High-Performance, Easy-to-Fabricate, Nanocomposite Heater for Life Sciences and Biomedical Applications
Abstract
1. Introduction
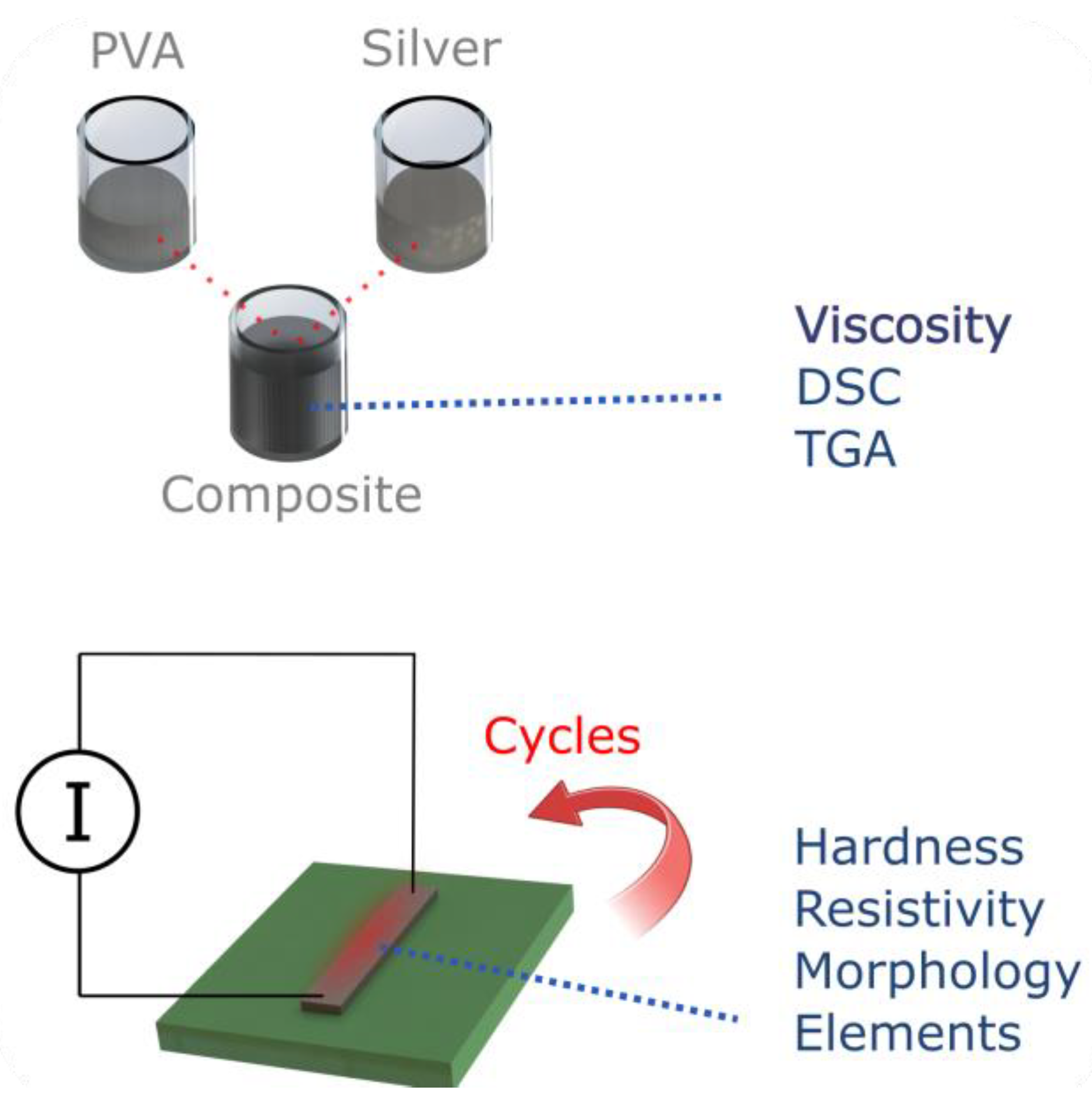
2. Materials and Methods
3. Results and Discussions
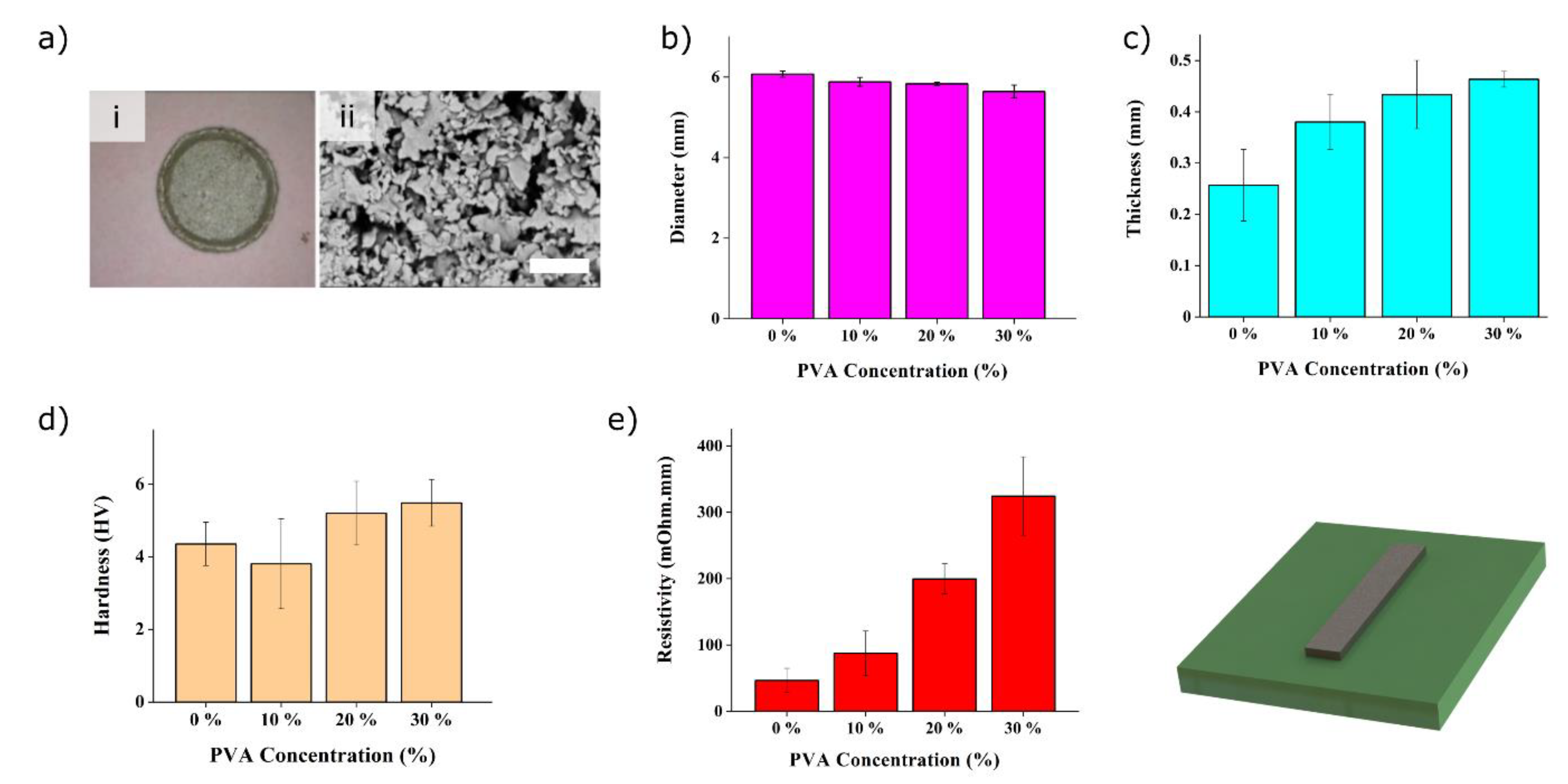
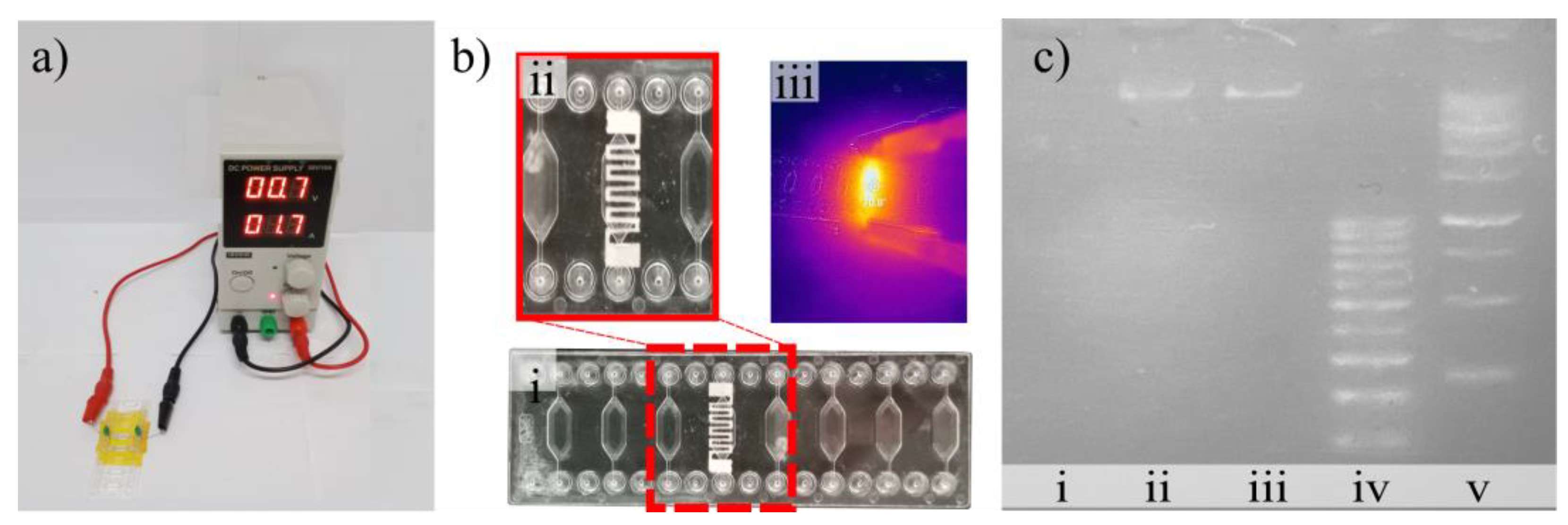
3.1. Fabrication of the Electrodes and Optimisation
3.2. Performances of the Nanocomposite Heater
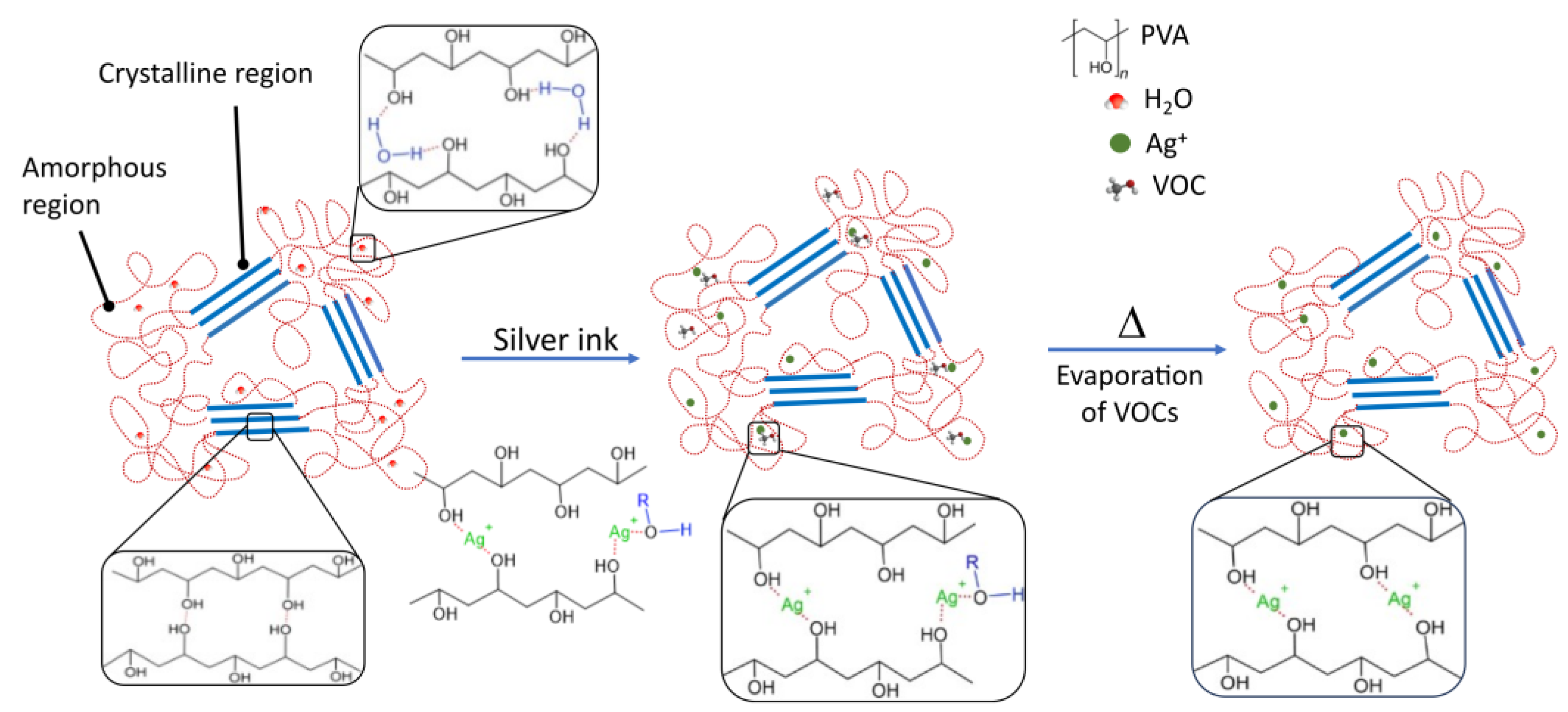
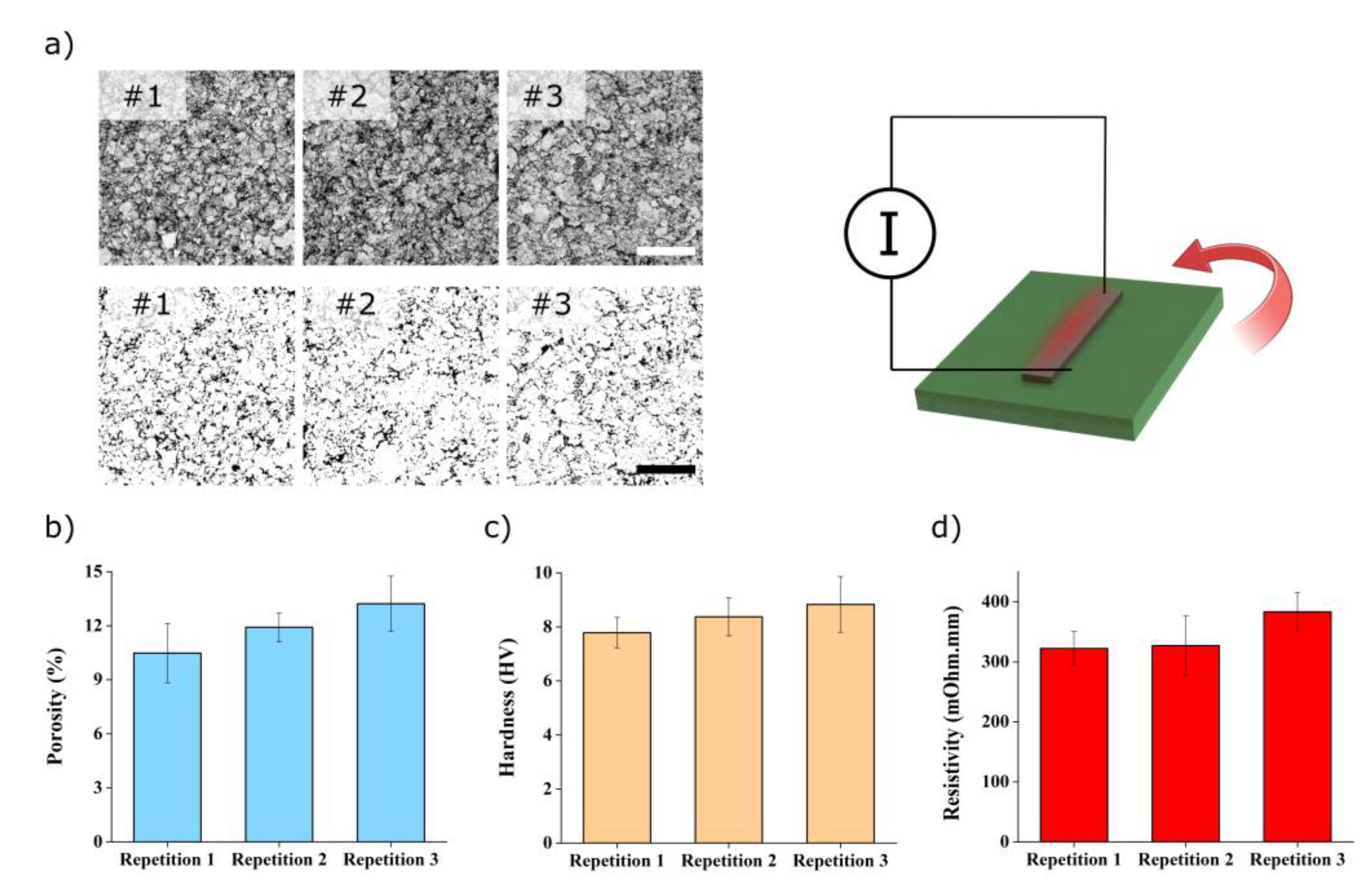
3.3. Study of the Raw Materials Properties
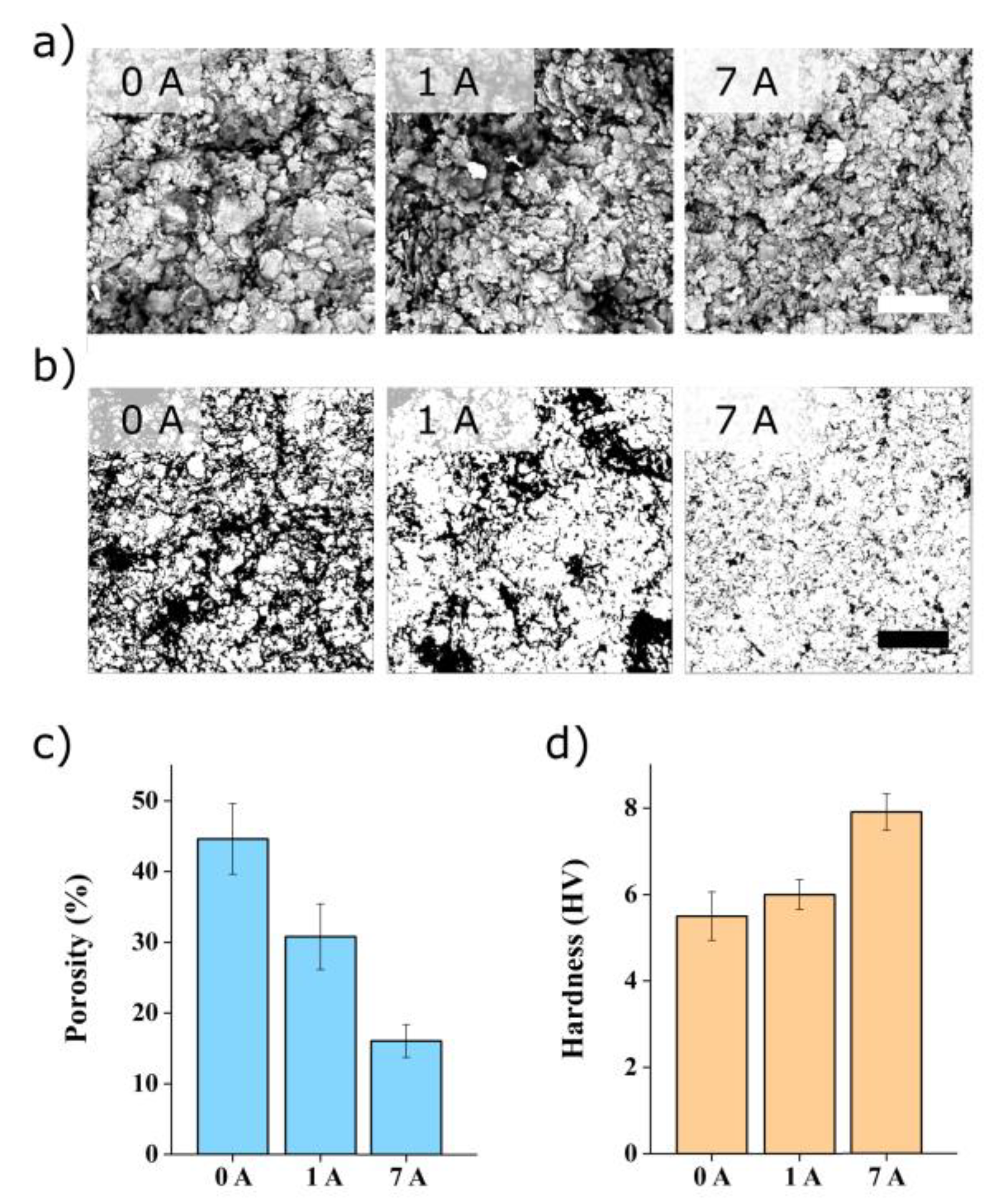
3.4. Morphology and Composition of Processed Samples
3.5. Validation Using DNA Amplification
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Byers, K.M.; Lin, L.K.; Moehling, T.J.; Stanciu, L.; Linnes, J.C. Versatile Printed Microheaters to Enable Low-Power Thermal Control in Paper Diagnostics. Analyst 2020, 145, 184–196. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, Z.; Liao, S.; Wang, J.; Wang, Y. A Light-Activated Microheater for the Remote Control of Enzymatic Catalysis. Chem.—A Eur. J. 2016, 22, 1152–1158. [Google Scholar] [CrossRef] [PubMed]
- Weng, C.-H.; Huang, C.-C.; Yeh, C.-S.; Lei, H.-Y.; Lee, G.-B. Synthesis of Hexagonal Gold Nanoparticles Using a Microfluidic Reaction System *. J. Micromech. Microeng. 2008, 18, 8. [Google Scholar] [CrossRef]
- Long, H.; Harley-Trochimczyk, A.; He, T.; Pham, T.; Tang, Z.; Shi, T.; Zettl, A.; Mickelson, W.; Carraro, C.; Maboudian, R. In Situ Localized Growth of Porous Tin Oxide Films on Low Power Microheater Platform for Low Temperature CO Detection. ACS Sensors 2016, 1, 339–343. [Google Scholar] [CrossRef]
- Lin, W.C.; Yang, Y.J.; Hsieh, G.W.; Tsai, C.H.; Chen, C.C.; Liang, C.C. Selective Local Synthesis of Nanowires on a Microreactor Chip. Sens. Actuators A Phys. 2006, 130–131, 625–632. [Google Scholar] [CrossRef]
- Darhuber, A.A.; Valentino, J.P.; Troian, S.M.; Wagner, S. Thermocapillary Actuation of Droplets on Chemically Patterned Surfaces by Programmable Microheater Arrays. J. Microelectromech. Syst. 2003, 12, 873–879. [Google Scholar] [CrossRef]
- Qaiser, N.; Khan, S.M.; Babatain, W.; Nour, M.; Joharji, L.; Shaikh, S.F.F.; El-Atab, N.; Hussain, M.M. A Thermal Microfluidic Actuator Based on a Novel Microheater. J. Micromech. Microeng. 2023, 33, 035001. [Google Scholar] [CrossRef]
- Vitale, W.A.; Petit, L.; Moldovan, C.F.; Fernández-Bolaños, M.; Paone, A.; Schüler, A.; Ionescu, A.M. Electrothermal Actuation of Vanadium Dioxide for Tunable Capacitors and Microwave Filters with Integrated Microheaters. Sens. Actuators A Phys. 2016, 241, 245–253. [Google Scholar] [CrossRef]
- Chuang, H.S.; Wereley, S. Design, Fabrication and Characterization of a Conducting PDMS for Microheaters and Temperature Sensors. J. Micromech. Microeng. 2009, 19, 045010. [Google Scholar] [CrossRef]
- Rao, A.; Long, H.; Harley-Trochimczyk, A.; Pham, T.; Zettl, A.; Carraro, C.; Maboudian, R. In Situ Localized Growth of Ordered Metal Oxide Hollow Sphere Array on Microheater Platform for Sensitive, Ultra-Fast Gas Sensing. ACS Appl. Mater. Interfaces 2017, 9, 2634–2641. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wu, Z.; Ding, H.; Wei, Y.; Yang, X.; Li, Z.; Yang, B.R.; Liu, C.; Qiu, L.; Wang, X. Multifunctional and High-Sensitive Sensor Capable of Detecting Humidity, Temperature, and Flow Stimuli Using an Integrated Microheater. ACS Appl. Mater. Interfaces 2019, 11, 43383–43392. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; Yoo, I.; Song, S.; Yoon, B.; Kim, J.M. A Thermoresponsive Fluorogenic Conjugated Polymer for a Temperature Sensor in Microfluidic Devices. J. Am. Chem. Soc. 2009, 131, 3800–3801. [Google Scholar] [CrossRef] [PubMed]
- Kan, W.; Chu, E.; Ren, W.; Liu, L.; Liu, W. Integrating NiCr-Based Thin Film Micro-Heater onto Electrode Header: A Review and Perspectives. In Proceedings of the 2021 IEEE International Conference on Advances in Electrical Engineering and Computer Applications (AEECA 2021), Dalian, China, 27–28 August 2021; pp. 382–385. [Google Scholar] [CrossRef]
- Yin, M.; Xiao, L.; Liu, Q.; Kwon, S.Y.; Zhang, Y.; Sharma, P.R.; Jin, L.; Li, X.; Xu, B. 3D Printed Microheater Sensor-Integrated, Drug-Encapsulated Microneedle Patch System for Pain Management. Adv. Healthc. Mater. 2019, 8, 1901170. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.L.; Wang, S.S.; Wu, M.H.; Oh-Yang, C.C. Development of an Integrated Microfluidic Perfusion Cell Culture System for Real-Time Microscopic Observation of Biological Cells. Sensors 2011, 11, 8395–8411. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.K.; Bhat, S.; Mahato, K.K. Design and Fabrication of Screen Printed Microheater. Microsyst. Technol. 2018, 24, 3273–3281. [Google Scholar] [CrossRef]
- Uddin, S.M.; Sayad, A.; Chan, J.; Huynh, D.H.; Skafidas, E.; Kwan, P. Heater Integrated Lab-on-a-Chip Device for Rapid HLA Alleles Amplification towards Prevention of Drug Hypersensitivity. Sensors 2021, 21, 3413. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kumar, D.; Vashishath, M.; Singh, J.; Hooda, M.K. Investigation of CMOS Compatible Titanium Nitride-Based Microheater for Microthrusters. ISSS J. Micro Smart Syst. 2022, 11, 417–426. [Google Scholar] [CrossRef]
- Moreira, I.P.; Sanivada, U.K.; Bessa, J.; Cunha, F.; Fangueiro, R. A Review of Multiple Scale Fibrous and Composite Systems for Heating Applications. Molecules 2021, 26, 3686. [Google Scholar] [CrossRef]
- Moschou, D.; Vourdas, N.; Kokkoris, G.; Papadakis, G.; Parthenios, J.; Chatzandroulis, S.; Tserepi, A. All-Plastic, Low-Power, Disposable, Continuous-Flow PCR Chip with Integrated Microheaters for Rapid DNA Amplification. Sens. Actuators B Chem. 2014, 199, 470–478. [Google Scholar] [CrossRef]
- Wu, J.; Cao, W.; Wen, W.; Chang, D.C.; Sheng, P. Polydimethylsiloxane Microfluidic Chip with Integrated Microheater and Thermal Sensor. Biomicrofluidics 2009, 3, 012005. [Google Scholar] [CrossRef]
- Nieto, D.; McGlynn, P.; de la Fuente, M.; Lopez-Lopez, R.; O’connor, G.M. Laser Microfabrication of a Microheater Chip for Cell Culture Outside a Cell Incubator. Colloids Surf. B Biointerfaces 2017, 154, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.L.; Wu, M.H.; Kuo, C.Y.; Da Lee, K.; Shen, Y.L. Application of Indium Tin Oxide (ITO)-Based Microheater Chip with Uniform Thermal Distribution for Perfusion Cell Culture Outside a Cell Incubator. Biomed. Microdevices 2010, 12, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Charmet, J.; Arosio, P.; Knowles, T.P.J. Microfluidics for Protein Biophysics. J. Mol. Biol. 2018, 430, 565–580. [Google Scholar] [CrossRef] [PubMed]
- Urbano-Gámez, J.D.; Perdigones, F.; Quero, J.M. Semi-Automatic Lab-on-PCB System for Agarose Gel Preparation and Electrophoresis for Biomedical Applications. Micromachines 2021, 12, 1071. [Google Scholar] [CrossRef] [PubMed]
- Jeroish, Z.E.; Bhuvaneshwari, K.S.; Samsuri, F.; Narayanamurthy, V. Microheater: Material, Design, Fabrication, Temperature Control, and Applications—A Role in COVID-19; Springer: New York, NY, USA, 2022; Volume 24. [Google Scholar] [CrossRef]
- Lin, W.C.; Lin, Y.C.; Esashi, M.; Seshia, A.A. In-Situ Hydrothermal Synthesis of Zinc Oxide Nanostructures Using Microheaters. IEEE Trans. Nanotechnol. 2015, 14, 1046–1053. [Google Scholar] [CrossRef]
- Charmet, J.; Rodrigues, R.; Yildirim, E.; Challa, P.K.; Roberts, B.; Dallmann, R.; Whulanza, Y. Low-Cost Microfabrication Tool Box. Micromachines 2020, 11, 135. [Google Scholar] [CrossRef] [PubMed]
- Whulanza, Y.; Ammarsyah, R.; Yatim, A. Characterization of Silver Resistive Electrode as Heating Module for Portable Thermocycler Device. In Proceedings of the 2021 IEEE International Biomedical Instrumentation and Technology Conference: The Improvement of Healthcare Technology to Achieve Universal Health Coverage, IBITeC, Yogyakarta, Indonesia, 20–21 October 2021; pp. 40–45. [Google Scholar] [CrossRef]
- Leong, C.K.; Chung, D.D.L. Improving the Electrical and Mechanical Behavior of Electrically Conductive Paint by Partial Replacement of Silver by Carbon Black. J. Electron. Mater. 2006, 35, 118–122. [Google Scholar] [CrossRef]
- Suriati, G.; Mariatti, M.; Azizan, A. Effects of Filler Shape and Size on the Properties of Silver Filled Epoxy Composite for Electronic Applications. J. Mater. Sci. Mater. Electron. 2011, 22, 56–63. [Google Scholar] [CrossRef]
- Mbhele, Z.H.; Salemane, M.G.; Van Sittert, C.G.C.E.; Nedeljković, J.M.; Djoković, V.; Luyt, A.S. Fabrication and Characterization of Silver-Polyvinyl Alcohol Nanocomposites. Chem. Mater. 2003, 15, 5019–5024. [Google Scholar] [CrossRef]
- Liu, L.; Peng, S.; Niu, X.; Wen, W. Microheaters Fabricated from a Conducting Composite. Appl. Phys. Lett. 2006, 89, 2–5. [Google Scholar] [CrossRef]
- Hojat, N.; Gentile, P.; Ferreira, A.M.; Šiller, L. Automatic Pore Size Measurements from Scanning Electron Microscopy Images of Porous Scaffolds. J. Porous Mater. 2023, 30, 93–101. [Google Scholar] [CrossRef]
- Subekti, D.T.; Ekawasti, F.; Azmi, Z.; Yuniarto, I.; Fong, S.; Fahrimal, Y. Does Trypanosoma Evansi Have the Maxicircle Gene, or Can Trypanosoma Equiperdum Be Isolated from Bovines? J. Parasitol. 2023, 109, 436–444. [Google Scholar] [PubMed]
- Walton, R.L.; Kupp, E.R.; Messing, G.L. Additive Manufacturing of Textured Ceramics: A Review. J. Mater. Res. 2021, 36, 3591–3606. [Google Scholar] [CrossRef]
- Gong, X.; Huang, K.; Wu, Y.-H.; Zhang, X.-S. Recent Progress on Screen-Printed Flexible Sensors for Human Health Monitoring. Sens. Actuators A Phys. 2022, 345, 113821. [Google Scholar] [CrossRef]
- Sau, S.; Kundu, S. Variation in Structure and Properties of Poly(Vinyl Alcohol) (PVA) Film in the Presence of Silver Nanoparticles Grown under Heat Treatment. J. Mol. Struct. 2022, 1250, 131699. [Google Scholar] [CrossRef]
- Vincent, M.; Gopalakrishnan, V.K.; Sivanandan, S.; Radhika, T.; Raghu, N. Factors Influencing Rheological Characteristics of Silver Thick Film Paste and Its Correlation to Multilayer Ceramic Processing. Adv. Appl. Ceram. 2018, 118, 106–113. [Google Scholar] [CrossRef]
- Devangamath, S.S.; Lobo, B.; Masti, S.P.; Narasagoudr, S. Thermal, Mechanical, and AC Electrical Studies of PVA–PEG–Ag2S Polymer Hybrid Material. J. Mater. Sci. Mater. Electron. 2020, 31, 2904–2917. [Google Scholar] [CrossRef]
- Huang, C.; Yen, C.; Chang, T. Studies on the Preparation and Properties of Conductive Polymers. III. Metallized Polymer Films by Retroplating Out. J. Appl. Polym. Sci. 1991, 42, 2237–2245. [Google Scholar] [CrossRef]
- Sherman, K.; Mackay, C.A. A Thermoanalytical Study of the Components and Formulation of a Rosin Based Flux. Solder. Surf. Mt. Technol. 1990, 2, 28–31. [Google Scholar] [CrossRef]
- Becherer, L.; Borst, N.; Bakheit, M.; Frischmann, S.; Zengerle, R.; von Stetten, F. Loop-Mediated Isothermal Amplification (LAMP)—Review and Classification of Methods for Sequence-Specific Detection. Anal. Methods 2020, 12, 717–746. [Google Scholar] [CrossRef]


| 0 A | 7 A | |
|---|---|---|
| Silver | 52.84% | 74.38% |
| Carbon | 25.16% | 11.54% |
| Oxygen | 17.69% | 8.58% |
| Gold | 4.31% | 5.50% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Whulanza, Y.; Ammar, H.; Haryadi, D.; Pangesty, A.I.; Widoretno, W.; Subekti, D.T.; Charmet, J. High-Performance, Easy-to-Fabricate, Nanocomposite Heater for Life Sciences and Biomedical Applications. Polymers 2024, 16, 1164. https://doi.org/10.3390/polym16081164
Whulanza Y, Ammar H, Haryadi D, Pangesty AI, Widoretno W, Subekti DT, Charmet J. High-Performance, Easy-to-Fabricate, Nanocomposite Heater for Life Sciences and Biomedical Applications. Polymers. 2024; 16(8):1164. https://doi.org/10.3390/polym16081164
Chicago/Turabian StyleWhulanza, Yudan, Husein Ammar, Deni Haryadi, Azizah Intan Pangesty, Widoretno Widoretno, Didik Tulus Subekti, and Jérôme Charmet. 2024. "High-Performance, Easy-to-Fabricate, Nanocomposite Heater for Life Sciences and Biomedical Applications" Polymers 16, no. 8: 1164. https://doi.org/10.3390/polym16081164
APA StyleWhulanza, Y., Ammar, H., Haryadi, D., Pangesty, A. I., Widoretno, W., Subekti, D. T., & Charmet, J. (2024). High-Performance, Easy-to-Fabricate, Nanocomposite Heater for Life Sciences and Biomedical Applications. Polymers, 16(8), 1164. https://doi.org/10.3390/polym16081164








