A New Approach to Implant Stability Using a Flexible Synthetic Silicate-Additive Beta-Tricalcium Phosphate-Poly(D,L-lactide-co-caprolactone) Bone Graft: An In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
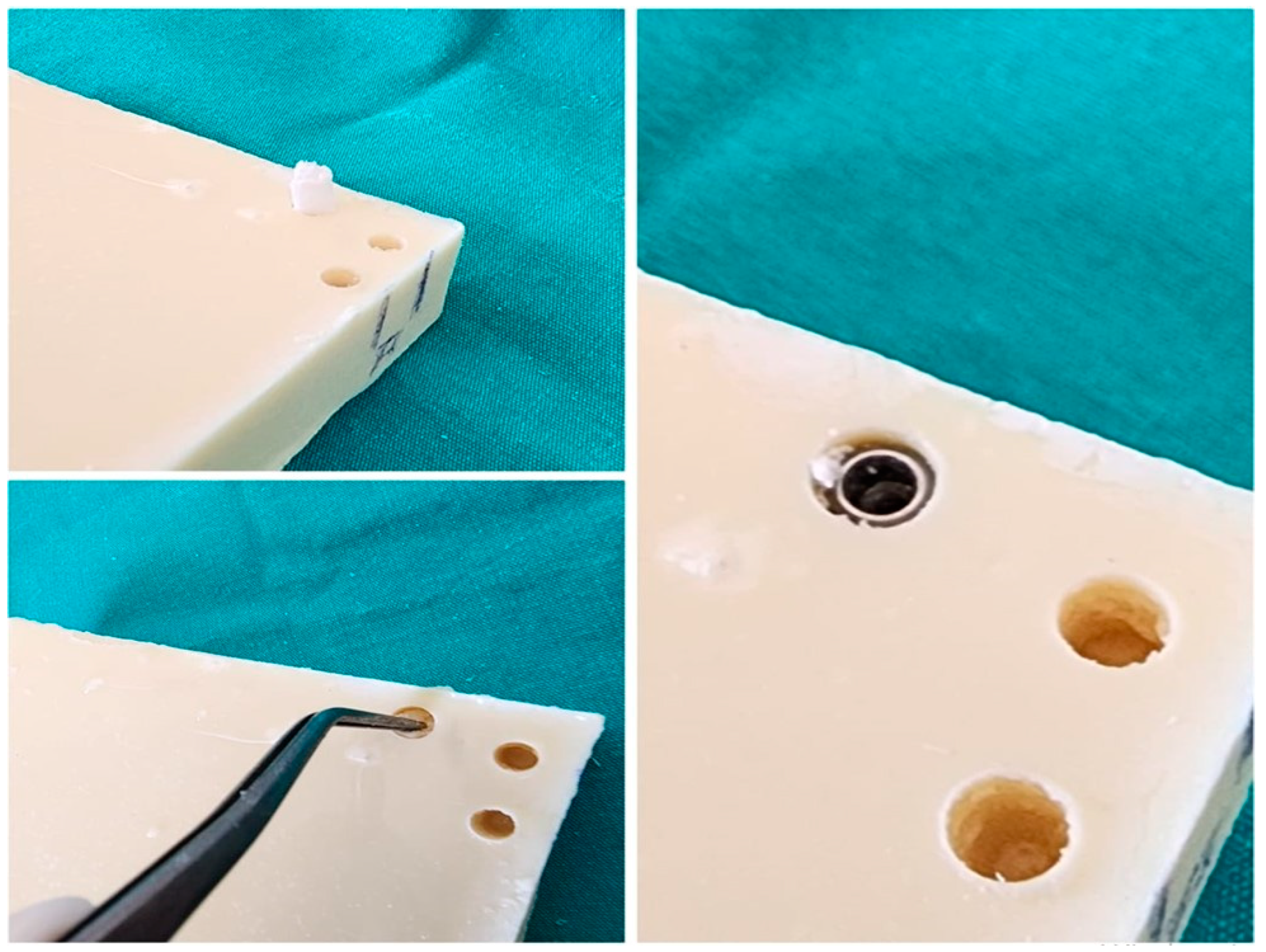
2.1. Drilling Protocol
2.2. Study Groups
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ustaoğlu, G.; Paksoy, T.; Gümüş, K. Evaluating the Effect of Design and Length of Implants on Primary Stability Using Resonance Frequency Analysis: An In Vitro Study. Selcuk Dent. J. 2020, 7, 265–272. [Google Scholar] [CrossRef]
- Sachdeva, A.; Dhawan, P.; Sindwani, S. Assessment of Implant Stability: Methods and Recent Advances. Br. J. Med. Med. Res. 2016, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Javed, F.; Almas, K.; Crespi, R.; Romanos, G.E. Implant Surface Morphology and Primary Stability: Is There a Connection? Implant Dent. 2011, 20, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, D.A.; Arosio, P.; Capparè, P.; Barbon, S.; Gherlone, E.F. Stability of Dental Implants and Thickness of Cortical Bone: Clinical Research and Future Perspectives. A Systematic Review. Mater. Basel Switz. 2021, 14, 7183. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, D.A.; Arosio, P.; Perrotti, V.; Iezzi, G.; Scarano, A.; Piattelli, A. Correlation between Implant Geometry, Bone Density, and the Insertion Torque/Depth Integral: A Study on Bovine Ribs. Dent. J. 2019, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Ebenezer, S.; Kumar, V.; Thor, A. Basics of Dental Implantology for the Oral Surgeon. In Oral and Maxillofacial Surgery for the Clinician; Springer: Singapore, 2021; pp. 385–405. ISBN 9789811513459. [Google Scholar]
- Aldahlawi, S.; Demeter, A.; Irinakis, T. The Effect of Implant Placement Torque on Crestal Bone Remodeling after 1 Year of Loading. Clin. Cosmet. Investig. Dent. 2018, 10, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Ziebart, J.; Fan, S.; Schulze, C.; Kämmerer, P.W.; Bader, R.; Jonitz-Heincke, A. Effects of Interfacial Micromotions on Vitality and Differentiation of Human Osteoblasts. Bone Jt. Res. 2018, 7, 187–195. [Google Scholar] [CrossRef]
- Barone, A.; Alfonsi, F.; Derchi, G.; Tonelli, P.; Toti, P.; Marchionni, S.; Covani, U. The Effect of Insertion Torque on the Clinical Outcome of Single Implants: A Randomized Clinical Trial. Clin. Implant Dent. Relat. Res. 2016, 18, 588–600. [Google Scholar] [CrossRef] [PubMed]
- Baldi, D.; Lombardi, T.; Colombo, J.; Cervino, G.; Perinetti, G.; Di Lenarda, R.; Stacchi, C. Correlation between Insertion Torque and Implant Stability Quotient in Tapered Implants with Knife-Edge Thread Design. BioMed Res. Int. 2018, 2018, 7201093. [Google Scholar] [CrossRef]
- Trisi, P.; Todisco, M.; Consolo, U.; Travaglini, D. High versus Low Implant Insertion Torque: A Histologic, Histomorphometric, and Biomechanical Study in the Sheep Mandible. Int. J. Oral Maxillofac. Implants 2011, 26, 837–849. [Google Scholar]
- Bashutski, J.D.; D’Silva, N.J.; Wang, H.-L. Implant Compression Necrosis: Current Understanding and Case Report. J. Periodontol. 2009, 80, 700–704. [Google Scholar] [CrossRef]
- Tabassum, A.; Meijer, G.J.; Wolke, J.G.C.; Jansen, J.A. Influence of Surgical Technique and Surface Roughness on the Primary Stability of an Implant in Artificial Bone with Different Cortical Thickness: A Laboratory Study. Clin. Oral Implants Res. 2010, 21, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Farré-Pagés, N.; Augé-Castro, M.L.; Alaejos-Algarra, F.; Mareque-Bueno, J.; Ferrés-Padró, E.; Hernández-Alfaro, F. Relation between Bone Density and Primary Implant Stability. Med. Oral Patol. Oral Cirugia Bucal 2011, 16, e62–e67. [Google Scholar] [CrossRef]
- Javed, F.; Ahmed, H.B.; Crespi, R.; Romanos, G.E. Role of Primary Stability for Successful Osseointegration of Dental Implants: Factors of Influence and Evaluation. Interv. Med. Appl. Sci. 2013, 5, 162–167. [Google Scholar] [CrossRef]
- Linetskiy, I.; Demenko, V.; Linetska, L.; Yefremov, O. Impact of Annual Bone Loss and Different Bone Quality on Dental Implant Success—A Finite Element Study. Comput. Biol. Med. 2017, 91, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Alsaadi, G.; Quirynen, M.; Michiels, K.; Jacobs, R.; van Steenberghe, D. A Biomechanical Assessment of the Relation between the Oral Implant Stability at Insertion and Subjective Bone Quality Assessment. J. Clin. Periodontol. 2007, 34, 359–366. [Google Scholar] [CrossRef]
- Molly, L. Bone Density and Primary Stability in Implant Therapy. Clin. Oral Implants Res. 2006, 17 (Suppl. S2), 124–135. [Google Scholar] [CrossRef] [PubMed]
- Ghosal, K.; Thomas, S.; Kalarikkal, N.; Gnanamani, A. Collagen Coated Electrospun Polycaprolactone (PCL) with Titanium Dioxide (TiO2) from an Environmentally Benign Solvent: Preliminary Physico-Chemical Studies for Skin Substitute. J. Polym. Res. 2014, 21, 410. [Google Scholar] [CrossRef]
- Maitz, M.F. Applications of Synthetic Polymers in Clinical Medicine. Biosurface Biotribology 2015, 1, 161–176. [Google Scholar] [CrossRef]
- Ghosal, K.; Agatemor, C.; Špitálsky, Z.; Thomas, S.; Kny, E. Electrospinning Tissue Engineering and Wound Dressing Scaffolds from Polymer-Titanium Dioxide Nanocomposites. Chem. Eng. J. 2019, 358, 1262–1278. [Google Scholar] [CrossRef]
- Liang, H.; Mirinejad, M.; Asefnejad, A.; Baharifar, H.; Li, X.; Saber-Samandari, S.; Toghraie, D.; Khandan, A. Fabrication of Tragacanthin Gum-Carboxymethyl Chitosan Bio-Nanocomposite Wound Dressing with Silver-Titanium Nanoparticles Using Freeze-Drying Method. Mater. Chem. Phys. 2022, 279, 125770. [Google Scholar] [CrossRef]
- Kurowiak, J.; Klekiel, T.; Będziński, R. Biodegradable Polymers in Biomedical Applications: A Review-Developments, Perspectives and Future Challenges. Int. J. Mol. Sci. 2023, 24, 16952. [Google Scholar] [CrossRef] [PubMed]
- von Burkersroda, F.; Schedl, L.; Göpferich, A. Why Degradable Polymers Undergo Surface Erosion or Bulk Erosion. Biomaterials 2002, 23, 4221–4231. [Google Scholar] [CrossRef] [PubMed]
- Nanda, H.S.; Yang, L.; Hu, J.-S.; Mao, H.; Jiang, S. Editorial: Biodegradable Polymers for Biomedical Applications. Front. Mater. 2022, 9, 944755. [Google Scholar] [CrossRef]
- Ulery, B.D.; Nair, L.S.; Laurencin, C.T. Biomedical Applications of Biodegradable Polymers. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 832–864. Available online: https://onlinelibrary.wiley.com/doi/full/10.1002/polb.22259 (accessed on 12 April 2024). [CrossRef] [PubMed]
- Tamada, J.A.; Langer, R. Erosion Kinetics of Hydrolytically Degradable Polymers. Proc. Natl. Acad. Sci. USA 1993, 90, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Salhi, S.; Mahfoudh, J.; Abid, S.; Atanase, L.I.; Popa, M.; Delaite, C. Random Poly(ε-caprolactone-L-alanine) by Direct Melt Copolymerization. Polym. Int. 2020, 69, 1161–1168. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/pi.6085 (accessed on 12 April 2024). [CrossRef]
- Zhang, M.; Chang, Z.; Wang, X.; Li, Q. Synthesis of Poly(l-lactide-co-ε-caprolactone) Copolymer: Structure, Toughness, and Elasticity. Polymers 2021, 13, 1270. [Google Scholar] [CrossRef] [PubMed]
- Matsuno, T.; Nakamura, T.; Kuremoto, K.; Notazawa, S.; Nakahara, T.; Hashimoto, Y.; Satoh, T.; Shimizu, Y. Development of Beta-Tricalcium Phosphate/Collagen Sponge Composite for Bone Regeneration. Dent. Mater. J. 2006, 25, 138–144. [Google Scholar] [CrossRef]
- Donate, R.; Monzon, M.; Alemán-Domínguez, M. Additive Manufacturing of PLA-based Scaffolds Intended for Bone Regeneration and Strategies to Improve Their Biological Properties. e-Polymers 2020, 20, 571–599. [Google Scholar] [CrossRef]
- Onak, G.; Gökmen, O.; Yaralı, Z.B.; Karaman, O. Enhanced Osteogenesis of Human Mesenchymal Stem Cells by Self-Assembled Peptide Hydrogel Functionalized with Glutamic Acid Templated Peptides. J. Tissue Eng. Regen. Med. 2020, 14, 1236–1249. [Google Scholar] [CrossRef]
- Jasser, R.A.; AlSarhan, M.; Alotaibi, D.; Aloraini, S.; Koppolu, P.; Andreana, S. Evaluation of Clinical Performance and Survival Rate of Straumann Dental Implants in Saudi Population Based on Cross-Sectional Study. Sci. Rep. 2021, 11, 9526. [Google Scholar] [CrossRef] [PubMed]
- Coathup, M.J.; Samizadeh, S.; Fang, Y.S.; Buckland, T.; Hing, K.A.; Blunn, G.W. The Osteoinductivity of Silicate-Substituted Calcium Phosphate. JBJS 2011, 93, 2219. [Google Scholar] [CrossRef]
- Peker, E.; Karaca, İ. Implant Tedavisinin Prognozunu Etkileyen Lokal Risk Faktörleri. Atatürk Üniversitesi Diş. Hekim. Fakültesi Derg. 2015, 25, 105–111. [Google Scholar] [CrossRef]
- Lee, C.-T.; Chen, Y.-W.; Starr, J.R.; Chuang, S.-K. Survival Analysis of Wide Dental Implant: Systematic Review and Meta-Analysis. Clin. Oral Implants Res. 2016, 27, 1251–1264. [Google Scholar] [CrossRef] [PubMed]
- Comuzzi, L.; Tumedei, M.; Pontes, A.E.; Piattelli, A.; Iezzi, G. Primary Stability of Dental Implants in Low-Density (10 and 20 pcf) Polyurethane Foam Blocks: Conical vs Cylindrical Implants. Int. J. Environ. Res. Public. Health 2020, 17, 2617. [Google Scholar] [CrossRef]
- Di Stefano, D.A.; Arosio, P.; Gastaldi, G.; Gherlone, E. The Insertion Torque-Depth Curve Integral as a Measure of Implant Primary Stability: An in Vitro Study on Polyurethane Foam Blocks. J. Prosthet. Dent. 2018, 120, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Liaw, K.; Delfini, R.H.; Abrahams, J.J. Dental Implant Complications. Semin. Ultrasound. CT MR 2015, 36, 427–433. [Google Scholar] [CrossRef]
- Ergüven, S.S.; Yıldızer, E.; Ozkan, A.; Şahin, Z.S.; Sarı, S.K.; Peker, F. Correlation between Preoperative Bone Quality and Primer Stability for Mandibular Posterior Implants. ADO Klin. Bilim. Derg. 2024, 13, 2–9. [Google Scholar] [CrossRef]
- Blanc-Sylvestre, N.; Bouchard, P.; Chaussain, C.; Bardet, C. Pre-Clinical Models in Implant Dentistry: Past, Present, Future. Biomedicines 2021, 9, 1538. [Google Scholar] [CrossRef]
- Okazaki, Y.; Hayakawa, E.; Tanahashi, K.; Mori, J. Mechanical Performance of Metallic Bone Screws Evaluated Using Bone Models. Materials 2020, 13, 4836. [Google Scholar] [CrossRef] [PubMed]
- Comuzzi, L.; Tumedei, M.; Covani, U.; Romasco, T.; Petrini, M.; Montesani, L.; Piattelli, A.; Di Pietro, N. Primary Stability Assessment of Conical Implants in Under-Prepared Sites: An In Vitro Study in Low-Density Polyurethane Foams. Appl. Sci. 2023, 13, 6041. [Google Scholar] [CrossRef]
- Stoilov, M.; Shafaghi, R.; Stark, H.; Marder, M.; Kraus, D.; Enkling, N. Influence of Implant Macro-Design, -Length, and -Diameter on Primary Implant Stability Depending on Different Bone Qualities Using Standard Drilling Protocols—An In Vitro Analysis. J. Funct. Biomater. 2023, 14, 469. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-M.; Chee, T.-J.; Lew, W.-Z.; Feng, S.-W. Modified Surgical Drilling Protocols Influence Osseointegration Performance and Predict Value of Implant Stability Parameters during Implant Healing Process. Clin. Oral Investig. 2020, 24, 3445–3455. [Google Scholar] [CrossRef] [PubMed]
- Yurttutan, M.E.; Kestane, R.; Keskin, A.; Dereci, O. Biomechanical Evaluation of Oversized Drilling on Implant Stability—An Experimental Study in Sheep. JPMA J. Pak. Med. Assoc. 2016, 66, 147–150. [Google Scholar] [PubMed]
- Cohen, O.; Ormianer, Z.; Tal, H.; Rothamel, D.; Weinreb, M.; Moses, O. Differences in Crestal Bone-to-Implant Contact Following an under-Drilling Compared to an over-Drilling Protocol. A Study in the Rabbit Tibia. Clin. Oral Investig. 2016, 20, 2475–2480. [Google Scholar] [CrossRef] [PubMed]
- Seleem, A.; Tawfik, O.K.; El-Nahass, H. Evaluation of Oversized Drilling on Implant Survival and Stability Versus Traditional Drilling Technique: A Randomized Clinical Trial. Int. J. Oral Maxillofac. Implants 2021, 36, 771–778. [Google Scholar] [CrossRef]
- Dündar, S.; Çakmak, Ö.; Solmaz, M.Y. Primer Stabilizasyon Olan Ve Olmayan Implantlarda Kemik Implant Kaynaşmasinin Biyomekanik Incelenmesi: In Vivo Bir Çalişma. Atatürk Üniversitesi Diş. Hekim. Fakültesi Derg. 2018, 28, 188–193. [Google Scholar] [CrossRef]


| n (%) | ||
|---|---|---|
| Group 1 (Control) | 25 (33.3) | |
| Group 2 (Oversized and Deepened) | 25 (33.3) | |
| Group 3 (Oversized only) | 25 (33.3) | |
| Insertion | Mean ± Standard deviation | 39.53 ± 13.46 |
| Torque Values (Nm) | Median (Min − Max) | 40 (10–65) |
| Torque Values (Nm) | a p | ||
|---|---|---|---|
| Mean ± Sd | Median (Min−Max) | ||
| Group 1 (Control) | 49.60 ± 7.63 | 50 (40–65) | 0.001 * |
| Group 2 (Oversized and Deepened) | 43.60 ± 7.84 | 45 (30–60) | |
| Group 3 (Oversized only) | 25.40 ± 10.4 | 25 (10–50) | |
| Torque Values (Nm) | b p | ||
|---|---|---|---|
| Mean ± Sd | Median (Min−Max) | ||
| Control | 49.60 ± 7.63 | 50 (40–65) | 0.001 * |
| Oversized Groups | 34.50 ± 12.95 | 35 (10–60) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orhan, Z.D.; Ciğerim, L. A New Approach to Implant Stability Using a Flexible Synthetic Silicate-Additive Beta-Tricalcium Phosphate-Poly(D,L-lactide-co-caprolactone) Bone Graft: An In Vitro Study. Polymers 2024, 16, 1101. https://doi.org/10.3390/polym16081101
Orhan ZD, Ciğerim L. A New Approach to Implant Stability Using a Flexible Synthetic Silicate-Additive Beta-Tricalcium Phosphate-Poly(D,L-lactide-co-caprolactone) Bone Graft: An In Vitro Study. Polymers. 2024; 16(8):1101. https://doi.org/10.3390/polym16081101
Chicago/Turabian StyleOrhan, Zeynep Dilan, and Levent Ciğerim. 2024. "A New Approach to Implant Stability Using a Flexible Synthetic Silicate-Additive Beta-Tricalcium Phosphate-Poly(D,L-lactide-co-caprolactone) Bone Graft: An In Vitro Study" Polymers 16, no. 8: 1101. https://doi.org/10.3390/polym16081101
APA StyleOrhan, Z. D., & Ciğerim, L. (2024). A New Approach to Implant Stability Using a Flexible Synthetic Silicate-Additive Beta-Tricalcium Phosphate-Poly(D,L-lactide-co-caprolactone) Bone Graft: An In Vitro Study. Polymers, 16(8), 1101. https://doi.org/10.3390/polym16081101






