The Removal of Formaldehyde from Urea Formaldehyde Adhesive by Sodium Borohydride Treatment and Its Application in Plywood
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Equipment
2.3. Determination of Free Formaldehyde in UF Resin
2.4. Experimental Methods
2.4.1. Synthesis of UF Adhesive
2.4.2. Modification of UF Adhesive with Sodium Borohydride
2.4.3. Viscosity Test
2.4.4. Solid Content Test
2.4.5. Determination of Free Formaldehyde in UF Adhesive
2.5. Plywood
2.5.1. Preparation of Plywood
2.5.2. Performance Tests of Plywood
2.6. Performance Tests of UF Resins
2.6.1. FTIR Characterization of UF Resins
2.6.2. TG Analysis of UF Resins
2.6.3. DSC Analysis of UF Resins
2.7. Statistical Analysis
3. Results
3.1. Effects of Sodium Borohydride Addition on the Properties of UF Resin Adhesive
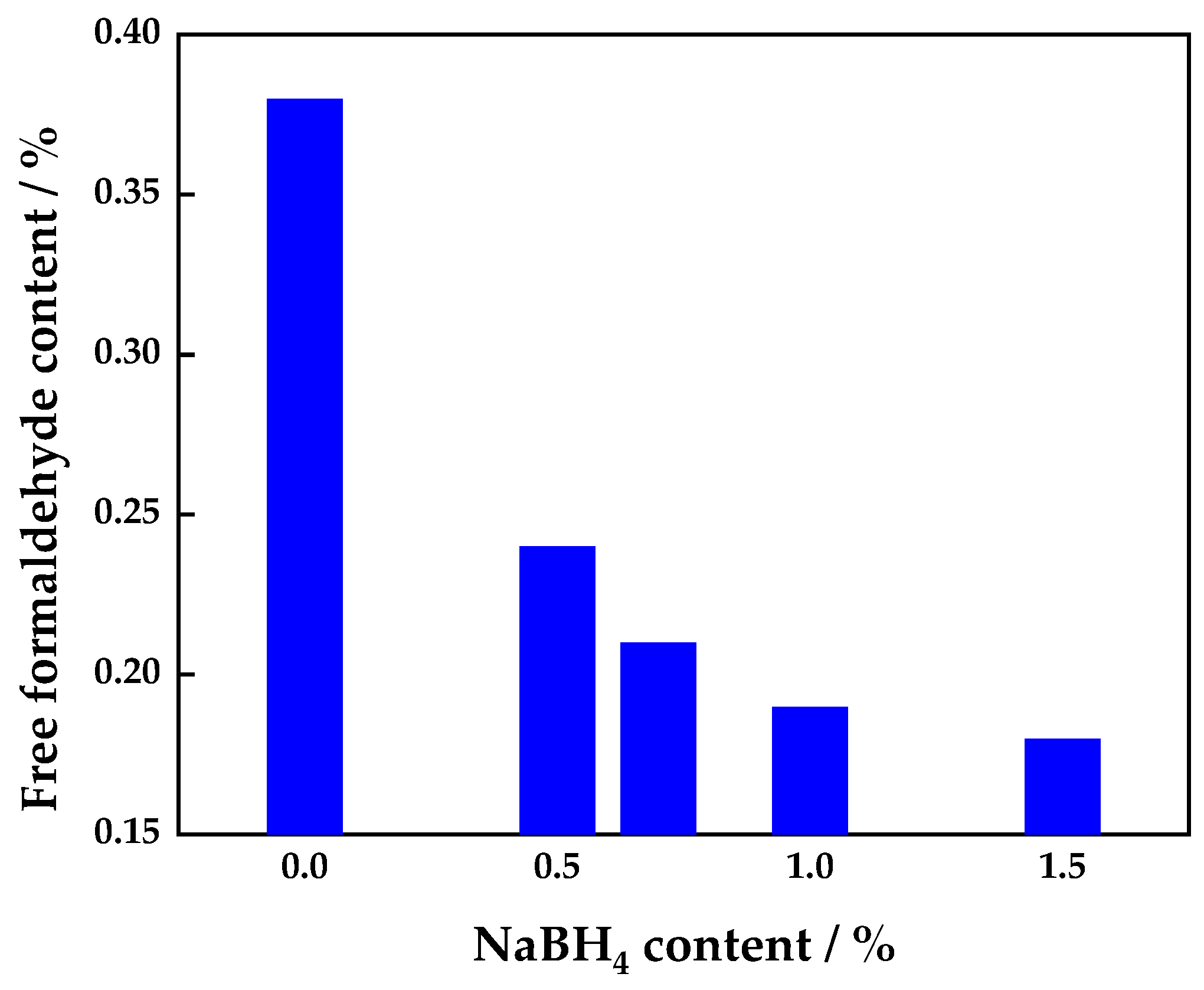
3.1.1. Effect of Addition of Sodium Borohydride on Free Formaldehyde Content of UF Adhesive
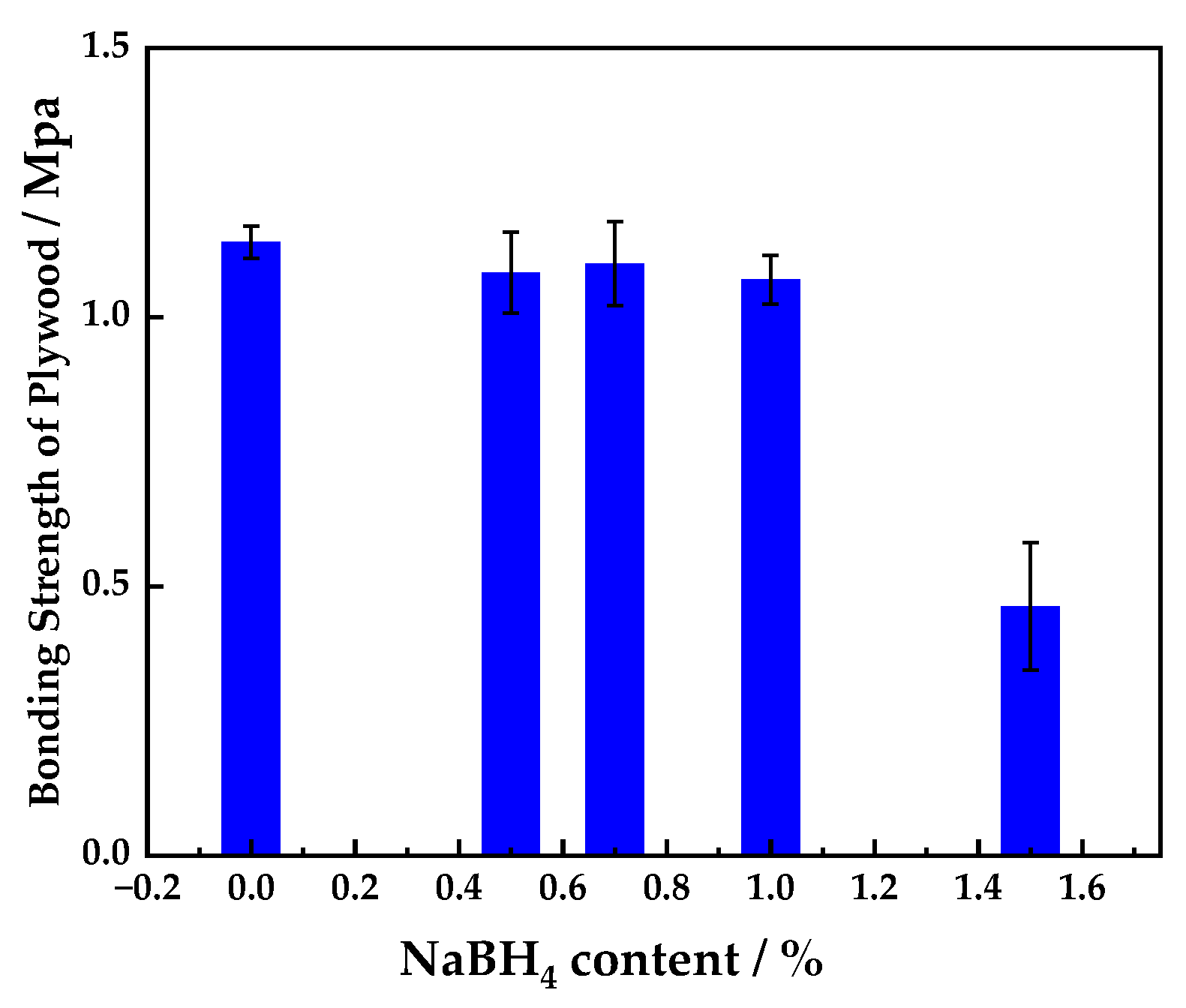
3.1.2. Effect of Sodium Borohydride Content on the Bonding Strength of Plywood
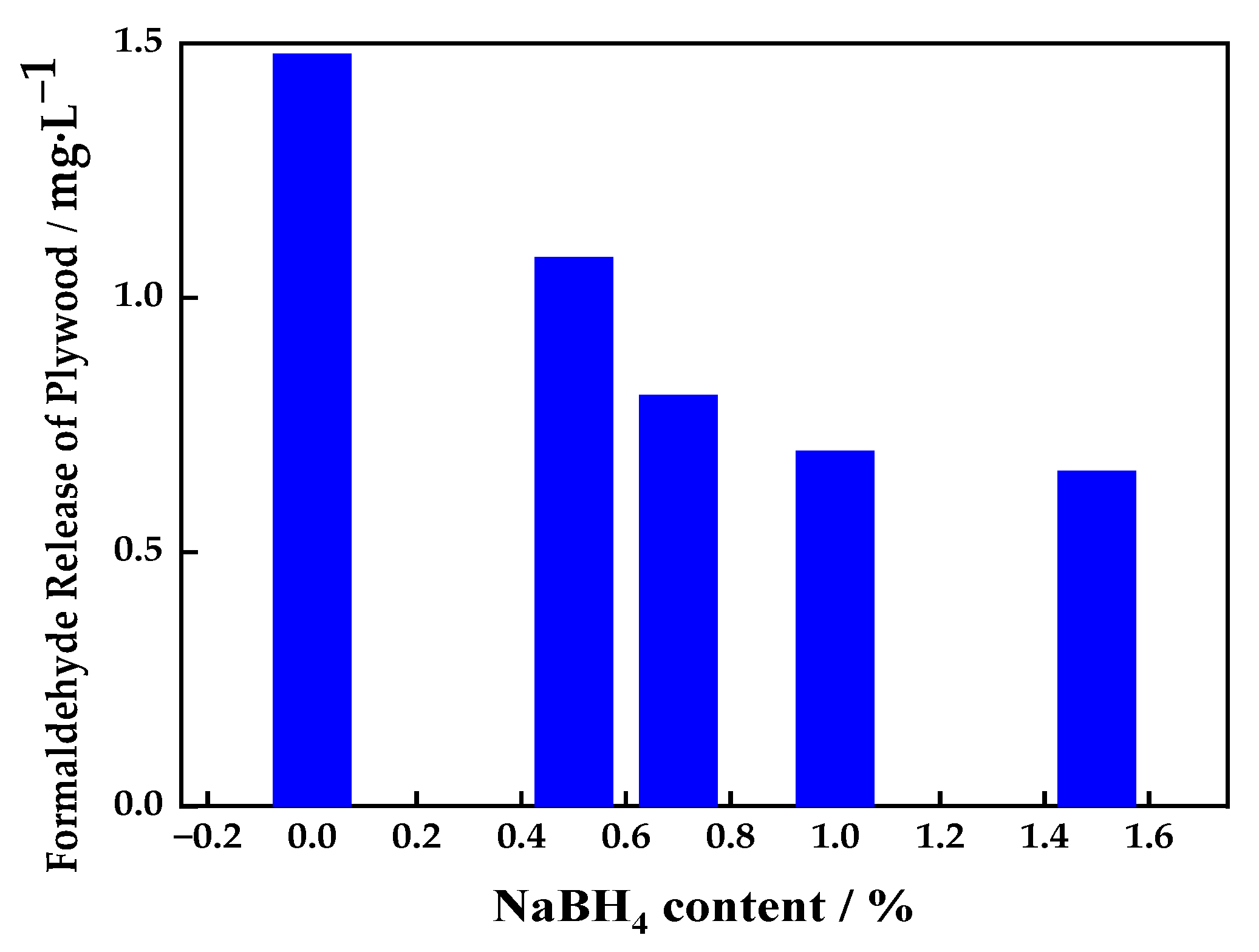
3.1.3. Effect of Sodium Borohydride Content on Release of Formaldehyde from Plywood
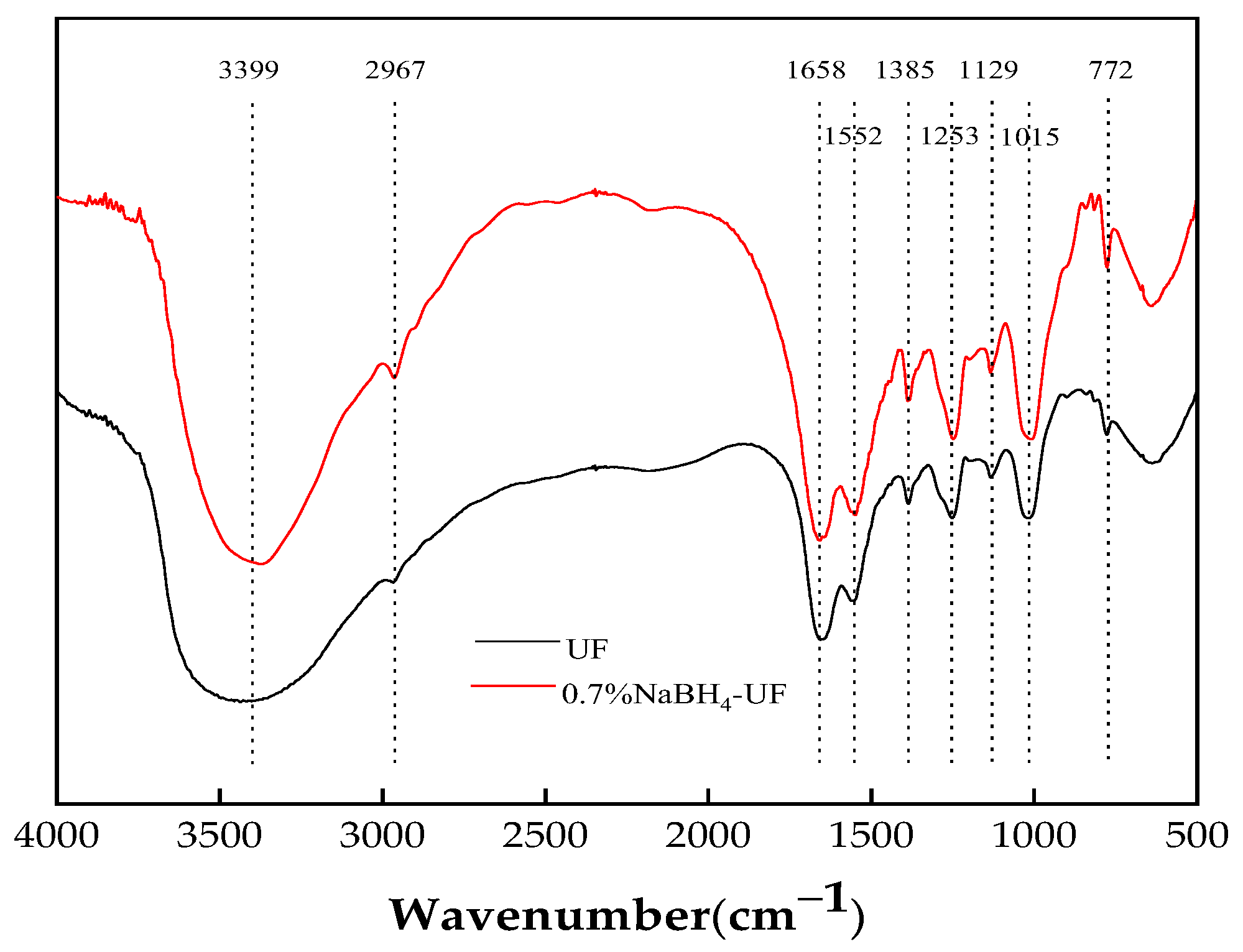
3.2. FTIR Characterization of UF Resin
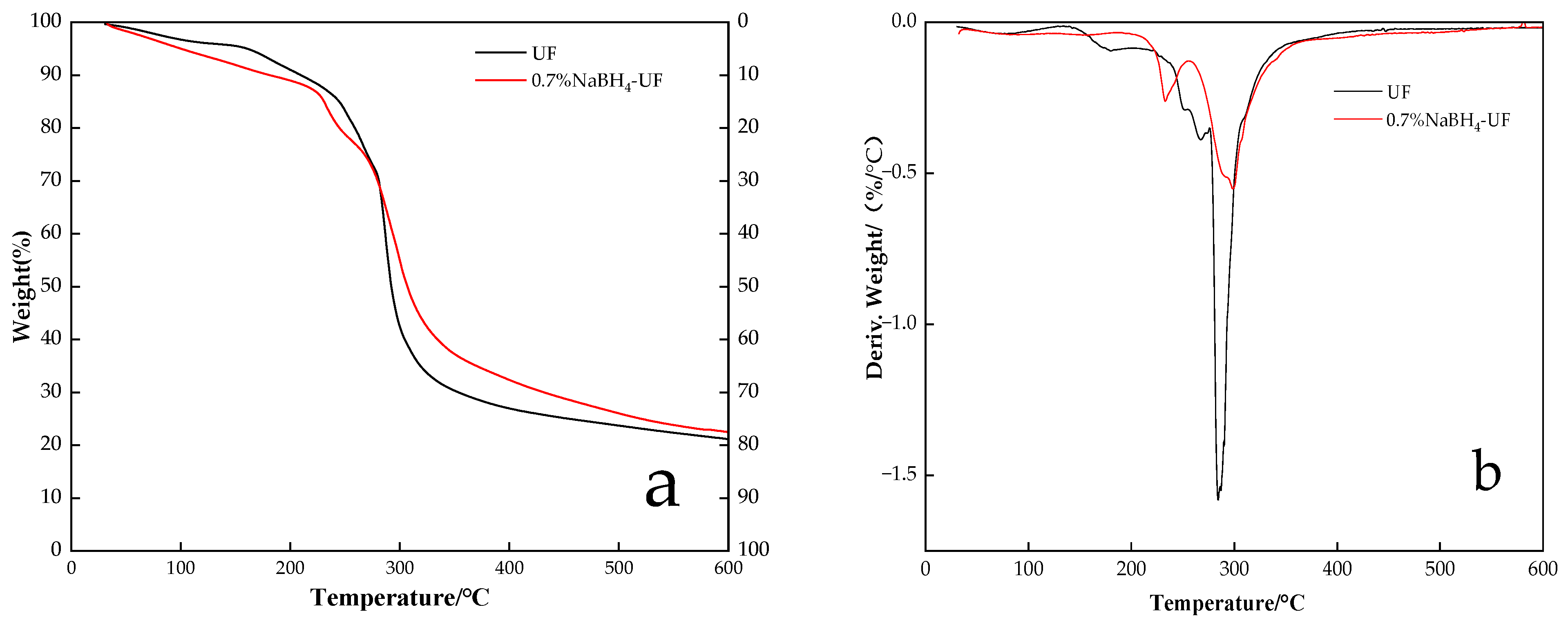
3.3. Thermogravimetric Analysis of UF Resin
- The first stage, in the range of 30–200 °C, was the evaporation of residual water and the release of formaldehyde from the UF resin, as well as the removal of water and formaldehyde produced by the condensation reaction of methylol and amine groups [33]. It can be seen from Figure 6a that the mass loss in the range of 30–200 °C from the control UF adhesive (9.2%) was lower than that from the 0.7% NaBH4-UF adhesive (11.1%). This may indicate that there was more water lost from hydrated boric acid during thermal decomposition at 30–200 °C (boric acid produced by reaction of sodium borohydride with formaldehyde [22]) in the 0.7% NaBH4-UF adhesive.
- The second stage, in the range of 200–350 °C, involved mass loss due to the breaking and decomposition of dimethyl ether and dimethyl bonds in the resin [34]. From the DTG traces, it can be seen that decomposition of the resin was extensive in this stage. The temperatures of maximum pyrolysis of the pure UF resin and 0.7% NaBH4-UF resin were determined to be 294 °C and 299 °C, respectively. Above 280 °C, the mass loss from the 0.7% NaBH4-UF resin was less than that from the pure UF resin.
- The third mass loss stage, in the range of 350–600 °C, involved the oxidative elimination of nitrogen, oxygen, hydrogen, and other elements, such that the resin was carbonized [29]. The amount of pyrolysis residue from the pure UF (21%) was lower than that from the 0.7% NaBH4-UF (23%) after the third stage at 600 °C. This may be due to the boron oxide formed by thermal decomposition of the boric acid that was produced by the reaction of sodium borohydride with formaldehyde. Boron oxide compounds can increase the amount of thermal decomposition residue from a resin in two ways: (1) they are inorganic compounds with low thermal decomposition and volatilization losses below 600 °C so the presence of these boron oxide compounds increases the amount of residue; (2) the presence of inorganic oxide particles creates a shielding effect. Their presence does not necessarily represent an increase in thermal stability.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Park, S.; Jeong, B.; Park, B.D. A comparison of adhesion behavior of urea-formaldehyde resins with melamine-urea-formaldehyde resins in bonding wood. Forests 2021, 12, 1037. [Google Scholar] [CrossRef]
- Cheng, T.; Hou, H.; Xu, G.; Hou, X.; Yang, R.; Zhang, L.; Wang, Q. Transformation of urea–formaldehyde resin waste into Pb(II) and Cu(II) adsorbent toward a circular materials economy approach. J. Polym. Environ. 2023, 31, 2612–2623. [Google Scholar] [CrossRef]
- Nuryawan, A.; Singh, A.P.; Park, B.; Causin, V. Micro-morphological features of cured urea-formaldehyde adhesives detected by transmission electron microscopy. J. Adhes. 2016, 92, 121–134. [Google Scholar] [CrossRef]
- Moslemi, A.; Koohi, M.Z.; Behzad, T.; Pizzi, A. Addition of cellulose nanofibers extracted from rice straw to urea formaldehyde resin; effect on the adhesive characteristics and medium density fiberboard properties. Int. J. Adhes. Adhes. 2020, 99, 102582. [Google Scholar] [CrossRef]
- Park, S.; Park, B.D. Sustainable bio-based dialdehyde cellulose for transforming crystalline urea–formaldehyde resins into amorphous ones to improve their performance. J. Ind. Eng. Chem. 2022, 113, 142–152. [Google Scholar] [CrossRef]
- Wibowo, E.S.; Lubis, M.A.R.; Park, B.D. In-situ modification of low molar ratio urea-formaldehyde resins with cellulose nanofibrils for plywood. J. Adhes. Sci. Technol. 2021, 35, 2452–2465. [Google Scholar] [CrossRef]
- Perminova, D.A.; Malkov, V.S.; Guschin, V.; Eisenreich, N. Influence of glyoxal on curing of urea-formaldehyde resins. Int. J. Adhes. Adhes. 2019, 92, 1–6. [Google Scholar] [CrossRef]
- Kristak, L.; Antov, P.; Bekhta, P.; Lubos, M.A.R.; Iswanto, A.H.; Reh, R.; Sedliacik, J.; Savov, V.; Taghiyari, H.R.; Papadopoulos, A.N.; et al. Recent progress in ultra-low formaldehyde emitting adhesive systems and formaldehyde scavengers in wood-based panels: A review. Wood Mater. Sci. Eng. 2022, 18, 763–782. [Google Scholar] [CrossRef]
- Zhao, H.; Li, X.; Wang, X.; Meng, M.; Wang, X.J.; Huang, S.; Gan, W. Effect of copper (II) sulfate on the properties of urea formaldehyde adhesive. Polymers 2022, 14, 94. [Google Scholar] [CrossRef]
- Basboga, I.H. Particleboard manufacturing with low formaldehyde content for indoor applications. Wood Mater. Sci. Eng. 2022, 18, 1134–1140. [Google Scholar] [CrossRef]
- Que, Z.; Furuno, T.; Katoh, S.; Nishino, Y. Effects of urea–formaldehyde resin mole ratio on the properties of particleboard. Build. Sci. 2007, 42, 1257–1263. [Google Scholar] [CrossRef]
- Kawalerczyk, J.; Siuda, J.; Mirski, R.; Ziurka, D. Hemp flour as a formaldehyde scavenger for melamine-urea-formaldehyde adhesive in plywood production. Bioresources 2020, 15, 4052–4064. [Google Scholar] [CrossRef]
- Chen, K.X.; Cheng, X.; Chen, Y.Z.; Qi, J.Q.; Xie, J.Q.; Huang, X.Y.; Jiang, Y.Z.; Xiao, H. Thermal degradation kinetics of urea–formaldehyde resins modified by almond shells. Am. Chem. J. 2021, 6, 25702–25709. [Google Scholar] [CrossRef]
- Cademartori, P.; Artner, M.; Freitas, R.; Magalhães, W. Alumina nanoparticles as formaldehyde scavenger for urea-formaldehyde resin: Rheological and in-situ cure performance. Compos. Part. B-Eng. 2019, 176, 17281. [Google Scholar] [CrossRef]
- Shao, H.X.; Tang, Q.G.; Liang, J.S.; Ding, Y. Effect of mineral composite fillers on properties of man-made plywood. Adv. Mat. Res. 2010, 178, 97–102. [Google Scholar] [CrossRef]
- Boran, S.; Usta, M.; Ondaral, S.; Gümüşkaya, E. The efficiency of tannin as a formaldehyde scavenger chemical in medium density fiberboard. Compos. Eng. 2012, 43, 2487–2491. [Google Scholar] [CrossRef]
- Ghani, A.; Ashaari, Z.; Bawon, P.; Lee, S.H. Reducing formaldehyde release of urea formaldehyde-bonded particleboard by addition of amines as formaldehyde scavenger. Build. Enviorn. 2018, 142, 188–194. [Google Scholar] [CrossRef]
- GB/T 14074-2017; Testing Methods for Wood Adhesives and Their Resins. China Standard Press: Beijing, China, 2017.
- GB/T 9846-2015; Ordinary Plywood. China Standard Press: Beijing, China, 2015.
- GB/T 17657-2013; Test Method for Physical and Chemical Properties of Wood-Based Panels and Facing Wood-Based Panels. China Standard Press: Beijing, China, 2013.
- GB/T 14732-2017; Wood Adhesives: Urea-Formaldehyde, Phenol-Formaldehyde and Melamine-Formaldehyde Resins. China Standard Press: Beijing, China, 2017.
- Chaikin, S.; Brown, W.G. Reduction of aldehydes, ketones and acid chlorides by sodium borohydride. J. Am. Chem. Soc. 1949, 71, 122–125. [Google Scholar] [CrossRef]
- Boran, S.; Usta, M.; Gümüşkaya, E. Decreasing formaldehyde release from medium density fiberboard panels produced by adding different amine compounds to urea formaldehyde resin. Int. J. Adhes. Adhes. 2011, 31, 674–678. [Google Scholar] [CrossRef]
- GB 18580-2001; Indoor Decorating and Refurbishing Materials Limit of Formal Dehyde Release of Wood Based Panels and Finishing Products. China Standard Press: Beijing, China, 2001.
- Ong, H.; Khan, M.; Prasad, D.; Yousuf, A.; Chowdhury, M. Palm kernel meal as a melamine urea formaldehyde adhesive filler for plywood applications. Int. J. Adhes. Adhes. 2018, 85, 8–14. [Google Scholar] [CrossRef]
- Li, C.; Zhang, J.; Yi, Z.; Yang, H.; Zhao, B.; Zhang, W.; Li, J. Preparation and characterization of a novel environmentally friendly phenol–formaldehyde adhesive modified with tannin and urea. Int. J. Adhes. Adhes. 2016, 66, 26–32. [Google Scholar] [CrossRef]
- Liu, M.; Wang, Y.; Wu, Y.; He, Z.; Wan, H. “Greener” adhesives composed of urea-formaldehyde resin and cottonseed meal for wood-based composites. J. Clean. Prod. 2018, 187, 361–371. [Google Scholar] [CrossRef]
- Song, J.; Chen, S.; Yi, X.; Zhao, X.; Zhang, J.; Liu, X.; Liu, B. Preparation and properties of the urea-formaldehyde res-in/reactive halloysite nanocomposites adhesive with low-formaldehyde release and good water resistance. Polymers 2021, 13, 2224. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, J.; Luo, J.; Li, J.; Gao, Q. Effect of melamine allocation proportion on chemical structures and properties of melamine-urea-formaldehyde resins. Bioresources 2015, 10, 3265–3276. [Google Scholar] [CrossRef]
- Liu, K.; Su, C.; Ma, W.; Li, H.; Zeng, Z.; Li, L. Free formaldehyde reduction in urea-formaldehyde resin adhesive: Modifier addition effect and physicochemical property characterization. Bioresources 2020, 15, 2339–2355. [Google Scholar] [CrossRef]
- Wang, H.; Wang, F.; Du, G. Enhanced performance of urea–glyoxal polymer with oxidized cassava starch as wood adhesive. Iran. Polym. J. 2019, 28, 1015–1021. [Google Scholar] [CrossRef]
- Huang, Z.; Yu, X.; Li, W.; Liu, S. Preparation of urea-formaldehyde paraffin microcapsules modified by carboxymethyl cellulose as a potential phase change material. J. For. Res. 2015, 26, 253–260. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, M.; Zheng, Y.; Tong, D.; Zhou, C.; Yu, W. Effect of a novel environmentally friendly additive of polyaspartic acid on the properties of urea formaldehyde resins/montmorillonite. J. Appl. Polym. Sci. 2019, 136, 48038–48044. [Google Scholar] [CrossRef]
- Roumeli, E.; Papadopoulou, E.; Pavlidou, E.; Vourlias, G.; Bikiaris, D.; Paraskevopoulos, K.M.; Chrissafis, K. Synthesis, characterization and thermal analysis of urea-formaldehyde/nanoSiO2 resins. Thermochim. Acta 2012, 527, 33–39. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Zhao, H.; Zhang, B.; Wen, X.; Huang, S.; Gan, W. The Removal of Formaldehyde from Urea Formaldehyde Adhesive by Sodium Borohydride Treatment and Its Application in Plywood. Polymers 2024, 16, 969. https://doi.org/10.3390/polym16070969
Wang X, Zhao H, Zhang B, Wen X, Huang S, Gan W. The Removal of Formaldehyde from Urea Formaldehyde Adhesive by Sodium Borohydride Treatment and Its Application in Plywood. Polymers. 2024; 16(7):969. https://doi.org/10.3390/polym16070969
Chicago/Turabian StyleWang, Xi, Hui Zhao, Bo Zhang, Xiuchan Wen, Siyu Huang, and Weixing Gan. 2024. "The Removal of Formaldehyde from Urea Formaldehyde Adhesive by Sodium Borohydride Treatment and Its Application in Plywood" Polymers 16, no. 7: 969. https://doi.org/10.3390/polym16070969
APA StyleWang, X., Zhao, H., Zhang, B., Wen, X., Huang, S., & Gan, W. (2024). The Removal of Formaldehyde from Urea Formaldehyde Adhesive by Sodium Borohydride Treatment and Its Application in Plywood. Polymers, 16(7), 969. https://doi.org/10.3390/polym16070969





