Rheological Behavior and Mechanical Performance of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/Natural Rubber Blends Modified with Coffee Oil Epoxide for Sustainable Packaging Applications
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Melt Processing
3. Characterization of PHBV and Its Blends
3.1. ATR-MIR
Chemometric Analysis
3.2. Optical Properties
3.3. Mechanical Testing
3.4. Rheological Properties
4. Results and Discussion
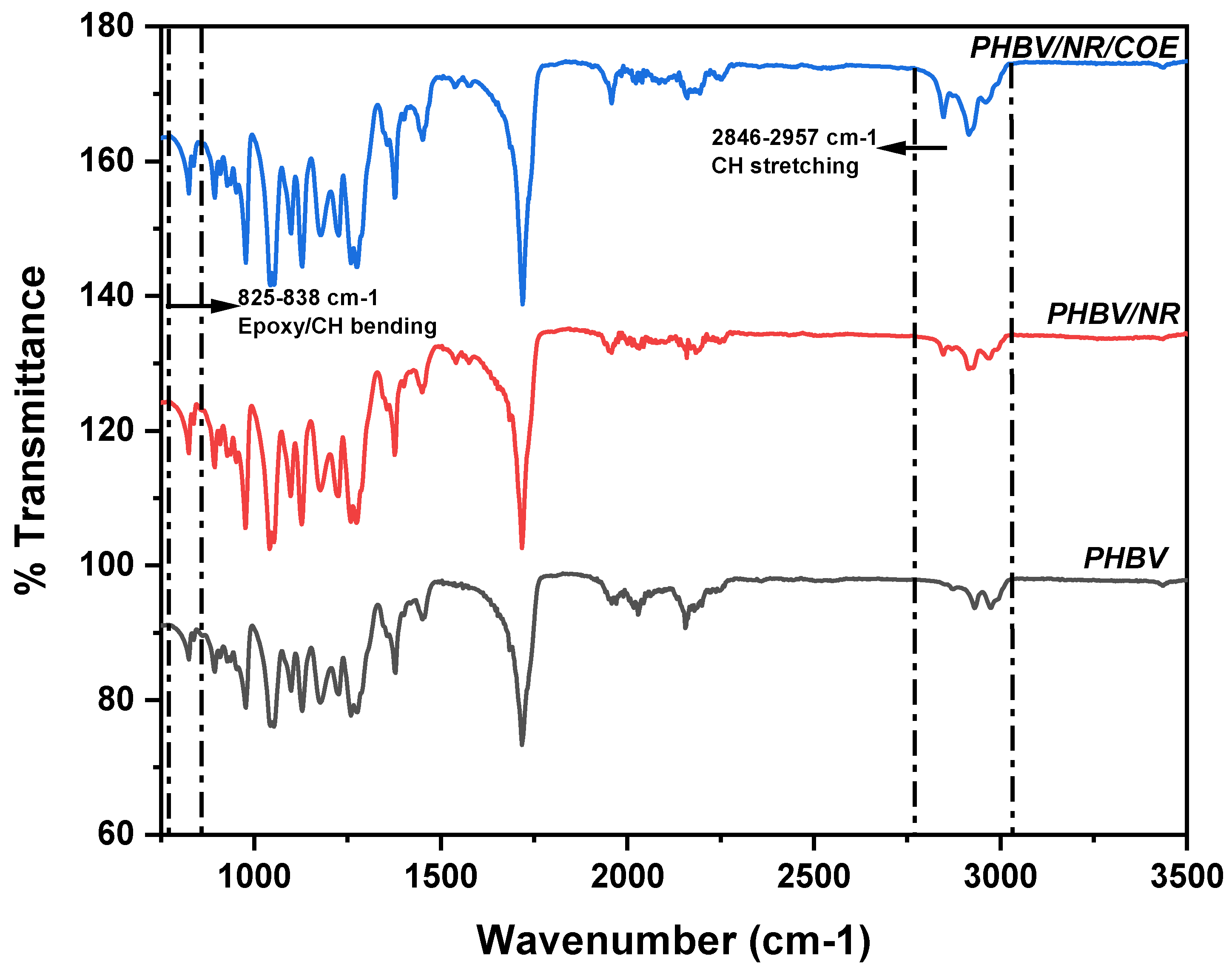
4.1. Effects of Coffee Oil Epoxide (COE) on the Structural Properties of PHBV/NR Blends
4.2. Optical Characteristics of Blended Films with and Without Plasticizers
4.3. Impact of Plasticization on the Rheological Properties of PHBV and Its Blends
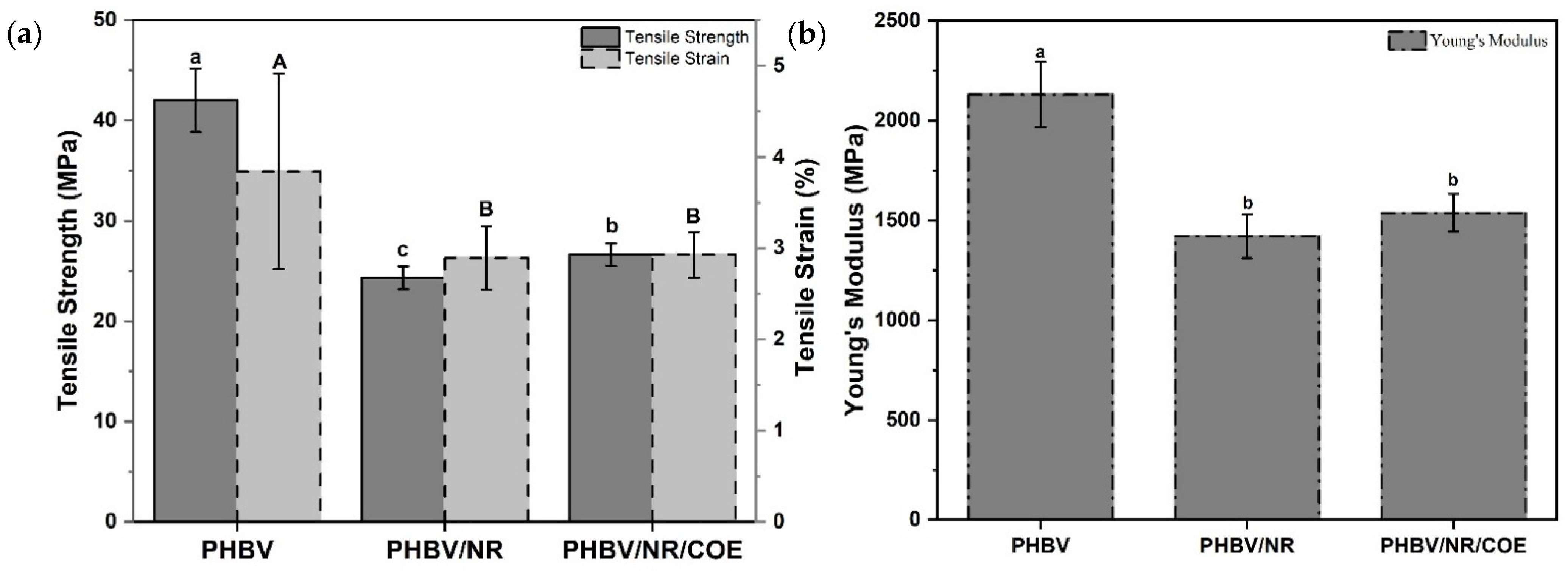
4.4. Mechanical Properties of the Blends
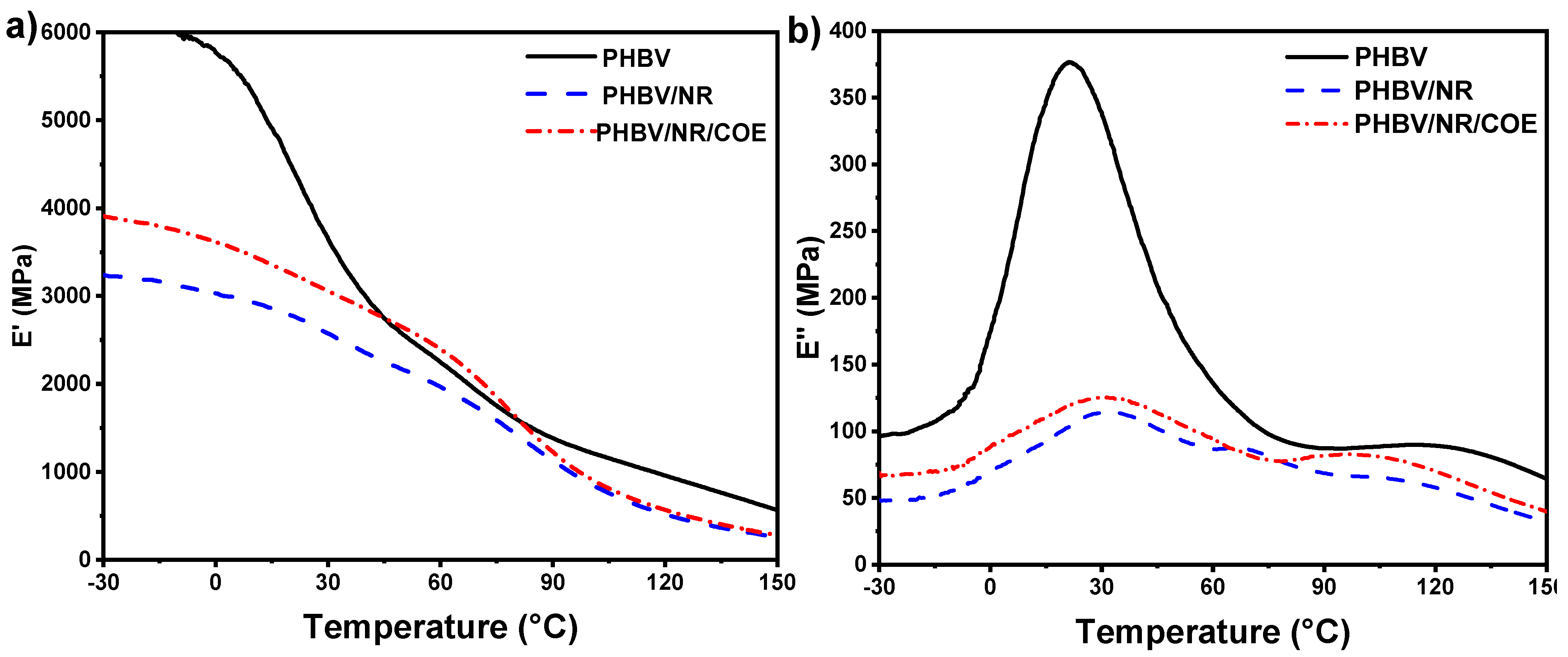
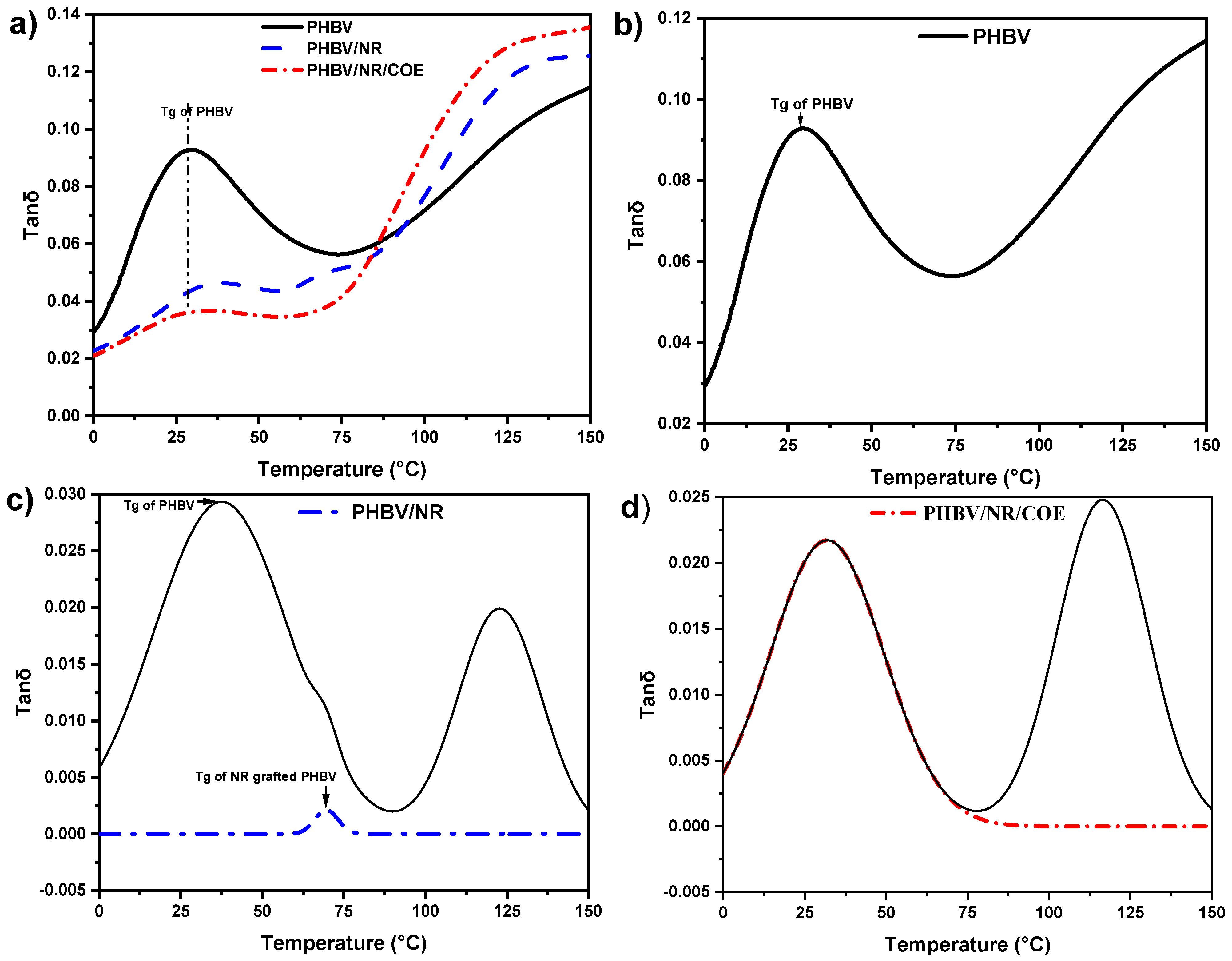
4.5. Dynamic Mechanical Analysis of the PHBV and Its Blends
4.6. Summary of Key Findings
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Da Costa, R.C.; Oliveira, P.R.; Nandi, L.G.; Bobermin, D.M.; Miotto, M.; Bellettini, I.C.; Da Silva Crespo, J.; Daitx, T.D.S.; Teixeira, C.D.S.; Carli, L.N. Green active food packaging films based on nanocomposites of PHBV/sepiolite/essential oils. Sustain. Food Technol. 2025, 3, 470–481. [Google Scholar] [CrossRef]
- Jahangiri, F.; Mohanty, A.K.; Misra, M. Sustainable biodegradable coatings for food packaging: Challenges and opportunities. Green Chem. 2024, 26, 4934–4974. [Google Scholar] [CrossRef]
- Westlake, J.R.; Tran, M.W.; Jiang, Y.; Zhang, X.; Burrows, A.D.; Xie, M. Biodegradable biopolymers for active packaging: Demand, development and directions. Sustain. Food Technol. 2023, 1, 50–72. [Google Scholar] [CrossRef]
- Moshood, T.D.; Nawanir, G.; Mahmud, F.; Mohamad, F.; Ahmad, M.H.; AbdulGhani, A. Sustainability of biodegradable plastics: New problem or solution to solve the global plastic pollution? Curr. Res. Green Sustain. Chem. 2022, 5, 100273. [Google Scholar] [CrossRef]
- Mohanty, A.K.; Wu, F.; Mincheva, R.; Hakkarainen, M.; Raquez, J.-M.; Mielewski, D.F.; Narayan, R.; Netravali, A.N.; Misra, M. Sustainable polymers. Nat. Rev. Methods Primers 2022, 2, 46. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, Y.; Chen, X.; Yu, X.; Li, W.; Zhang, S.; Meng, X.; Zhao, Z.-M.; Dong, T.; Anderson, A.; et al. Sustainable bioplastics derived from renewable natural resources for food packaging. Matter 2023, 6, 97–127. [Google Scholar] [CrossRef]
- Bernabé, I.; Amarilla, E.; De La Orden, M.U.; Martínez Urreaga, J.; Beltrán, F.R. Effect of oligomeric lactic acid plasticizer on the mechanical recycling of poly(3-hydroxybutyrate-co-3-hydroxyvalerate). Environ. Sci. Pollut. Res. 2024. [CrossRef]
- Ibrahim, M.I.; Alsafadi, D.; Alamry, K.A.; Hussein, M.A. Properties and Applications of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Biocomposites. J. Polym. Environ. 2021, 29, 1010–1030. [Google Scholar] [CrossRef]
- Ling, C.; Clarke, R.W.; Rosetto, G.; Xu, S.; Cywar, R.M.; Kim, D.H.; Hamernik, L.J.; Haugen, S.J.; Michener, W.E.; Woodworth, S.P.; et al. Tunable and Degradable Dynamic Thermosets from Compatibilized Polyhydroxyalkanoate Blends. ACS Sustain. Chem. Eng. 2025, 13, 3817–3829. [Google Scholar] [CrossRef]
- Meereboer, K.W.; Misra, M.; Mohanty, A.K. Review of recent advances in the biodegradability of polyhydroxyalkanoate (PHA) bioplastics and their composites. Green Chem. 2020, 22, 5519–5558. [Google Scholar] [CrossRef]
- Da Costa, R.C.; Daitx, T.S.; Mauler, R.S.; Da Silva, N.M.; Miotto, M.; Crespo, J.S.; Carli, L.N. Poly(hydroxybutyrate-co-hydroxyvalerate)-based nanocomposites for antimicrobial active food packaging containing oregano essential oil. Food Packag. Shelf Life 2020, 26, 100602. [Google Scholar] [CrossRef]
- Siracusa, V.; Rocculi, P.; Romani, S.; Rosa, M.D. Biodegradable polymers for food packaging: A review. Trends Food Sci. Technol. 2008, 19, 634–643. [Google Scholar] [CrossRef]
- Global Market Insights Inc. Polyhydroxyalkanoate Market to Surpass USD 200 Million by 2030, Says Global Market Insights Inc. 2022. Available online: https://www.globenewswire.com/news-release/2022/10/04/2527501/0/en/Polyhydroxyalkanoate-Market-to-surpass-USD-200-Million-by-2030-says-Global-Market-Insights-Inc.html (accessed on 9 May 2025).
- Meereboer, K.W.; Pal, A.K.; Cisneros-López, E.O.; Misra, M.; Mohanty, A.K. The effect of natural fillers on the marine biodegradation behaviour of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV). Sci. Rep. 2021, 11, 911. [Google Scholar] [CrossRef]
- Bugnicourt, E.; Cinelli, P.; Lazzeri, A.; Alvarez, V. Polyhydroxyalkanoate (PHA): Review of synthesis, characteristics, processing and potential applications in packaging. Express Polym. Lett. 2014, 8, 791–808. [Google Scholar] [CrossRef]
- Feijoo, P.; Mohanty, A.K.; Rodriguez-Uribe, A.; Gámez-Pérez, J.; Cabedo, L.; Misra, M. Biodegradable blends from bacterial biopolyester PHBV and bio-based PBSA: Study of the effect of chain extender on the thermal, mechanical and morphological properties. Int. J. Biol. Macromol. 2023, 225, 1291–1305. [Google Scholar] [CrossRef]
- Yolacan, O.; Deniz, S. Effects of different biopolymers and additives on mechanical and barrier properties of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) blend films. J. Dispers. Sci. Technol. 2024, 1–10. [Google Scholar] [CrossRef]
- Pesaranhajiabbas, E.; Mohanty, A.K.; Misra, M. Reactive Extrusion of Biodegradable Poly(3-hydroxybutyrate-co-3-hyrdoxyvalerate) and Poly(butylene adipate-co-terephthalate) Blends: Toward a Toughening Approach. ACS Appl. Polym. Mater. 2025, 7, 2439–2450. [Google Scholar] [CrossRef]
- Hedrick, M.M.; Wu, F.; Mohanty, A.K.; Misra, M. Morphology and performance relationship studies on biodegradable ternary blends of poly(3-hydroxybutyrate-co-3-hydroxyvalerate), polylactic acid, and polypropylene carbonate. RSC Adv. 2020, 10, 44624–44632. [Google Scholar] [CrossRef]
- Kennouche, S.; Le Moigne, N.; Kaci, M.; Quantin, J.-C.; Caro-Bretelle, A.-S.; Delaite, C.; Lopez-Cuesta, J.-M. Morphological characterization and thermal properties of compatibilized poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV)/poly(butylene succinate) (PBS)/halloysite ternary nanocomposites. Eur. Polym. J. 2016, 75, 142–162. [Google Scholar] [CrossRef]
- Zhao, X.; Ji, K.; Kurt, K.; Cornish, K.; Vodovotz, Y. Optimal mechanical properties of biodegradable natural rubber-toughened PHBV bioplastics intended for food packaging applications. Food Packag. Shelf Life 2019, 21, 100348. [Google Scholar] [CrossRef]
- Zhao, X.; Cornish, K.; Vodovotz, Y. Synergistic Mechanisms Underlie the Peroxide and Coagent Improvement of Natural-Rubber-Toughened Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Mechanical Performance. Polymers 2019, 11, 565. [Google Scholar] [CrossRef] [PubMed]
- Le Delliou, B.; Vitrac, O.; Benihya, A.; Guinault, A.; Domenek, S. Development of extrusion blown films of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) blends for flexible packaging. J. Appl. Polym. Sci. 2024, 141, e55240. [Google Scholar] [CrossRef]
- Ghosh, R.; Zhao, X.; Vodovotz, Y. Addition of Coffee Waste-Derived Plasticizer Improves Processability and Barrier Properties of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate)-Natural Rubber Bioplastic. Polymers 2024, 16, 2164. [Google Scholar] [CrossRef]
- Audic, J.; Lemiègre, L.; Corre, Y. Thermal and mechanical properties of a polyhydroxyalkanoate plasticized with biobased epoxidized broccoli oil. J. Appl. Polym. Sci. 2014, 131, 1–7. [Google Scholar] [CrossRef]
- Choi, J.S.; Park, W.H. Thermal and mechanical properties of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) plasticized by biodegradable soybean oils. Macromol. Symp. 2003, 197, 65–76. [Google Scholar] [CrossRef]
- Slongo, M.D.; Brandolt, S.D.F.; Daitx, T.S.; Mauler, R.S.; Giovanela, M.; Crespo, J.S.; Carli, L.N. Comparison of the Effect of Plasticizers on PHBV—And Organoclay—Based Biodegradable Polymer Nanocomposites. J. Polym. Environ. 2018, 26, 2290–2299. [Google Scholar] [CrossRef]
- Jost, V.; Langowski, H.-C. Effect of different plasticisers on the mechanical and barrier properties of extruded cast PHBV films. Eur. Polym. J. 2015, 68, 302–312. [Google Scholar] [CrossRef]
- Campos-Vega, R.; Loarca-Piña, G.; Vergara-Castañeda, H.A.; Oomah, B.D. Spent coffee grounds: A review on current research and future prospects. Trends Food Sci. Technol. 2015, 45, 24–36. [Google Scholar] [CrossRef]
- Delgado, P.A.; Vignoli, J.A.; Siika-aho, M.; Franco, T.T. Sediments in coffee extracts: Composition and control by enzymatic hydrolysis. Food Chem. 2008, 110, 168–176. [Google Scholar] [CrossRef]
- Tabassum, Z.; Girdhar, M.; Anand, A.; Kumari, N.; Sood, B.; Malik, T.; Kumar, A.; Mohan, A. Trash to treasure: Advancing resource efficiency using waste-derived fillers as sustainable reinforcing agents in bioplastics. Mater. Adv. 2025, 6, 527–546. [Google Scholar] [CrossRef]
- Williamson, K.; Banker, T.; Zhao, X.; Ortega-Anaya, J.; Jimenez-Flores, R.; Vodovotz, Y.; Hatzakis, E. Spent coffee ground oil as a valuable source of epoxides and epoxidation derivatives: Quantitation and characterization using low-field NMR. LWT 2022, 165, 113719. [Google Scholar] [CrossRef]
- Bomfim, A.; Oliveira, D.; Voorwald, H.; Benini, K.; Dumont, M.-J.; Rodrigue, D. Valorization of Spent Coffee Grounds as Precursors for Biopolymers and Composite Production. Polymers 2022, 14, 437. [Google Scholar] [CrossRef] [PubMed]
- Pathare, P.B.; Opara, U.L.; Al-Said, F.A.-J. Colour Measurement and Analysis in Fresh and Processed Foods: A Review. Food Bioprocess Technol. 2013, 6, 36–60. [Google Scholar] [CrossRef]
- ASTM D790-15; Standard Test Methods for Flexural Properties of Unreinforced and Reinforced Plastics and Electrical Insulating Materials. ASTM International: West Conshohocken, PA, USA, 2015.
- Tosakul, T.; Chanthot, P.; Pattamaprom, C. High toughness and fast home-compost biodegradable packaging films derived from polylactic acid/thermoplastic starch/para-rubber ternary blends. Sci. Rep. 2024, 14, 18603. [Google Scholar] [CrossRef]
- Frank, C.; Emmerstorfer-Augustin, A.; Rath, T.; Trimmel, G.; Nachtnebel, M.; Stelzer, F. Bio-Polyester/Rubber Compounds: Fabrication, Characterization, and Biodegradation. Polymers 2023, 15, 2593. [Google Scholar] [CrossRef]
- Gerard, T.; Budtova, T.; Podshivalov, A.; Bronnikov, S. Polylactide/poly(hydroxybutyrate-co-hydroxyvalerate) blends: Morphology and mechanical properties. Express Polym. Lett. 2014, 8, 609–617. [Google Scholar] [CrossRef]
- Montes, M.L.I.; Luzi, F.; Dominici, F.; Torre, L.; Cyras, V.P.; Manfredi, L.B.; Puglia, D. Design and Characterization of PLA Bilayer Films Containing Lignin and Cellulose Nanostructures in Combination With Umbelliferone as Active Ingredient. Front. Chem. 2019, 7, 157. [Google Scholar] [CrossRef]
- Olewnik-Kruszkowska, E.; Gierszewska, M.; Richert, A.; Grabska-Zielińska, S.; Rudawska, A.; Bouaziz, M. Antibacterial Films Based on Polylactide with the Addition of Quercetin and Poly(Ethylene Glycol). Materials 2021, 14, 1643. [Google Scholar] [CrossRef]
- Adekunte, A.O.; Tiwari, B.K.; Cullen, P.J.; Scannell, A.G.M.; O’Donnell, C.P. Effect of sonication on colour, ascorbic acid and yeast inactivation in tomato juice. Food Chem. 2010, 122, 500–507. [Google Scholar] [CrossRef]
- La Fuente Arias, C.I.; González-Martínez, C.; Chiralt, A. Active Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) Films Containing Phenolic Compounds with Different Molecular Structures. Polymers 2024, 16, 1574. [Google Scholar] [CrossRef]
- Zhao, X.; Venoor, V.; Koelling, K.; Cornish, K.; Vodovotz, Y. Bio-based blends from poly(3-hydroxybutyrate- co -3-hydroxyvalerate) and natural rubber for packaging applications. J. Appl. Polym. Sci. 2019, 136, 47334. [Google Scholar] [CrossRef]
- Sadiku-Agboola, O.; Sadiku, E.R.; Adegbola, A.T.; Biotidara, O.F. Rheological Properties of Polymers: Structure and Morphology of Molten Polymer Blends. Mater. Sci. Appl. 2011, 02, 30–41. [Google Scholar] [CrossRef]
- Eslami, Z.; Elkoun, S.; Robert, M.; Adjallé, K. A Review of the Effect of Plasticizers on the Physical and Mechanical Properties of Alginate-Based Films. Molecules 2023, 28, 6637. [Google Scholar] [CrossRef]
- Li, H.; Huneault, M.A. Comparison of sorbitol and glycerol as plasticizers for thermoplastic starch in TPS/PLA blends. J. Appl. Polym. Sci. 2011, 119, 2439–2448. [Google Scholar] [CrossRef]
- Khamzin, A.A.; Nigmatullin, R.R.; Popov, I.I. Log-periodic corrections to the Cole–Cole expression in dielectric relaxation. Phys. A: Stat. Mech. Its Appl. 2013, 392, 136–148. [Google Scholar] [CrossRef]
- Singla, R.K.; Zafar, M.T.; Maiti, S.N.; Ghosh, A.K. Physical blends of PLA with high vinyl acetate containing EVA and their rheological, thermo-mechanical and morphological responses. Polym. Test. 2017, 63, 398–406. [Google Scholar] [CrossRef]
- Alberola, N.; Bergeret, A. Physical modeling of the interphase in amorphous thermoplastic/glass bead composites. Polym. Compos. 1994, 15, 442–452. [Google Scholar] [CrossRef]
- Perez, J.; Cavaille, J.Y.; Etienne, S.; Jourdan, C. Physical interpretation of the rheological behaviour of amorphous polymers through the glass transition. Rev. De Phys. Appliquée 1988, 23, 125–135. [Google Scholar] [CrossRef]
- Rolere, S.; Cartault, M.; Sainte-Beuve, J.; Bonfils, F. A rheological method exploiting Cole-Cole plot allows gel quantification in Natural Rubber. Polym. Test. 2017, 61, 378–385. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, Z.; Leng, X.; Shen, K.; Li, Y. Highly toughened polylactide with epoxidized polybutadiene by in-situ reactive compatibilization. Polymer 2016, 92, 74–83. [Google Scholar] [CrossRef]
- Ahmed, J.; Varshney, S.K.; Auras, R. Rheological and Thermal Properties of Polylactide/Silicate Nanocomposites Films. J. Food Sci. 2010, 75, N17–N24. [Google Scholar] [CrossRef] [PubMed]
- Fauziyah, N.A.; Hilmi, A.R.; Zainuri, M.; Asrori, M.Z.; Mashuri, M.; Jawaid, M.; Pratapa, S. Thermal and dynamic mechanical properties of polyethylene glycol/quartz composites for phase change materials. J. Appl. Polym. Sci. 2019, 136, 48130. [Google Scholar] [CrossRef]
- Han, C.D.; Chuang, H. Criteria for rheological compatibility of polymer blends. J. Appl. Polym. Sci. 1985, 30, 4431–4454. [Google Scholar] [CrossRef]
- Kuntanoo, K.; Promkotra, S.; Kaewkannetra, P. Physical-biopolymer characterization of polyhydroxybutyrate-co-hydroxyvalerate (PHBV) blended with natural rubber latex. In Proceedings of the 4th International Congress in Advances in Applied Physics and Materials, Fethiye, Turkey, 24–27 April 2014; p. 020063. [Google Scholar] [CrossRef]
- Kosenko, I.N.; Urman, O.S.; Metelkin, E.K.; Shurygin, B.N.; Igolnikov, A.E. New Data on the Litho- and Biostratigraphy of the J/K Boundary Interval of the Lower Reaches of the Lena River (Eastern Siberia). Open J. Geol. 2019, 9, 554–557. [Google Scholar] [CrossRef]
- Platzer, N. The technology of plasticizers, J. Kern Sears and Joseph R. Darby, SPE Monograph Series, Wiley, New York, 1982, 1166 pp. Price: $130.00. J. Polym. Sci. Polym. Lett. Ed. 1982, 20, 459. [Google Scholar] [CrossRef]
- Choi, J.S.; Park, W.H. Effect of biodegradable plasticizers on thermal and mechanical properties of poly(3-hydroxybutyrate). Polym. Test. 2004, 23, 455–460. [Google Scholar] [CrossRef]
- Phromma, W.; Magaraphan, R. Fabrication of Admicelled Natural Rubber by Polycaprolactone for Toughening Poly(lactic acid). J. Polym. Environ. 2018, 26, 2268–2280. [Google Scholar] [CrossRef]
- Barbosa, J.L.; Perin, G.B.; Felisberti, M.I. Plasticization of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) with an Oligomeric Polyester: Miscibility and Effect of the Microstructure and Plasticizer Distribution on Thermal and Mechanical Properties. ACS Omega 2021, 6, 3278–3290. [Google Scholar] [CrossRef]
- Park, H.-M.; Misra, M.; Drzal, L.T.; Mohanty, A.K. “Green” Nanocomposites from Cellulose Acetate Bioplastic and Clay: Effect of Eco-Friendly Triethyl Citrate Plasticizer. Biomacromolecules 2004, 5, 2281–2288. [Google Scholar] [CrossRef]
- Qiu, J.; Xing, C.; Cao, X.; Wang, H.; Wang, L.; Zhao, L.; Li, Y. Miscibility and Double Glass Transition Temperature Depression of Poly(l-lactic acid) (PLLA)/Poly(oxymethylene) (POM) Blends. Macromolecules 2013, 46, 5806–5814. [Google Scholar] [CrossRef]
- ASTM D256-00; Standard Test Methods for Determining the Izod Pendulum Impact Resistance of Plastics. ASTM International: West Conshohocken, PA, USA, 2000.
- Tomano, N.; Boondamnoen, O.; Aumnate, C.; Potiyaraj, P. Enhancing impact resistance and biodegradability of PHBV by melt blending with ENR. Sci. Rep. 2022, 12, 22633. [Google Scholar] [CrossRef] [PubMed]



| Zone | Barrel Temperature (°C) |
|---|---|
| T1 | 180 |
| T2 | 180 |
| T3 | 178 |
| T4 | 178 |
| T5 | 175 |
| T6 | 175 |
| T7 | 167 |
| T8 | 165 |
| T9 | 165 |
| Die | 162 |
| Colorimeter | |||||||
|---|---|---|---|---|---|---|---|
| Sample | L* | a* | b* | ΔE | hue | Chroma | Yellowness Index (YI) |
| PHBV | 80.33 ± 1.79 b | 1.86 ± 0.67 b | 30.42 ± 0.64 a | 85.87 ± 1.45 b | 86.52 ± 1.16 b | 30.48 ± 0.68 a | 54.10 ± 0.52 a |
| PHBV/NR | 79.52 ± 0.33 b | 3.04 ± 0.04 a | 27.53 ± 0.36 b | 84.14 ± 0.27 c | 83.71 ± 0.10 c | 27.77 ± 0.38 b | 49.46 ± 0.71 b |
| PHBV/NR/COE | 86.54 ± 0.36 a | −0.15 ± 0.01 c | 25.61 ± 0.04 c | 90.39 ± 0.04 a | 90.34 ± 0.02 a | 25.61 ± 0.04 c | 42.27 ± 0.63 c |
| Sample | h Parameter | Han Plot Slope | R2 Value |
|---|---|---|---|
| PHBV | - | 0.42 ± 0.02 | 0.95695 |
| PHBV/NR | 8.89 | 0.80 ± 0.03 | 0.97978 |
| PHBV/NR/COE | 6.15 | 1.12 ± 0.04 | 0.98421 |
| Analytical Technique | Parameter Measured | PHBV/NR | PHBV/NR/COE | % Change | Significance |
|---|---|---|---|---|---|
| Structural Properties | CH₂ stretching intensity (2847, 2920 cm−1) | Low | High | 67.9% (PCA variance) | Confirms successful COE incorporation |
| Mechanical Properties | Cole–Cole Plot: Relaxation time distribution parameter (h) | 8.89 | 6.15 | ~30.8% | Enhanced crosslinking density |
| Han plot: Slope linearity | Non-linear | Near linear | — | Reduced interfacial tension | |
| Tensile strength (MPa) | 24.33 ± 1.15 | 26.63 ± 1.10 | ~9.4% | Enhanced interfacial adhesion | |
| Young’s modulus (MPa) | 1421.283 ± 110.1 | 1538.793 ± 94.13 | ~8.3% | Compatibilizing effect | |
| Flexural strength (MPa) | 54.69 ± 19.66 MPa | 45.65 ± 1.58 MPa | ~16.5% | Plasticization effect | |
| Flexural modulus (MPa) | 8341.80 ± 3978.63 | 5274.27 ± 510.42 | ~36.8% | Enhanced chain mobility | |
| DMA: Glass transition temperatures (°C) | 37 and 69 °C | ~32 °C | — | Improved phase miscibility | |
| tan δ peak profile | Bimodal | Single broad | — | Enhanced interfacial interactions | |
| Optical Properties | Lightness (L*) | 79.52 ± 0.33 | 86.54 ± 0.36 | ~8.83% | Improved visual appearance |
| Yellowness Index | 49.46 ± 0.71 | 42.27 ± 0.63 | ~14.54% | Enhanced thermal stability | |
| Barrier 1 Properties | WVTR (g/m2/day) | 4.05 ± 0.54 | 1.55 ± 0.20 | ~61.7% | Enhanced barrier properties |
| OTR (cc/m2/day) | 2025.29 ± 35.69 | 21.51 ± 1.68 | ~98.9% | Significantly improved barrier | |
| Water uptake (%) | 3.54 | 1.36 | ~61.6% | Reduced hydrophilicity | |
| Contact angle (°) | 69.47 | 71.12 | ~2.3% | Increased surface hydrophobicity | |
| Crystalline Properties 1 | Crystallinity (%) | 63.52 | 68.85 | ~8.4% | Enhanced crystalline structure |
| Crystal size (nm) | 22.67 | 20.32 | ~10.4% | More numerous, smaller crystallites | |
| d-spacing (020) plane (Å) | 5.86 | 6.51 | ~11.1% | More perfect crystals with improved long-range order | |
| Morphological Properties 1 (SEM) | Phase dispersion | Distinct phases | Homogeneous | — | Elimination of microdroplets |
| Processability 1 | Extrusion homogeneity | Inconsistent | Good | — | Improved industrial processability |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghosh, R.; Zhao, X.; Vodovotz, Y. Rheological Behavior and Mechanical Performance of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/Natural Rubber Blends Modified with Coffee Oil Epoxide for Sustainable Packaging Applications. Polymers 2025, 17, 1324. https://doi.org/10.3390/polym17101324
Ghosh R, Zhao X, Vodovotz Y. Rheological Behavior and Mechanical Performance of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/Natural Rubber Blends Modified with Coffee Oil Epoxide for Sustainable Packaging Applications. Polymers. 2025; 17(10):1324. https://doi.org/10.3390/polym17101324
Chicago/Turabian StyleGhosh, Rinky, Xiaoying Zhao, and Yael Vodovotz. 2025. "Rheological Behavior and Mechanical Performance of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/Natural Rubber Blends Modified with Coffee Oil Epoxide for Sustainable Packaging Applications" Polymers 17, no. 10: 1324. https://doi.org/10.3390/polym17101324
APA StyleGhosh, R., Zhao, X., & Vodovotz, Y. (2025). Rheological Behavior and Mechanical Performance of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/Natural Rubber Blends Modified with Coffee Oil Epoxide for Sustainable Packaging Applications. Polymers, 17(10), 1324. https://doi.org/10.3390/polym17101324






