Effects on the Enthalpy of Microsynthesis Calorimetry of the Graft Copolymer Starch-g-Polycaprolactone for Five Starch Sources
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of the Graft Copolymer
2.3. Characterization of Starch–g–PCL
2.3.1. DSC Measurements
2.3.2. Analysis of Starch–g–PCL by Fourier Transform Infrared Spectroscopy
2.3.3. Pasting Properties
2.3.4. Statistical Analysis
3. Results and Discussion
3.1. Synthesis of the Graft Copolymer
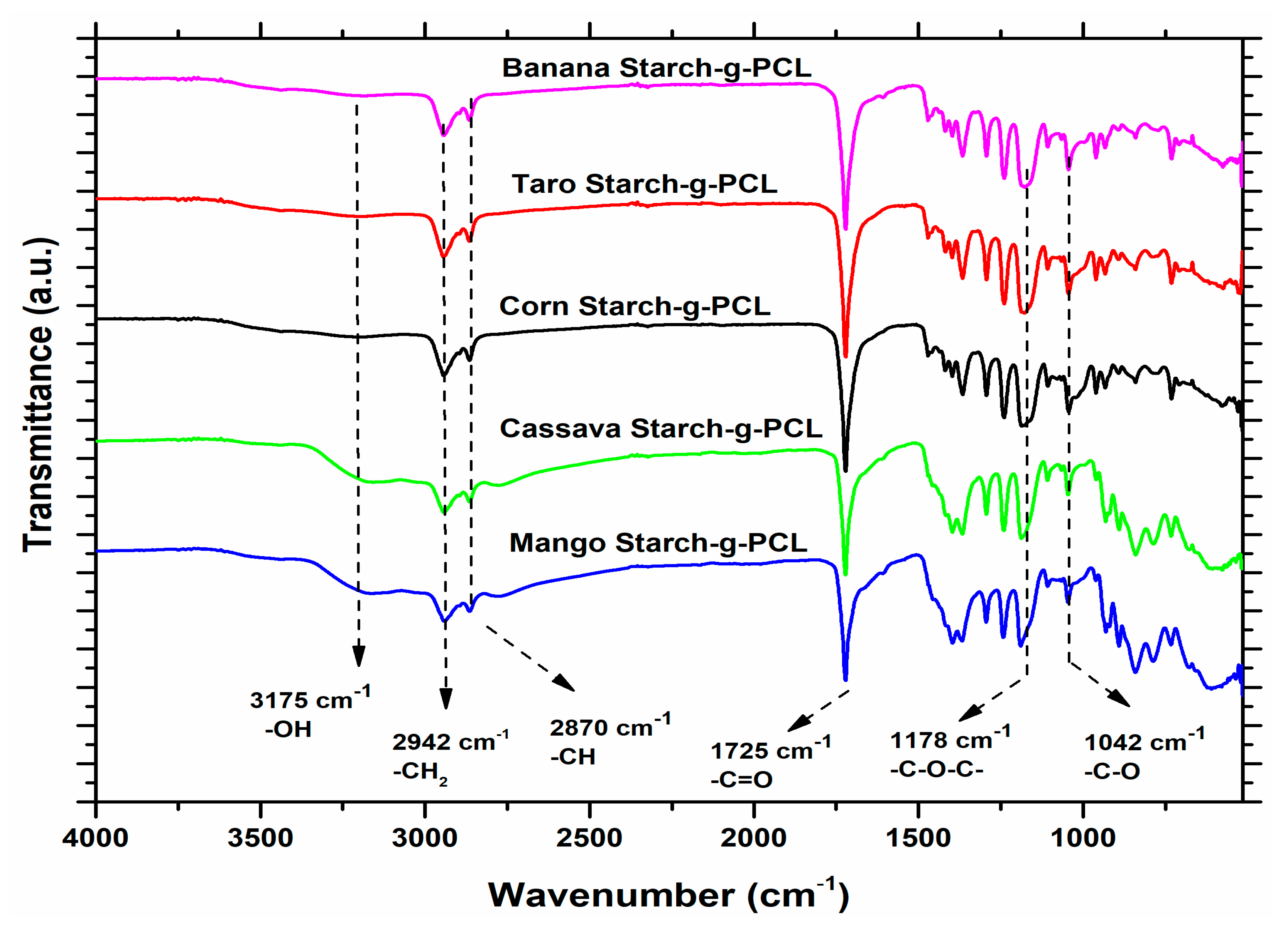
3.2. FTIR Analysis
3.3. Starch–g–PCL Paste Formation Process
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stanley, J.; Culliton, D.; Jovani-Sancho, A.J.; Neves, A.C. The Journey of Plastics: Historical Development, Environmental Challenges, and the Emergence of Bioplastics for Single-Use Products. Eng 2025, 6, 17. [Google Scholar] [CrossRef]
- Oluwafemi, S.O.; Ajiboye, T.O.; Mhlanga, S.D.; Mufhandu, H.T. Biomedical applications of biodegradable polycaprolactone-functionalized magnetic iron oxides nanoparticles and their polymer nanocomposites. Colloids Surf. B Biointerfaces 2023, 227, 113342. [Google Scholar]
- Wu, D.; Lv, Y.; Guo, R.; Li, J.; Habadati, A.; Lu, B.; Wang, H.; Wei, Z. Kinetics of Sn(Oct)2-catalyzed ring opening polymerization of ε-caprolactone. Macromol. Res. 2017, 25, 1070–1075. [Google Scholar] [CrossRef]
- Hai-Bo, W.; Xian-Tai, Z.; Xiao-Wu, Z.; Yan-Xiong, F. Ring-opening polymerization of ϵ-caprolactone by benzoic acid in the presence of benzyl alcohol as initiator. ChemistrySelect 2023, 8, e202301948. [Google Scholar]
- Kayan, G.Ö.; Kayan, A. Polycaprolactone composites/blends and their applications especially in water treatment. ChemEngineering 2023, 7, 104. [Google Scholar] [CrossRef]
- Báez, J.E.; Martínez-Rosales, M.; Martínez-Richa, A. Ring-opening polymerization of lactones catalyzed by decamolybdate anion. Polymer 2003, 44, 6767–6772. [Google Scholar] [CrossRef]
- Ramírez-Hernández, A.; Crisanto-Contreras, O.; Conde-Acevedo, J.; Navarro-Moreno, L.G. Poly (ε-caprolactone) degradation under acidic and alkaline conditions. Am. J. Polym. Sci. 2013, 3, 70–75. [Google Scholar]
- Christen, M.O.; Vercesi, F. Polycaprolactone: How a Well-Known and Futuristic Polymer Has Become an Innovative Collagen-Stimulator in Esthetics. Clin. Cosmet. Investig. Dermatol. 2020, 13, 31–48. [Google Scholar] [CrossRef]
- Munzeiwa, W.A.; Omondi, B.O.; Nyamori, V.O. A perspective into ring-opening polymerization of ε-caprolactone and lactides: Effect of, ligand, catalyst structure and system dynamics, on catalytic activity and polymer properties. Polym. Bull. 2024, 81, 9419–9464. [Google Scholar] [CrossRef]
- Pitts, J.; Hänsch, R.; Roger, Y.; Hoffmann, A.; Menzel, H. 3D Porous Polycaprolactone with Chitosan-Graft-PCL Modified Surface for In Situ Tissue Engineering. Polymers 2025, 17, 383. [Google Scholar] [CrossRef]
- Chen, L.; Ni, Y.; Bian, X.; Qiu, X.; Zhuang, X.; Chen, X.; Jing, X. A novel approach to grafting polymerization of ε-caprolactone onto starch granules. Carbohydr. Polym. 2005, 60, 103–109. [Google Scholar] [CrossRef]
- Ramírez-Hernández, A.; Aparicio-Saguilán, A.; Mata-Mata, J.L.; González-García, G.; Hernández-Mendoza, H.; Gutiérrez-Fuentes, A.; Báez-García, E. Chemical modification of banana starch by the in situ polymerization of e-caprolactone in one step. Starch/Starke 2017, 69, 1600197. [Google Scholar] [CrossRef]
- Purohit, P.; Bhatt, A.; Mittal, R.K.; Abdellattif, M.H.; Farghaly, T.A. Polymer Grafting and its chemical reactions. Front. Bioeng. Biotechnol. 2023, 11, 1044927. [Google Scholar] [CrossRef]
- Ramírez-Hernández, A.; Mata-Mata, J.L.; Aparicio-Saguilán, A.; González-García, G.; Hernández-Mendoza, H.; Gutiérrez-Fuentes, A.; Báez-García, E. The effect of ethylene glycol on starch-g-PCL graft copolymer synthesis. Starch/Starke 2016, 68, 1148–1157. [Google Scholar] [CrossRef]
- Ramírez-Hernández, A.; Mata-Mata, J.L.; Aparicio-Saguilán, A.; González-García, G.; Hernández-Mendoza, H.; Báez-García, E.; Conde-Acevedo, C. Clusters of starch-g-PCL and their effect on the physicochemical properties of films. Starch/Starke 2017, 70, 1700135. [Google Scholar] [CrossRef]
- Singh, G.P.; Bangar, S.P.; Yang, T.; Trif, M.; Kumar, V.; Kumar, D. Effect on the properties of edible starch-based films by the Incorporation of additives: A review. Polymers 2022, 14, 1987. [Google Scholar] [CrossRef]
- Muñoz-Gimena, P.F.; Oliver-Cuenca, V.; Peponi, L.; López, D. A Review on Reinforcements and Additives in Starch-Based Composites for Food Packaging. Polymers 2023, 15, 2972. [Google Scholar] [CrossRef]
- Ditimoni, D.; Nandan, S. Comprehensive review on developments in starch-based films along with active ingredients for sustainable food packaging. Sustain. Chem. Pharm. 2024, 39, 101534. [Google Scholar]
- Ramírez-Hernández, A.; Martı, A. Ring opening polymerization of ε-caprolactone initiated by decamolybdate anion: Determination of kinetic and thermodynamic parameters by DSC and 1H-NMR. J. Appl. Polym. Sci. 2009, 115, 2288–2295. [Google Scholar] [CrossRef]
- Taylor, C.J.; Pomberger, A.; Felton, K.C.; Grainger, R.; Barecka, M.; Chamberlain, T.W.; Bourne, R.A.; Johnson, C.N.; Lapkin, A.A. A Brief Introduction to Chemical Reaction Optismization. Chem. Rev. 2023, 123, 3089–3126. [Google Scholar] [CrossRef]
- Bicking, M.K.L. Extraction: Analytical extractions. In Encyclopedia of Separation Science; Wilson, I.D., Ed.; Academic Press: Woodbury, NY, USA, 2000; pp. 1371–1382. [Google Scholar]
- Rincón-Londoño, N.; Vega-Rojas, L.J.; Contreras-Padilla, M.; Acosta-Osorio, A.A.; Rodríguez-García, M.E. Analysis of the pasting profile in corn starch: Structural, morphological, and thermal transformations, Part I. Int. J. Biol. Macromol. 2016, 91, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Aoyagi, T.; Oshima, T.; Imaizumi, T. Quantitative characterization of individual starch grain morphology using a particle flow analyzer. LWT Food Sci. Technol. 2021, 139, 110589. [Google Scholar] [CrossRef]
- Singla, D.; Singh, A.; Dhull, S.B.; Kumar, P.; Malik, T.; Kumar, P. Taro starch: Isolation, morphology, modification and novel applications concern—A review. Int. J. Biol. Macromol. 2020, 163, 1283–1290. [Google Scholar] [CrossRef] [PubMed]
- Aboubakar, A.; Njintang, Y.N.; Scher, J.; Mbofung, C.M.F. Physicochemical, thermal properties and microstructure of six varieties of taro (Colocasia esculenta L. Schott) flours and starches. J. Food Eng. 2008, 86, 294–305. [Google Scholar] [CrossRef]
- Kouřimská, H.; Skuppin, L.; Springborn, B. A variational principle for cyclic polygons with prescribed edge lengths. In Advances in Discrete Differential Geometry; Bobenko, A., Ed.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 177–195. [Google Scholar]
- Chang, D.; Park, J. Describing the geometric difference of architectural forms in three primary shapes of circle, triangle and square. J. Asian Archit. Build. Eng. 2021, 21, 1–21. [Google Scholar] [CrossRef]
- Singh, N.; Singh, J.; Kaur, L.; Singh, N.S.; Singh, B.G. Morphological, thermal and rheological properties of starches from different botanical sources. Food Chem. 2003, 81, 219–231. [Google Scholar] [CrossRef]
- Nagar, C.K.; Dash, S.K.; Rayaguru, K.; Pal, U.S.; Nedunchezhiyan, M. Isolation, characterization, modification and uses of taro starch: A review. Int. J. Biol. Macromol. 2021, 192, 574–589. [Google Scholar] [CrossRef]
- Dong, L.; Mazzarino, I.; Alexiadis, A. Development of solid-fluid reaction models—A literature review. Chemengineering 2021, 5, 36. [Google Scholar] [CrossRef]
- Jingjing, C.; Chao, C.; Niu, B.; Copeland, L.; Yu, J.; Wang, S.; Wang, S. New insight into the interactions among starch, lipid and protein in model systems with different starches. Food Hydrocoll. 2021, 112, 106323. [Google Scholar]
- Chaudhary, A.L.; Miler, M.; Torley, P.J.; Sopade, P.A.; Halley, P.J. Amylose content and chemical modification effects on the extrusion of thermoplastic starch from maize. Carbohydr. Polym. 2008, 74, 907–913. [Google Scholar] [CrossRef]
- Rashwan, A.K.; Younis, H.A.; Abdelshafy, A.M.; Osman, A.I.; Eletmany, M.R.; Hafouda, M.A.; Chen, W. Plant starch extraction, modification, and green applications: A review. Environ. Chem. Lett. 2024, 22, 2483–2530. [Google Scholar] [CrossRef]
- Rostamabadi, H.; Demirkesen, I.; Mert, B.; Barua, S.; Colussi, R.; Frasson, S.F.; Wang, Y.; Falsafi, S.R. Starch digestibility: How single, double, and multiple physicochemical modifications change nutritional attributes of starch? Food Front. 2024, 5, 1410–1444. [Google Scholar] [CrossRef]
- Herlina, M.; Cahyana, Y.; Djali, M.; Pramafisi, G. The properties, modification, and application of banana starch. Polymers 2022, 14, 3092. [Google Scholar] [CrossRef] [PubMed]
- Goryunova, P.E.; Sologubov, S.S.; Markin, A.V.; Mirnova, N.N.; Mochalova, A.E.; Zaitsev, S.D.; Smirnova, L.A. Calorimetric study of chitosan-graft-poly(2-ethylhexyl acrylate) copolymer. Thermochim. Acta 2018, 670, 136–141. [Google Scholar] [CrossRef]
- Maldonado-Celis, M.E.; Yahia, E.M.; Bedoya, R.; Landázuri, P.; Loango, N.; Aguillón, J.; Restrepo, B.; Guerrero-Ospina, J.C. Chemical Composition of Mango (Mangifera indica L.) Fruit: Nutritional and Phytochemical Compounds. Front. Plant Sci. 2019, 10, 1073. [Google Scholar] [CrossRef]
- Mishra, K.; Siwal, S.S.; Sithole, T.; Singh, N.; Hart, P.; Thakur, V.K. Biorenewable materials for water remediation: The central role of cellulose in achieving sustainability. J. Bioresour. Bioprod. 2024, 9, 253–282. [Google Scholar] [CrossRef]
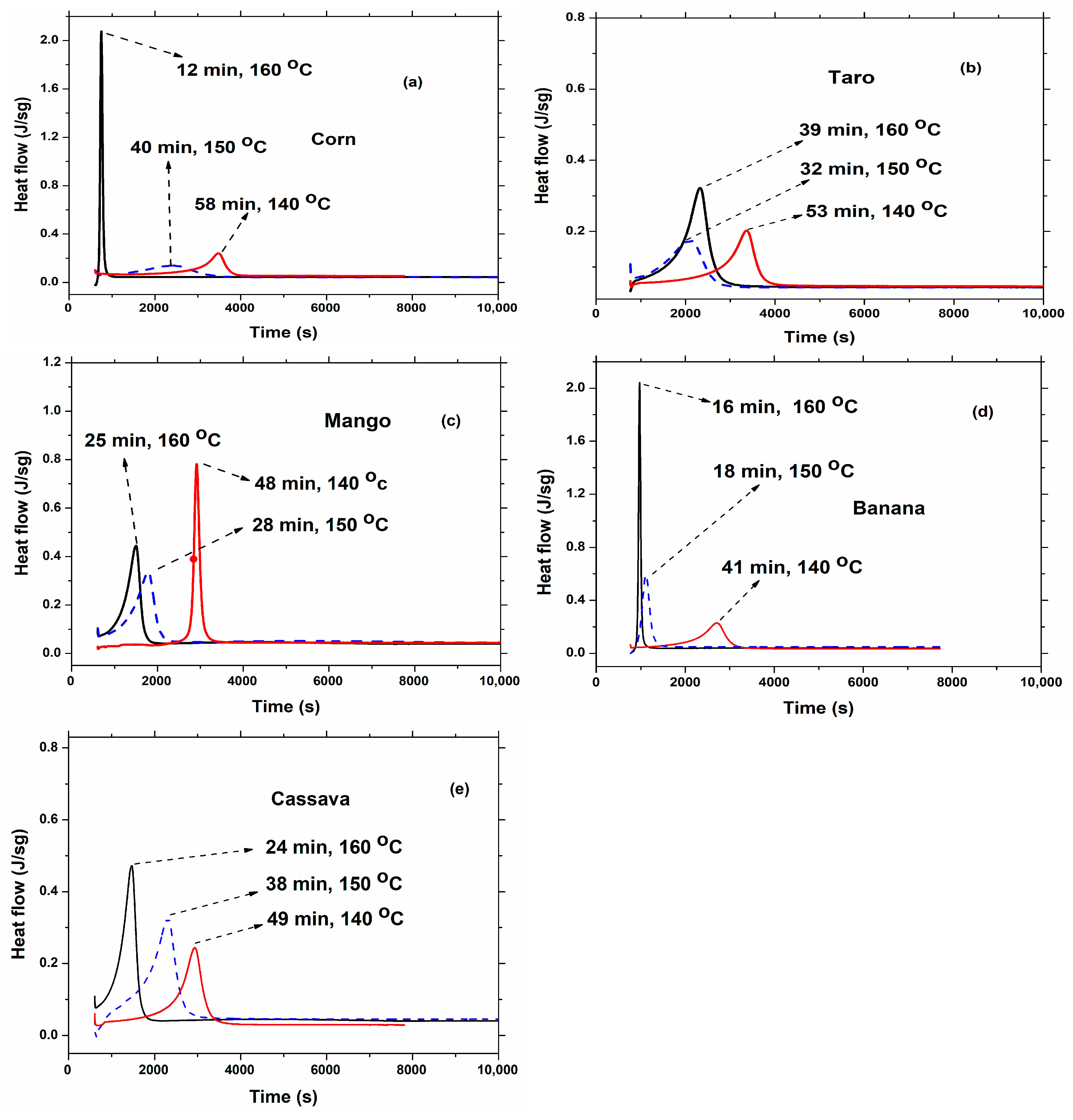


| Source | Synthesis Temperature (°C) | Time (min) | Enthalpy (J/g) | Tm (°C) |
|---|---|---|---|---|
| Banana | 160 | 16 | 118.0 | 57.0 |
| 150 | 18 | 112.9 | 57.0 | |
| 140 | 41 | 130.7 | 44.0 | |
| Taro | 160 | 39 | 179.8 | 45.6 |
| 150 | 32 | 104.7 | 37.4 | |
| 140 | 53 | 108.2 | 35.6 | |
| Corn | 160 | 12 | 153.6 | 57.3 |
| 150 | 40 | 113.4 | 45.0 | |
| 140 | 58 | 108.1 | 37.6 | |
| Cassava | 160 | 24 | 163.1 | 40.4 |
| 150 | 38 | 199.4 | 44.0 | |
| 140 | 49 | 110.1 | 39.4 | |
| Mango | 160 | 25 | 151.7 | 56.8 |
| 150 | 28 | 151.5 | 39.3 | |
| 140 | 48 | 119.9 | 39.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendoza-Morales, N.F.; Aparicio-Saguilán, A.; Páramo-Calderón, D.E.; García-Muñoz, M.A.; Carrillo-Ahumada, J.; Baéz-García, J.E.; Saldaña-Herrera, J.; Flores-Munguía, E.J.; Ramírez-Hernández, A. Effects on the Enthalpy of Microsynthesis Calorimetry of the Graft Copolymer Starch-g-Polycaprolactone for Five Starch Sources. Polymers 2025, 17, 1311. https://doi.org/10.3390/polym17101311
Mendoza-Morales NF, Aparicio-Saguilán A, Páramo-Calderón DE, García-Muñoz MA, Carrillo-Ahumada J, Baéz-García JE, Saldaña-Herrera J, Flores-Munguía EJ, Ramírez-Hernández A. Effects on the Enthalpy of Microsynthesis Calorimetry of the Graft Copolymer Starch-g-Polycaprolactone for Five Starch Sources. Polymers. 2025; 17(10):1311. https://doi.org/10.3390/polym17101311
Chicago/Turabian StyleMendoza-Morales, Noé Francisco, Alejandro Aparicio-Saguilán, Delia E. Páramo-Calderón, Miguel A. García-Muñoz, Jesús Carrillo-Ahumada, José Eduardo Baéz-García, Javier Saldaña-Herrera, Enrique J. Flores-Munguía, and Aurelio Ramírez-Hernández. 2025. "Effects on the Enthalpy of Microsynthesis Calorimetry of the Graft Copolymer Starch-g-Polycaprolactone for Five Starch Sources" Polymers 17, no. 10: 1311. https://doi.org/10.3390/polym17101311
APA StyleMendoza-Morales, N. F., Aparicio-Saguilán, A., Páramo-Calderón, D. E., García-Muñoz, M. A., Carrillo-Ahumada, J., Baéz-García, J. E., Saldaña-Herrera, J., Flores-Munguía, E. J., & Ramírez-Hernández, A. (2025). Effects on the Enthalpy of Microsynthesis Calorimetry of the Graft Copolymer Starch-g-Polycaprolactone for Five Starch Sources. Polymers, 17(10), 1311. https://doi.org/10.3390/polym17101311







