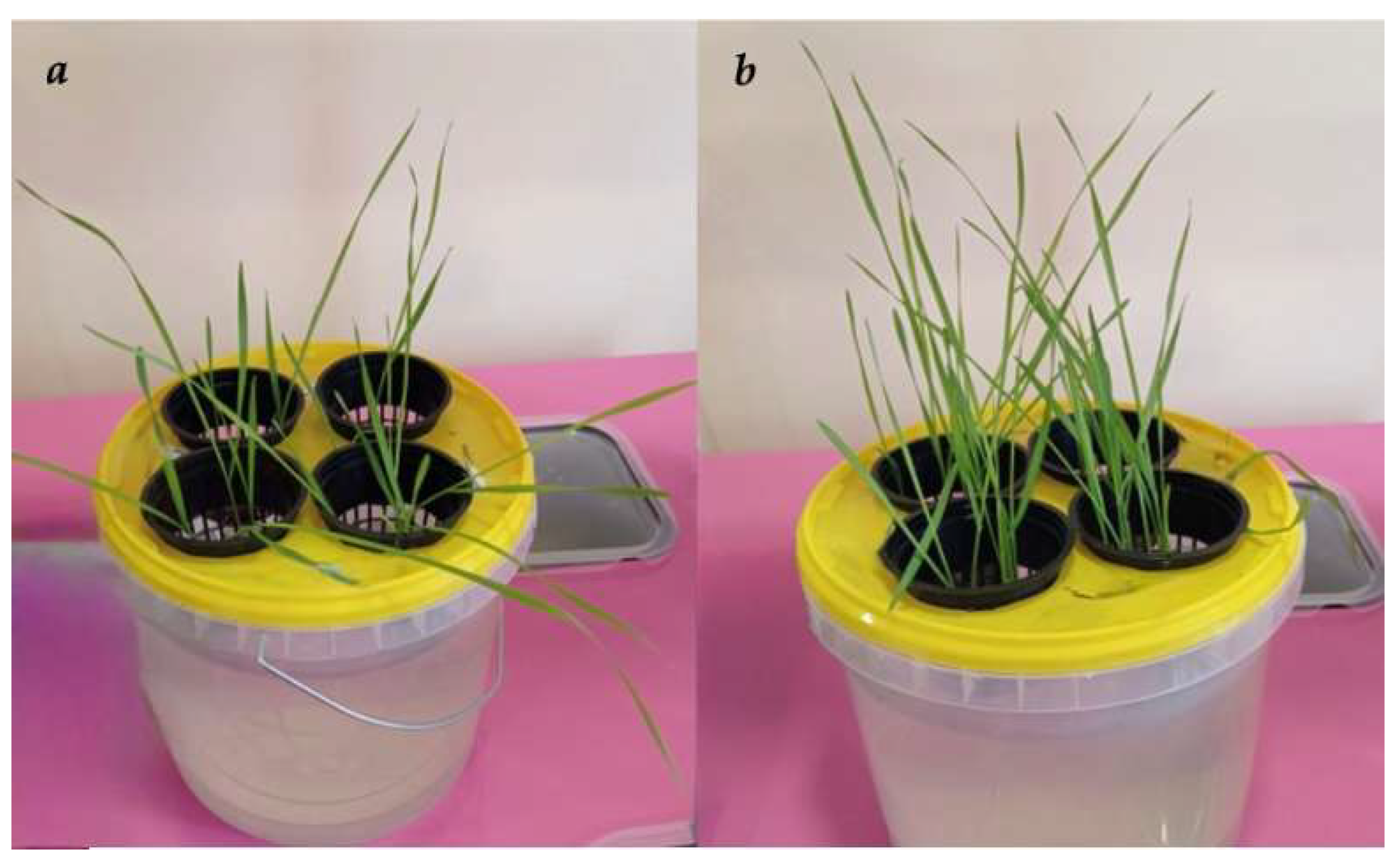
| Observation Day | 5th Day of Observation | 10th Day of Observation |
|---|---|---|
| Control: Uncoated seeds in the presence of Kaz-6 |  |  |
| Coated with gellan hydrogel and Kaz-6 |  |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tursynova, B.; Zharkynbek, T.; Mangazbayeva, R.; Mukhamadiyev, N.; Koizhaiganova, R.; Mengdibayeva, G.; Ten, A.; Yermukhambetova, B.; Mun, G.; Yu, V. Application of Gellan Hydrogel and Kaz-6 in Wheat Seed Coating for Improved Productivity and Environmental Resilience. Polymers 2025, 17, 1330. https://doi.org/10.3390/polym17101330
Tursynova B, Zharkynbek T, Mangazbayeva R, Mukhamadiyev N, Koizhaiganova R, Mengdibayeva G, Ten A, Yermukhambetova B, Mun G, Yu V. Application of Gellan Hydrogel and Kaz-6 in Wheat Seed Coating for Improved Productivity and Environmental Resilience. Polymers. 2025; 17(10):1330. https://doi.org/10.3390/polym17101330
Chicago/Turabian StyleTursynova, Bagila, Tolganay Zharkynbek, Rauash Mangazbayeva, Nurzhan Mukhamadiyev, Raushan Koizhaiganova, Gulnaz Mengdibayeva, Assel Ten, Bayana Yermukhambetova, Grigoriy Mun, and Valentina Yu. 2025. "Application of Gellan Hydrogel and Kaz-6 in Wheat Seed Coating for Improved Productivity and Environmental Resilience" Polymers 17, no. 10: 1330. https://doi.org/10.3390/polym17101330
APA StyleTursynova, B., Zharkynbek, T., Mangazbayeva, R., Mukhamadiyev, N., Koizhaiganova, R., Mengdibayeva, G., Ten, A., Yermukhambetova, B., Mun, G., & Yu, V. (2025). Application of Gellan Hydrogel and Kaz-6 in Wheat Seed Coating for Improved Productivity and Environmental Resilience. Polymers, 17(10), 1330. https://doi.org/10.3390/polym17101330





