Increasing the Permeability of Polyphenylene Sulfone Hollow Fiber Ultrafiltration Membranes by Switching the Polymer End Groups
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of PPSU
2.3. Characterization of Synthesized PPSUs
2.3.1. Gel Permeation Chromatography (GPC)
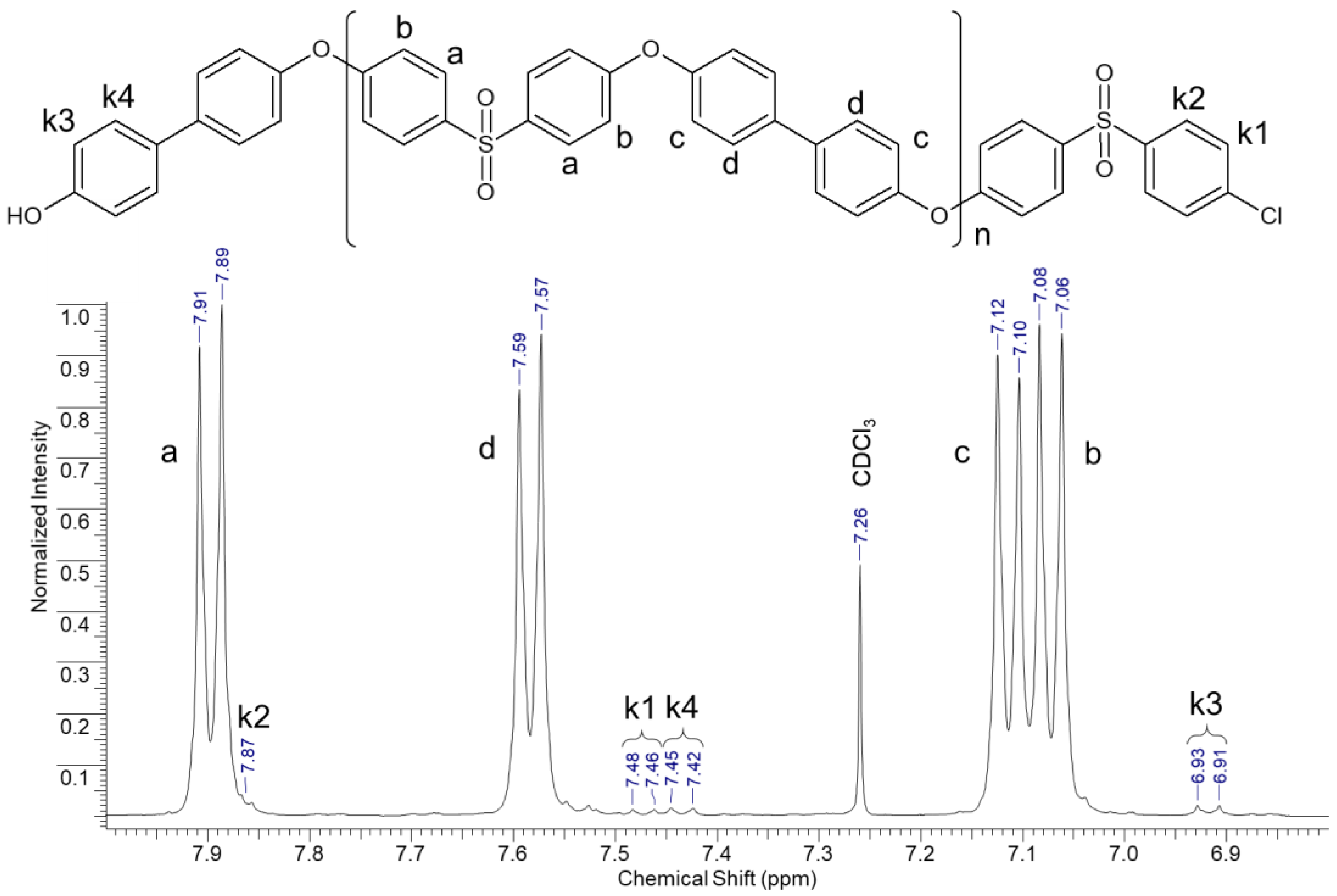
2.3.2. Nuclear Magnetic Resonance (NMR)
2.3.3. Differential Scanning Calorimetry (DSC)
2.3.4. Determination of Coagulation Values
2.4. Preparation of PPSU Solutions and Measurement of Viscosity
2.5. Preparation of Hollow Fiber PPSU Membranes
2.6. Measurements of Transport and Separation Properties of Hollow Fiber PPSU Membranes
2.7. Scanning Electron Microscopy (SEM)
3. Results
3.1. Chemical Structure of the Synthesized PPSUs
3.2. Glass Transition Temperature of PPSUs
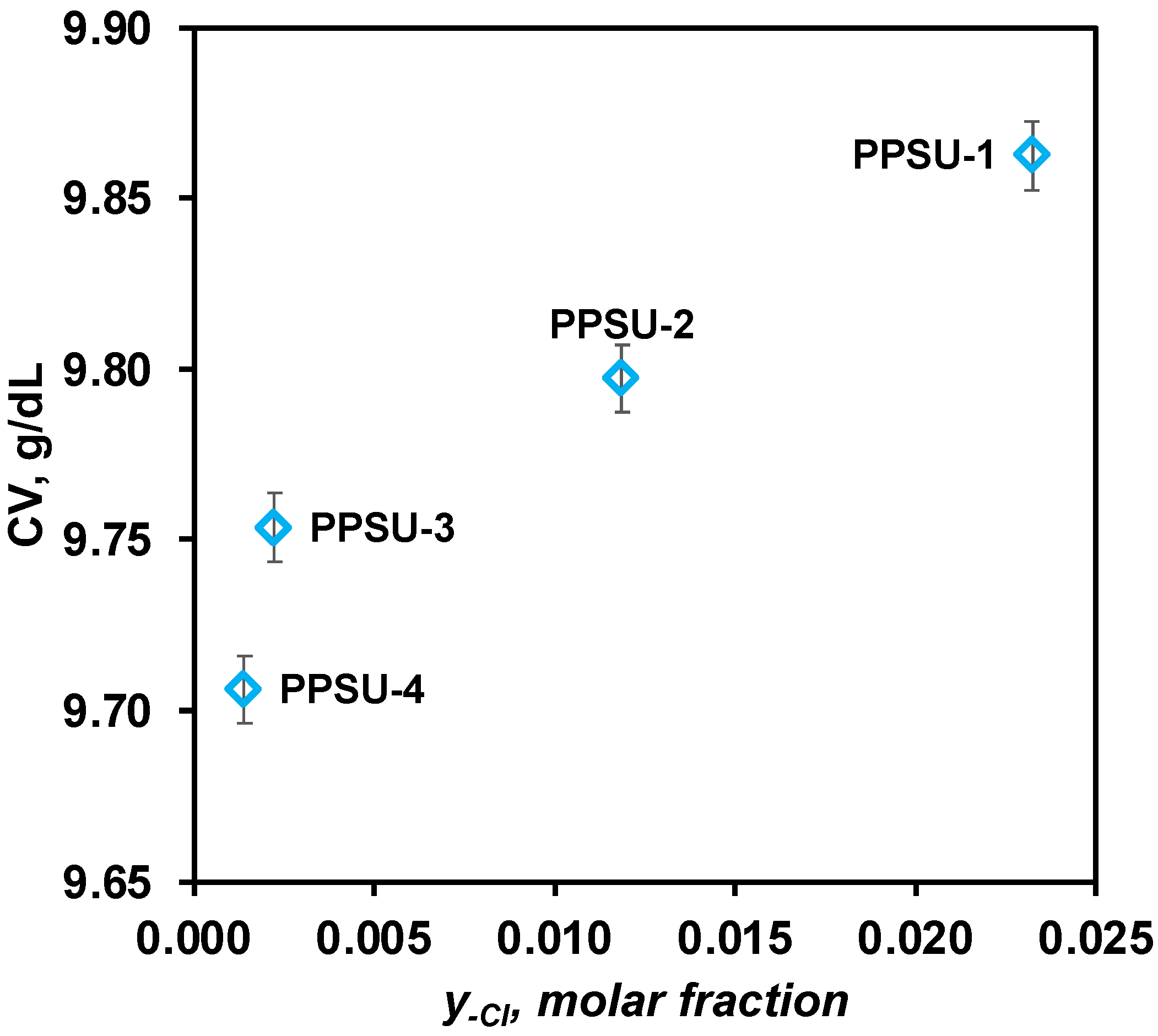
3.3. Coagulation Values
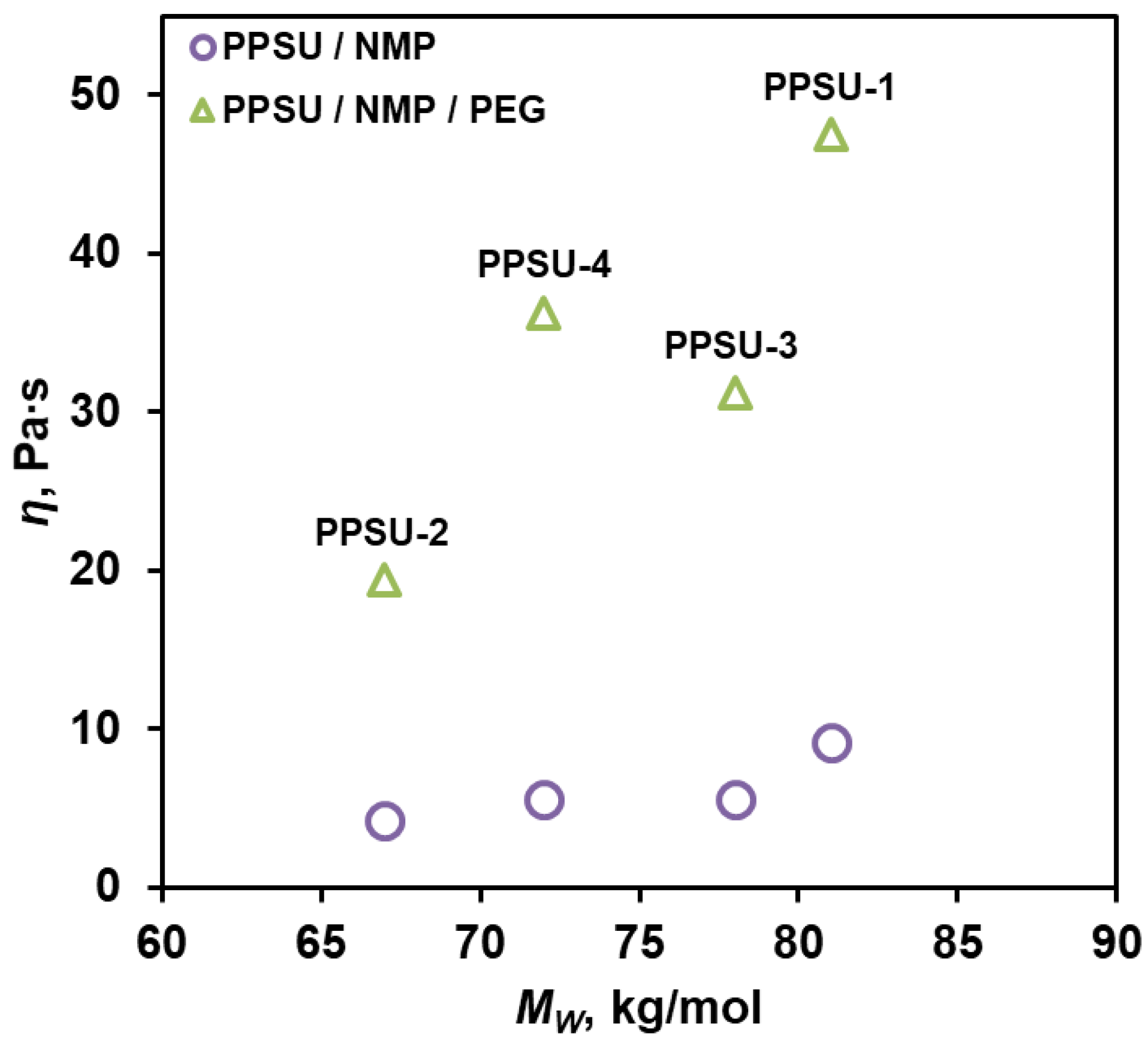
3.4. Properties of Dope Solutions
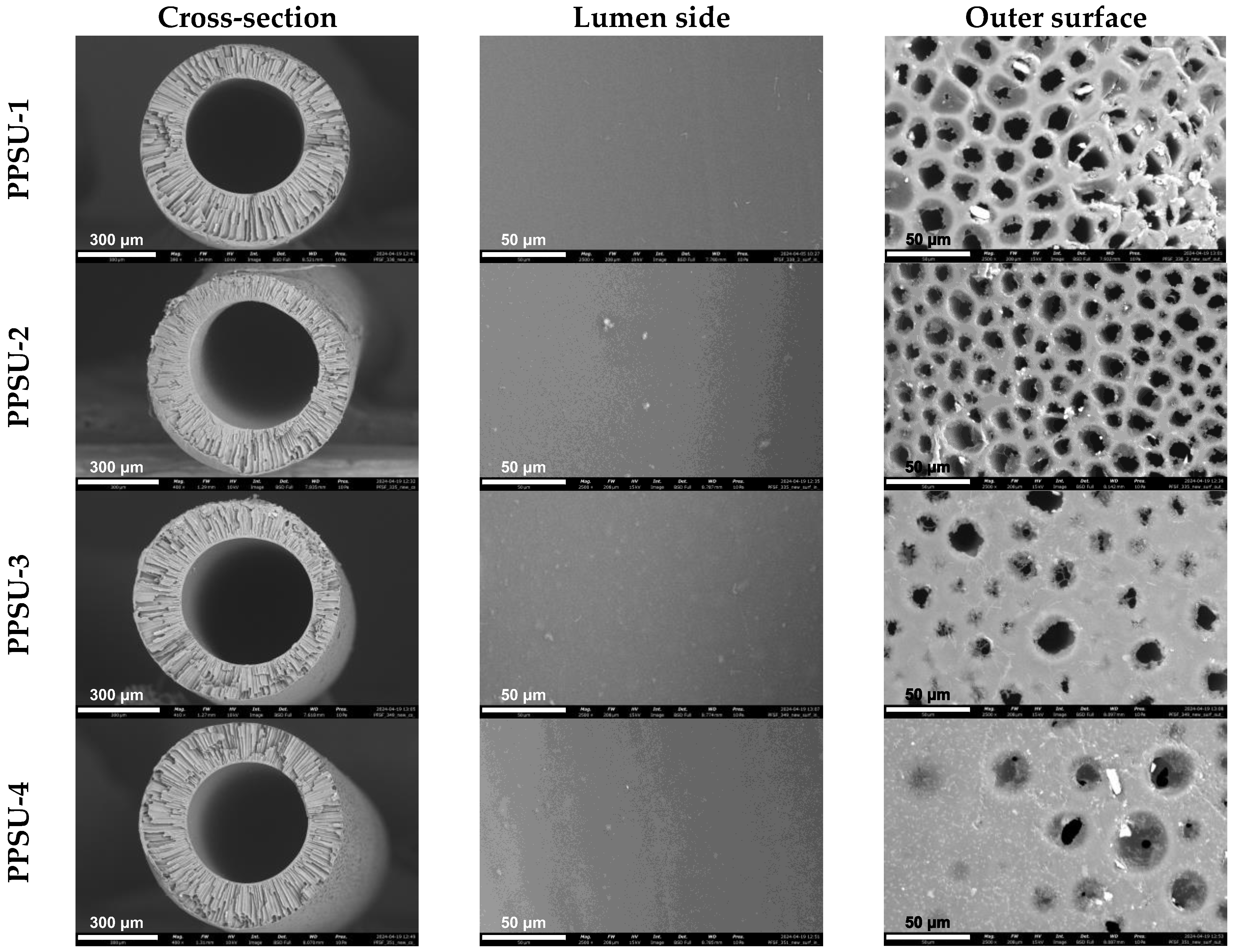
3.5. Characterization of Hollow Fiber Membranes from Synthesized PPSUs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, J.K.; Pandit, A.B. Drinking Water Disinfection Techniques; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- World Health Organization—Drinking-Water. Available online: https://www.who.int/news-room/fact-sheets/detail/drinking-water (accessed on 20 September 2024).
- Chen, J.P.; Mou, H.; Wang, L.K.; Matsuura, T. Membrane filtration. In Advanced Physicochemical Treatment Processes. Handbook of Environmental Engineering; Wang, L.K., Hung, Y.T., Shammas, N.K., Eds.; Humana Press: Totowa, NJ, USA, 2006; Volume 4, pp. 203–259. [Google Scholar] [CrossRef]
- RESEARCH AND MARKETS. Membrane Filtration Market Global Market Report 2024. Available online: https://www.researchandmarkets.com/reports/5783028/membrane-filtration-market-global-market-report#tag-pos-4 (accessed on 21 September 2024).
- Fiksdal, L.; Leiknes, T. The effect of coagulation with MF/UF membrane filtration for the removal of virus in drinking water. J. Memb. Sci. 2006, 279, 364–371. [Google Scholar] [CrossRef]
- Goswami, K.P.; Pugazhenthi, G. Credibility of polymeric and ceramic membrane filtration in the removal of bacteria and virus from water: A review. J. Environ. Manag. 2020, 268, 110583. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.F.; Yang, T.; Lipscomb, G.G.; Stookey, D.J.; Chung, T.S. Design and fabrication of hollow fiber membrane modules. In Hollow Fiber Membranes. Fabrication and Applications; Chung, T.S., Feng, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 11, pp. 225–252. [Google Scholar] [CrossRef]
- Liu, F.; Hashim, N.A.; Liu, Y.; Abed, M.M.; Li, K. Progress in the production and modification of PVDF membranes. J. Memb. Sci. 2011, 375, 1–27. [Google Scholar] [CrossRef]
- Zodrow, K.; Brunet, L.; Mahendra, S.; Li, D.; Zhang, A.; Li, Q.; Alvarez, P.J. Polysulfone ultrafiltration membranes impregnated with silver nanoparticles show improved biofouling resistance and virus removal. Water Res. 2009, 43, 715–723. [Google Scholar] [CrossRef]
- Eray, E.; Candelario, V.M.; Boffa, V.; Safafar, H.; Østedgaard-Munck, D.N.; Zahrtmann, N.; Kadrispahic, H.; Jørgensen, M.K. A roadmap for the development and applications of silicon carbide membranes for liquid filtration: Recent advancements, challenges, and perspectives. Chem. Eng. J. 2021, 414, 128826. [Google Scholar] [CrossRef]
- Golli-Bennour, E.E.; Kouidhi, B.; Dey, M.; Younes, R.; Bouaziz, C.; Zaied, C.; Bacha, H.; Achour, A. Cytotoxic effects exerted by polyarylsulfone dialyser membranes depend on different sterilization processes. Int. Urol. Nephrol. 2011, 43, 483–490. [Google Scholar] [CrossRef]
- Massey, L.K. The Effect of Sterilization Methods on Plastics and Elastomers, 2nd ed.; William Andrew: Norwich, NY, USA, 2004. [Google Scholar]
- Kubyshkina, G.; Marion, L.; Emri, I. The influence of different sterilization techniques on the time-dependent behavior of polyamides. J. Biomater. Nanobiotechnol. 2011, 2, 361. [Google Scholar] [CrossRef]
- Told, R.; Ujfalusi, Z.; Pentek, A.; Kerenyi, M.; Banfai, K.; Vizi, A.; Szabo, P.; Melegh, S.; Bovari-Biri, J.; Pongracz, J.E.; et al. A state-of-the-art guide to the sterilization of thermoplastic polymers and resin materials used in the additive manufacturing of medical devices. Mater. Des. 2022, 223, 111119. [Google Scholar] [CrossRef]
- Guo, Q.; Huang, Y.; Xu, M.; Huang, Q.; Cheng, J.; Yu, S.; Zhang, Y.; Xiao, C. PTFE porous membrane technology: A comprehensive review. J. Memb. Sci. 2022, 664, 121115. [Google Scholar] [CrossRef]
- Wingham, J.R.; Omran, M.; Shepherd, J.; Majewski, C. Effect of steam autoclaving on laser sintered polyamide 12. Rapid Prototyp. J. 2021, 27, 45–52. [Google Scholar] [CrossRef]
- Ultrason® E, S, P Product Brochure—Plastics & Rubber. Available online: https://plastics-rubber.basf.com/southamerica/en/performance_polymers/products/ultrason (accessed on 1 October 2024).
- Taha, Y.R.; Zrelli, A.; Hajji, N.; Al-Juboori, R.A.; Alsalhy, Q. Impact of PCLNPG Nanopolymeric Additive on the Surface and Structural Properties of PPSU Ultrafiltration Membranes for Enhanced Protein Rejection. Processes 2024, 12, 1930. [Google Scholar] [CrossRef]
- Plisko, T.V.; Bildyukevich, A.V.; Karslyan, Y.A.; Ovcharova, A.A.; Volkov, V.V. Development of high flux ultrafiltration polyphenylsulfone membranes applying the systems with upper and lower critical solution temperatures: Effect of polyethylene glycol molecular weight and coagulation bath temperature. J. Membr. Sci. 2018, 565, 266–280. [Google Scholar] [CrossRef]
- Praneeth, K.; James, T.; Sridhar, S. Design of novel ultrafiltration systems based on robust polyphenylsulfone hollow fiber membranes for treatment of contaminated surface water. Chem. Eng. J. 2014, 248, 297–306. [Google Scholar] [CrossRef]
- Nafti-Mateur, M.; Jaouadi, M.; Van der Bruggen, B.; Naifer, K.H.; Jellouli Ennigrou, D. Development and characterization of porous membranes based on PPSU/PES using polyethylene glycol as porogen: Application for cobalt removal by polyelectrolyte-enhanced ultrafiltration. J. Chem. Technol. Biotechnol. 2024, 99, 343–354. [Google Scholar] [CrossRef]
- Burts, K.; Plisko, T.; Makarava, M.; Krasnova, M.; Penkova, A.; Ermakov, S.; Grigoryev, E.; Komolkin, A.; Bildyukevich, A. The effect of PEG-content and molecular weight of PEG-PPG-PEG block copolymers on the structure and performance of polyphenylsulfone ultra-and nanofiltration membranes. J. Membr. Sci. 2024, 704, 122869. [Google Scholar] [CrossRef]
- Lin, Y.C.; Zhuang, G.L.; Tasi, P.F.; Tseng, H.H. Removal of protein, histological dye and tetracycline from simulated bioindustrial wastewater with a dual pore size PPSU membrane. J. Hazard. Mater. 2022, 431, 128525. [Google Scholar] [CrossRef]
- Nayak, M.C.; Isloor, A.M.; Lakshmi, B.; Marwani, H.M.; Khan, I. Polyphenylsulfone/multiwalled carbon nanotubes mixed ultrafiltration membranes: Fabrication, characterization and removal of heavy metals Pb2+, Hg2+, and Cd2+ from aqueous solutions. Arab. J. Chem. 2020, 13, 4661–4672. [Google Scholar] [CrossRef]
- Sani, N.A.A.; Lau, W.J.; Ismail, A.F. Influence of polymer concentration in casting solution and solvent-solute-membrane interactions on performance of polyphenylsulfone (PPSU) nanofiltration membrane in alcohol solvents. J. Polym. Eng. 2014, 34, 489–500. [Google Scholar] [CrossRef]
- Darvishmanesh, S.; Jansen, J.C.; Tasselli, F.; Tocci, E.; Luis, P.; Degrève, J.; Drioli, E.; Van der Bruggen, B. Novel polyphenylsulfone membrane for potential use in solvent nanofiltration. J. Membr. Sci. 2011, 379, 60–68. [Google Scholar] [CrossRef]
- Najafipour, M.; Mousavi, S.M.; Saljoughi, E.; Kiani, S. Hybrid membranes of polyphenylsulfone/functionalized MXene nanosheets for the pervaporation dehydration of acetone and isopropyl alcohol. Polym. Compos. 2023, 44, 4771–4783. [Google Scholar] [CrossRef]
- Tang, Y.; Widjojo, N.; Shi, G.M.; Chung, T.S.; Weber, M.; Maletzko, C. Development of flat-sheet membranes for C1–C4 alcohols dehydration via pervaporation from sulfonated polyphenylsulfone (sPPSU). J. Membr. Sci. 2012, 415, 686–695. [Google Scholar] [CrossRef]
- Çalhan, A.; Deniz, S.; Kujawski, W.; Kujawa, J.; Knozowska, K.; Hasanoğlu, A. Silica filled polyphenylsulfone/polydimethylsiloxane composite membranes for pervaporation separation of biobutanol from ABE mixtures. Chem. Eng. Process. Process Intensif. 2020, 156, 108099. [Google Scholar] [CrossRef]
- Kujawski, W.; Li, G.; Van der Bruggen, B.; Pedišius, N.; Tonkonogij, J.; Tonkonogovas, A.; Stankevicius, A.; Sereika, J.; Jullok, N.; Kujawa, J. Preparation and characterization of polyphenylsulfone (PPSU) membranes for biogas upgrading. Materials 2020, 13, 2847. [Google Scholar] [CrossRef]
- Feng, F.; Liang, C.Z.; Wu, J.; Weber, M.; Maletzko, C.; Zhang, S.; Chung, T.S. Polyphenylsulfone (PPSU)-based copolymeric membranes: Effects of chemical structure and content on gas permeation and separation. Polymers 2021, 13, 2745. [Google Scholar] [CrossRef]
- Luo, L.; Han, G.; Chung, T.S.; Weber, M.; Staudt, C.; Maletzko, C. Oil/water separation via ultrafiltration by novel triangle-shape tri-bore hollow fiber membranes from sulfonated polyphenylenesulfone. J. Membr. Sci. 2015, 476, 162–170. [Google Scholar] [CrossRef]
- Arumugham, T.; Kaleekkal, N.J.; Doraiswamy, M. Development of new hybrid ultrafiltration membranes by entanglement of macromolecular PPSU-SO3H chains: Preparation, morphologies, mechanical strength, and fouling resistant properties. J. Appl. Polym. Sci. 2015, 132, 41986. [Google Scholar] [CrossRef]
- Shukla, A.K.; Alam, J.; Alhoshan, M. Recent advancements in polyphenylsulfone membrane modification methods for separation applications. Membranes 2022, 12, 247. [Google Scholar] [CrossRef]
- Agalloco, J. Steam Sterilization-in-Place. In Handbook of Validation in Pharmaceutical Processes, Fourth Edition; Agalloco, J., DeSantis, P., Grilli, A., Pavell, A., Eds.; CRC Press: Boca Raton, FL, USA, 2021; pp. 245–270. [Google Scholar] [CrossRef]
- Anokhina, T.; Raeva, A.; Sokolov, S.; Storchun, A.; Filatova, M.; Zhansitov, A.; Kurdanova, Z.; Shakhmurzova, K.; Khashirova, S.; Borisov, I. Effect of Composition and Viscosity of Spinning Solution on Ultrafiltration Properties of Polyphenylene Sulfone Hollow-Fiber Membranes. Membranes 2022, 12, 1113. [Google Scholar] [CrossRef]
- Plisko, T.V.; Burts, K.S.; Bildyukevich, A.V. Development of high flux nanocomposite polyphenylsulfone/oxidized multiwalled carbon nanotubes membranes for ultrafiltration using the systems with critical solution temperatures. Membranes 2022, 12, 724. [Google Scholar] [CrossRef]
- Dai, F.; Qian, G.; Ke, Z.; Xu, K.; Wang, M.; Li, D.; Deng, Z.; Yu, Y.; Chen, C. Antifouling polyphenylene sulfone tight-ultrafiltration membrane by co-depositing dopamine and zwitterionic polymer for efficient dye/salt separation. Sep. Purif. Technol. 2024, 345, 127403. [Google Scholar] [CrossRef]
- Dai, F.; Xu, K.; Ke, Z.; Wang, M.; Chen, C.; Qian, G.; Yu, Y. Poly (ionic liquid) blended polyphenylene sulfone ultrafiltration membranes with enhanced surface hydrophilicity and antifouling performance. Sep. Purif. Technol. 2023, 325, 124708. [Google Scholar] [CrossRef]
- Zhansitov, A.; Kurdanova, Z.; Shakhmurzova, K.; Slonov, A.; Borisov, I.; Khashirova, S. Effect of Solvent and Monomer Ratio on the Properties of Polyphenylene Sulphone. Polymers 2023, 15, 2279. [Google Scholar] [CrossRef] [PubMed]
- Anokhina, T.; Raeva, A.; Makaev, S.; Borisov, I.; Vasilevsky, V.; Volkov, A. Express method of preparation of hollow fiber membrane samples for spinning solution optimization: Polysulfone as example. Membranes 2021, 11, 396. [Google Scholar] [CrossRef] [PubMed]
- Izunobi, J.U.; Higginbotham, C.L. Polymer molecular weight analysis by 1H NMR spectroscopy. J. Chem. Educ. 2011, 88, 1098–1104. [Google Scholar] [CrossRef]

| Sample | DHBP:DCDPS |
|---|---|
| PPSU-1 | 1:1.02 |
| PPSU-2 | 1:1 |
| PPSU-3 | 1.005:1 |
| PPSU-4 | 1.008:1 |
| Sample | Cp, wt.% | Csolv, wt.% | Pore-Forming Agent | Cpor, wt.% |
|---|---|---|---|---|
| PPSU-1 | 20 | 80 | - | - |
| PPSU-2 | 20 | 80 | ||
| PPSU-3 | 20 | 80 | ||
| PPSU-4 | 20 | 80 | ||
| PPSU-1 | 20 | 50 | PEG | 30 |
| PPSU-2 | 20 | 50 | 30 | |
| PPSU-3 | 20 | 50 | 30 | |
| PPSU-4 | 20 | 50 | 30 |
| Sample | Mp, kg/mol | Mw, kg/mol | Mn, kg/mol | Mw/Mn | MNMR | DHBP:DCDPS in Synthesis | OH:Cl in Polymers | y-OH | y-Cl |
|---|---|---|---|---|---|---|---|---|---|
| PPSU-1 | 75 | 81 | 24 | 3.4 | 12.7 | 1:1.02 | 0.43 | 0.010 | 0.023 |
| PPSU-2 | 56 | 67 | 25 | 2.6 | 10.5 | 1:1 | 1.7 | 0.020 | 0.012 |
| PPSU-3 | 76 | 78 | 33 | 2.4 | 15.3 | 1.005:1 | 11 | 0.022 | 0.002 |
| PPSU-4 | 64 | 72 | 44 | 1.6 | 15.7 | 1.008:1 | 17 | 0.017 | 0.001 |
| Sample | Glass Transition Temperature Tg, °C |
|---|---|
| PPSU-1 | 227.0 |
| PPSU-2 | 225.9 |
| PPSU-3 | 226.2 |
| PPSU-4 | 228.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raeva, A.; Matveev, D.; Anokhina, T.; Zhansitov, A.A.; Khashirova, S.; Volkov, V.; Borisov, I. Increasing the Permeability of Polyphenylene Sulfone Hollow Fiber Ultrafiltration Membranes by Switching the Polymer End Groups. Polymers 2025, 17, 53. https://doi.org/10.3390/polym17010053
Raeva A, Matveev D, Anokhina T, Zhansitov AA, Khashirova S, Volkov V, Borisov I. Increasing the Permeability of Polyphenylene Sulfone Hollow Fiber Ultrafiltration Membranes by Switching the Polymer End Groups. Polymers. 2025; 17(1):53. https://doi.org/10.3390/polym17010053
Chicago/Turabian StyleRaeva, Alisa, Dmitry Matveev, Tatyana Anokhina, Azamat A. Zhansitov, Svetlana Khashirova, Vladimir Volkov, and Ilya Borisov. 2025. "Increasing the Permeability of Polyphenylene Sulfone Hollow Fiber Ultrafiltration Membranes by Switching the Polymer End Groups" Polymers 17, no. 1: 53. https://doi.org/10.3390/polym17010053
APA StyleRaeva, A., Matveev, D., Anokhina, T., Zhansitov, A. A., Khashirova, S., Volkov, V., & Borisov, I. (2025). Increasing the Permeability of Polyphenylene Sulfone Hollow Fiber Ultrafiltration Membranes by Switching the Polymer End Groups. Polymers, 17(1), 53. https://doi.org/10.3390/polym17010053









