Recrystallization of Cellulose, Chitin and Starch in Their Individual and Native Forms
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of β-Chitin
2.3. Preparation of Potato Flour
2.4. Mechanical Treatment
2.5. Recrystallization
2.6. Scanning Electron Microscopy (SEM)
2.7. Particle Size
2.8. Specific Surface Area (SSA)
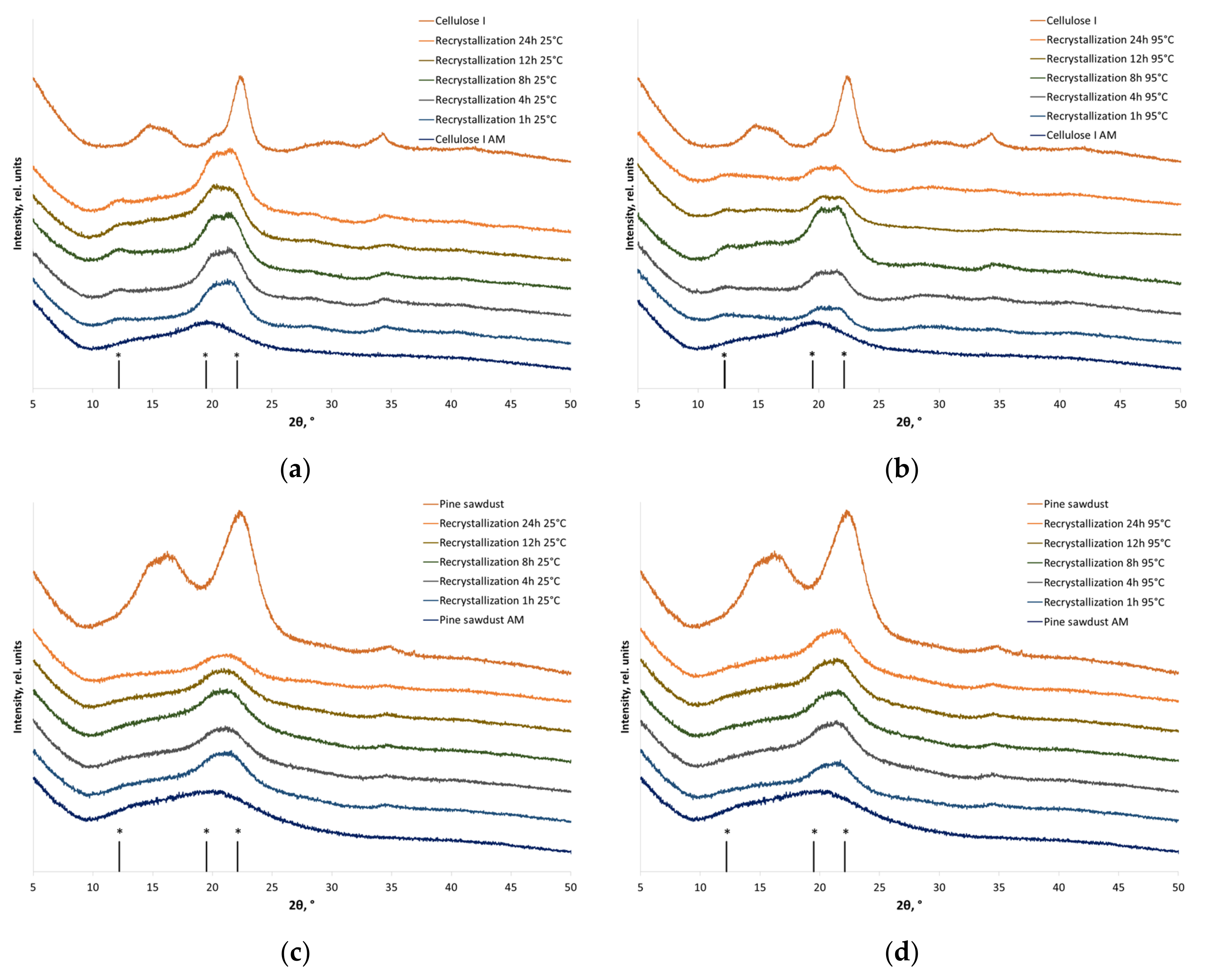
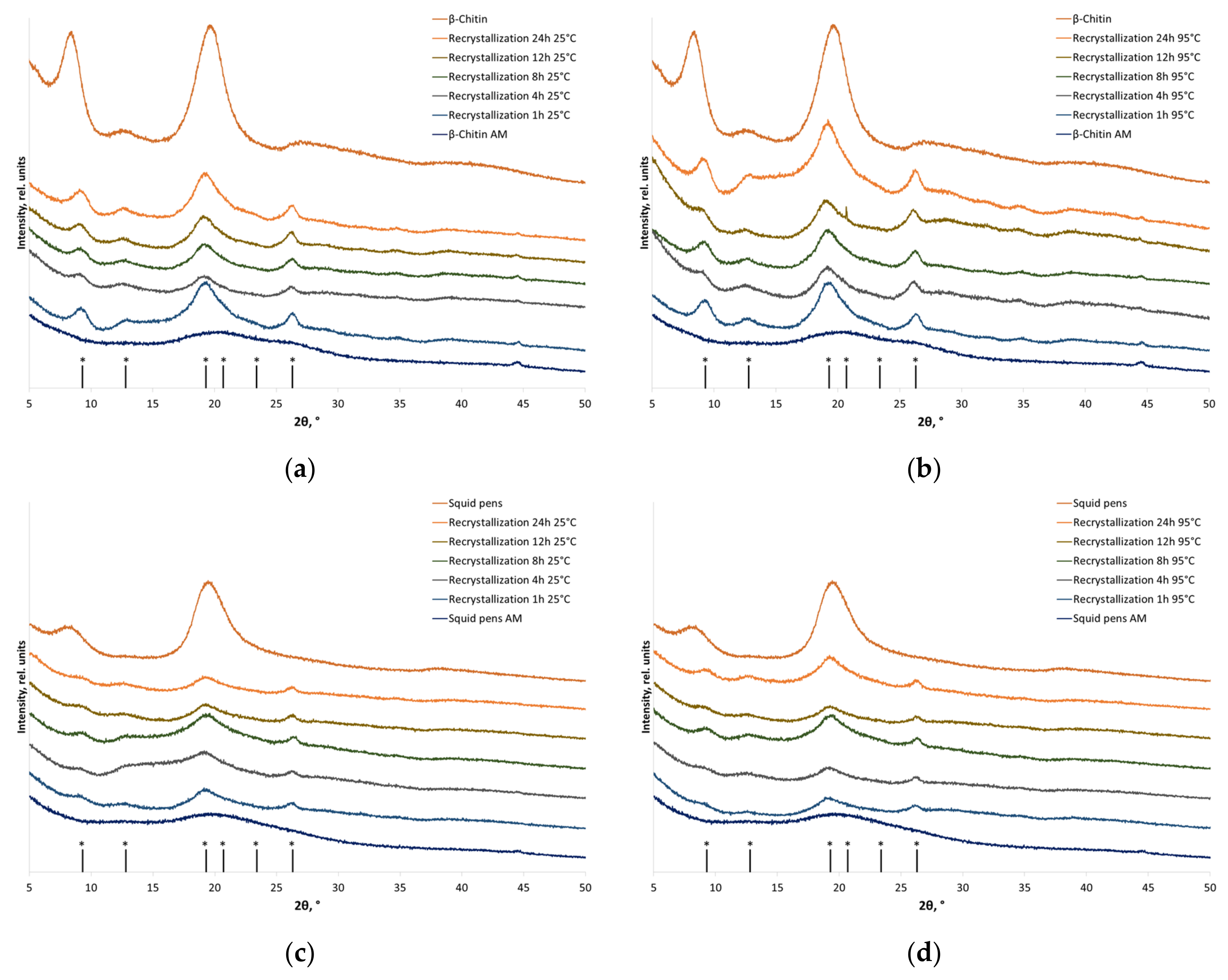
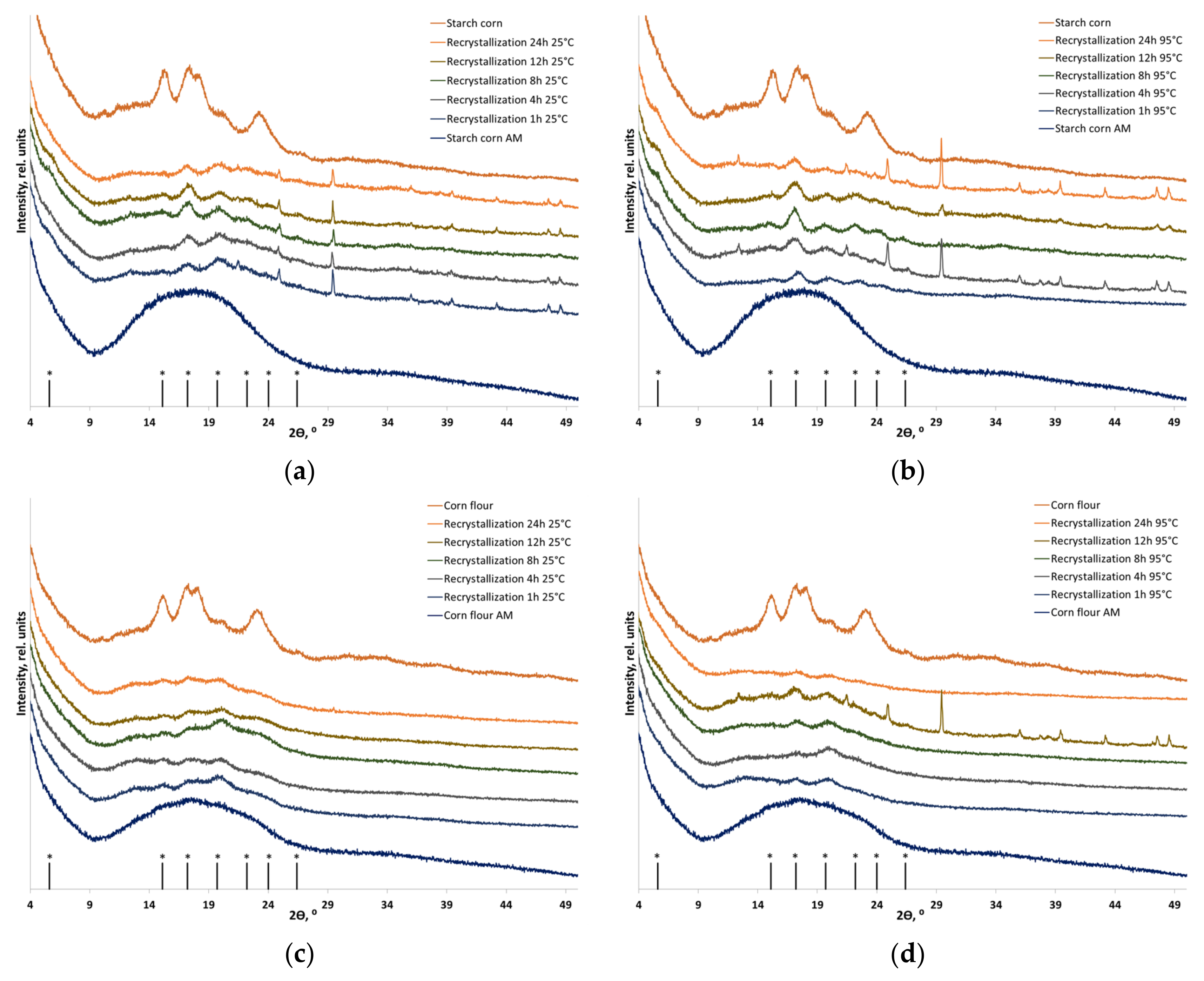
2.9. X-ray Diffraction (XRD) Analysis
2.10. Statistical Analysis
3. Results and Discussion
3.1. Mechanical Treatment
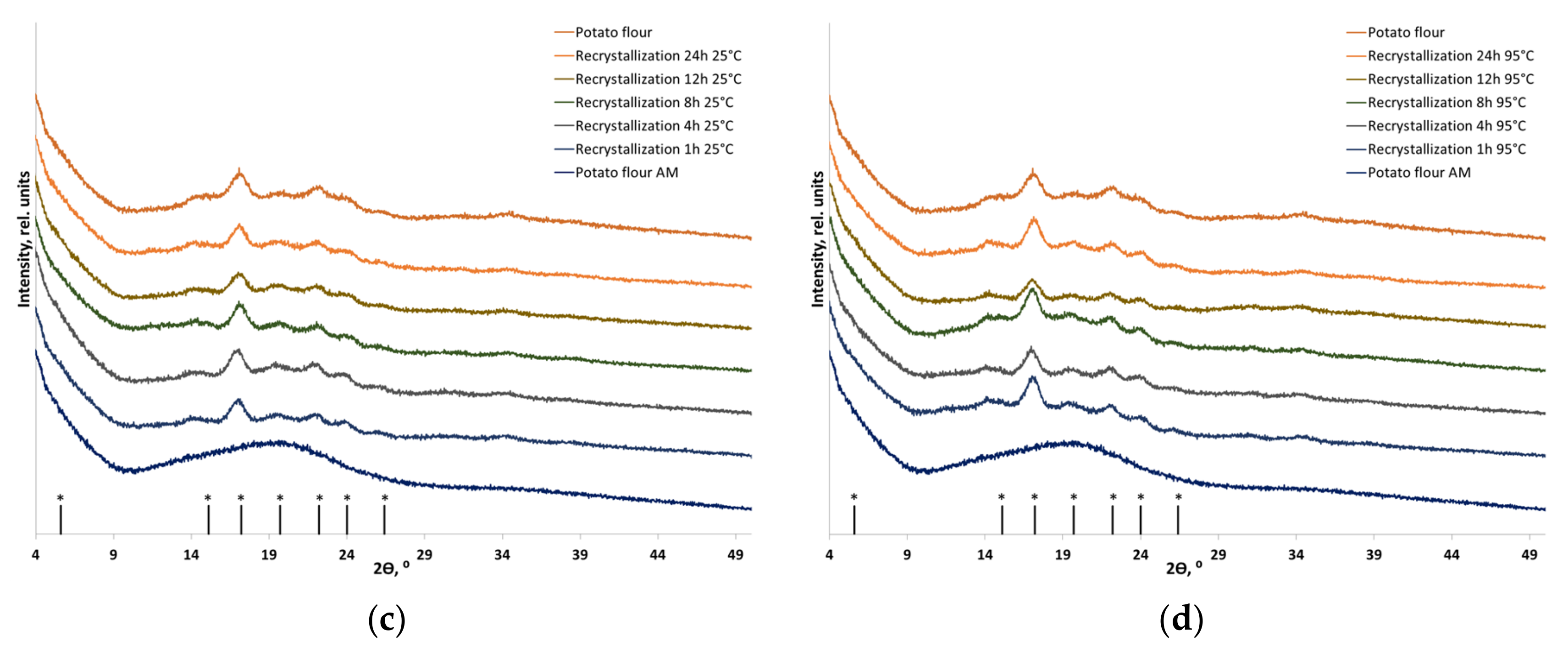
3.2. Recrystallization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baláž, P.; Achimovičová, M.; Baláž, M.; Billik, P.; Cherkezova-Zheleva, Z.; Criado, J.M.; Delogu, F.; Dutková, E.; Gaffet, E.; Gotor, F.J.; et al. Hallmarks of mechanochemistry: From nanoparticles to technology. Chem. Soc. Rev. 2013, 42, 7571–7637. [Google Scholar] [CrossRef] [PubMed]
- Ardila-Fierro, K.J.; Hernández, J.G. Sustainability Assessment of Mechanochemistry by Using the Twelve Principles of Green Chemistry. Chem. Sus. Chem. 2021, 14, 2145–2162. [Google Scholar] [CrossRef] [PubMed]
- Di Nardo, T.; Moores, A. Mechanochemical amorphization of chitin: Impact of apparatus material on performance and contamination. Beilstein J. Org. Chem. 2019, 15, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Merchán, A.M.; Rodríguez-Carballo, G.; Torres-Olea, B.; García-Sancho, C.; Maireles-Torres, P.J.; Mérida-Robles, J.; Moreno-Tost, R. Recent Advances in Mechanochemical Pretreatment of Lignocellulosic Biomass. Energies 2022, 15, 5948. [Google Scholar] [CrossRef]
- Bychkov, A.L.; Podgorbunskikh, E.M.; Bychkova, E.S.; Lomovsky, O.I. Current achievements in the mechanically pretreated conversion of plant biomass. Biotechnol. Bioeng. 2019, 116, 1234–1244. [Google Scholar] [CrossRef] [PubMed]
- Kuga, S.; Wu, M. Mechanochemistry of cellulose. Cellulose 2019, 26, 215–225. [Google Scholar] [CrossRef]
- Barbash, V.A.; Yaschenko, O.V.; Alushkin, S.V.; Kondratyuk, A.S.; Posudievsky, O.Y.; Koshenko, V.G. The Effect of Mechanochemical Treatment of the Cellulose on Characteristics of Nanocellulose Films. Nanoscale Res. Lett. 2016, 11, 410. [Google Scholar] [CrossRef] [PubMed]
- Andrade, I.H.P.; Otoni, C.G.; Amorim, T.S.; Camilloto, G.P.; Cruz, R.S. Ultrasound-assisted extraction of starch nanoparticles from breadfruit (Artocarpus altilis (Parkinson) Fosberg). Colloids Surf. A Physicochem. Eng. 2020, 586, 124277. [Google Scholar] [CrossRef]
- Anusha, J.R.; Fleming, A.T.; Valan Arasu, M.; Chul Kim, B.; Abdullah Al-Dhabi, N.; Yu, K.-H.; Justin Raj, C. Mechanochemical synthesis of chitosan submicron particles from the gladius of Todarodes pacificus. J. Adv. Res. 2016, 7, 863–871. [Google Scholar] [CrossRef]
- Akopova, T.A.; Popyrina, T.N.; Demina, T.S. Mechanochemical Transformations of Polysaccharides: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 10458. [Google Scholar] [CrossRef]
- Chen, X.; Yang, H.; Zhong, Z.; Yan, N. Base-catalysed, one-step mechanochemical conversion of chitin and shrimp shells into low molecular weight chitosan. Green Chem. 2017, 19, 2783–2792. [Google Scholar] [CrossRef]
- Di Nardo, T.; Hadad, C.; Van Nhien, A.N.; Moores, A. Synthesis of high molecular weight chitosan from chitin by mechanochemistry and aging. Green Chem. 2019, 21, 3276–3285. [Google Scholar] [CrossRef]
- Hou, D.-F.; Li, M.-L.; Li, P.-Y.; Zhou, L.; Zhang, K.; Liu, Z.-Y.; Yang, W.; Yang, M.-B. Efficient Conversion of Cellulose to Thermoplastics by Mechanochemical Esterification. ACS Sustain. Chem. Eng. 2023, 11, 7655–7663. [Google Scholar] [CrossRef]
- Vakili, M.; Qiu, W.; Cagnetta, G.; Huang, J.; Yu, G. Mechanochemically oxidized chitosan-based adsorbents with outstanding Penicillin G adsorption capacity. J. Environ. Chem. Eng. 2021, 9, 105454. [Google Scholar] [CrossRef]
- Zhao, H.; Kwak, J.H.; Wang, Y.; Franz, J.A.; White, J.M.; Holladay, J.E. Effects of Crystallinity on Dilute Acid Hydrolysis of Cellulose by Cellulose Ball-Milling Study. Energy Fuels 2006, 20, 807–811. [Google Scholar] [CrossRef]
- Hall, M.; Bansal, P.; Lee, J.H.; Realff, M.J.; Bommarius, A.S. Cellulose crystallinity—A key predictor of the enzymatic hydrolysis rate. FEBS J. 2010, 277, 1571–1582. [Google Scholar] [CrossRef]
- Lemos, P.V.F.; Barbosa, L.S.; Ramos, I.G.; Coelho, R.E.; Druzian, J.I. The important role of crystallinity and amylose ratio in thermal stability of starches. J. Therm. Anal. Calorim. 2018, 131, 2555–2567. [Google Scholar] [CrossRef]
- Zhu, X.; He, Q.; Hu, Y.; Huang, R.; Shao, N. A comparative study of structure, thermal degradation, and combustion behavior of starch from different plant sources. J. Therm. Anal. Calorim. 2018, 132, 927–935. [Google Scholar] [CrossRef]
- Li, Y.; Yan, C.; Chen, Y.; Han, X.; Shao, Z.; Qi, H.; Li, H.; Nishiyama, Y.; Hu, T.; Chen, P. The major role of London dispersion interaction in the assembly of cellulose, chitin, and chitosan. Cellulose 2023, 30, 8127–8138. [Google Scholar] [CrossRef]
- O’Sullivan, A.C. Cellulose: The structure slowly unravels. Cellulose 1997, 4, 173–207. [Google Scholar] [CrossRef]
- Emenike, E.C.; Iwuozor, K.O.; Saliu, O.D.; Ramontja, J.; Adeniyi, A.G. Advances in the extraction, classification, modification, emerging and advanced applications of crystalline cellulose: A review. Carbohydr. Polym. Technol. Appl. 2023, 6, 100337. [Google Scholar] [CrossRef]
- Hermans, P.H.; Weidinger, A. On the recrystallization of amorphous cellulose. J. Am. Chem. Soc. 1946, 68, 2547–2552. [Google Scholar] [CrossRef]
- Ago, M.; Endo, T.; Hirotsu, T. Crystalline transformation of native cellulose from cellulose I to cellulose II polymorph by a ball-milling method with a specific amount of water. Cellulose 2004, 11, 163–167. [Google Scholar] [CrossRef]
- Ago, M.; Endo, T.; Okajima, K. Effect of solvent on morphological and structural change of cellulose under ball-milling. Polym. J. 2007, 39, 435–441. [Google Scholar] [CrossRef]
- Kocherbitov, V.; Ulvenlund, S.; Kober, M.; Jarring, K.; Arnebrant, T. Hydration of microcrystalline cellulose and milled cellulose studied by sorption calorimetry. J. Phys. Chem. B. 2008, 112, 3728–3734. [Google Scholar] [CrossRef] [PubMed]
- Klemm, D.; Schmauder, H.P.; Heinze, T. Cellulose. In Biopolymers. Polysaccharides II: Polysaccharides from Eukaryotes, 6th ed.; DeBaets, S., Vandamme, E.J., Steinbüchel, A., Eds.; Wiley: Weinheim, Germany, 2002; Volume 6, pp. 275–287. [Google Scholar]
- Rosenau, T.; Potthast, A.; Sixta, H.; Kosma, P. The chemistry of side reactions and byproduct formation in the system NMMO/cellulose (Lyocell process). Prog. Polym. Sci. 2001, 26, 1763–1837. [Google Scholar] [CrossRef]
- Ahn, Y.; Song, Y.; Kwak, S.Y.; Kim, H. Highly ordered cellulose II crystalline regenerated from cellulose hydrolyzed by 1-butyl-3-methylimidazolium chloride. Carbohydr. Polym. 2016, 137, 321–327. [Google Scholar] [CrossRef]
- Holland, S.; Tuck, C.; Foster, T. Selective recrystallization of cellulose composite powders and microstructure creation through 3D binder jetting. Carbohydr. Polym. 2018, 200, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Pita-Vilar, M.; Concheiro, A.; Alvarez-Lorenzo, C.; Diaz-Gomez, L. Recent advances in 3D printed cellulose-based wound dressings: A review on in vitro and in vivo achievements. Carbohydr. Polym. 2023, 321, 121298. [Google Scholar] [CrossRef]
- Qiao, Y.; Zhai, C.; Liu, F.; Chen, L.; Na, H.; Chen, J.; Zhu, J. Highly efficient microwave driven assisted hydrolysis of cellulose to sugar with the utilization of ZrO2 to inhibit recrystallization of cellulose. Carbohydr. Polym. 2020, 228, 115358. [Google Scholar] [CrossRef]
- Tsurkan, M.V.; Voronkina, A.; Khunyk, Y.; Wysokowski, M.; Petrenko, I.; Ehrlich, H. Progress in chitin analytics. Carbohydr. Polym. 2021, 252, 117204. [Google Scholar] [CrossRef] [PubMed]
- Fernando, L.D.; Dickwella Widanage, M.C.; Penfield, J.; Lipton, A.S.; Washton, N.; Latgé, J.-P.; Wang, P.; Zhang, L.; Wang, T. Structural Polymorphism of Chitin and Chitosan in Fungal Cell Walls From Solid-State NMR and Principal Component Analysis. Front. Mol. Biosci. 2021, 8, 727053. [Google Scholar] [CrossRef] [PubMed]
- Noishiki, Y.; Takami, H.; Nishiyama, Y.; Wada, M.; Okada, S.; Kuga, S. Alkali-induced conversion of β-chitin to α-chitin. Biomacromolecules 2003, 4, 896–899. [Google Scholar] [CrossRef] [PubMed]
- Faria, R.R.; Guerra, R.F.; de Sousa Neto, L.R.; Motta, L.F.; de Faria Franca, E. Computational study of polymorphic structures of α- and β-chitin and chitosan in aqueous solution. J. Mol. Graph. 2016, 63, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhong, Y.; Weia, P.; Cai, J. Rapid dissolution of β-chitin and hierarchical self-assembly of chitin chains in aqueous KOH/urea solution. Green Chem. 2021, 23, 3048–3060. [Google Scholar] [CrossRef]
- Zobel, H.F. Starch crystal transformations and their industrial importance. Starch 1988, 40, 1–7. [Google Scholar] [CrossRef]
- Dome, K.; Podgorbunskikh, E.; Bychkov, A.; Lomovsky, O. Changes in the crystallinity degree of starch having different types of crystal structure after mechanical pretreatment. Polymers 2020, 12, 641. [Google Scholar] [CrossRef] [PubMed]
- Robyt, J.F. Starch: Structure, properties, chemistry, and enzymology. In Glycoscience; Fraser-Reid, B., Tatsuta, K., Thiem, J., Eds.; Springer-Verlag: Berlin, Germany, 2008; Volume 6, pp. 1437–1472. [Google Scholar] [CrossRef]
- Trinh, K.S. Recrystallization of starches by hydrothermal treatment: Digestibility, structural, and physicochemical properties. J. Food Sci. Technol. 2015, 52, 7640–7654. [Google Scholar] [CrossRef] [PubMed]
- Miao, M.; Jiang, B.; Zhang, T. Effect of pullulanase debranching and recrystallization on structure and digestibility of waxy maize starch. Carbohydr. Polym. 2009, 76, 214–221. [Google Scholar] [CrossRef]
- Pratiwi, M.; Nur Faridah, D.; Lioe, H.N. Structural changes to starch after acid hydrolysis, debranching, autoclaving-cooling cycles, and heat moisture treatment (HMT): A review. Starch 2017, 70, 1700028. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Wang, G.S.; Pan, X.J.; Gleisner, R. Specific surface to evaluate the efficiencies of milling and pretreatment of wood for enzymatic saccharification. Chem. Eng. Sci. 2009, 64, 474–485. [Google Scholar] [CrossRef]
- Tyufekchiev, M.; Kolodziejczak, A.; Duan, P.; Foston, M.; Schmidt-Rohrb, K.; Timko, M.T. Reaction engineering implications of cellulose crystallinity and water-promoted recrystallization. Green Chem. 2019, 21, 5541–5555. [Google Scholar] [CrossRef]
- Salem, K.S.; Kasera, N.K.; Rahman, M.A.; Jameel, H.; Habibi, Y.; Eichhorn, S.J.; French, A.D.; Pal, L.; Lucia, L.A. Comparison and evaluation of methods for cellulose crystallinity determination. Chem. Soc. Rev. 2023, 52, 6417. [Google Scholar] [CrossRef] [PubMed]
- Cuong, H.N.; Minh, N.C.; Hoa, N.V.; Trung, T.S. Preparation and characterization of high purity β-chitin from squid pens (Loligo chenisis). Int. J. Biol. Macromol. 2016, 93, 442–447. [Google Scholar] [CrossRef] [PubMed]
- ISO 13322-2; Particle Size Analysis Image Analysis Methods—Part 2: Dynamic Image Analysis Methods. ISO: Geneva, Switzerland, 2021.
- Krumbein, W.C.; Sloss, L.L. Stratigraphy and Sedimentation, 2nd ed.; W.H. Freeman and Company: San Francisco, CA, USA, 1963. [Google Scholar]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Nara, S.; Mori, A.; Komiya, T. Study on Relative Crystallinity of Moist Potato Starch. Starch 1978, 4, 111–114. [Google Scholar] [CrossRef]
- Podgorbunskikh, E.M.; Dome, K.V.; Bukhtoyarov, V.; Bychkov, A.L. X-ray Diffraction for Detecting Starch Adulteration and Measuring the Crystallinity Indices of the Polymorphic Modifications of Starch. Health Food Biotechnol. 2022, 4, 62–70. [Google Scholar] [CrossRef]
- Focher, B.; Beltrame, P.L.; Naggi, A.; Torri, G. Alkaline N-deacetylation of chitin enhanced by flash treatments. Reaction kinetics and structure modifications. Carbohydr. Polym. 1990, 12, 405–418. [Google Scholar] [CrossRef]
- Podgorbunskikh, E.M.; Bychkov, A.L.; Bulina, N.V.; Lomovskii, O.I. Disordering of the crystal structure of cellulose under mechanical activation. J. Struct. Chem. 2018, 59, 201–208. [Google Scholar] [CrossRef]
- Podgorbunskikh, E.; Kuskov, T.; Matveeva, A.; Ulihin, A.; Bychkov, A.; Lomovsky, I.; Polienko, Y. Disordering of Starch Films as a Factor Influencing the Release Rate of Biologically Active Substances. Polymers 2023, 15, 2303. [Google Scholar] [CrossRef] [PubMed]
- French, A.D. Idealized powder diffraction patterns for cellulose Polymorphs. Cellulose 2014, 21, 885–896. [Google Scholar] [CrossRef]
- Liang, X.H.; Gu, L.Z.; Ding, E.Y. Recrystallization behavior of cellulose and lignocellulose from Pinus massoniana. Wood Sci. Technol. 1993, 27, 461–467. [Google Scholar] [CrossRef]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Cryst. 1969, 2, 65–71. [Google Scholar] [CrossRef]
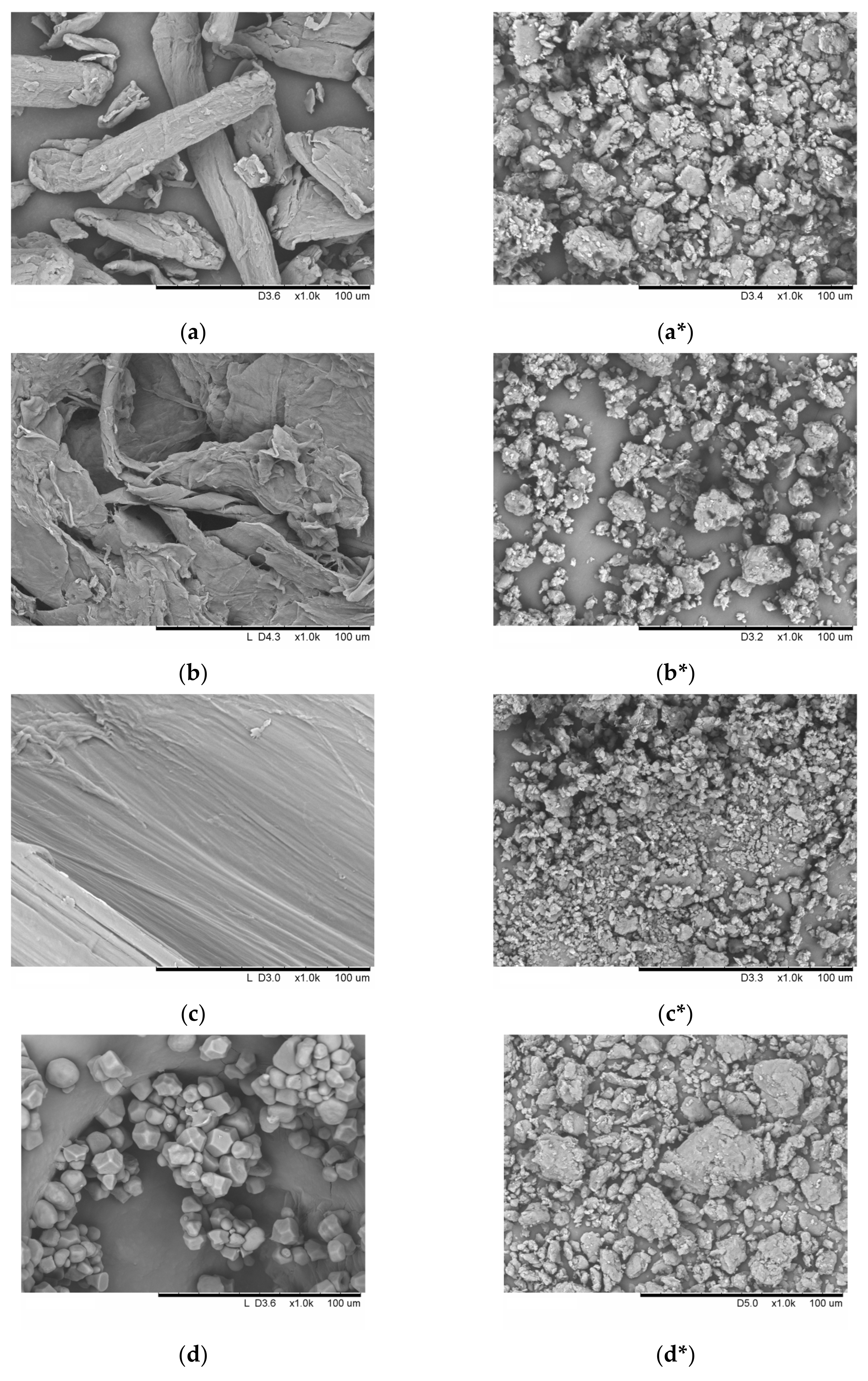




| Sample | Average Particle Size, μm | Sphericity, b/l | SSA 1, m2/g | Ref. |
|---|---|---|---|---|
| Individual cellulose | ||||
| Before amorphization | 39.0 ± 0.3 | 0.562 ± 0.001 | 1.6 ± 0.2 | present work |
| After amorphization | 29.4 ± 0.1 | 0.768 ± 0.003 | 2.1 ± 0.2 | present work |
| Individual α-chitin | ||||
| Before amorphization | 120.3 ± 0.4 | 0.606 ± 0.001 | 6.3 ± 0.3 | present work |
| After amorphization | 19.8 ± 0.1 | 0.779 ± 0.002 | 2.7 ± 0.2 | present work |
| Individual β-chitin | ||||
| Before amorphization | 52.5 ± 0.3 | 0.599 ± 0.001 | 6.6 ± 0.1 | present work |
| After amorphization | 19.3 ± 0.1 | 0.778 ± 0.002 | 3.1 ± 0.2 | present work |
| Individual starch (A-type) | ||||
| Before amorphization | 19.0 ± 0.1 | 0.798 ± 0.001 | 0.7 ± 0.1 | [55] |
| After amorphization | 23.8 ± 0.1 | 0.772 ± 0.001 | 1.6 ± 0.1 | [55] |
| Individual starch (B-type) | ||||
| Before amorphization | 28.4 ± 0.1 | 0.780 ± 0.001 | 0.5 ± 0.1 | [55] |
| After amorphization | 29.7 ± 0.1 | 0.753 ± 0.001 | 1.5 ± 0.1 | [55] |
| Individual starch (C-type) | ||||
| Before amorphization | 16.6 ± 0.1 | 0.839 ± 0.001 | 0.8 ± 0.1 | [55] |
| After amorphization | 27.5 ± 0.1 | 0.758 ± 0.001 | 1.5 ± 0.1 | [55] |
| Sample | CrI, % | |
|---|---|---|
| Individual cellulose | ||
| Before amorphization | 90 ± 2 | |
| After amorphization | <<13 | |
| Recrystallized individual cellulose | ||
| 25 °C | 95 °C | |
| Recrystallization for 1 h | 71 ± 3 | 75 ± 2 |
| Recrystallization for 4 h | 69 ± 3 | 77 ± 2 |
| Recrystallization for 8 h | 64 ± 2 | 67 ± 4 |
| Recrystallization for 12 h | 53 ± 4 | 68 ± 2 |
| Recrystallization for 24 h | 65 ± 4 | 67 ± 3 |
| Cellulose in pine sawdust | ||
| Before amorphization | 60 ± 2 | |
| After amorphization | <<13 | |
| Recrystallized cellulose in pine sawdust | ||
| 25 °C | 95 °C | |
| Recrystallization for 1 h | 39 ± 3 | 43 ± 3 |
| Recrystallization for 4 h | 38 ± 4 | 42 ± 2 |
| Recrystallization for 8 h | 37 ± 4 | 43 ± 2 |
| Recrystallization for 12 h | 41 ± 2 | 44 ± 1 |
| Recrystallization for 24 h | 46 ± 3 | 43 ± 3 |
| Sample | CrI, % | |
|---|---|---|
| Individual α-chitin | ||
| Before amorphization | 80 ± 1 | |
| After amorphization | <<16 | |
| Recrystallized individual α-chitin | ||
| 25 °C | 95 °C | |
| Recrystallization for 1 h | 65 ± 2 | 65 ± 4 |
| Recrystallization for 4 h | 64 ± 2 | 65 ± 2 |
| Recrystallization for 8 h | 67 ± 2 | 67 ± 2 |
| Recrystallization for 12 h | 65 ± 3 | 65 ± 2 |
| Recrystallization for 24 h | 65 ± 2 | 65 ± 2 |
| α-Chitin in crab shells | ||
| Before amorphization | 42 ± 4 | |
| After amorphization | <<10 | |
| Recrystallized α-chitin in crab shells | ||
| 25 °C | 95 °C | |
| Recrystallization for 1 h | 11 ± 4 | 10 ± 4 |
| Recrystallization for 4 h | 13 ± 3 | 10 ± 3 |
| Recrystallization for 8 h | 10 ± 2 | 18 ± 3 |
| Recrystallization for 12 h | 12 ± 4 | 19 ± 3 |
| Recrystallization for 24 h | 12 ± 4 | 14 ± 4 |
| Individual β-chitin | ||
| Before amorphization | 62 ± 2 | |
| After amorphization | <<12 | |
| Recrystallized individual β-chitin | ||
| 25 °C | 95 °C | |
| Recrystallization for 1 h | 47 ± 4 | 55 ± 4 |
| Recrystallization for 4 h | 36 ± 3 | 40 ± 3 |
| Recrystallization for 8 h | 45 ± 2 | 55 ± 3 |
| Recrystallization for 12 h | 52 ± 3 | 50 ± 4 |
| Recrystallization for 24 h | 55 ± 4 | 41 ± 4 |
| β-Chitin in squid pens | ||
| Before amorphization | 48 ± 2 | |
| After amorphization | <<12 | |
| Recrystallized β-chitin in squid pens | ||
| 25 °C | 95 °C | |
| Recrystallization for 1 h | 35 ± 3 | 40 ± 4 |
| Recrystallization for 4 h | 21 ± 4 | 28 ± 4 |
| Recrystallization for 8 h | 32 ± 3 | 37 ± 3 |
| Recrystallization for 12 h | 33 ± 2 | 33 ± 2 |
| Recrystallization for 24 h | 29 ± 3 | 39 ± 3 |
| Sample | CrI, % | |
|---|---|---|
| Individual starch (A-type) | ||
| Before amorphization | 34 ± 2 | |
| After amorphization | <<2 | |
| Recrystallized individual starch (A-type) | ||
| 25 °C | 95 °C | |
| Recrystallization for 1 h | 8 ± 3 | 14 ± 4 |
| Recrystallization for 4 h | 9 ± 2 | 12 ± 4 |
| Recrystallization for 8 h | 15 ± 3 | 18 ± 3 |
| Recrystallization for 12 h | 15 ± 2 | 18 ± 2 |
| Recrystallization for 24 h | 12 ± 3 | 13 ± 3 |
| A-starch in corn flour | ||
| Before amorphization | 29 ± 2 | |
| After amorphization | <<2 | |
| Recrystallized A-starch in corn flour | ||
| 25 °C | 95 °C | |
| Recrystallization for 1 h | 5 ± 3 | 8 ± 4 |
| Recrystallization for 4 h | 8 ± 3 | 9 ± 3 |
| Recrystallization for 8 h | 7 ± 2 | 9 ± 3 |
| Recrystallization for 12 h | 9 ± 3 | 8 ± 4 |
| Recrystallization for 24 h | 9 ± 4 | 9 ± 4 |
| Individual starch (B-type) | ||
| Before amorphization | 27 ± 2 | |
| After amorphization | <<2 | |
| Recrystallized individual starch (B-type) | ||
| 25 °C | 95 °C | |
| Recrystallization for 1 h | 12 ± 2 | 14 ± 2 |
| Recrystallization for 4 h | 13 ± 2 | 14 ± 2 |
| Recrystallization for 8 h | 13 ± 2 | 15 ± 3 |
| Recrystallization for 12 h | 13 ± 3 | 15 ± 2 |
| Recrystallization for 24 h | 14 ± 2 | 15 ± 3 |
| B-starch in potato flour | ||
| Before amorphization | 18 ± 4 | |
| After amorphization | <<2 | |
| Recrystallized B-starch in potato flour | ||
| 25 °C | 95 °C | |
| Recrystallization for 1 h | 10 ± 2 | 10 ± 3 |
| Recrystallization for 4 h | 11 ± 2 | 12 ± 2 |
| Recrystallization for 8 h | 8 ± 3 | 8 ± 2 |
| Recrystallization for 12 h | 10 ± 3 | 13 ± 2 |
| Recrystallization for 24 h | 10 ± 2 | 11 ± 3 |
| Individual starch (C-type) | ||
| Before amorphization | 40 ± 2 | |
| After amorphization | <<2 | |
| Recrystallized individual starch (C-type) | ||
| 25 °C | 95 °C | |
| Recrystallization for 1 h | 8 ± 2 | 6 ± 3 |
| Recrystallization for 4 h | 10 ± 2 | 8 ± 2 |
| Recrystallization for 8 h | 10 ± 2 | 9 ± 2 |
| Recrystallization for 12 h | 9 ± 3 | 10 ± 2 |
| Recrystallization for 24 h | 10 ± 2 | 13 ± 2 |
| C-starch in cassava flour | ||
| Before amorphization | 32 ± 2 | |
| After amorphization | <<2 | |
| Recrystallized C-starch in cassava flour | ||
| 25 °C | 95 °C | |
| Recrystallization for 1 h | 2 ± 1 | 4 ± 1 |
| Recrystallization for 4 h | 2 ± 1 | 6 ± 2 |
| Recrystallization for 8 h | 2 ± 1 | 9 ± 1 |
| Recrystallization for 12 h | 4 ± 2 | 7 ± 2 |
| Recrystallization for 24 h | 3 ± 1 | 8 ± 2 |
| Before Amorphization and Recrystallization | More (+) and Less (−) Stable Polymorphic Modification | After Recrystallization | |
|---|---|---|---|
| In Individual Form | In Native Form | ||
| Linear polysaccharides | |||
| Cellulose I | − | I + II | I + II |
| Cellulose II | + | II | II |
| α-Chitin | + | α-chitin | α-chitin |
| β-Chitin | − | α-chitin | α-chitin |
| Branched polysaccharides | |||
| Starch (A-type) | − | B-type * | B-type * |
| Starch (B-type) | + | B-type * | B-type * |
| Starch (C-type) | − | B-type * | B-type * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Podgorbunskikh, E.; Kuskov, T.; Bukhtoyarov, V.; Lomovsky, O.; Bychkov, A. Recrystallization of Cellulose, Chitin and Starch in Their Individual and Native Forms. Polymers 2024, 16, 980. https://doi.org/10.3390/polym16070980
Podgorbunskikh E, Kuskov T, Bukhtoyarov V, Lomovsky O, Bychkov A. Recrystallization of Cellulose, Chitin and Starch in Their Individual and Native Forms. Polymers. 2024; 16(7):980. https://doi.org/10.3390/polym16070980
Chicago/Turabian StylePodgorbunskikh, Ekaterina, Timofei Kuskov, Vladimir Bukhtoyarov, Oleg Lomovsky, and Aleksey Bychkov. 2024. "Recrystallization of Cellulose, Chitin and Starch in Their Individual and Native Forms" Polymers 16, no. 7: 980. https://doi.org/10.3390/polym16070980
APA StylePodgorbunskikh, E., Kuskov, T., Bukhtoyarov, V., Lomovsky, O., & Bychkov, A. (2024). Recrystallization of Cellulose, Chitin and Starch in Their Individual and Native Forms. Polymers, 16(7), 980. https://doi.org/10.3390/polym16070980







