Characterization of Waste Nicotiana rustica L. (Tobacco) Fiber Having a Potential in Textile and Composite Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Fiber Extraction
2.2. Physical Analysis
2.3. Chemical Composition Quantification
2.4. Categorization of Chemical Functional Groups in NRL
2.5. X-ray Photoelectron Spectroscopy Analysis (XPS)
2.6. XRD Analysis and Crystallinity Index Determination
2.7. Investigation of the Thermal Degradation Behavior
2.8. Examination of Surface Morphology
2.9. Single Fiber Test
2.10. Fiber Yield Analysis
2.11. Moisture Content
3. Results and Discussion
3.1. Physical and Chemical Properties of NRL Fiber
| Fiber | Length (mm) | Diameter (μm) | Density (g/cm3) | Ref. |
|---|---|---|---|---|
| Nicotiana rustica L. | 16–55 | 26–46 | 1.50 | In this study |
| Cotton | 10–50 | 14–21 | 1.52–1.56 | [50,51] |
| Hemp | 15–25 | 15–30 | 1.48–1.49 | [50] |
| Flax | Up to 900 | 17–20 | 1.50 | [50,51] |
| Jute | Up to 4000 | 14–20 | 1.44–1.49 | [50] |
| Sisal | 800–1200 | 7–47 | 1.20–1.22 | [50] |
| Ramie | Up to 1900 | 40–60 | 1.51–1.55 | [50,51] |
| Fiber | Cellulose (%) | Hemicellulose (%) | Lignin (%) | Moisture Uptake | Ref. |
|---|---|---|---|---|---|
| Nicotiana rustica L. | 56.6 | 11.8 | 14.97 | 13.15 | In this study |
| Hemp | 67–75 | 16–18 | 3–5 | 6.2–12 | [42] |
| Flax | 62–71 | 16–18 | 3–4.5 | 8–12 | [42] |
| Jute | 59–71 | 12–13 | 11.8–12.9 | 12.5–13.7 | [42] |
| Kenaf | 45–57 | 21.5 | 12–13 | 6–12 | [60] |
| Ramie | 68–76 | 13–14 | 0.6–2 | 7.5–17 | [60] |
3.2. Mechanical Properties of NRL Fiber
3.3. Surface Morphology of NRL Fiber
3.4. FTIR Analysis
3.5. XPS Analysis of NRL Fiber
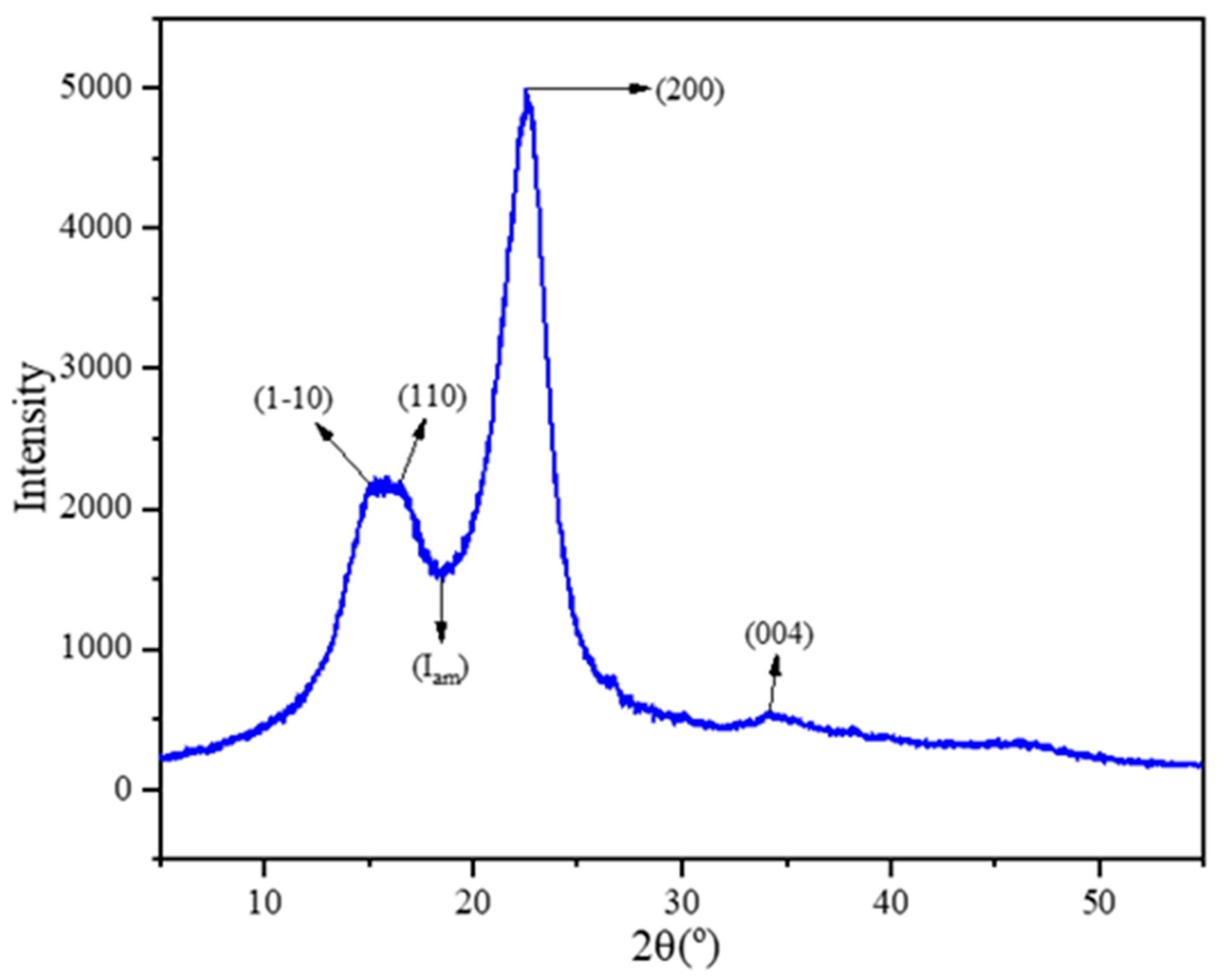
3.6. XRD Analysis of NRL Fiber
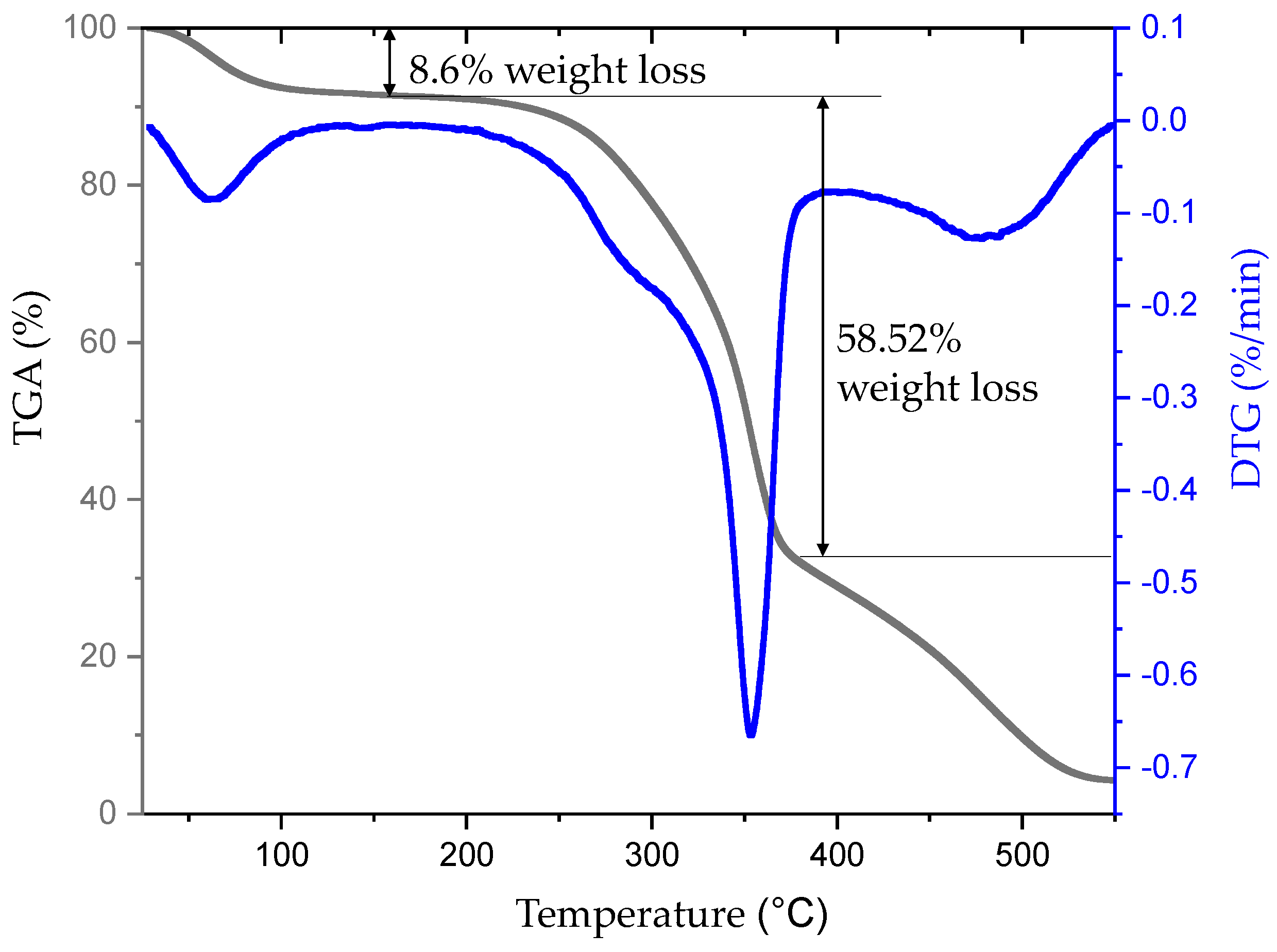
3.7. TGA Analysis of NRL Fiber
4. Conclusions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Karabacak, K. Tobacco Agriculture and Geographical Distribution in Turkey. CBD 2017, 15, 27–48. [Google Scholar] [CrossRef]
- Berlowitz, I.; García Torres, E.; Maake, C.; Wolf, U.; Martin-Soelch, C. Indigenous-Amazonian Traditional Medicine’s Usage of the Tobacco Plant: A Transdisciplinary Ethnopsychological Mixed-Methods Case Study. Plants 2023, 12, 346. [Google Scholar] [CrossRef]
- Kishore, K. Monograph of Tobacco (Nicotiana tabacum). Indian J. Drugs 2014, 2, 5–23. [Google Scholar]
- Şahïn, G.; Taşligïl, N. Türkiye’de Tütün (Nicotiana tabacum L.) Yetiştiriciliğinin Tarihsel Gelişimi ve Coğrafi Dağilimi. East. Geogr. Rev. 2014, 18, 71–102. [Google Scholar]
- Adıyaman Tobacco Report; İpekyolu Kalkınma Ajansı: Adıyaman, Turkey, 2013; p. 44. Available online: https://www.ika.org.tr/assets/upload/dosyalar/adiyaman-tutun-raporu.pdf (accessed on 8 March 2024).
- Sifola, M.I.; Postiglione, L. The Effect of Increasing NaCl in Irrigation Water on Growth, Gas Exchange and Yield of Tobacco Burley Type. Field Crops Res. 2002, 74, 81–91. [Google Scholar] [CrossRef]
- Sifola, M.I.; del Piano, L.; Todisco, D.; Graziani, G.; Faugno, S.; Sannino, M.; Piscopo, R.; Salluzzo, A.; Cozzolino, E. A Multipurpose Sustainable Farming System for Tobacco Crops in the Mediterranean Area. Sustainability 2023, 15, 16636. [Google Scholar] [CrossRef]
- Tian, W.-W.; Xu, F.; Xing, S.-J.; Wu, R.; Yuan, Z.-Y. Comprehensive Study on the Thermal Decomposition Process of Waste Tobacco Leaves and Stems to Investigate Their Bioenergy Potential: Kinetic, Thermodynamic, and Biochar Analysis. Thermochim. Acta 2023, 723, 179473. [Google Scholar] [CrossRef]
- Kumar, K.; Goh, K.M. Crop Residues and Management Practices: Effects on Soil Quality, Soil Nitrogen Dynamics, Crop Yield, and Nitrogen Recovery. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 1999; Volume 68, pp. 197–319. [Google Scholar]
- Hirel, B.; Tétu, T.; Lea, P.J.; Dubois, F. Improving Nitrogen Use Efficiency in Crops for Sustainable Agriculture. Sustainability 2011, 3, 1452–1485. [Google Scholar] [CrossRef]
- Zou, X.; Bk, A.; Abu-Izneid, T.; Aziz, A.; Devnath, P.; Rauf, A.; Mitra, S.; Emran, T.B.; Mujawah, A.A.H.; Lorenzo, J.M.; et al. Current Advances of Functional Phytochemicals in Nicotiana Plant and Related Potential Value of Tobacco Processing Waste: A Review. Biomed. Pharmacother. 2021, 143, 112191. [Google Scholar] [CrossRef]
- Banožić, M.; Babić, J.; Jokić, S. Recent Advances in Extraction of Bioactive Compounds from Tobacco Industrial Waste—a Review. Ind. Crops Prod. 2020, 144, 112009. [Google Scholar] [CrossRef]
- Sha, Y.; Yu, H.; Xiong, J.; Wang, J.; Fei, T.; Wu, D.; Yang, K.; Zhang, L. Separation and Purification of Active Ingredients in Tobacco by Free-Flow Electrophoresis. Anal. Methods 2023, 15, 5885–5890. [Google Scholar] [CrossRef]
- Shi, W.; Li, H.; Zeng, X.; Zhang, H.; Qin, X. The Extraction of Tobacco Protein from Discarded Tobacco Leaf by Hollow Fiber Membrane Integrated Process. Innov. Food Sci. Emerg. Technol. 2019, 58, 102245. [Google Scholar] [CrossRef]
- Muvhiiwa, R.; Mawere, E.; Moyo, L.B.; Tshuma, L. Utilization of Cellulose in Tobacco (Nicotiana Tobacum) Stalks for Nitrocellulose Production. Heliyon 2021, 7, e07598. [Google Scholar] [CrossRef]
- Ahmed, M.J.; Hameed, B.H. Recent Progress on Tobacco Wastes–Derived Adsorbents for the Remediation of Aquatic Pollutants: A Review. Environ. Res. 2024, 247, 118203. [Google Scholar] [CrossRef]
- Gargol, M.; Klepka, T.; Klapiszewski, Ł.; Podkościelna, B. Synthesis and Thermo-Mechanical Study of Epoxy Resin-Based Composites with Waste Fibers of Hemp as an Eco-Friendly Filler. Polymers 2021, 13, 503. [Google Scholar] [CrossRef]
- AL-Oqla, F.M.; Sapuan, S.M.; Ishak, M.R.; Nuraini, A.A. Predicting the Potential of Agro Waste Fibers for Sustainable Automotive Industry Using a Decision Making Model. Comput. Electron. Agric. 2015, 113, 116–127. [Google Scholar] [CrossRef]
- Kulić, G.; Radojičić, V. Analysis of Cellulose Content in Stalks and Leaves of Large Leaf Tobacco. J. Agric. Sci. 2011, 56, 207–215. [Google Scholar] [CrossRef]
- Tuzzin, G.; Godinho, M.; Dettmer, A.; Zattera, A.J. Nanofibrillated Cellulose from Tobacco Industry Wastes. Carbohydr. Polym. 2016, 148, 69–77. [Google Scholar] [CrossRef]
- Lavanya, D.; Kulkarni, P.K.; Dixit, M.; Raavi, P.K.; Krishna, L.N.V. Sources of Cellulose and Their Applications—A Review. Int. J. Drug Formul. Res. 2011, 2, 19–38. [Google Scholar]
- Hinterstoisser, B.; Salmén, L. Application of Dynamic 2D FTIR to Cellulose. Vib. Spectrosc. 2000, 22, 111–118. [Google Scholar] [CrossRef]
- Bochek, A.M. Effect of Hydrogen Bonding on Cellulose Solubility in Aqueous and Nonaqueous Solvents. Russ. J. Appl. Chem. 2003, 76, 1711–1719. [Google Scholar] [CrossRef]
- Salem, K.S.; Kasera, N.K.; Rahman, M.A.; Jameel, H.; Habibi, Y.; Eichhorn, S.J.; French, A.D.; Pal, L.; Lucia, L.A. Comparison and Assessment of Methods for Cellulose Crystallinity Determination. Chem. Soc. Rev. 2023, 52, 6417–6446. [Google Scholar] [CrossRef]
- Felgueiras, C.; Azoia, N.G.; Gonçalves, C.; Gama, M.; Dourado, F. Trends on the Cellulose-Based Textiles: Raw Materials and Technologies. Front. Bioeng. Biotechnol. 2021, 9, 608826. [Google Scholar] [CrossRef]
- Ramamoorthy, S.K.; Skrifvars, M.; Persson, A. A Review of Natural Fibers Used in Biocomposites: Plant, Animal and Regenerated Cellulose Fibers. Polym. Rev. 2015, 55, 107–162. [Google Scholar] [CrossRef]
- Rech, F.; da Silva, F.P.; Roldo, L.; Duarte, L. Morphological, Chemical, And Thermal Characterization Of Tobacco Stalk For Application In Composite Materials. Eng. Technol. J. 2023, 08, 2299–2305. [Google Scholar] [CrossRef]
- Ashori, A.; Ornelas, M.; Sheshmani, S.; Cordeiro, N. Influence of Mild Alkaline Treatment on the Cellulosic Surfaces Active Sites. Carbohydr. Polym. 2012, 88, 1293–1298. [Google Scholar] [CrossRef]
- Garcia, K.R.; Weiss-Angeli, V.; Koester, L.S.; dos Santos, V.; Brandalise, R.N. Tobacco Stalk Lignocellulosic Nanofibers Characterization for Pharmaceutical Applications. Res. Soc. Dev. 2021, 10, e522101422261. [Google Scholar] [CrossRef]
- Çerçioğlu, M. The Usage Possibility of Tobacco Waste in Sustainable Agriculture. J. Agric. Fac. Uludag Univ. 2011, 25, 101–107. [Google Scholar]
- Prima Indahsari, O.; Suryo Negoro, A.H. Erratum to: Contribution of Tobacco Waste for Agriculture. In Proceedings of the E3S Web of Conferences, Jember, Indonesia, 31 July–2 August 2019; Soeparjono, S., Addy, H.S., Eds.; EDP Sciences: Les Ulis, France, 2020; Volume 142, p. 04006. [Google Scholar]
- Popova, V.; Tumbarski, Y.; Ivanova, T.; Hadjikinova, R.; Stoyanova, A. Tobacco Resinoid (Nicotiana tabacum L.) as an Active Ingredient of Cosmetic Gels. J. Appl. Pharm. Sci. 2019, 9, 111–118. [Google Scholar] [CrossRef]
- Popova, V.; Ivanova, T.; Prokopov, T.; Nikolova, M.; Stoyanova, A.; Zheljazkov, V.D. Carotenoid-Related Volatile Compounds of Tobacco (Nicotiana Tabacum L.) Essential Oils. Molecules 2019, 24, 3446. [Google Scholar] [CrossRef]
- Fortunati, E.; Luzi, F.; Puglia, D.; Torre, L. Chapter 1—Extraction of Lignocellulosic Materials From Waste Products. In Multifunctional Polymeric Nanocomposites Based on Cellulosic Reinforcements; Puglia, D., Fortunati, E., Kenny, J.M., Eds.; Elsevier B.V.: Amsterdam, The Netherland, 2016; pp. 1–38. ISBN 978-0-323-44248-0. [Google Scholar]
- Acda, M.N.; Cabangon, R.J. Termite Resistance and Physico-Mechanical Properties of Particleboard Using Waste Tobacco Stalk and Wood Particles. Int. Biodeterior. Biodegrad. 2013, 85, 354–358. [Google Scholar] [CrossRef]
- Wang, S.; Cheng, Q. A Novel Process to Isolate Fibrils from Cellulose Fibers by High-Intensity Ultrasonication, Part 1: Process Optimization. J. Appl. Polym. Sci. 2009, 113, 1270–1275. [Google Scholar] [CrossRef]
- Shadhin, M.; Rahman, M.; Jayaraman, R.; Chen, Y.; Mann, D.; Zhong, W. Natural Biomass & Waste Biomass Fibers—Structures, Environmental Footprints, Sustainability, Degumming Methods, & Surface Modifications. Ind. Crops Prod. 2023, 204, 117252. [Google Scholar] [CrossRef]
- Tang, Y.; Yang, S.; Zhang, N.; Zhang, J. Preparation and Characterization of Nanocrystalline Cellulose via Low-Intensity Ultrasonic-Assisted Sulfuric Acid Hydrolysis. Cellulose 2014, 21, 335–346. [Google Scholar] [CrossRef]
- Taherdanak, M.; Zilouei, H. Improving Biogas Production from Wheat Plant Using Alkaline Pretreatment. Fuel 2014, 115, 714–719. [Google Scholar] [CrossRef]
- Satyamurthy, P.; Vigneshwaran, N. A Novel Process for Synthesis of Spherical Nanocellulose by Controlled Hydrolysis of Microcrystalline Cellulose Using Anaerobic Microbial Consortium. Enzym. Microb. Technol. 2013, 52, 20–25. [Google Scholar] [CrossRef]
- Pekpazar, Y.K.; Kilic, U. Effects of Different Additives on Methane Productions and Feed Values of Tobacco Straws. Int. Multiling. J. Sci. Technol. (IMJST) 2020, 5, 2149–2158. [Google Scholar]
- Lee, C.H.; Khalina, A.; Lee, S.H.; Liu, M. A Comprehensive Review on Bast Fibre Retting Process for Optimal Performance in Fibre-Reinforced Polymer Composites. Adv. Mater. Sci. Eng. 2020, 2020, e6074063. [Google Scholar] [CrossRef]
- Tahir, P.; Ahmed, A.B.; SaifulAzry, S.O.A.; Ahmed, Z. Rettıng Process Of Some Bast Plant Fıbres And Its Effect On Fıbre Qualıty: A Revıew. BioResources 2011, 6, 5260–5281. [Google Scholar] [CrossRef]
- Duman, M.N.; Kocak, E.D.; Merdan, N.; Mistik, I. Nonwoven Production from Agricultural Okra Wastes and Investigation of Their Thermal Conductivities. IOP Conf. Ser. Mater. Sci. Eng. 2017, 254, 192007. [Google Scholar] [CrossRef]
- ASTM D8171-18; Standard Test Methods for Density Determination of Flax Fiber. ASTM International: West Conshohocken, PA, USA, 2018.
- Mylsamy, K.; Rajendran, I. Investigation on Physio-Chemical and Mechanical Properties of Raw and Alkali-Treated Agave Americana Fiber. J. Reinf. Plast. Compos. 2010, 29, 2925–2935. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- ASTM D3822-07; Standard Test Method for Tensile Properties of Single Textile Fibers. ASTM International: West Conshohocken, PA, USA, 2007.
- Dalmis, R.; Kilic, G.B.; Seki, Y.; Koktas, S.; Keskin, O.Y. Characterization of a Novel Natural Cellulosic Fiber Extracted from the Stem of Chrysanthemum Morifolium. Cellulose 2020, 27, 8621–8634. [Google Scholar] [CrossRef]
- Shaker, K.; Waseem Ullah Khan, R.M.; Jabbar, M.; Umair, M.; Tariq, A.; Kashif, M.; Nawab, Y. Extraction and Characterization of Novel Fibers from Vernonia Elaeagnifolia as a Potential Textile Fiber. Ind. Crops Prod. 2020, 152, 112518. [Google Scholar] [CrossRef]
- Ali, A.; Shaker, K.; Nawab, Y.; Jabbar, M.; Hussain, T.; Militky, J.; Baheti, V. Hydrophobic Treatment of Natural Fibers and Their Composites—A Review. J. Ind. Text. 2018, 47, 2153–2183. [Google Scholar] [CrossRef]
- Keskin, O.Y.; Dalmis, R.; Balci Kilic, G.; Seki, Y.; Koktas, S. Extraction and Characterization of Cellulosic Fiber from Centaurea Solstitialis for Composites. Cellulose 2020, 27, 9963–9974. [Google Scholar] [CrossRef]
- Arthanarieswaran, V.P.; Kumaravel, A.; Saravanakumar, S.S. Characterization of New Natural Cellulosic Fiber from Acacia Leucophloea Bark. Int. J. Polym. Anal. Charact. 2015, 20, 367–376. [Google Scholar] [CrossRef]
- Rusdianto, A.S.; Amilia, W.; Sinta, V.J.D. The Optimization Of Cellulose Content In Tobacco Stems (Nicotiana Tabaccum L.) With Acid Extraction Method And Alkaline Extraction Method. Int. J. Food Agric. Nat. Resour. 2021, 2, 13–19. [Google Scholar] [CrossRef]
- Sun, D.; Sun, S.-C.; Wang, B.; Sun, S.-F.; Shi, Q.; Zheng, L.; Wang, S.-F.; Liu, S.-J.; Li, M.-F.; Cao, X.-F.; et al. Effect of Various Pretreatments on Improving Cellulose Enzymatic Digestibility of Tobacco Stalk and the Structural Features of Co-Produced Hemicelluloses. Bioresour. Technol. 2020, 297, 122471. [Google Scholar] [CrossRef]
- Gedik, G. Extraction of New Natural Cellulosic Fiber from Trachelospermum jasminoides (Star Jasmine) and Its Characterization for Textile and Composite Uses. Cellulose 2021, 28, 6899–6915. [Google Scholar] [CrossRef]
- Shakhes, J.; Marandi, M.A.B.; Zeinaly, F.; Saraian, A.; Saghafi, T. Tobacco Residuals as Promising Lignocellulosic Materials for Pulp and Paper Industry. BioResources 2011, 6, 4481–4493. [Google Scholar] [CrossRef]
- Placet, V.; Day, A.; Beaugrand, J. The Influence of Unintended Field Retting on the Physicochemical and Mechanical Properties of Industrial Hemp Bast Fibres. J. Mater. Sci. 2017, 52, 5759–5777. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, Y.; Yu, J.; Zhuang, J.; Wu, S.; Tong, J. Development and Characterization of Alkali Treated Abaca Fiber Reinforced Friction Composites. Compos. Interfaces 2019, 26, 67–82. [Google Scholar] [CrossRef]
- Lampke, A.B. Supriya Mishra, Thomas Plant Fibers as Reinforcement for Green Composites. In Natural Fibers, Biopolymers, and Biocomposites; CRC Press: Boca Raton, FL, USA, 2005; ISBN 978-0-429-21160-7. [Google Scholar]
- Hyness, N.R.J.; Vignesh, N.J.; Senthamaraikannan, P.; Saravanakumar, S.S.; Sanjay, M.R. Characterization of New Natural Cellulosic Fiber from Heteropogon Contortus Plant. J. Nat. Fibers 2018, 15, 146–153. [Google Scholar] [CrossRef]
- Rajkumar, R.; Manikandan, A.; Saravanakumar, S.S. Physicochemical Properties of Alkali-Treated New Cellulosic Fiber from Cotton Shell. Int. J. Polym. Anal. Charact. 2016, 21, 359–364. [Google Scholar] [CrossRef]
- Dawit, J.B.; Regassa, Y.; Lemu, H.G. Property Characterization of Acacia Tortilis for Natural Fiber Reinforced Polymer Composite. Results Mater. 2020, 5, 100054. [Google Scholar] [CrossRef]
- Belouadah, Z.; Ati, A.; Rokbi, M. Characterization of New Natural Cellulosic Fiber from Lygeum spartum L. Carbohydr. Polym. 2015, 134, 429–437. [Google Scholar] [CrossRef]
- Senthamaraikannan, P.; Kathiresan, M. Characterization of Raw and Alkali Treated New Natural Cellulosic Fiber from Coccinia grandis L. Carbohydr. Polym. 2018, 186, 332–343. [Google Scholar] [CrossRef]
- Liu, D.; Han, G.; Huang, J.; Zhang, Y. Composition and Structure Study of Natural Nelumbo Nucifera Fiber. Carbohydr. Polym. 2009, 75, 39–43. [Google Scholar] [CrossRef]
- Reddy, K.O.; Maheswari, C.U.; Shukla, M.; Rajulu, A.V. Chemical Composition and Structural Characterization of Napier Grass Fibers. Mater. Lett. 2012, 67, 35–38. [Google Scholar] [CrossRef]
- Jayaramudu, J.; Guduri, B.R.; Varada Rajulu, A. Characterization of New Natural Cellulosic Fabric Grewia Tilifolia. Carbohydr. Polym. 2010, 79, 847–851. [Google Scholar] [CrossRef]
- Saravanakumar, S.S.; Kumaravel, A.; Nagarajan, T.; Moorthy, I.G. Investigation of Physico-Chemical Properties of Alkali-Treated Prosopis Juliflora Fibers. Int. J. Polym. Anal. Charact. 2014, 19, 309–317. [Google Scholar] [CrossRef]
- Bulut, Y.; Aksit, A. A Comparative Study on Chemical Treatment of Jute Fiber: Potassium Dichromate, Potassium Permanganate and Sodium Perborate Trihydrate. Cellulose 2013, 20, 3155–3164. [Google Scholar] [CrossRef]
- Manimaran, P.; Saravanan, S.P.; Prithiviraj, M. Investigation of Physico Chemical Properties and Characterization of New Natural Cellulosic Fibers from the Bark of Ficus Racemosa. J. Nat. Fibers 2021, 18, 274–284. [Google Scholar] [CrossRef]
- Sgriccia, N.; Hawley, M.C.; Misra, M. Characterization of Natural Fiber Surfaces and Natural Fiber Composites. Compos. Part A Appl. Sci. Manuf. 2008, 39, 1632–1637. [Google Scholar] [CrossRef]
- Šernek, M.; Kamke, F.A.; Glasser, W.G. Comparative Analysis of Inactivated Wood Surfaces. Holzforschung 2004, 58, 22–31. [Google Scholar] [CrossRef]
- French, A.D.; Santiago Cintrón, M. Cellulose Polymorphy, Crystallite Size, and the Segal Crystallinity Index. Cellulose 2013, 20, 583–588. [Google Scholar] [CrossRef]
- Kılınç, A.Ç.; Köktaş, S.; Seki, Y.; Atagür, M.; Dalmış, R.; Erdoğan, Ü.H.; Göktaş, A.A.; Seydibeyoğlu, M.Ö. Extraction and Investigation of Lightweight and Porous Natural Fiber from Conium Maculatum as a Potential Reinforcement for Composite Materials in Transportation. Compos. Part B Eng. 2018, 140, 1–8. [Google Scholar] [CrossRef]
- Kim, U.-J.; Eom, S.H.; Wada, M. Thermal Decomposition of Native Cellulose: Influence on Crystallite Size. Polym. Degrad. Stab. 2010, 95, 778–781. [Google Scholar] [CrossRef]
- Diyana, Z.N.; Jumaidin, R.; Selamat, M.Z.; Alamjuri, R.H.; Md Yusof, F.A. Extraction and Characterization of Natural Cellulosic Fiber from Pandanus Amaryllifolius Leaves. Polymers 2021, 13, 4171. [Google Scholar] [CrossRef]
- Sheltami, R.M.; Abdullah, I.; Ahmad, I.; Dufresne, A.; Kargarzadeh, H. Extraction of Cellulose Nanocrystals from Mengkuang Leaves (Pandanus tectorius). Carbohydr. Polym. 2012, 88, 772–779. [Google Scholar] [CrossRef]
- Boumediri, H.; Bezazi, A.; Del Pino, G.G.; Haddad, A.; Scarpa, F.; Dufresne, A. Extraction and Characterization of Vascular Bundle and Fiber Strand from Date Palm Rachis as Potential Bio-Reinforcement in Composite. Carbohydr. Polym. 2019, 222, 114997. [Google Scholar] [CrossRef]
- Ovalı, S. Characterization of Lignocellulosic Glycyrrhiza Glabra Fibers as a Potential Reinforcement for Polymer Composites. J. Thermoplast. Compos. Mater. 2023, 36, 08927057231151928. [Google Scholar] [CrossRef]
- Yao, F.; Wu, Q.; Lei, Y.; Guo, W.; Xu, Y. Thermal Decomposition Kinetics of Natural Fibers: Activation Energy with Dynamic Thermogravimetric Analysis. Polym. Degrad. Stab. 2008, 93, 90–98. [Google Scholar] [CrossRef]
- Maache, M.; Bezazi, A.; Amroune, S.; Scarpa, F.; Dufresne, A. Characterization of a Novel Natural Cellulosic Fiber from Juncus effusus L. Carbohydr. Polym. 2017, 171, 163–172. [Google Scholar] [CrossRef]
- Manfredi, L.B.; Rodríguez, E.S.; Wladyka-Przybylak, M.; Vázquez, A. Thermal Degradation and Fire Resistance of Unsaturated Polyester, Modified Acrylic Resins and Their Composites with Natural Fibres. Polym. Degrad. Stab. 2006, 91, 255–261. [Google Scholar] [CrossRef]
- Kaya, A.I. Extraction of Lightweight Platanus orientalis L. Fruit’s Stem Fiber and Determination of Its Mechanical and Physico-Chemical Properties and Potential of Its Use in Composites. Polymers 2024, 16, 657. [Google Scholar] [CrossRef]





| Fiber | Tensile Strength (MPa) | Tensile Modulus (GPa) | Elongation (%) | Ref. |
|---|---|---|---|---|
| Nicotiana rustica L. | 113.40 | 2.87 | 3.94 | In this study |
| Cotton | 400 | 12 | 3–10 | [62] |
| Hemp | 690 | 70 | 2–4 | [42] |
| Flax | 500–1500 | 27.6 | 2.7–3.2 | [42] |
| Jute | 93–773 | 26.5 | 1.5–1.8 | [42] |
| Fiber | Cls (%) | O1s (%) | N1s (%) | C/O (%) | O/C (%) |
|---|---|---|---|---|---|
| Nicotiana rustica L. | 68.37 | 18.33 | 1.59 | 3.72 | 0.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ovalı, S. Characterization of Waste Nicotiana rustica L. (Tobacco) Fiber Having a Potential in Textile and Composite Applications. Polymers 2024, 16, 1117. https://doi.org/10.3390/polym16081117
Ovalı S. Characterization of Waste Nicotiana rustica L. (Tobacco) Fiber Having a Potential in Textile and Composite Applications. Polymers. 2024; 16(8):1117. https://doi.org/10.3390/polym16081117
Chicago/Turabian StyleOvalı, Sabih. 2024. "Characterization of Waste Nicotiana rustica L. (Tobacco) Fiber Having a Potential in Textile and Composite Applications" Polymers 16, no. 8: 1117. https://doi.org/10.3390/polym16081117
APA StyleOvalı, S. (2024). Characterization of Waste Nicotiana rustica L. (Tobacco) Fiber Having a Potential in Textile and Composite Applications. Polymers, 16(8), 1117. https://doi.org/10.3390/polym16081117





