Spray-Coated MoO3 Hole Transport Layer for Inverted Organic Photovoltaics
Abstract
1. Introduction
2. Experiments
2.1. Materials
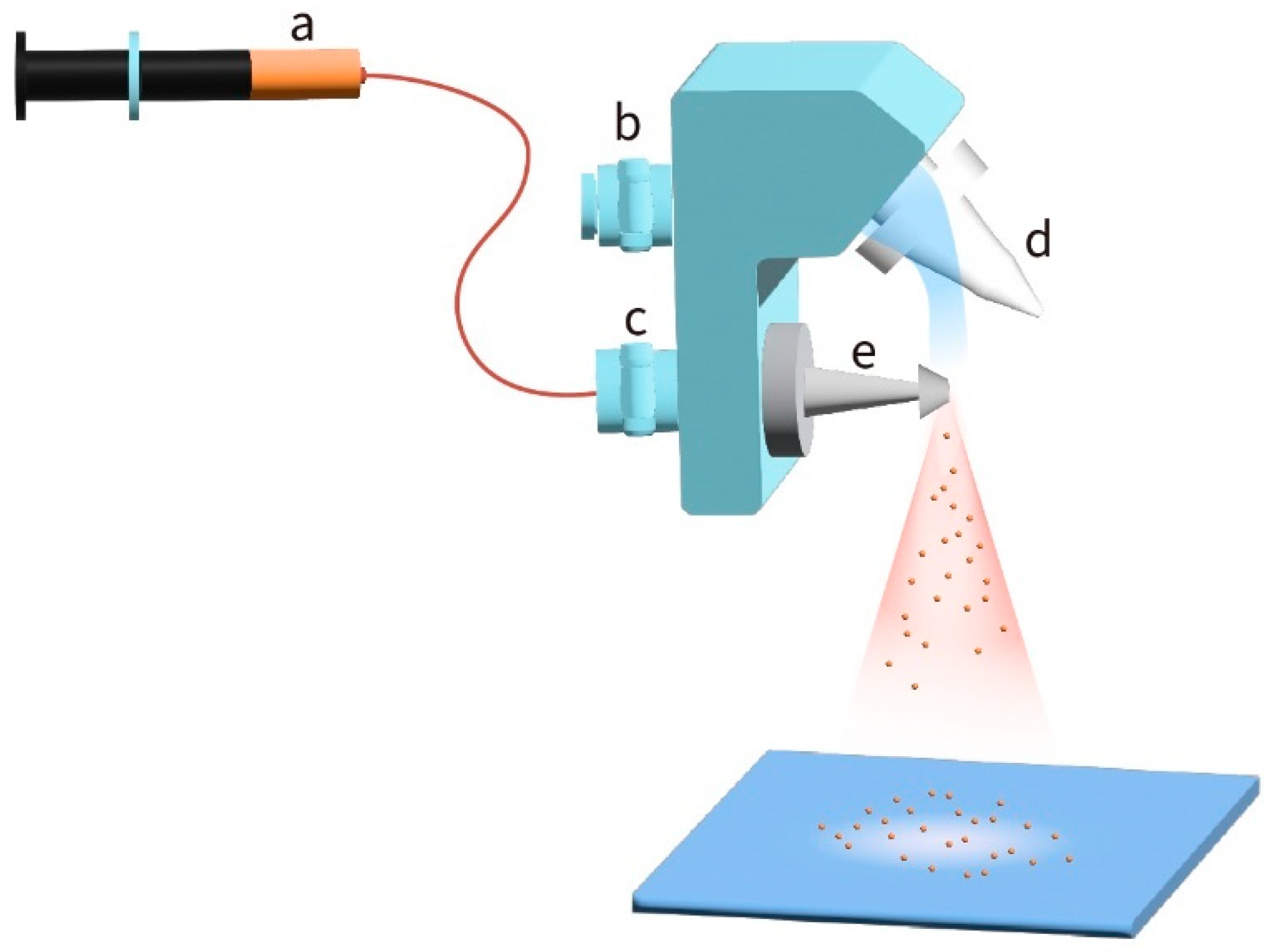
2.2. Device Fabrication
2.3. Device Characterization
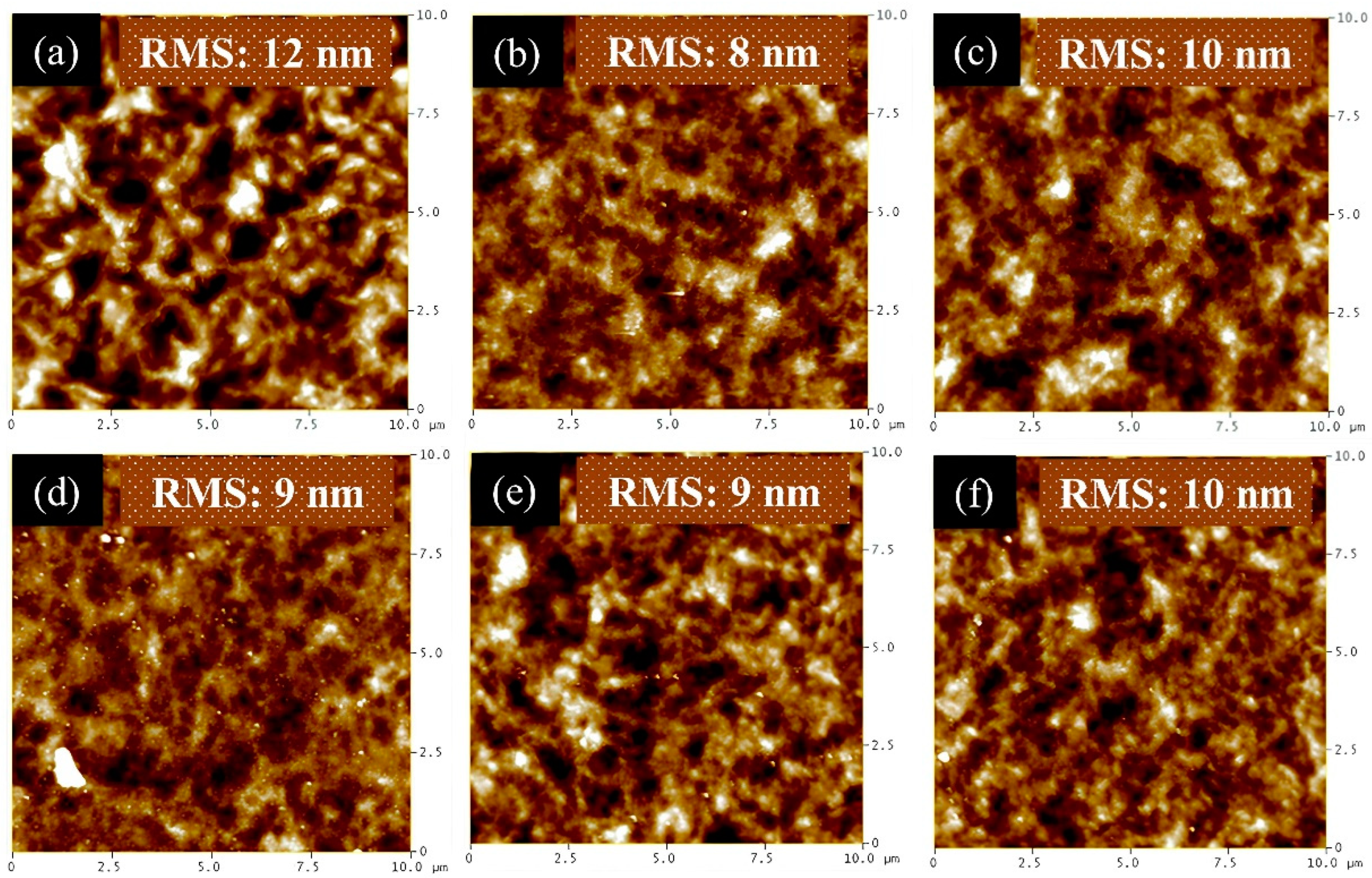
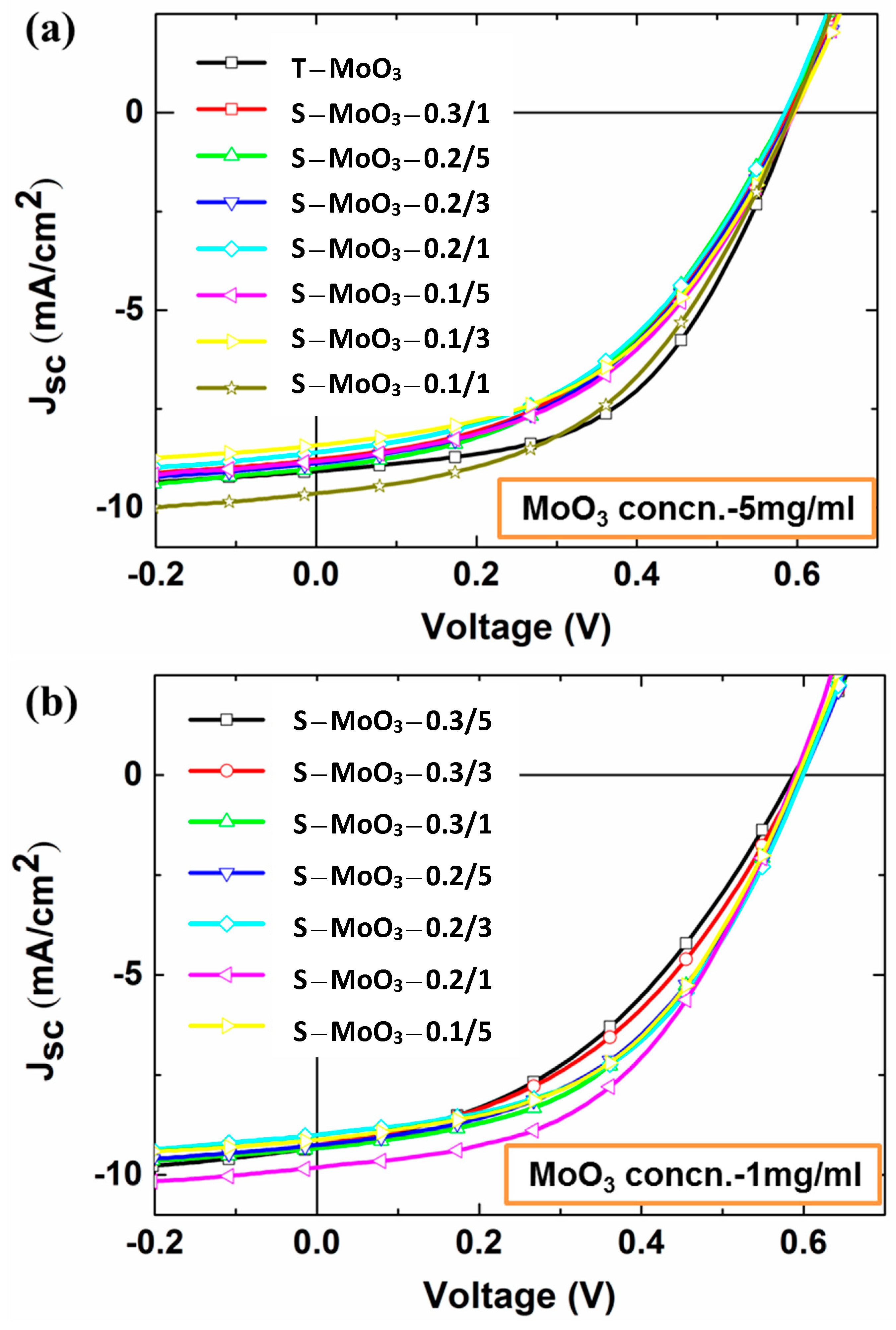
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lewis, N.S.; Crabtree, G.; Nozik, A.J.; Wasielewski, M.R.; Alivisatos, P.; Kung, H.; Tsao, J.; Chandler, E.; Walukiewicz, W.; Spitler, M.; et al. Basic Research Needs for Solar Energy Utilization. Report of the Basic Energy Sciences Workshop on Solar Energy Utilization, 18–21 April 2005. Available online: https://www.osti.gov/biblio/899136 (accessed on 27 February 2024).
- So, F. Organic Electronics; Materials, Processing, Devices and Applications; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Boca Raton, FL, USA, 2009. [Google Scholar]
- Kaltenbrunner, M.; White, M.; Głowacki, E.; Sekitani, T.; Someya, T.; Sariciftci, N.S.; Bauer, S. Ultrathin and lightweight organic solar cells with high flexibility. Nat Commun. 2012, 3, 770. [Google Scholar] [CrossRef] [PubMed]
- Dennler, G.; Scharber, M.C.; Brabec, C.J. Polymer-Fullerene Bulk-Heterojunction Solar Cells. Adv. Materials. 2009, 21, 1323–1338. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, M.; Xu, J.; Li, C.; Yan, J.; Zhou, G.; Liu, F. Single-junction organic solar cells with over 19% efficiency enabled by a refined double-fibril network morphology. Nat. Mater. 2022, 21, 656–663. [Google Scholar] [CrossRef]
- Ling, Z.; Nugraha, M.I.; Hadmojo, W.T.; Lin, Y.; Jeong, S.Y.; Yengel, E.; Anthopoulos, T.D. Over 19% Efficiency in Ternary Organic Solar Cells Enabled by n-Type Dopants. ACS Energy Lett. 2023, 8, 4104–4112. [Google Scholar] [CrossRef]
- Lee, S.J.; Kim, H.P.; bin Mohd Yusoff, A.R.; Jang, J. Organic photovoltaic with PEDOT: PSS and V2O5 mixture as hole transport layer. Sol. Energy Mater. Sol. Cells 2014, 120, 238–243. [Google Scholar] [CrossRef]
- Chen, M.H.; Kuo, Y.C.; Lin, H.H.; Chao, Y.P.; Wong, M.S. Highly stable inverted organic photovoltaics using aluminum-doped zinc oxide as electron transport layers. J. Power Sources 2015, 275, 274–278. [Google Scholar] [CrossRef]
- Chu, C.W.; Li, S.H.; Chen, C.W.; Shrotriya, V.; Yang, Y. High-performance organic thin-film transistors with metal oxide/metal bilayer electrode. Appl. Phys. Lett. 2005, 87, 193508. [Google Scholar] [CrossRef]
- Das, S.; Choi, J.Y.; Alford, T.L. P3HT: PC61BM based solar cells employing solution processed copper iodide as the hole transport layer. Sol. Energy Mater. Sol. Cells 2015, 133, 255–259. [Google Scholar] [CrossRef]
- Shrotriya, V.; Li, G.; Yao, Y.; Chu, C.-W.; Yang, Y. Transition metal oxides as the buffer layer for polymer photovoltaic cells. Appl. Phys. Lett. 2006, 88, 073508. [Google Scholar] [CrossRef]
- Irwin, M.D.; Buchholz, D.B.; Hains, A.W.; Chang, R.P.H.; Marks, T.J. p-Type semiconducting nickel oxide as an efficiency-enhancing anode interfacial layer in polymer bulk-heterojunction solar cells. Proc. Natl. Acad. Sci. USA 2008, 105, 2783–2787. [Google Scholar] [CrossRef]
- Tao, C.; Ruan, S.; Xie, G.; Kong, X.; Shen, L.; Meng, F.; Liu, C.; Zhang, X.; Dong, W.; Chen, W. Role of tungsten oxide in inverted polymer solar cells. Appl. Phys. Lett. 2009, 94, 043311. [Google Scholar] [CrossRef]
- Motaung, D.E.; Makgwane, P.R.; Ray, S.S. Metal oxide nanostructures-containing organic polymer hybrid solar cells: Optimization of processing parameters on cell performance. Appl. Surf. Sci. 2015, 355, 484–494. [Google Scholar] [CrossRef]
- Liu, F.; Shao, S.; Guo, X.; Zhao, Y.; Xie, Z. Efficient polymer photovoltaic cells using solution-processed MoO3 as anode buffer layer. Sol. Energy Mater. Sol. Cells 2010, 94, 842–845. [Google Scholar] [CrossRef]
- Girotto, C.; Voroshazi, E.; Cheyns, D.; Heremans, P.; Rand, B.P. Solution-processed MoO3 thin films as a hole-injection layer for organic solar cells. ACS Appl. Mater. Interfaces 2011, 3, 3244–3247. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Wang, M.; Cao, Y.; Huang, F.; Huang, L.; Peng, J.; Cao, Y. Polymer solar cells with a low-temperature-annealed sol–gel-derived MoOx film as a hole extraction layer. Adv. Energy Mater. 2012, 2, 523–527. [Google Scholar] [CrossRef]
- Zilberberg, K.; Gharbi, H.; Behrendt, A.; Trost, S.; Riedl, T. Low-temperature, solution-processed MoOx for efficient and stable organic solar cells. ACS Appl. Mater. Interfaces 2012, 4, 1164–1168. [Google Scholar] [CrossRef]
- Jasieniak, J.J.; Seifter, J.; Jo, J.; Mates, T.; Heeger, A.J. A solution-processed MoOx anode interlayer for use within organic photovoltaic devices. Adv. Funct. Mater. 2012, 22, 2594–2605. [Google Scholar] [CrossRef]
- Yang, K.; Chen, S.; Zhou, Y.; Odunmbaku, G.O.; Xiong, Z.; Yang, Q.; Wang, M.; Kan, Z.; Xiao, Z.; Lu, S.; et al. Annealing-free alcohol-processable MoOX anode interlayer enables efficient light utilization in organic photovoltaics. J. Energy Chem. 2021, 61, 141–146. [Google Scholar] [CrossRef]
- Song, C.; Huang, X.; Zhan, T.; Ding, L.; Li, Y.; Xue, X.; Peng, H.; Cai, P.; Duan, C.; Chen, J. Annealing-Insensitive, Alcohol-Processed MoOx Hole Transport Layer for Universally Enabling High-Performance Conventional and Inverted Organic Solar Cells. ACS Appl. Mater. Interfaces 2022, 14, 40851–40861. [Google Scholar] [CrossRef]
- Sung, Y.M.; Tsao, C.S.; Lin, H.K.; Cha, H.C.; Jiang, P.C.; Liu, T.C.; Chang, K.W.; Huang, Y.C.; Tsay, J.S. Dramatic improvement in the stability and mechanism of high-performance inverted polymer solar cells featuring a solution-processed buffer layer. Nanoscale 2023, 15, 3375–3386. [Google Scholar] [CrossRef]
- Ioakeimidis, A.; Hauser, A.; Rossier, M.; Linardi, F.; Choulis, S.A. High-performance non-fullerene acceptor inverted organic photovoltaics incorporating solution processed doped metal oxide hole selective contact. Appl. Phys. Lett. 2022, 120, 233301. [Google Scholar] [CrossRef]
- Teng, N.W.; Li, C.H.; Lo, W.C.; Tsai, Y.S.; Liao, C.Y.; You, Y.W.; Ho, S.L.; Li, W.L.; Lee, C.C.l.; Lin, W.C.; et al. Highly Efficient Nonfullerene Organic Photovoltaic Devices with 10% Power Conversion Efficiency Enabled by a Fine-Tuned and Solution-Processed Hole-Transporting Layer. Solar RRL 2020, 4, 2000223. [Google Scholar] [CrossRef]
- Søndergaard, R.; Hösel, M.; Angmo, D.; Larsen-Olsen, T.T.; Krebs, F.C. Roll-to-roll fabrication of polymer solar cells. Mater. Today 2012, 15, 36–49. [Google Scholar] [CrossRef]
- Krebs, F.C. Fabrication and processing of polymer solar cells: A review of printing and coating techniques. Sol. Energy Mater. Sol. Cells 2009, 93, 394–412. [Google Scholar] [CrossRef]
- Susanna, G.; Salamandra, L.; Brown, T.M.; Di Carlo, A.; Brunetti, F.; Reale, A. Airbrush spray-coating of polymer bulk-heterojunction solar cells. Sol. Energy Mater. Sol. Cells 2011, 95, 1775–1778. [Google Scholar] [CrossRef]
- Huang, Y.C.; Tsao, C.S.; Cha, H.C.; Chuang, C.M.; Su, C.J.; Jeng, U.S.; Chen, C.Y. Correlation between hierarchical structure and processing control of large-area spray-coated polymer solar cells toward high performance. Sci. Rep. 2016, 6, 20062. [Google Scholar] [CrossRef]
- Kumar, P.; Bilen, C.; Vaughan, B.; Zhou, X.; Dastoor, P.C.; Belcher, W.J. Comparing the degradation of organic photovoltaic devices under ISOS testing protocols. Sol. Energy Mater. Sol. Cells 2016, 149, 179–186. [Google Scholar] [CrossRef]



| Thermally Evaporated MoO3 Layer | ||||||
|---|---|---|---|---|---|---|
| JSC (mA/cm2) | Voc (V) | FF (%) | PCE (%) | PCEmax(%) | ||
| 9.10 ± 0.120 | 0.59 ± 0.003 | 50.30 ± 1.40 | 2.72 ± 0.071 | 2.81 | ||
| Sprayed MoO3 Concentration = 5 mg/mL | ||||||
| spray flux (mL/min) | spray cycle | JSC (mA/cm2) | Voc (V) | FF (%) | PCE (%) | PCEmax (%) |
| 0.3 | 1 | 8.54 ± 0.19 | 0.594 ± 0.006 | 45.20 ± 0.50 | 2.29 ± 0.077 | 2.42 |
| 0.2 | 5 | 9.20 ± 0.17 | 0.588 ± 0.007 | 42.25 ± 1.13 | 2.28 ± 0.035 | 2.33 |
| 3 | 9.05 ± 0.26 | 0.588 ± 0.008 | 43.21 ± 0.98 | 2.30 ± 0.091 | 2.49 | |
| 1 | 8.48 ± 0.13 | 0.591 ± 0.004 | 45.18 ± 1.69 | 2.27 ± 0.092 | 2.42 | |
| 0.1 | 5 | 8.94 ± 0.22 | 0.589 ± 0.006 | 43.91 ± 1.16 | 2.31 ± 0.093 | 2.50 |
| 3 | 8.41 ± 0.25 | 0.591 ± 0.005 | 45.23 ± 1.23 | 2.24 ± 0.052 | 2.35 | |
| 1 | 9.80 ± 0.30 | 0.596 ± 0.004 | 46.13 ± 1.36 | 2.69 ± 0.059 | 2.79 | |
| Sprayed MoO3 Concentration = 1 mg/mL | ||||||
| spray flux (mL/min) | spray cycle | JSC (mA/cm2) | Voc (V) | FF (%) | PCE (%) | PCEmax (%) |
| 0.3 | 5 | 9.35 ± 0.101 | 0.59 ± 0.002 | 41.05 ± 0.45 | 2.26 ± 0.020 | 2.28 |
| 3 | 8.88 ± 0.424 | 0.59 ± 0.004 | 44.45 ± 1.56 | 2.34 ± 0.129 | 2.68 | |
| 1 | 9.11 ± 0.329 | 0.60 ± 0.005 | 47.70 ± 1.30 | 2.59 ± 0.063 | 2.67 | |
| 0.2 | 5 | 9.54 ± 0.339 | 0.59 ± 0.007 | 45.40 ± 1.57 | 2.57 ± 0.153 | 2.97 |
| 3 | 9.26 ± 0.339 | 0.60 ± 0.003 | 47.10 ± 1.35 | 2.61 ± 0.119 | 2.81 | |
| 1 | 9.64 ± 0.124 | 0.59 ± 0.008 | 49.60 ± 0.67 | 2.80 ± 0.063 | 2.85 | |
| 0.1 | 5 | 9.22 ± 0.233 | 0.59 ± 0.004 | 47.01 ± 1.59 | 2.56 ± 0.081 | 2.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cha, H.-C.; Li, C.-F.; Chung, T.-Y.; Ma, W.-Y.; Tsao, C.-S.; Huang, Y.-C. Spray-Coated MoO3 Hole Transport Layer for Inverted Organic Photovoltaics. Polymers 2024, 16, 981. https://doi.org/10.3390/polym16070981
Cha H-C, Li C-F, Chung T-Y, Ma W-Y, Tsao C-S, Huang Y-C. Spray-Coated MoO3 Hole Transport Layer for Inverted Organic Photovoltaics. Polymers. 2024; 16(7):981. https://doi.org/10.3390/polym16070981
Chicago/Turabian StyleCha, Hou-Chin, Chia-Feng Li, Tsui-Yun Chung, Wei-Yang Ma, Cheng-Si Tsao, and Yu-Ching Huang. 2024. "Spray-Coated MoO3 Hole Transport Layer for Inverted Organic Photovoltaics" Polymers 16, no. 7: 981. https://doi.org/10.3390/polym16070981
APA StyleCha, H.-C., Li, C.-F., Chung, T.-Y., Ma, W.-Y., Tsao, C.-S., & Huang, Y.-C. (2024). Spray-Coated MoO3 Hole Transport Layer for Inverted Organic Photovoltaics. Polymers, 16(7), 981. https://doi.org/10.3390/polym16070981







