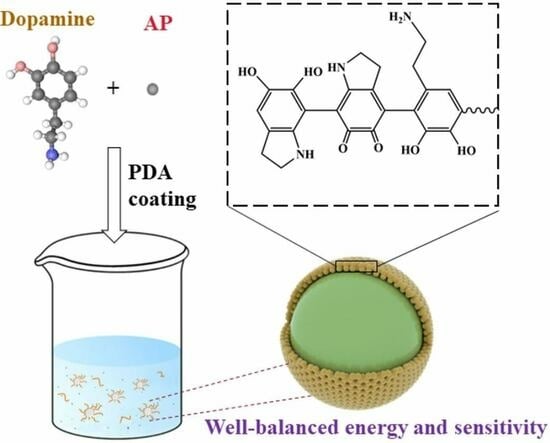
Bioinspired Fabrication of an Insensitive Ammonium Perchlorate Core–Shell Composite with Polydopamine Coating
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
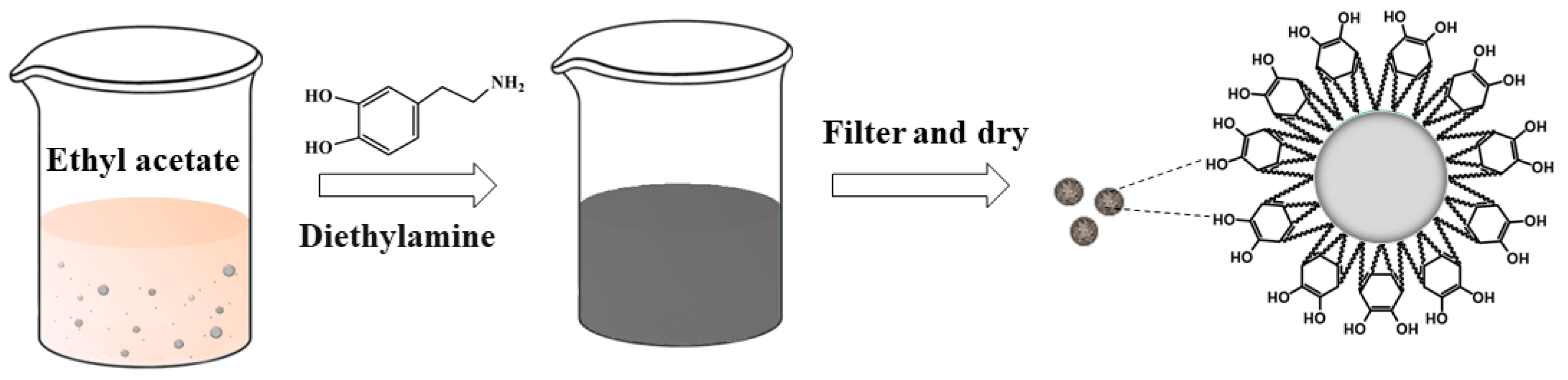
2.2. Preparation of the AP/PDA Core-Shell Composite
2.3. Characterization
2.4. Mechanical Sensitivity Tests
3. Results and Discussions
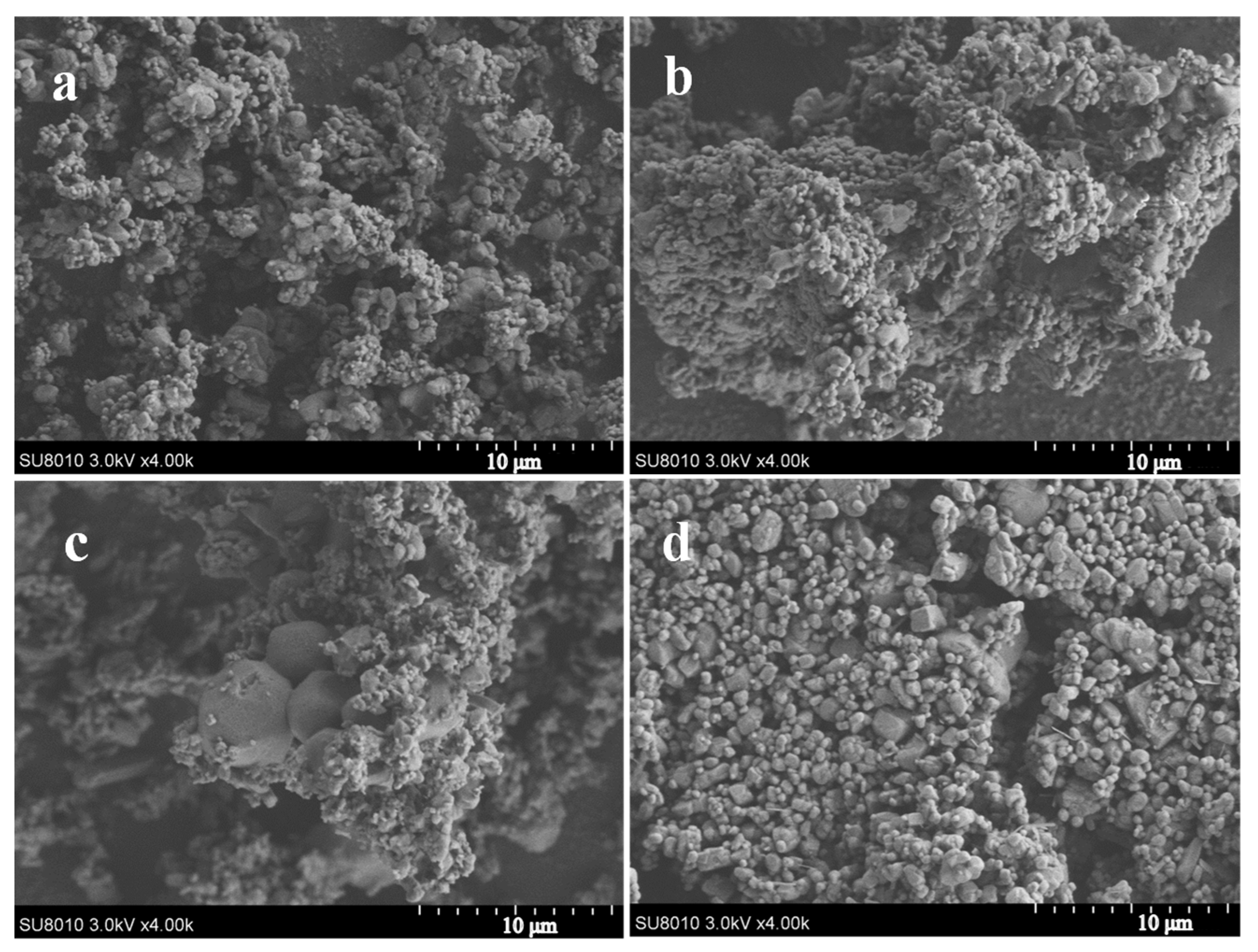
3.1. Preparation of the AP/PDA Core-Shell Composite
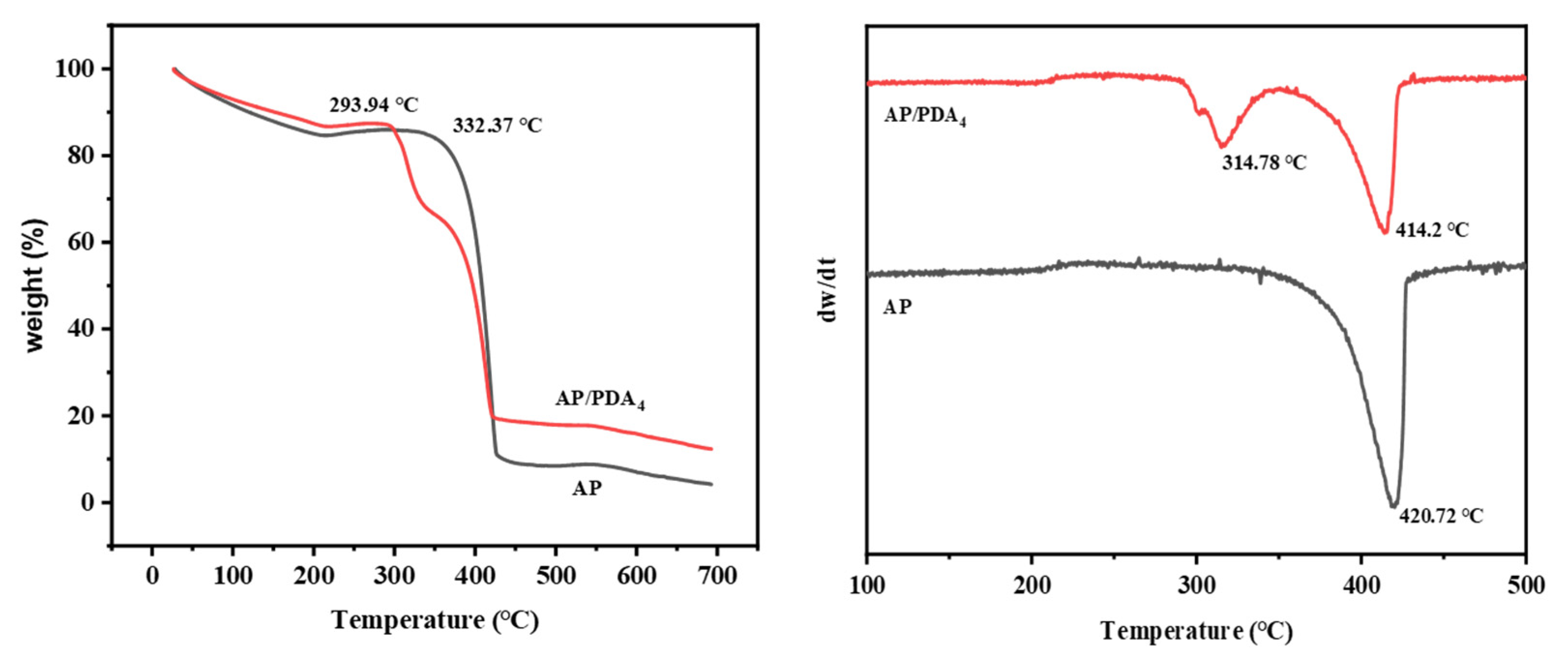
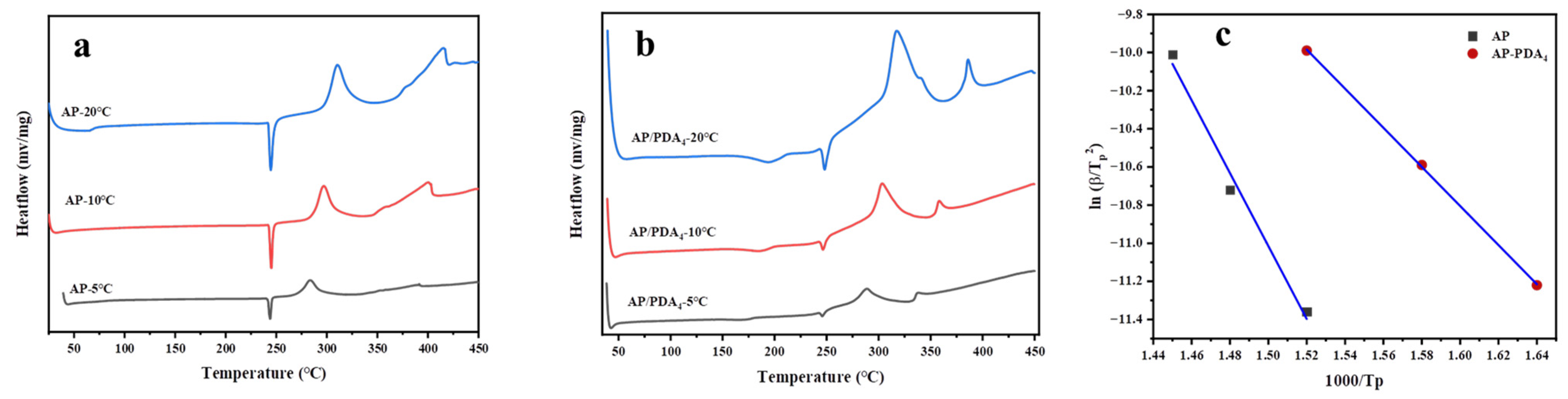
3.2. Thermal Decomposition Properties of the AP/PDA Core-Shell Composite
3.3. Mechanical Sensitivity of the AP/PDA Core-Shell Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Shusser, M.; Culick, F.E.C.; Cohen, N.S. Combustion response of ammonium perchlorate composite propellants. J. Propuls. Power 2002, 18, 1093–1100. [Google Scholar] [CrossRef]
- Hu, Y.; Yang, S.; Tao, B.; Liu, X.; Lin, K.; Yang, Y.; Fan, R.; Xia, D.; Hao, D. Catalytic decomposition of ammonium perchlorate on hollow mesoporous CuO microspheres. Vacuum 2019, 159, 105–111. [Google Scholar] [CrossRef]
- Yang, Y.; Bai, Y.; Zhao, F.; Yao, E.; Yi, J.; Xuan, C.; Chen, S. Effects of metal organic framework Fe-BTC on the thermal decomposition of ammonium perchlorate. RSC Adv. 2016, 6, 67308–67314. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, F.; Wang, Y.; Chen, X.; Pei, Q.; Xu, H.; Hao, H.; Yang, Y.; Li, H. Evaluation of graphene-ferrocene nanocomposite as multifunctional combustion catalyst in AP-HTPB propellant. Fuel 2021, 302, 121229. [Google Scholar] [CrossRef]
- Fitzgerald, R.P.; Brewster, M.Q. Flame and surface structure of laminate propellants with coarse and fine ammonium perchlorate. Combust. Flame 2004, 136, 313–326. [Google Scholar] [CrossRef]
- Kohga, M. Safe preparations of fine ammonium perchlorate particles. Chin. J. Energetic Mater. 2006, 14, 471–474. [Google Scholar]
- Kohga, M. Burning characteristics of AP/HTPB composite propellants prepared with fine porous or fine hollow ammonium perchlorate. Propellants Explos. Pyrotech. 2006, 31, 50–55. [Google Scholar] [CrossRef]
- Sunil, J.; Mehilal, M.; Nandagopal, S.; Singh, P.P.; Radhakrishnan, K.K.; Bhattacharya, B. Size and Shape of Ammonium Perchlorate and their Influence on Properties of Composite Propellant. Def. Sci. J. 2009, 59, 294–299. [Google Scholar] [CrossRef]
- Jiang, B.Z.; Zhang, W.; Yang, J.; Yu, Y.; Bao, T.; Zhou, X. Low-Temperature Oxidation of Catocene and Its Influence on the Mechanical Sensitivities of a Fine-AP/Catocene Mixture. Propellants Explos. Pyrotech. 2015, 40, 854–859. [Google Scholar] [CrossRef]
- Zhang, Z.; Xie, W.; Liu, Y.; Liu, X.; Sun, B.; Shen, Y. Research Progress of the Desensitization Technology of Ammonium Perchlorate. Explos. Mater. 2023, 52, 16. [Google Scholar]
- Song, J.; Guo, X.D.; Li, F.S. Preparation and property characterization of superfine spherical ammonium perchlorate. J. Solid Rocket Technol. 2014, 37, 521–524. [Google Scholar] [CrossRef]
- Wan, X.J.; Guo, X.D.; Ou, Y.G.; Wang, G.Y.; Zheng, D. Preparation and characterization of ultra-fine spherical AP particles. Chin. J. Explos. Propellants 2015, 38, 51–55. [Google Scholar] [CrossRef]
- Liu, Q.H.; An, W.Q.; He, J.Y.; Leng, J.P.; Zhao, Q.; Pan, H.X.; Chen, J.; Guo, J.C.; Li, Y.X.; Cao, D.L. Crystallization and Characterization of Spherical Ammonium Perchlorate. Chin. J. Explos. Propellants 2021, 44, 139–146. [Google Scholar] [CrossRef]
- Hu, J.; Guo, X.D.; Liu, H.Y.; Li, F.S. Preparation and study on properties of resorcinol/AP composite oxidizer. Chin. J. Explos. Propellants 2013, 36, 28–30, 35. [Google Scholar] [CrossRef]
- Sun, S.; Cao, X.; Yu, H.; Zhang, Z. Preparation and properties of spherical nano CuO@AP core-shell particles. Chin. J. Explos. Propellants 2021, 44, 844–850. [Google Scholar] [CrossRef]
- Cheng, J.; Yan, J.C.; Wang, L.; Zhang, R.X.; Liu, Z.L.; Wang, R.; Li, Z.M. Functionalization graphene oxide with energetic groups as a new family of metal-free and energetic burning rate catalysts and desensitizers for ammonium perchlorate. J. Therm. Anal. Calorim. 2020, 140, 2111–2122. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, L.; Gong, S.; Guang, C.; Li, L.; Hu, S.-q.; Deng, P. Study of Ammonium Perchlorate-based Molecular Perovskite (H2DABCO)[NH4(ClO4)3]/Graphene Energetic Composite with Insensitive Performance. Cent. Eur. J. Energetic Mater. 2020, 17, 451–469. [Google Scholar] [CrossRef]
- Li, Y.B.; Huang, H.; Pan, L.P.; Zhang, J.H.; Li, J.S.; Zheng, B.H. Desensitizing technology of AP by coating and its application. Chin. J. Energetic Mater. 2014, 22, 792–797. [Google Scholar] [CrossRef]
- Zhou, T.; Liu, F.; Hua, X.; Li, B. Preparation and Properties of the F2604/AP Composite. Explos. Mater. 2017, 46, 21–25. [Google Scholar]
- Hai, N.; Xin, G.; Pei-Pei, S.; Yu-Lei, N.; Xuan, T. The Influence of Coating Material on the Impact Sensitivity of AP Powder. Initiat. Pyrotech. 2013, 6, 39–41. [Google Scholar]
- Nandagopal, S.; Mehilal, M.; Tapaswi, M.A.; Jawalkar, S.N.; Radhakrishnan, K.K.; Bhattacharya, B. Effect of Coating of Ammonium Perchlorate with Fluorocarbon on Ballistic and Sensitivity Properties of AP/Al/HTPB Propellant. Propellants Explos. Pyrotech. 2009, 34, 526–531. [Google Scholar] [CrossRef]
- Yang, Z.J.; Gong, F.Y.; Ding, L.; Li, Y.B.; Yang, G.C.; Nie, F.D. Efficient Sensitivity Reducing and Hygroscopicity Preventing of Ultra-Fine Ammonium Perchlorate for High Burning-Rate Propellants. Propellants Explos. Pyrotech. 2017, 42, 809–815. [Google Scholar] [CrossRef]
- Liu, Y.L.; Ai, K.L.; Lu, L.H. Polydopamine and Its Derivative Materials: Synthesis and Promising Applications in Energy, Environmental, and Biomedical Fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef]
- Lin, C.; He, W.; Gong, F.; He, G.; Liu, J.; Zeng, C.; Yang, Z.; Yan, Q. Research Progress of Bioinspired Interface Design in Energetic Materials Based on Polydopamine. Chin. J. Energetic Mater. 2020, 28, 577–588. [Google Scholar] [CrossRef]
- Lin, C.M.; Wen, Y.S.; Wei, L.Y.; Liu, R.Q.; Tu, X.Q.; Huang, S.L.; Zhang, C.; Qian, W.; Bai, L.F.; Chen, L.; et al. Construction of zirconium tungstate modified polymer bonded energetic composites with highly inhibited thermal expansion via bioinspired interfacial reinforcement. Compos. Part A Appl. Sci. Manuf. 2023, 175, 107794. [Google Scholar] [CrossRef]
- He, G.S.; Tian, X.; Dai, Y.; Li, X.; Lin, C.M.; Yang, Z.J.; Liu, S.J. Bioinspired interfacial engineering of polymer based energetic composites towards superior thermal conductivity via reducing thermal resistance. Appl. Surf. Sci. 2019, 493, 679–690. [Google Scholar] [CrossRef]
- Yang, X.; Zeng, C.; Gong, F.; Yang, W.; Yang, Z. Mechanical properties and thermal stabilities of CL-20 and FOX-7 explosives modified by polydopamine. Chin. J. Energetic Mater. 2021, 29, 1049–1060. [Google Scholar] [CrossRef]
- He, G.S.; Yang, Z.J.; Pan, L.P.; Zhang, J.H.; Liu, S.J.; Yan, Q.L. Bioinspired interfacial reinforcement of polymer based energetic composites with a high loading of solid explosive crystals. J. Mater. Chem. A 2017, 5, 13499–13510. [Google Scholar] [CrossRef]
- Zhu, Q.; Wu, S.; Xiao, C.; Li, X.; Xie, X.; Li, S.; Luo, G. Bioinspired Improving Interfacial Performances of HMX, TATB and Aluminum Powders with Polydopamine Coating. Chin. J. Energetic Mater. 2019, 27, 949–954. [Google Scholar]
- Zhang, H.L.; Jiao, Q.J.; Zhao, W.J.; Guo, X.Y.; Li, D.Y.; Sun, X.L. Enhanced Crystal Stabilities of ε-CL-20 via Core-Shell Structured Energetic Composites. Appl. Sci. 2020, 10, 2663. [Google Scholar] [CrossRef]
- GJB/772A-97; Experimental Methods of Sensitivity and Safety. National Military Standard of China: Beijing, China,, 1997.
- Kang, L.; Li, S.R.; Wang, B.; Li, X.S.; Zeng, Q.G. Exploration of the Energetic Material Ammonium Perchlorate at High Pressures: Combined Raman Spectroscopy and X-ray Diffraction Study. J. Phys. Chem. C 2018, 122, 15937–15944. [Google Scholar] [CrossRef]
- Chen, D.Q.; Mei, Y.H.; Hu, W.H.; Li, C.M. Electrochemically enhanced antibody immobilization on polydopamine thin film for sensitive surface plasmon resonance immunoassay. Talanta 2018, 182, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.L.; Dai, W.; Yang, M.; Wei, X.R.; Ma, K.; Song, B.T.; Jia, P.X.; Gong, Y.K.; Yang, J.F.; Zhao, J. Cell membrane mimetic copolymer coated polydopamine nanoparticles for combined pH-sensitive drug release and near-infrared photothermal therapeutic. Colloids Surf. B Biointerfaces 2019, 176, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kissinger, H. Reaction kinetics in different thermal analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]



| Sample | PDA Content (%) | AP Recovery Rate (%) |
|---|---|---|
| AP/PDA1 | 0.34 | 87.2% |
| AP/PDA3 | 0.67 | 86.9% |
| AP/PDA4 | 0.76 | 86.7% |
| AP/PDA5 | 0.47 | 86.4% |
| AP/PDA4 in ethanol | 0.56 | 31.2% |
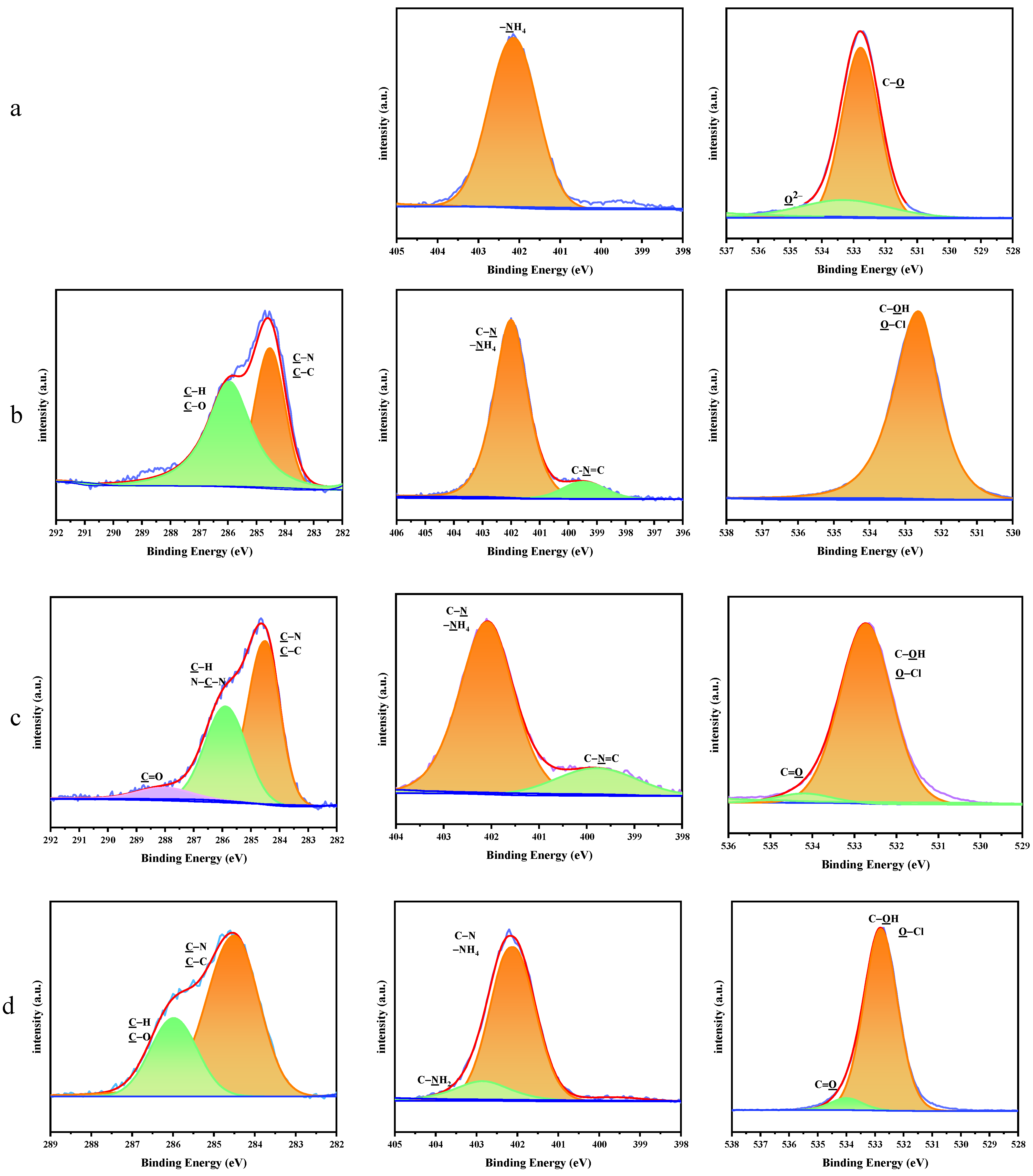
| Sample | C 1s (%) | N 1s (%) | O 1s (%) | Cl 2p (%) |
|---|---|---|---|---|
| AP | 50.53 | 6.16 | 37.29 | 6.02 |
| AP/PDA1 | 15.36 | 18.44 | 59.16 | 7.04 |
| AP/PDA4 | 30.85 | 16.91 | 48.83 | 3.41 |
| AP/PDA4 in ethanol | 6.15 | 20.24 | 72.17 | 1.44 |
| Sample | β/(K∙min−1) | Tp/°C | Ea/(KJ∙mol−1) | R2 |
|---|---|---|---|---|
| AP | 5 | 381.4 | 175.5 | 0.9875 |
| 10 | 400.5 | |||
| 20 | 415.5 | |||
| AP/PDA4 | 5 | 337.4 | 84.4 | 0.9998 |
| 10 | 358.1 | |||
| 20 | 386.1 |
| Sample | Impact Sensitivity (H50, cm) | Friction Sensitivity (P, %) |
|---|---|---|
| AP | 69.6 | 76 |
| AP/PDA1 | 85.4 | 24 |
| AP/PDA4 | ≥125.9 | 8 |
| AP/PDA4 in ethanol | 79.5 | 24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Tian, X.; Wang, J.; Zhong, K.; Chen, Y.; Li, C.; Jia, P. Bioinspired Fabrication of an Insensitive Ammonium Perchlorate Core–Shell Composite with Polydopamine Coating. Polymers 2024, 16, 1069. https://doi.org/10.3390/polym16081069
Huang Y, Tian X, Wang J, Zhong K, Chen Y, Li C, Jia P. Bioinspired Fabrication of an Insensitive Ammonium Perchlorate Core–Shell Composite with Polydopamine Coating. Polymers. 2024; 16(8):1069. https://doi.org/10.3390/polym16081069
Chicago/Turabian StyleHuang, Yafeng, Xuan Tian, Jinfei Wang, Kejun Zhong, Yuan Chen, Chenglong Li, and Pengxiang Jia. 2024. "Bioinspired Fabrication of an Insensitive Ammonium Perchlorate Core–Shell Composite with Polydopamine Coating" Polymers 16, no. 8: 1069. https://doi.org/10.3390/polym16081069
APA StyleHuang, Y., Tian, X., Wang, J., Zhong, K., Chen, Y., Li, C., & Jia, P. (2024). Bioinspired Fabrication of an Insensitive Ammonium Perchlorate Core–Shell Composite with Polydopamine Coating. Polymers, 16(8), 1069. https://doi.org/10.3390/polym16081069