Mechanical and Physical Changes in Bio-Polybutylene-Succinate Induced by UVC Ray Photodegradation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Employed
2.2. Irradiation Set-Up
2.3. Mechanical Characterization Tests
2.4. Physical Characterization Tests
3. Results and Discussion
3.1. Mechanical Characterization Tests
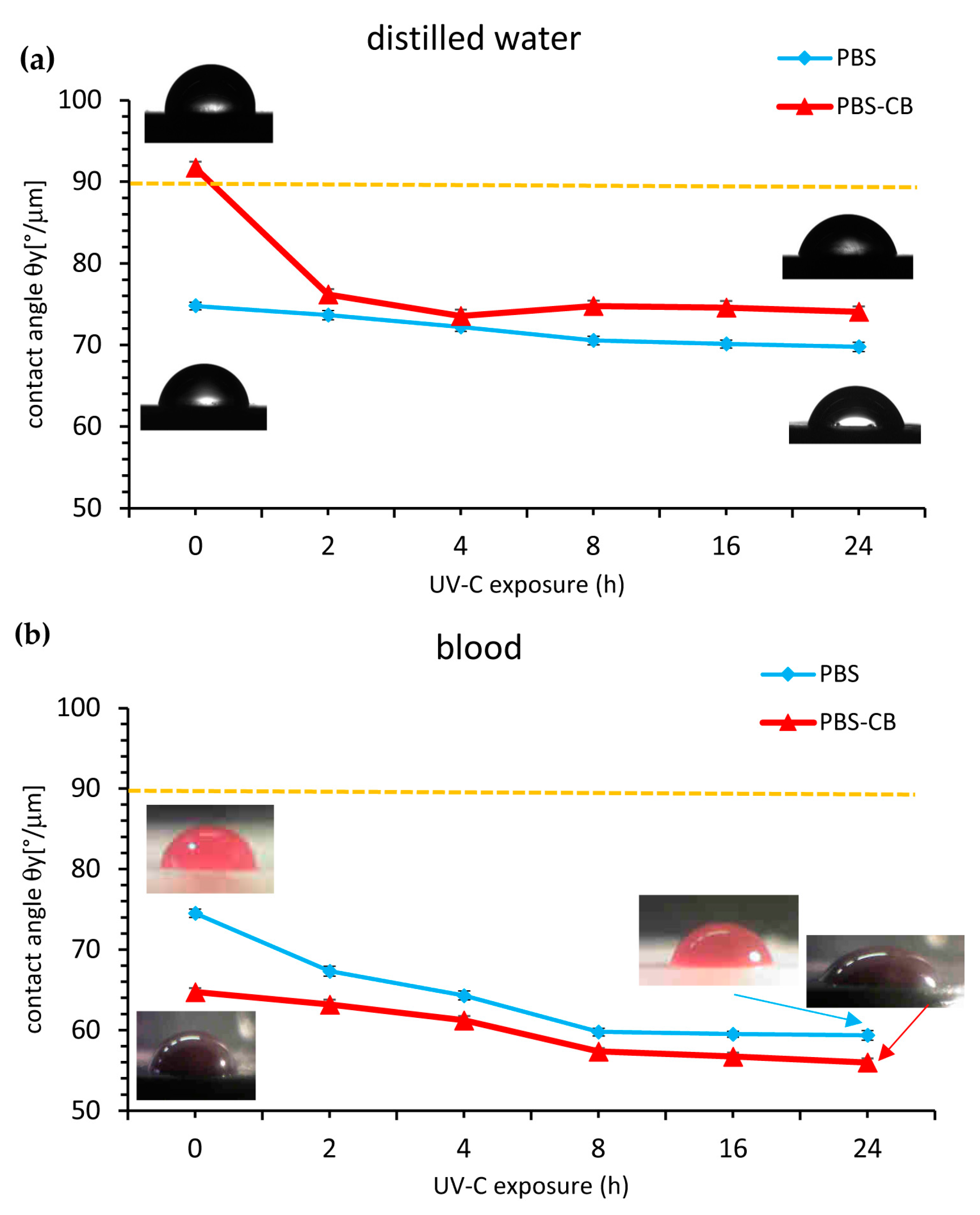
3.2. Physical Characterization Tests
3.3. Literature Comparison of Operative Parameters Employed for UVC Irradiation of Polymers
- (i)
- Adsorption capacity as a function of material thickness.
- (ii)
- Possible variations in the power of the UV source affecting the electromagnetic wavelength.
- (iii)
- The presence of dust particles capable of protecting microorganisms from UV rays.
- (iv)
- The ability of microorganisms to resist radiation during exposure.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Di Bartolo, A.; Infurna, G.; Dintcheva, N.T. A Review of Bioplastics and Their Adoption in the Circular Economy. Polymers 2021, 13, 1229. [Google Scholar] [CrossRef]
- Visco, A.; Scolaro, C.; Facchin, M.; Brahimi, S.; Belhamdi, H.; Gatto, V.; Beghetto, V. Agri-Food Wastes for Bioplastics: European Prospective on Possible Applications in Their Second Life for a Circular Economy. Polymers 2022, 14, 2752. [Google Scholar] [CrossRef]
- Beghetto, V.; Gatto, V.; Samiolo, R.; Scolaro, C.; Brahimi, S.; Facchin, M.; Visco, A. Plastics Today: Key Challenges and EU Strategies towards Carbon Neutrality: A Review. Environ. Pollut. 2023, 334, 122102. [Google Scholar] [CrossRef]
- Moshood, T.D.; Nawanir, G.; Mahmud, F.; Mohamad, F.; Ahmad, M.H.; AbdulGhani, A. Sustainability of Biodegradable Plastics: New Problem or Solution to Solve the Global Plastic Pollution? Curr. Res. Green Sustain. Chem. 2022, 5, 100273. [Google Scholar] [CrossRef]
- Kibria, M.G.; Masuk, N.I.; Safayet, R.; Nguyen, H.Q.; Mourshed, M. Plastic Waste: Challenges and Opportunities to Mitigate Pollution and Effective Management. Int. J. Environ. Res. 2023, 17, 20. [Google Scholar] [CrossRef] [PubMed]
- Fritz, M.; Lauschke, T.; Schlebrowski, T.; Beucher, L.; Schweyen, P.; Alenezi, B.; Hahn, B.; Dierkes, G.; Ternes, T.; Fischer, C.B. Photoaging Phenomena of Biodegradable Polybutylene Succinate and Conventional Low Density Polyethylene by Artificial Weathering—A Comparative Surface Study. Appl. Surf. Sci. 2022, 590, 153058. [Google Scholar] [CrossRef]
- Rosenboom, J.-G.; Langer, R.; Traverso, G. Bioplastics for a Circular Economy. Nat. Rev. Mater. 2022, 7, 117–137. [Google Scholar] [CrossRef] [PubMed]
- Aliotta, L.; Seggiani, M.; Lazzeri, A.; Gigante, V.; Cinelli, P. A Brief Review of Poly (Butylene Succinate) (PBS) and Its Main Copolymers: Synthesis, Blends, Composites, Biodegradability, and Applications. Polymers 2022, 14, 844. [Google Scholar] [CrossRef]
- Barletta, M.; Aversa, C.; Ayyoob, M.; Gisario, A.; Hamad, K.; Mehrpouya, M.; VAHABI, H. Poly(Butylene Succinate) (PBS): Materials, Processing, and Industrial Applications. Prog. Polym. Sci. 2022, 132, 101579. [Google Scholar] [CrossRef]
- Rafiqah, S.A.; Khalina, A.; Harmaen, A.S.; Tawakkal, I.A.; Zaman, K.; Asim, M.; Nurrazi, M.N.; Lee, C.H. A Review on Properties and Application of Bio-Based Poly(Butylene Succinate). Polymers 2021, 13, 1436. [Google Scholar] [CrossRef]
- Colucci, G.; Piano, M.; Lupone, F.; Baruffaldi, D.; Frascella, F.; Bondioli, F.; Messori, M. Printability Study by Selective Laser Sintering of Bio-Based Samples Obtained by Using PBS as Polymeric Matrix. Polym. Test. 2024, 131, 108327. [Google Scholar] [CrossRef]
- Platnieks, O.; Gaidukovs, S.; Thakur, V.; Barkāne, A.; Beluns, S. Bio-Based Poly (Butylene Succinate): Recent Progress, Challenges and Future Opportunities. Eur. Polym. J. 2021, 161, 110855. [Google Scholar] [CrossRef]
- Kim, J.; Park, S.; Jung, S.; Yun, H.; Choi, K.; Heo, G.; Jin, H.-J.; Park, S.; Kwak, H.W. Biodegradation Behavior of Polybutylene Succinate (PBS) Fishing Gear in Marine Sedimentary Environments for Ghost Fishing Prevention. Polym. Degrad. Stab. 2023, 216, 110490. [Google Scholar] [CrossRef]
- Huysman, S. The Potential of Bio-Based PBS for the Textile Industry. Unitex 2018, 2018, 4–5. [Google Scholar]
- Mtibe, A.; Muniyasamy, S.; Teboho, M.; Ofosu, O.; Ojijo, V.; John, M. Recent Insight into the Biomedical Applications of Polybutylene Succinate and Polybutylene Succinate-Based Materials. Express Polym. Lett. 2023, 17, 2–28. [Google Scholar] [CrossRef]
- Gigli, M.; Fabbri, M.; Lotti, N.; Gamberini, R.; Rimini, B.; Munari, A. Poly(Butylene Succinate)-Based Polyesters for Biomedical Applications: A Review. Eur. Polym. J. 2016, 75, 431–460. [Google Scholar] [CrossRef]
- Huang, Z.; Qian, L.; Yin, Q.; Yu, N.; Liu, T.; Tian, D. Biodegradability Studies of Poly(Butylene Succinate) Composites Filled with Sugarcane Rind Fiber. Polym. Test. 2018, 66, 319–326. [Google Scholar] [CrossRef]
- Liu, L.; Yu, J.; Cheng, L.; Yang, X. Biodegradability of Poly(Butylene Succinate) (PBS) Composite Reinforced with Jute Fibre. Polym. Degrad. Stab. 2009, 94, 90–94. [Google Scholar] [CrossRef]
- Bahl, S.; Dolma, J.; Singh, J.; Sehgal, S. Biodegradation of Plastics: A State of the Art Review. Mater. Today Proc. 2020, 39, 31–34. [Google Scholar] [CrossRef]
- European Environment Agency—Biodegradable and Compostable Plastics—Challenges and Opportunities. Available online: https://www.eea.europa.eu/publications/biodegradable-and-compostable-plastics (accessed on 20 March 2024).
- Visco, A.; Scolaro, C.; Bellhamdi, H.; Grasso, A. Photo-Degradation of a Biopolyester Blend under UV-C Rays. Macromol. Symp. 2022, 404, 2100330. [Google Scholar] [CrossRef]
- Belhamdi, H.; Kouini, B.; Grasso, A.; Scolaro, C.; Sili, A.; Visco, A. Tribological Behavior of Biomedical Grade UHMWPE with Graphite-based Fillers against EBM-Ti6Al4V Pin under Various Lubricating Conditions. J. Appl. Polym. Sci. 2022, 139, 52313. [Google Scholar] [CrossRef]
- Alavian Petroody, S.S.; Hashemi, S.H.; Škrlep, L.; Mušič, B.; van Gestel, C.A.M.; Sever Škapin, A. UV Light Causes Structural Changes in Microplastics Exposed in Bio-Solids. Polymers 2023, 15, 4322. [Google Scholar] [CrossRef] [PubMed]
- Orihuela, G.; Reyes, E.; Huamanchahua, D. Automated Disinfection System for Polyethylene Terephthalate Bottles for Bacteria, Fungi, and Viruses Using UVC LED Camera, Proceedings of the 12th International Conference on Manufacturing Engineering and Processes, ICMEP 2022, Seoul, Republic of Korea, 6–8 June 2022; Recent Advances in Manufacturing Engineering and Processes—Proceedings of ICMEP 2022; Springer: Singapore, 2023; pp. 11–23. [Google Scholar] [CrossRef]
- Sahu, A.; Sudhakar, K.; Sarviya, R. U.V Light Effect on the Mechanical Behaviour of HDPE/Carbon Black Composites. IOP Conf. Ser. Mater. Sci. Eng. 2020, 788, 012054. [Google Scholar] [CrossRef]
- Lin, J.; Yan, D.; Fu, J.; Chen, Y.; Ou, H.-S. Ultraviolet-C and Vacuum Ultraviolet Inducing Surface Degradation of Microplastics. Water Res. 2020, 186, 116360. [Google Scholar] [CrossRef]
- Storm, N.; McKay, L.G.A.; Downs, S.N.; Johnson, R.I.; Birru, D.; de Samber, M.; Willaert, W.; Cennini, G.; Griffiths, A. Rapid and Complete Inactivation of SARS-CoV-2 by Ultraviolet-C Irradiation. Sci. Rep. 2020, 10, 22421. [Google Scholar] [CrossRef] [PubMed]
- Amza, C.G.; Zapciu, A.; Baciu, F.; Vasile, M.I.; Popescu, D. Aging of 3D Printed Polymers under Sterilizing UV-C Radiation. Polymers 2021, 13, 4467. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Li, Y.; Yu, J. Photo-Stabilization of Linear Low Density Polyethylene by Inorganic Nano-Particles. Polym. Degrad. Stab. 2005, 88, 168–174. [Google Scholar] [CrossRef]
- Cai, L.; Qi, Z.; Xu, J.; Guo, B.; Huang, Z. Study on the Photodegradation Stability of Poly(Butylene Succinate- Co -Butylene Adipate)/TiO2 Nanocomposites. J. Chem. 2019, 2019, 5036019. [Google Scholar] [CrossRef]
- Ge, F.; Wang, X.; Ran, X. Properties of Biodegradable Poly(Butylene Succinate) (PBS) Composites with Carbon Black. Polym. Sci. Ser. A 2017, 59, 416–424. [Google Scholar] [CrossRef]
- Catauro, M.; Scolaro, C.; Dal Poggetto, G.; Pacifico, S.; Visco, A. Wear Resistant Nanocomposites Based on Biomedical Grade UHMWPE Paraffin Oil and Carbon Nano-Filler: Preliminary Biocompatibility and Antibacterial Activity Investigation. Polymers 2020, 12, 978. [Google Scholar] [CrossRef]
- Veranitisagul, C.; Wattanathana, W.; Wannapaiboon, S.; Hanlumyuang, Y.; Sukthavorn, K.; Nootsuwan, N.; Chotiwan, S.; Phuthong, W.; Jongrungruangchok, S.; Laobuthee, A. Antimicrobial, Conductive, and Mechanical Properties of AgCB/PBS Composite System. J. Chem. 2019, 2019, 3487529. [Google Scholar] [CrossRef]
- Amza, C.G.; Zapciu, A.; Baciu, F.; Radu, C. Effect of UV-C Radiation on 3D Printed ABS-PC Polymers. Polymers 2023, 15, 1966. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Horrocks, A.R. Effect of Carbon Black on UV Stability of LLDPE Films under Artificial Weathering Conditions. Polym. Degrad. Stab. 2002, 75, 485–499. [Google Scholar] [CrossRef]
- Collins, R.J.; Shin, H.; DeGuire, M.R.; Heuer, A.H.; Sukenik, C.N. Low Temperature Deposition of Patterned TiO2 Thin Films Using Photopatterned Self-assembled Monolayers. Appl. Phys. Lett. 1996, 69, 860–862. [Google Scholar] [CrossRef]
- Phua, Y.J.; Lau, N.S.; Sudesh, K.; Chow, W.S.; Ishak, Z.A.M. Biodegradability Studies of Poly(Butylene Succinate)/Organo-Montmorillonite Nanocomposites under Controlled Compost Soil Conditions: Effects of Clay Loading and Compatibiliser. Polym. Degrad. Stab. 2012, 97, 1345–1354. [Google Scholar] [CrossRef]
- He, X.; Tang, L.; Zheng, J.; Jin, Y.; Chang, R.; Yu, X.; Song, Y.; Huang, R. A Novel UV Barrier Poly(Lactic Acid)/Poly(Butylene Succinate) Composite Biodegradable Film Enhanced by Cellulose Extracted from Coconut Shell. Polymers 2023, 15, 3000. [Google Scholar] [CrossRef] [PubMed]
- Marmur, A. A Guide to the Equilibrium Contact Angles Maze. Contact Angle Wettability Adhes. 2009, 6, 3–18. [Google Scholar]
- Bertina, R.M.; Koeleman, B.P.C.; Koster, T.; Rosendaal, F.R.; Dirven, R.J.; de Ronde, H.; van der Velden, P.A.; Reitsma, P.H. Mutation in Blood Coagulation Factor V Associated with Resistance to Activated Protein C. Nature 1994, 369, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Askeland, D.R.; Fulay, P.P. Essentials of Materials Science & Engineering—SI Version; Cengage Learning: Boston, MA, USA, 2009; ISBN 978-1-111-78094-4. [Google Scholar]
- Chen, C.; Zhang, X.-W.; Ye, H.-M. Dependence of Crystallization Behavior of Interacting Telechelic Poly(Butylene Succinate) Oligomer on Molecular Weight. Crystals 2021, 11, 1530. [Google Scholar] [CrossRef]
- Ainali, N.M.; Bikiaris, D.; Lambropoulou, D. Aging Effects on Low- and High-Density Polyethylene, Polypropylene and Polystyrene under UV Irradiation: An Insight into Decomposition Mechanism by Py-GC/MS for Microplastic Analysis. J. Anal. Appl. Pyrolysis 2021, 158, 105207. [Google Scholar] [CrossRef]
- Visco, A.; Richaud, E.; Scolaro, C. Ageing Of Uhmwpe In Presence of Simulated Synovial Fluid. Polym. Degrad. Stab. 2021, 189, 109605. [Google Scholar] [CrossRef]
- Rasouli, D.; Dintcheva, N.T.; Faezipour, M.; Mantia, F.P.L.; Farahani, M.R.M.; Tajvidi, M. Effect of Nano Zinc Oxide as UV Stabilizer on the Weathering Performance of Wood-Polyethylene Composite. Polym. Degrad. Stab. 2016, 133, 85–91. [Google Scholar] [CrossRef]
- Cai, L.; Wang, J.; Wu, Z.; Tan, X. Observation of the Degradation of Three Types of Plastic Pellets Exposed to UV Irradiation in Three Different Environments. Sci. Total Environ. 2018, 628–629, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Torrisi, L.; Scolaro, C. Blood Wettability of Haemocompatible Carbon-Based Materials. J. Adv. Chem. Eng. 2017, 7, 1000179. [Google Scholar] [CrossRef]
- Bak, J.; Ladefoged, S.; Tvede, M.; Begovic, T.; Gregersen, A. Dose Requirements for UVC Disinfection of Catheter Biofilm. Biofouling 2009, 25, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Olewnik-Kruszkowska, E. Effect of UV Irradiation on Thermal Properties of Nanocomposites Based on Polylactide. J. Therm. Anal. Calorim. 2014, 119, 219–228. [Google Scholar] [CrossRef][Green Version]
- Kowalski, W. The Ultraviolet Germicidal Irradiation Handbook—UVGI for Air and Surface Disinfection; Springer: Berlin/Heidelberg, Germany, 2010; ISBN 978-3-642-01999-9. [Google Scholar]
- Buhr, T.L.; Borgers-Klonkowski, E.; Gutting, B.W.; Hammer, E.E.; Hamilton, S.M.; Huhman, B.M.; Jackson, S.L.; Kennihan, N.L.; Lilly, S.D.; Little, J.D.; et al. Ultraviolet Dosage and Decontamination Efficacy Were Widely Variable across 14 UV Devices after Testing a Dried Enveloped Ribonucleic Acid Virus Surrogate for SARS-CoV-2. Front. Bioeng. Biotechnol. 2022, 10, 875817. [Google Scholar] [CrossRef]
- Achieving Effective Germicidal Action with UVC Radiation: A Comprehensive Guide. Available online: https://www.ledrise.eu/blog/uv-fluence-for-disinfection/ (accessed on 14 March 2024).
- Malayeri, A.; Mohseni, M.; Cairns, B. Fluence (UV Dose) Required to Achieve Incremental Log Inactivation of Bacteria, Protozoa, Viruses and Algae. IUVA News 2016, 18, 4–6. [Google Scholar]
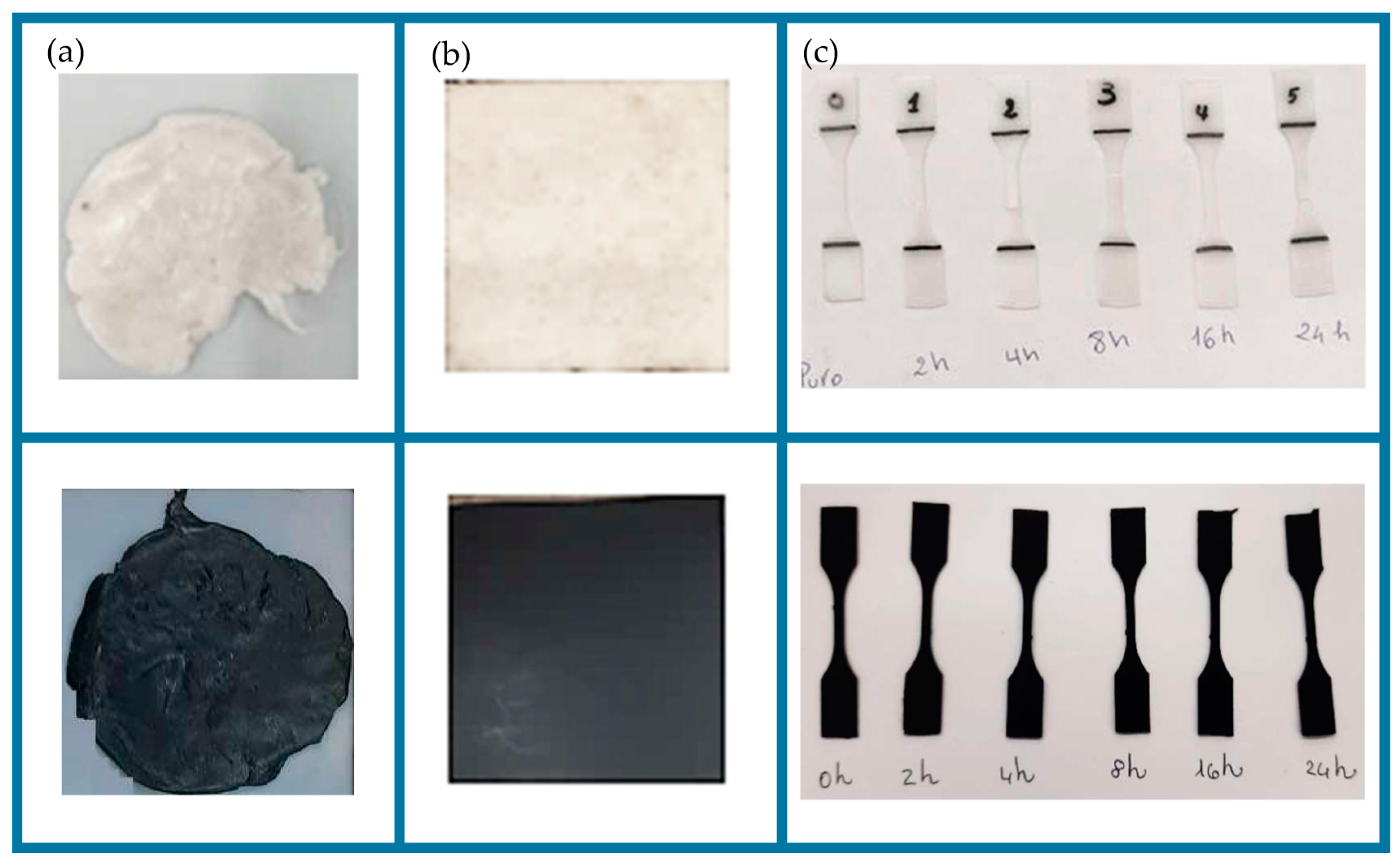
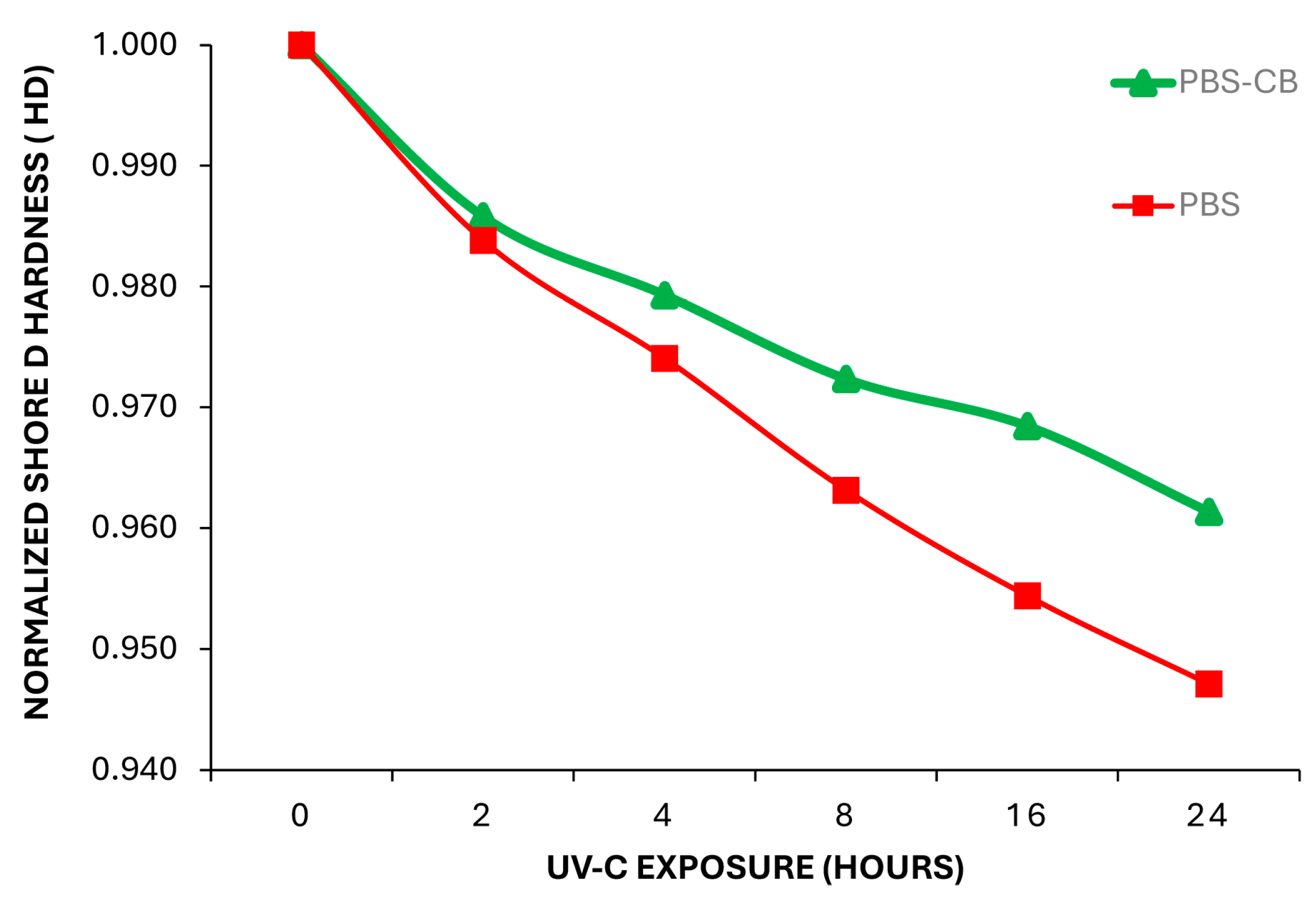
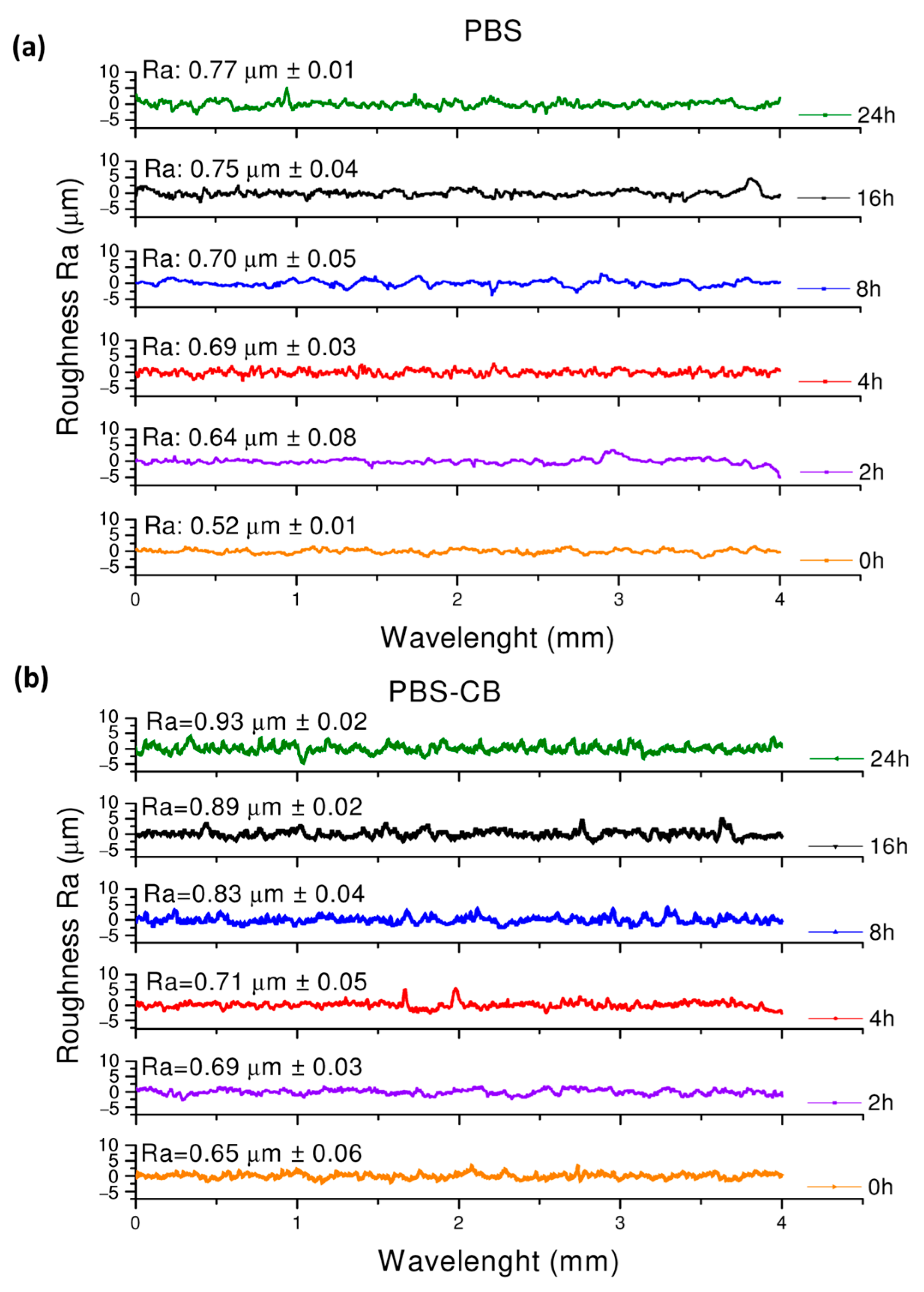
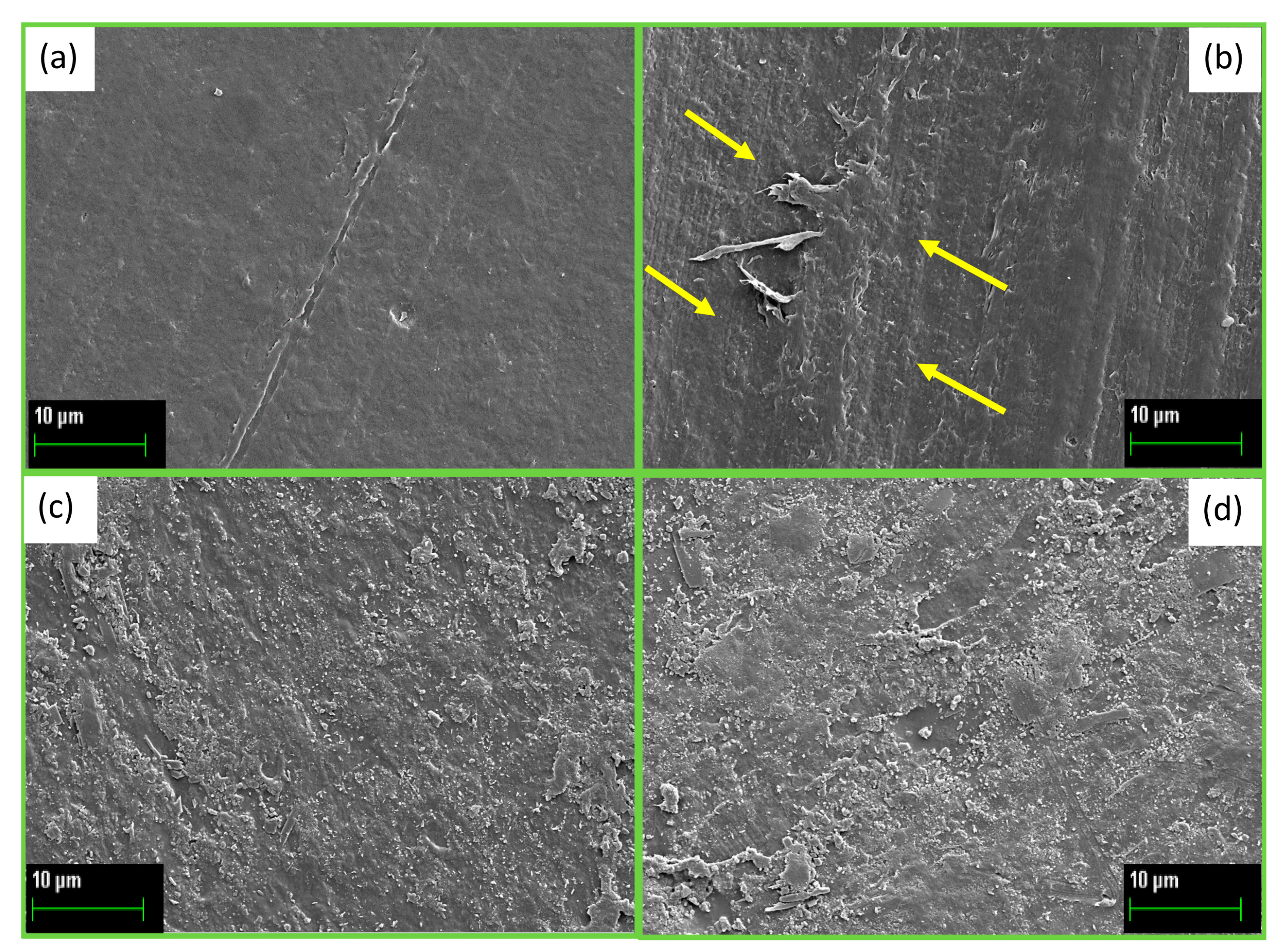
| UVC Exposure Time (h) | Fluence Dose [J·cm2] | Shore D PBS (HD) | Shore D PBS-CB (HD) |
|---|---|---|---|
| 0 | - | 68.6 ± 0.03 | 69.1 ± 0.03 |
| 2 | 6.2 | 67.5 ± 0.02 | 68.1 ± 0.05 |
| 4 | 12.8 | 66.8 ± 0.07 | 67.6 ± 0.01 |
| 8 | 24.8 | 66.1 ± 0.06 | 67.1 ± 0.03 |
| 16 | 49.6 | 65.5 ± 0.01 | 66.8 ± 0.03 |
| 24 | 74.3 | 65.0 ± 0.05 | 66.4 ± 0.07 |
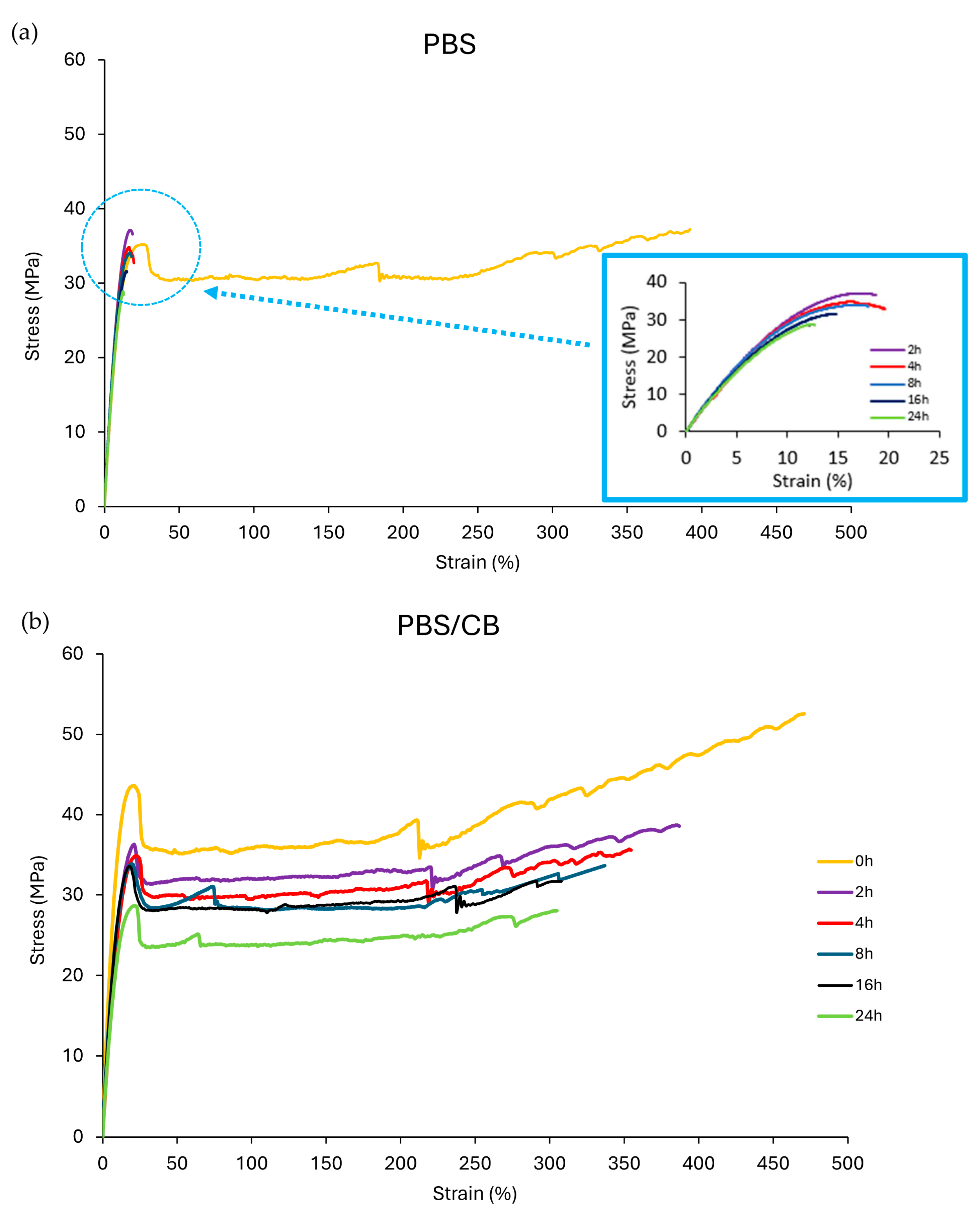
| UVC Exposure | Mechanical Parameter–Tensile Test of PBS | ||||||
| Time (h) | E [MPa] | σy [MPa] | εy [%] | Load r [N] | σr [MPa] | εr [%] | Lr [J] |
| 0 | 337.49 ± 4.71 | 35.31 ± 1.58 | 25.61 ± 1.33 | 88.90 ± 2.90 | 37.74 ± 2.23 | 411.61 ± 5.67 | 4.76 ± 0.09 |
| 2 | 346.11 ± 10.05 | - | - | 78.46 ± 5.03 | 37.44 ± 1.33 | 18.75 ± 1.67 | 0.15 ± 0.02 |
| 4 | 337.11 ± 14.84 | - | - | 76.96 ± 5.24 | 34.40 ± 2.77 | 18.19 ± 0.93 | 0.15 ± 0.02 |
| 8 | 332.87 ± 12.04 | - | - | 75.96 ± 2.42 | 33.70 ± 1.64 | 17.85 ± 1.11 | 0.14 ± 0.02 |
| 16 | 328.63 ± 7.10 | - | - | 72.48 ± 1.02 | 31.82 ± 1.94 | 14.03 ± 0.45 | 0.11 ± 0.01 |
| 24 | 201.68 ± 11.43 | - | - | 66.92 ± 0.47 | 28.57 ± 0.37 | 12.71 ± 0.32 | 0.11 ± 0.02 |
| UVC Exposure | Mechanical Parameter–Tensile Test of PBS-CB | ||||||
| Time (h) | E [MPa] | σy [MPa] | εy [%] | Load r [N] | σr [MPa] | εr [%] | Lr [J] |
| 0 | 449.20 ± 17.23 | 43.86 ± 1.26 | 20.82 ± 1.69 | 85.41 ± 3.47 | 52.28 ± 5.12 | 470.48 ± 27.55 | 4.64 ± 1.34 |
| 2 | 301.19 ± 16.97 | 36.48 ± 1.30 | 21.33 ± 1.16 | 93.71 ± 14.23 | 38.71 ± 4.10 | 387.54 ± 18.79 | 4.15 ± 0.74 |
| 4 | 294.94 ± 19.30 | 34.13 ± 0.49 | 22.22 ± 1.07 | 87.59 ± 10.00 | 35.12 ± 4.31 | 345.27 ± 30.12 | 3.96 ± 1.02 |
| 8 | 277.55 ± 21.24 | 33.86 ± 3.22 | 19.41 ± 1.16 | 83.88 ± 14.89 | 33.81 ± 2.26 | 336.82 ± 15.78 | 3.78 ± 0.42 |
| 16 | 272.41 ± 26.46 | 33.75 ± 0.83 | 18.39 ± 1.56 | 74.63 ± 2.49 | 31.77 ± 1.93 | 307.90 ± 22.19 | 3.22 ± 0.85 |
| 24 | 264.02 ± 23.11 | 28.72 ± 2.21 | 21.82 ± 0.41 | 71.38 ± 3.85 | 28.33 ± 2.59 | 304.05 ± 11.48 | 2.83 ± 0.67 |
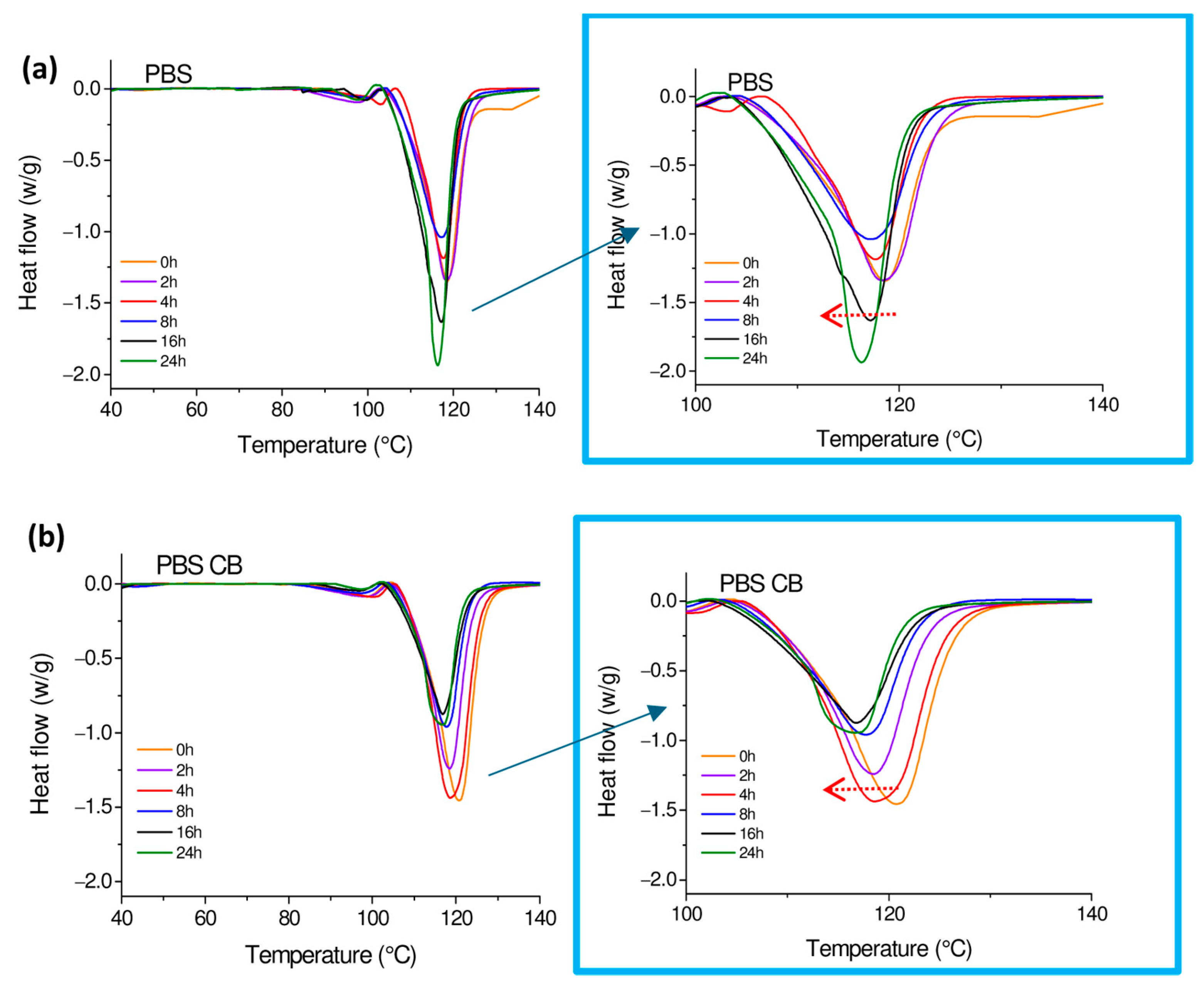
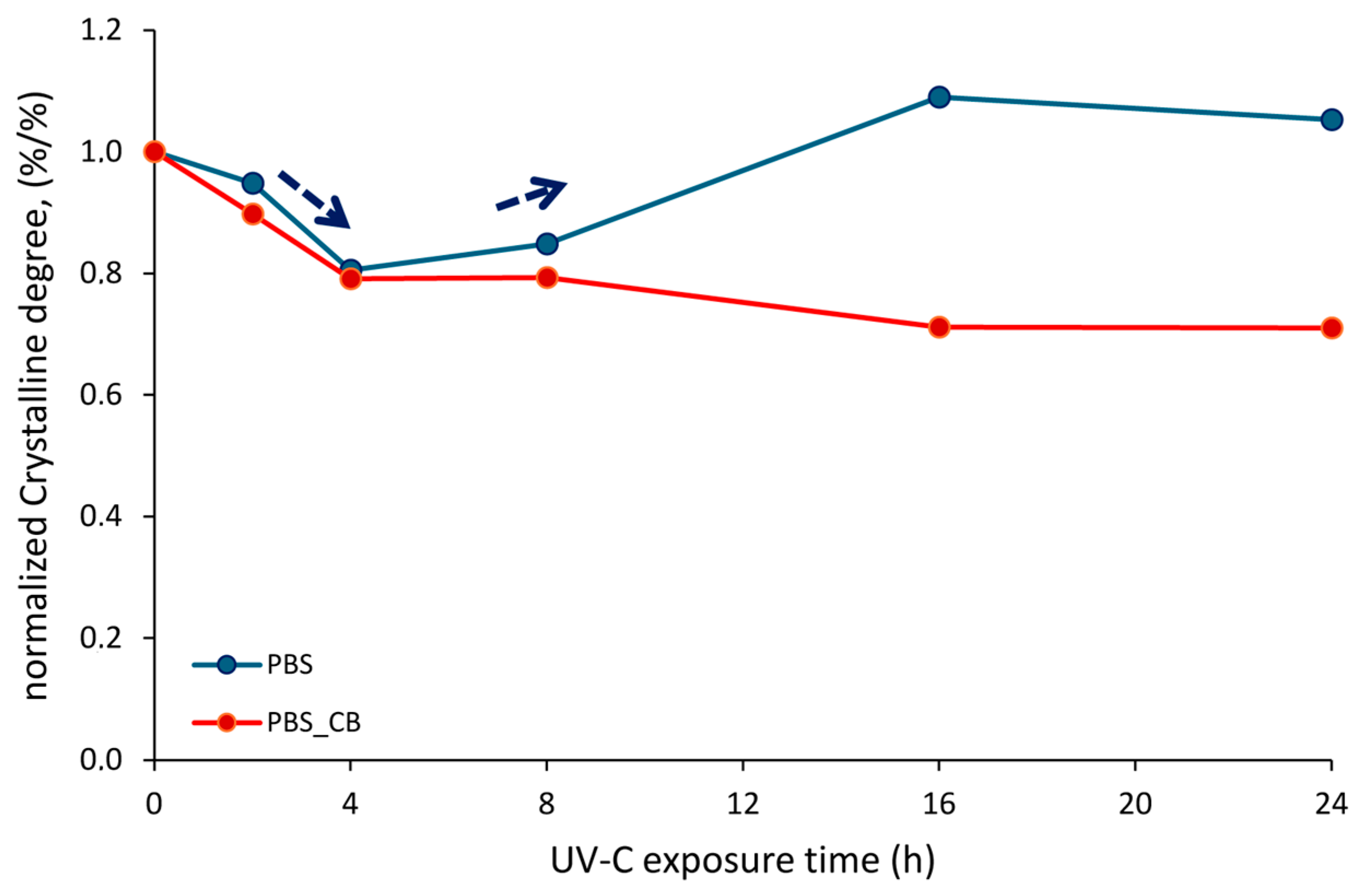
| UVC Rays | Melting Enthalpy | Melting Temperature | Crystalline Degree | |||
|---|---|---|---|---|---|---|
| Exposure Time (h) | PBS ΔHm (J/g) | PBS-CB ΔHm (J/g) | PBS (°C) | PBS-CB (°C) | PBS (%) | PBS-CB (%) |
| 0 | 74.75 ± 0.40 | 80.62 ± 0.71 | 118.73 ± 0.32 | 119.63 ± 0.24 | 67.76 ± 0.65 | 74.54 ± 0.46 |
| 2 | 74.62 ± 0.04 | 72.32 ± 0.54 | 118.29 ± 0.24 | 118.79 ± 0.68 | 64.25 ± 0.34 | 66.92 ± 0.73 |
| 4 | 60.16 ± 0.46 | 83.66 ± 0.71 | 118.22 ± 0.53 | 118.73 ± 0.43 | 54.57 ± 0.42 | 58.98 ± 0.56 |
| 8 | 63.37 ± 0.45 | 63.91 ± 0.34 | 117.41 ± 0.19 | 117.55 ± 0.39 | 57.46 ± 0.37 | 59.09 ± 0.39 |
| 16 | 81.44 ± 0.28 | 57.32 ± 0.29 | 117.06 ± 0.89 | 117.23 ± 0.27 | 73.85 ± 0.85 | 53.04 ± 0.53 |
| 24 | 78.71 ± 0.38 | 57.17 ± 0.71 | 116.60 ± 0.12 | 116.72 ± 0.64 | 71.34 ± 0.64 | 52.93 ± 0.55 |
| UVC Exposure | Wenzel Contact Angle, (θw) [°] | |||
|---|---|---|---|---|
| PBS | PBS-CB | |||
| Time (h) | Distilled Water | Blood | Distilled Water | Blood |
| 0 | 82.2 ± 0.95 | 82.1 ± 1.02 | 91.1 ± 1.53 | 73.9 ± 0.99 |
| 2 | 79.7 ± 1.14 | 75.8 ± 1.22 | 80.5 ± 1.38 | 71.8 ± 1.28 |
| 4 | 77.8 ± 1.10 | 72.5 ± 1.11 | 78.4 ± 1.62 | 69.5 ± 1.09 |
| 8 | 76.2 ± 1.04 | 69.3 ± 0.96 | 77.4 ± 1.33 | 67.8 ± 0.86 |
| 16 | 75.5 ± 0.98 | 68.4 ± 0.77 | 76.3 ± 1.66 | 63.2 ± 0.96 |
| 24 | 74.6 ± 1.13 | 67.0 ± 1.18 | 75.2 ± 1.34 | 62.8 ± 1.00 |
| Refs. | Power (W) | Intensity (10−3 W/cm2) | Fluence Dose (J/cm2) | Time (min) | Distance Sample–Lamp (cm) | Polymer/Killed Microorganisms (%) |
|---|---|---|---|---|---|---|
| [34] | n.a. * | 1.0 | 20 × 10−3 | 1440 | n.a. * | ABS-PC/90 |
| [48] | 6 | 0.4 | 2.16 | 90 | 9 | Silicone/98.7 |
| 1.4 × 103 | 60 | 9 | Silicone/99.9 | |||
| [49] | n.a. * | 3.12 | n.a. * | 120–960 | 5 | PLA/n.a. * |
| [50] | 30 | 0.125 | 2.24 × 10−4 | 30 | 100 | n.a. */90 |
| [51] | 18 | 10.12 | 729 × 10−3 | 2 | 17 | PMMA-ABS-PU/99.99 |
| This work | 6 | 0.86 | 6.2–74.3 | 120–1440 | 15 | PBS/n.a. * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scolaro, C.; Brahimi, S.; Falcone, A.; Beghetto, V.; Visco, A. Mechanical and Physical Changes in Bio-Polybutylene-Succinate Induced by UVC Ray Photodegradation. Polymers 2024, 16, 1288. https://doi.org/10.3390/polym16091288
Scolaro C, Brahimi S, Falcone A, Beghetto V, Visco A. Mechanical and Physical Changes in Bio-Polybutylene-Succinate Induced by UVC Ray Photodegradation. Polymers. 2024; 16(9):1288. https://doi.org/10.3390/polym16091288
Chicago/Turabian StyleScolaro, Cristina, Salim Brahimi, Aurora Falcone, Valentina Beghetto, and Annamaria Visco. 2024. "Mechanical and Physical Changes in Bio-Polybutylene-Succinate Induced by UVC Ray Photodegradation" Polymers 16, no. 9: 1288. https://doi.org/10.3390/polym16091288
APA StyleScolaro, C., Brahimi, S., Falcone, A., Beghetto, V., & Visco, A. (2024). Mechanical and Physical Changes in Bio-Polybutylene-Succinate Induced by UVC Ray Photodegradation. Polymers, 16(9), 1288. https://doi.org/10.3390/polym16091288








