Synthesis of Soluble High Molar Mass Poly(Phenylene Methylene)-Based Polymers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Poly(Phenylene Methylene) Catalyzed by Complexes Based on W and Mo
2.3. Synthesis of PPM with Durene Units (PPM-D)
2.4. Fractionation
2.5. Characterization
3. Results and Discussions
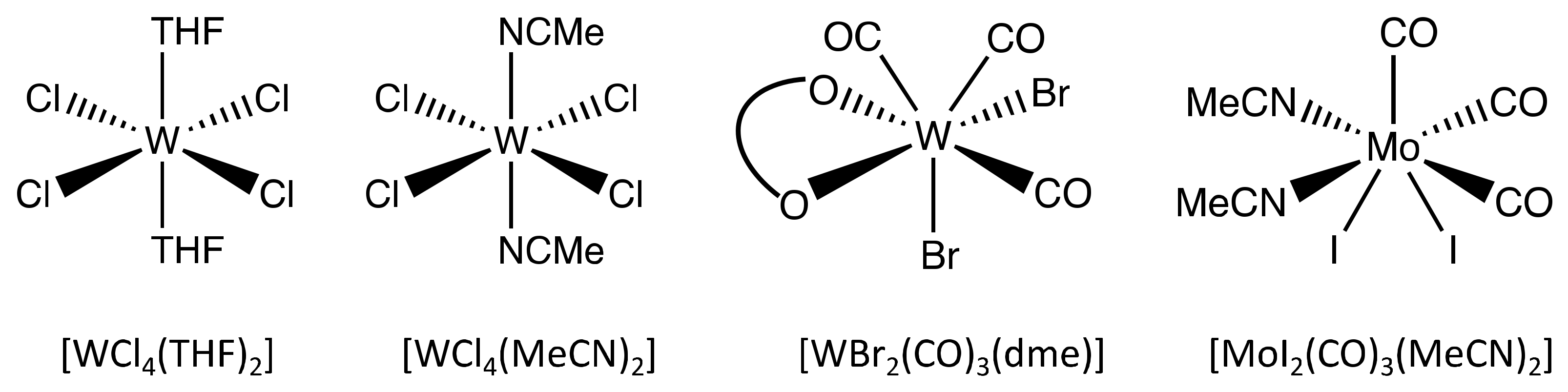
3.1. Homopolymerization of Benzyl Chloride with Different Catalysts
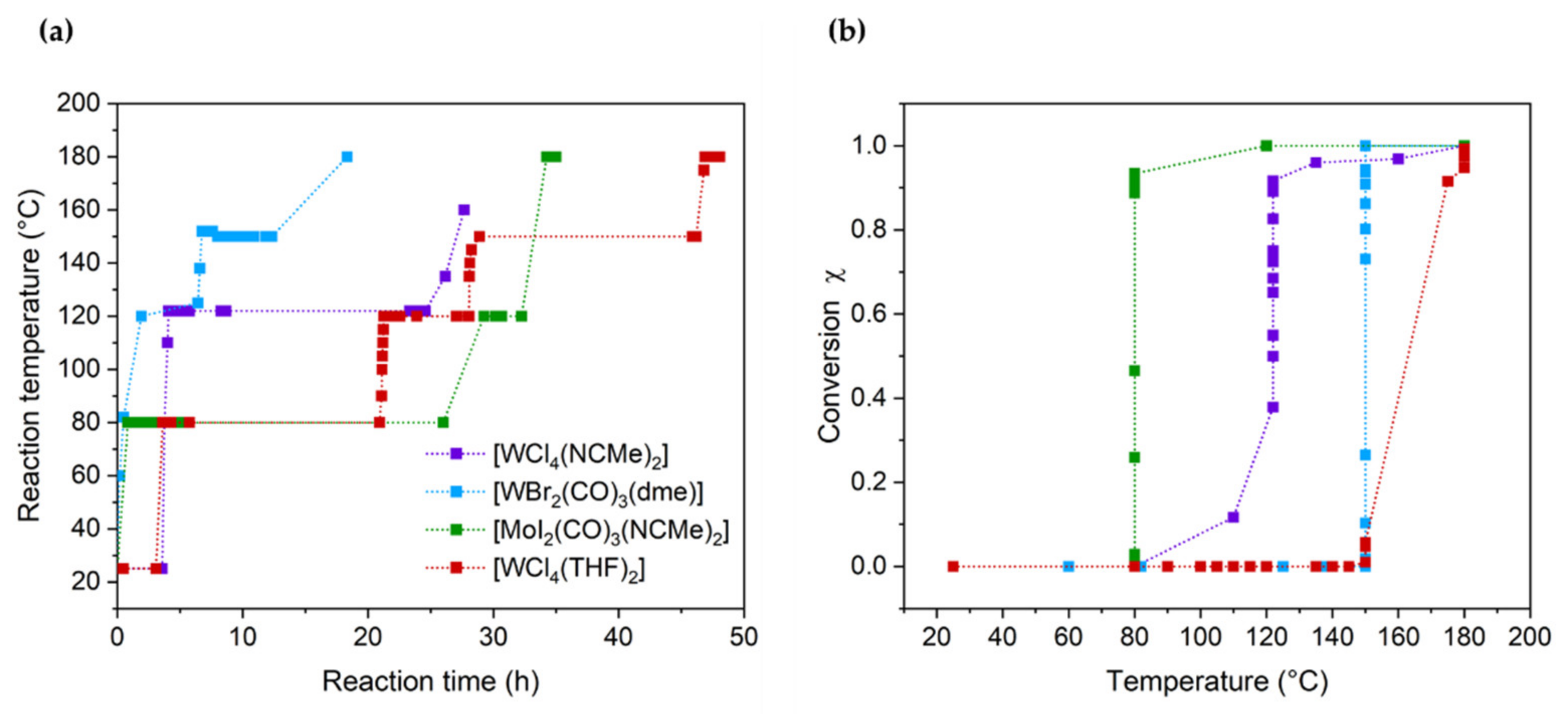
| Catalyst | Mn (g mol−1) | Mw (g mol−1) | PDI | Yield of Reaction |
|---|---|---|---|---|
| [WCl4(MeCN)2] | 3118 | 7013 | 2.2 | 68% |
| [WBr2(CO)3(dme)] | 3325 | 13,170 | 3.9 | 72% |
| [MoI2(CO)3(MeCN)2] | 4538 | 11,480 | 2.5 | 77% |
| [WCl4(THF)2] | 4090 | 63,760 | 15.6 | 69% |
3.2. Copolymerization of Benzyl Chloride with BCMD
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Has, H.C.; Livingston, D.I.; Saunders, M. Polybenzyl type polymers. J. Polym. Sci. 1955, 15, 503–514. [Google Scholar] [CrossRef]
- Parker, D.B. The structure of polybenzyls and related polymers. Eur. Polym. J. 1969, 5, 93–104. [Google Scholar] [CrossRef]
- Valentine, L.; Winter, R.W. Valentine and Winter: The Polycondensation of 931. The Polycondensation of Benzyl Chloride Catalysed by Stannic Chloride. J. Chem. Soc. Resumed 1956, 4768–4779. [Google Scholar] [CrossRef]
- Ellis, B.; White, P.G. Thermal degradation of polybenzyl. J. Polym. Sci. Polym. Chem. Ed. 1973, 11, 801–821. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Jarvis, K.A.; Ferreira, P.J.; Bielawski, C.W. Graphite Oxide as a Dehydrative Polymerization Catalyst: A One-Step Synthesis of Carbon-Reinforced Poly(phenylene methylene) Composites. Macromolecules 2011, 44, 7659–7667. [Google Scholar] [CrossRef]
- Hino, M.; Arata, K. Iron oxide as an effective catalyst for the polycondensation of benzyl chloride, the formation of para-substituted polybenzyl. Chem. Lett. 1979, 8, 1141–1144. [Google Scholar] [CrossRef]
- Teng, D.-G.; Wei, X.-Y.; Yang, Z.; Zong, Z.-M. α-Fe2 O3 /Attapulgite-mediated reaction of benzyl chloride: Synthesis of poly(phenylene methylene). J. Polym. Sci. Part A Polym. Chem. 2018, 56, 2280–2285. [Google Scholar] [CrossRef]
- Perevedentsev, A.; Francisco-López, A.; Shi, X.; Braendle, A.; Caseri, W.R.; Goñi, A.R.; Campoy-Quiles, M. Homoconjugation in Light-Emitting Poly(phenylene methylene)s: Origin and Pressure-Enhanced Photoluminescence. Macromolecules 2020, 53, 7519–7527. [Google Scholar] [CrossRef]
- Braendle, A.; Perevedentsev, A.; Cheetham, N.J.; Stavrinou, P.N.; Schachner, J.A.; Mösch-Zanetti, N.C.; Niederberger, M.; Caseri, W.R. Homoconjugation in poly(phenylene methylene)s: A case study of non-π-conjugated polymers with unexpected fluorescent properties. J. Polym. Sci. Part B Polym. Phys. 2017, 55, 707–720. [Google Scholar] [CrossRef]
- Tsonis, C.P. Homogeneous catalytic polymerization of benzyl chloride leading to linear high molecular weight polymers: An elusive goal. J. Mol. Catal. 1990, 57, 313–323. [Google Scholar] [CrossRef]
- D’elia, M.F.; Braendle, A.; Schweizer, T.B.; Ortenzi, M.A.; Trasatti, S.P.M.; Niederberger, M.; Caseri, W. Poly(Phenylene Methylene): A Multifunctional Material for Thermally Stable, Hydrophobic, Fluorescent, Corrosion-Protective Coatings. Coatings 2018, 8, 274. [Google Scholar] [CrossRef]
- Cannizzaro, S. Ueber den der Benzoësäure entsprechenden Alkohol. Eur. J. Org. Chem. 1853, 88, 129–130. [Google Scholar] [CrossRef]
- M’Hiri, T.; Catusse, C.; Catusse, R.; Janier Dubry, J.L. Polymerization of benzyl alcohol and its derivative compounds with an ion exchange resin. React. Kinet. Catal. Lett. 1983, 22, 425–428. [Google Scholar] [CrossRef]
- Parker, D.B.V.; Davies, W.G.; South, K.D. The polycondensation of benzyl chloride in the presence of Lewis acids. J. Chem. Soc. B Phys. Org. 1967, 471–477. [Google Scholar] [CrossRef]
- Kuo, J.; Lenz, R.W. Linear polybenzyls. II. Lack of structure control in benzyl chloride polymerization. J. Polym. Sci. Polym. Chem. Ed. 1976, 14, 2749–2761. [Google Scholar] [CrossRef]
- Jacobson, R.A. Polymers from benzyl chloride and related compounds. J. Am. Chem. Soc. 1932, 54, 1513–1518. [Google Scholar] [CrossRef]
- Montaudo, G.; Finocchiaro, P.; Caccamese, S.; Bottino, F. Polycondensation of benzyl chloride and its derivatives: A study of the reaction at different temperatures. J. Polym. Sci. Part A-1 Polym. Chem. 1970, 8, 2475–2490. [Google Scholar] [CrossRef]
- Banks, R.; François, P.-Y.; Preston, P.N. Polymerization of benzyl alcohol in anhydrous hydrogen fluoride: An efficient synthesis of poly(phenylenemethylene). Polymer 1992, 33, 3974–3975. [Google Scholar] [CrossRef]
- Perkins, W.G.; Capiati, N.J.; Porter, R.S. The effect of molecular weight on the physical and mechanical properties of ultra-drawn high density polyethylene. Polym. Eng. Sci. 1976, 16, 200–203. [Google Scholar] [CrossRef]
- Hubert, L.; David, L.; Séguéla, R.; Vigier, G.; Corfias-Zuccalli, C.; Germain, Y. Physical and mechanical properties of polyethylene for pipes in relation to molecular architecture. II. Short-term creep of isotropic and drawn materials. J. Appl. Polym. Sci. 2002, 84, 2308–2317. [Google Scholar] [CrossRef]
- Braendle, A.; Schwendimann, P.; Niederberger, M.; Caseri, W.R. Synthesis and fractionation of poly(phenylene methylene). J. Polym. Sci. Part A Polym. Chem. 2017, 56, 309–318. [Google Scholar] [CrossRef]
- Tsonis, C.P.; Hwang, J.S. Catalytic activity and selectivity of group vib metal carbonyl complexes in dehydrohalogenation reactions. J. Mol. Catal. 1984, 26, 219–229. [Google Scholar] [CrossRef]
- Dermer, O.C.; Hooper, E. Catalysts for the Polymerization of Benzyl Chloride. J. Am. Chem. Soc. 1941, 63, 3525–3526. [Google Scholar] [CrossRef]
- Braendle, A.; Vidovič, C.; Mösch-Zanetti, N.C.; Niederberger, M.; Caseri, W. Synthesis of High Molar Mass Poly(phenylene methylene) Catalyzed by Tungsten(II) Compounds. Polymers 2018, 10, 881. [Google Scholar] [CrossRef] [PubMed]
- Martín, C.F.; Stöckel, E.; Clowes, R.; Adams, D.J.; Cooper, A.I.; Pis, J.J.; Rubiera, F.; Pevida, C. Hypercrosslinked organic polymer networks as potential adsorbents for pre-combustion CO2 capture. J. Mater. Chem. 2011, 21, 5475–5483. [Google Scholar] [CrossRef]
- Wood, C.D.; Tan, B.; Trewin, A.; Niu, H.; Bradshaw, D.; Rosseinsky, M.J.; Khimyak, Y.Z.; Campbell, N.L.; Kirk, R.; Stöckel, E.; et al. Hydrogen Storage in Microporous Hypercrosslinked Organic Polymer Networks. Chem. Mater. 2007, 19, 2034–2048. [Google Scholar] [CrossRef]
- James, A.M.; Reynolds, J.; Reed, D.G.; Styring, P.; Dawson, R. A Pressure Swing Approach to Selective CO2 Sequestration Using Functionalized Hypercrosslinked Polymers. Materials 2021, 14, 1605. [Google Scholar] [CrossRef] [PubMed]
- D’elia, M.F.; Magni, M.; Romanò, T.; Trasatti, S.P.M.; Niederberger, M.; Caseri, W.R. Smart Anticorrosion Coatings Based on Poly(phenylene methylene): An Assessment of the Intrinsic Self-Healing Behavior of the Copolymer. Polymers 2022, 14, 3457. [Google Scholar] [CrossRef] [PubMed]
- Peschel, L.M.; Schachner, J.A.; Sala, C.H.; Belaj, F.; Mösch-Zanetti, N.C. An Update on WII and MoII Carbonyl Precursors and Their Application in the Synthesis of Potentially Bio-Inspired Thiophenolate-Oxazoline Complexes. Z. Anorg. Allg. Chem. 2013, 639, 1559–1567. [Google Scholar] [CrossRef]
- Belaj, F.; Vidovic, C.; Mösch-Zanetti, N. Structural Mimics of Acetylene Hydratase: Tungsten Complexes Capable of Intramolecular Nucleophilic Attack on Acetylene. CSD Commun. 2021, 25, 14267–14272. [Google Scholar] [CrossRef]
- Baker, P.K.; Fraser, S.G.; Keys, E.M. The synthesis and spectral properties of some highly reactive new seven-coordinate molybdenum(II) and tungsten(II) bisacetonitrile dihalogenotricarbonyl complexes. J. Organomet. Chem. 1986, 309, 319–321. [Google Scholar] [CrossRef]
- Kolesnichenko, V.; Swenson, D.C.; Messerle, L. Facile Reduction of Tungsten Halides with Nonconventional, Mild Reductants. I. Tungsten Tetrachloride: Several Convenient Solid-State Syntheses, a Solution Synthesis of Highly Reactive (WCl4)x, and the Molecular Structure of Polymeric Tungsten Tetrachloride. Inorg. Chem. 1998, 37, 3257–3262. [Google Scholar]
- Persson, C.; Andersson, C. Reduction of tungsten(VI) and molybdenum(V) by allyltrimethylsilane and cyclopentene. Simple high yield syntheses of MoCl4(OEt2)2, MoCl4(dme), WCl4(thf)2, WCl4(dme) and WOCl3(thf)2. Inorg. Chim. Acta 1993, 203, 235–238. [Google Scholar] [CrossRef]

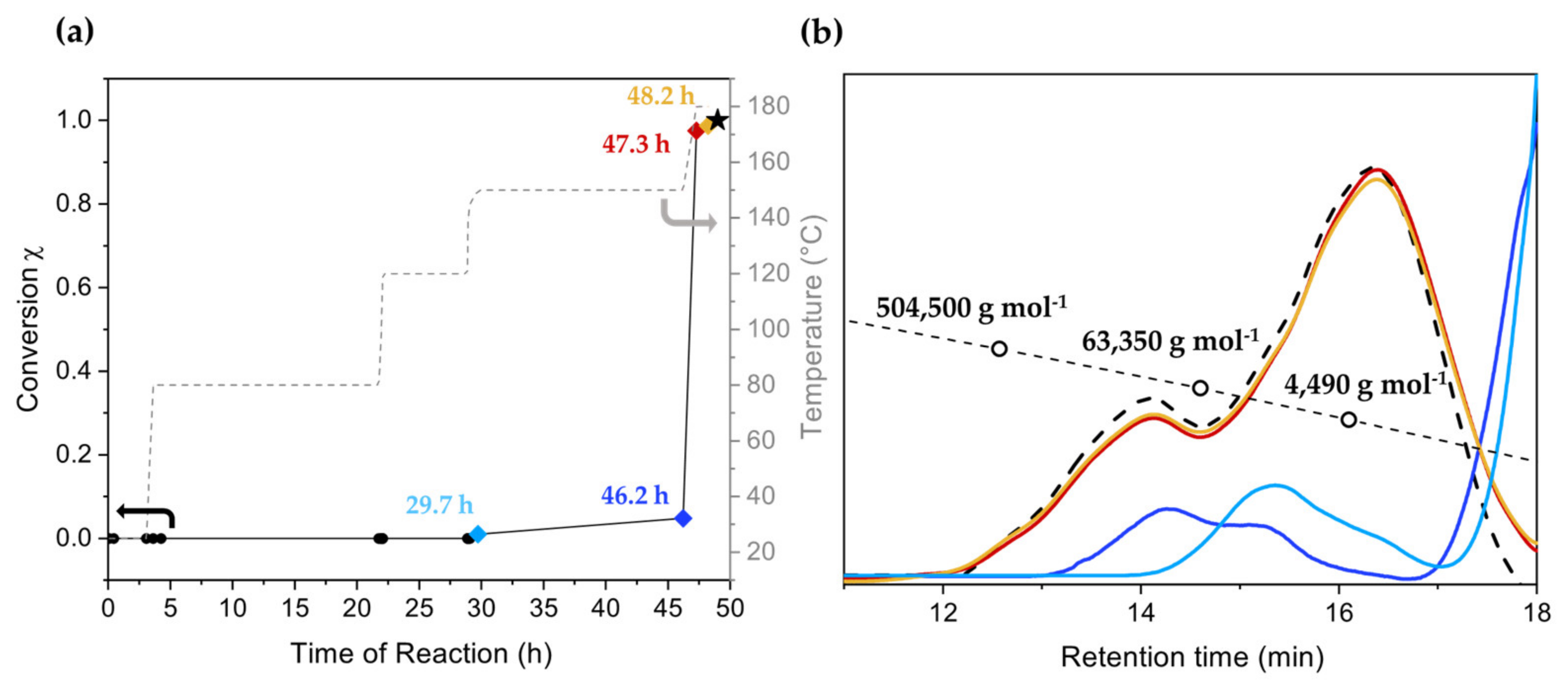
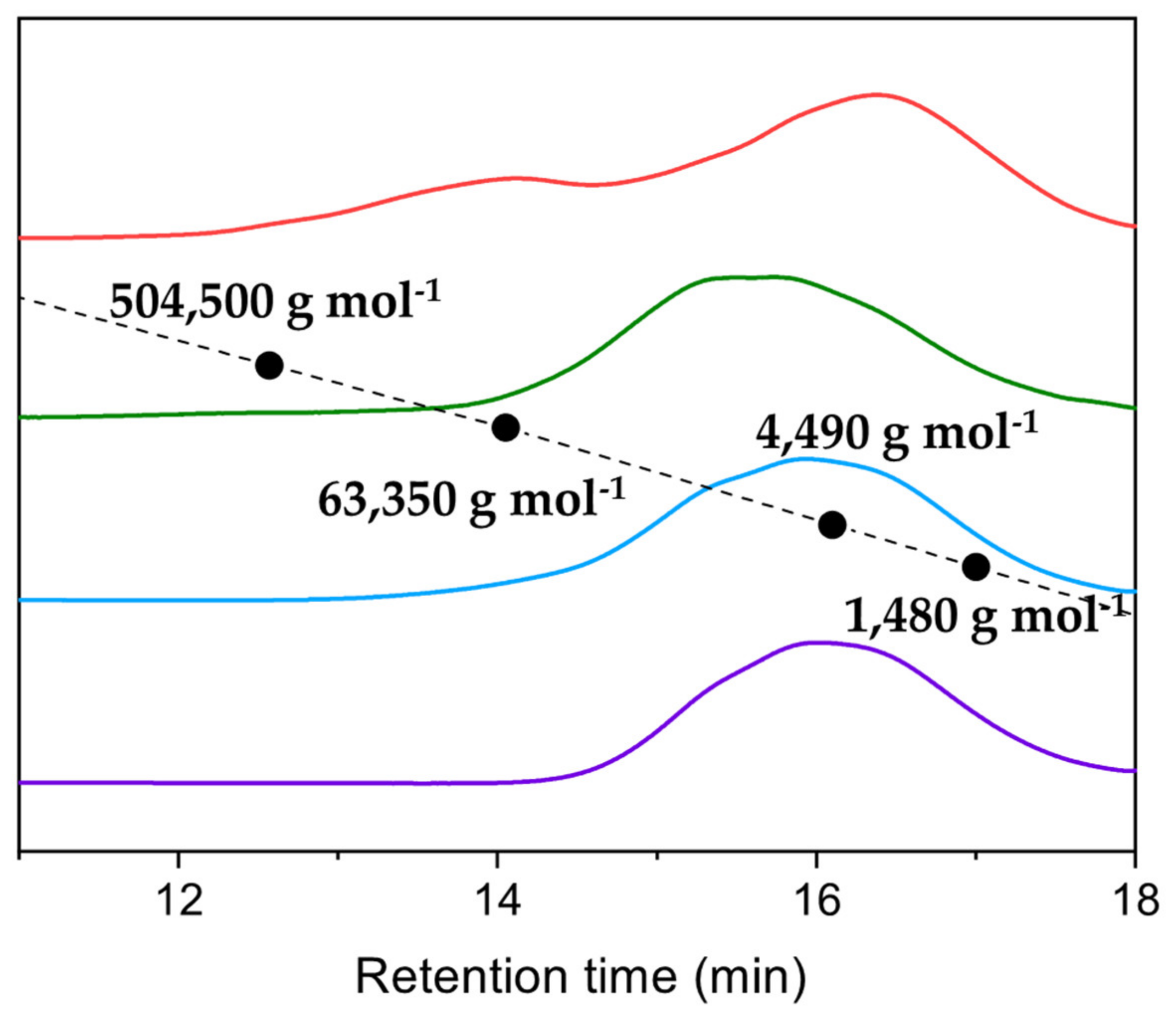
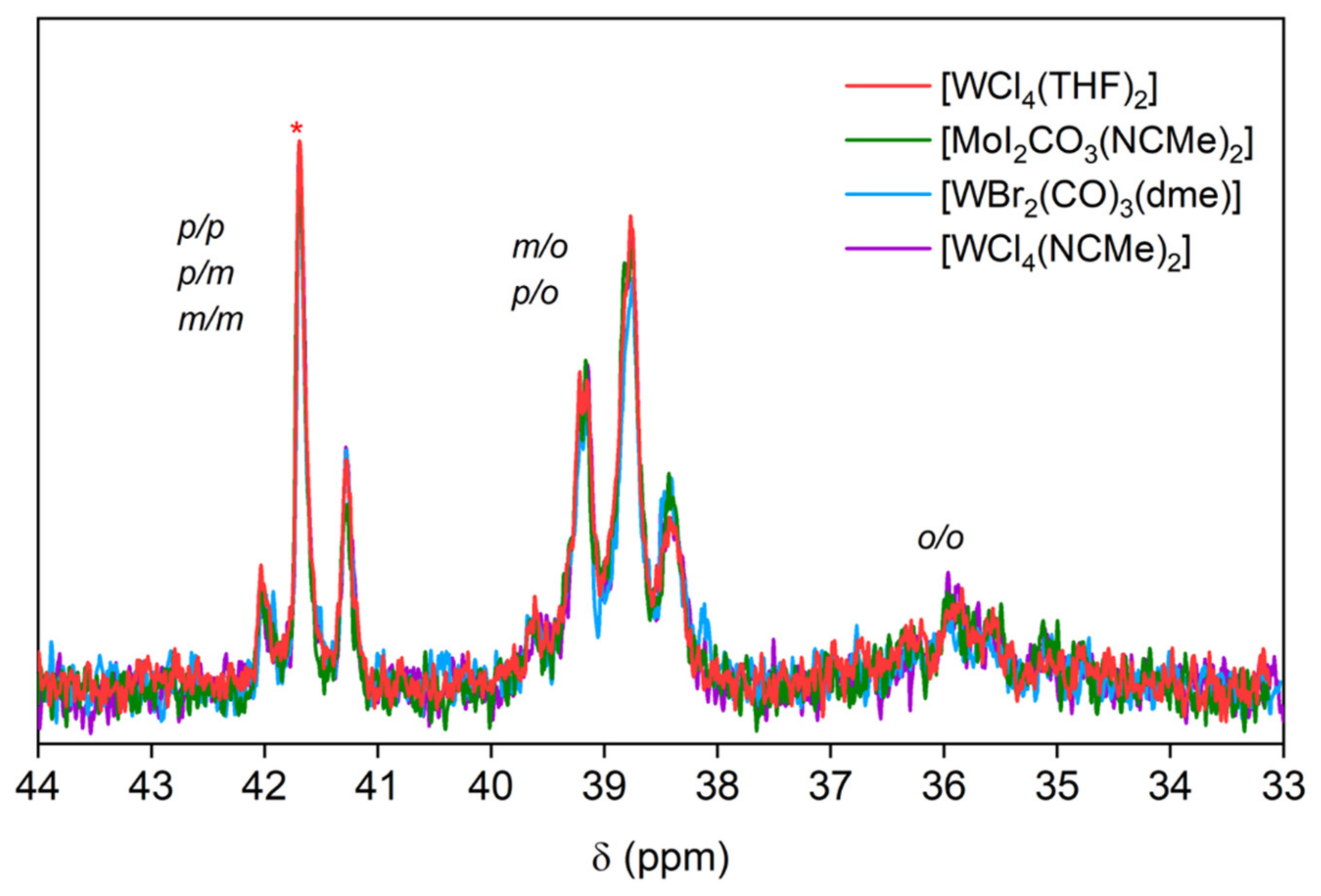

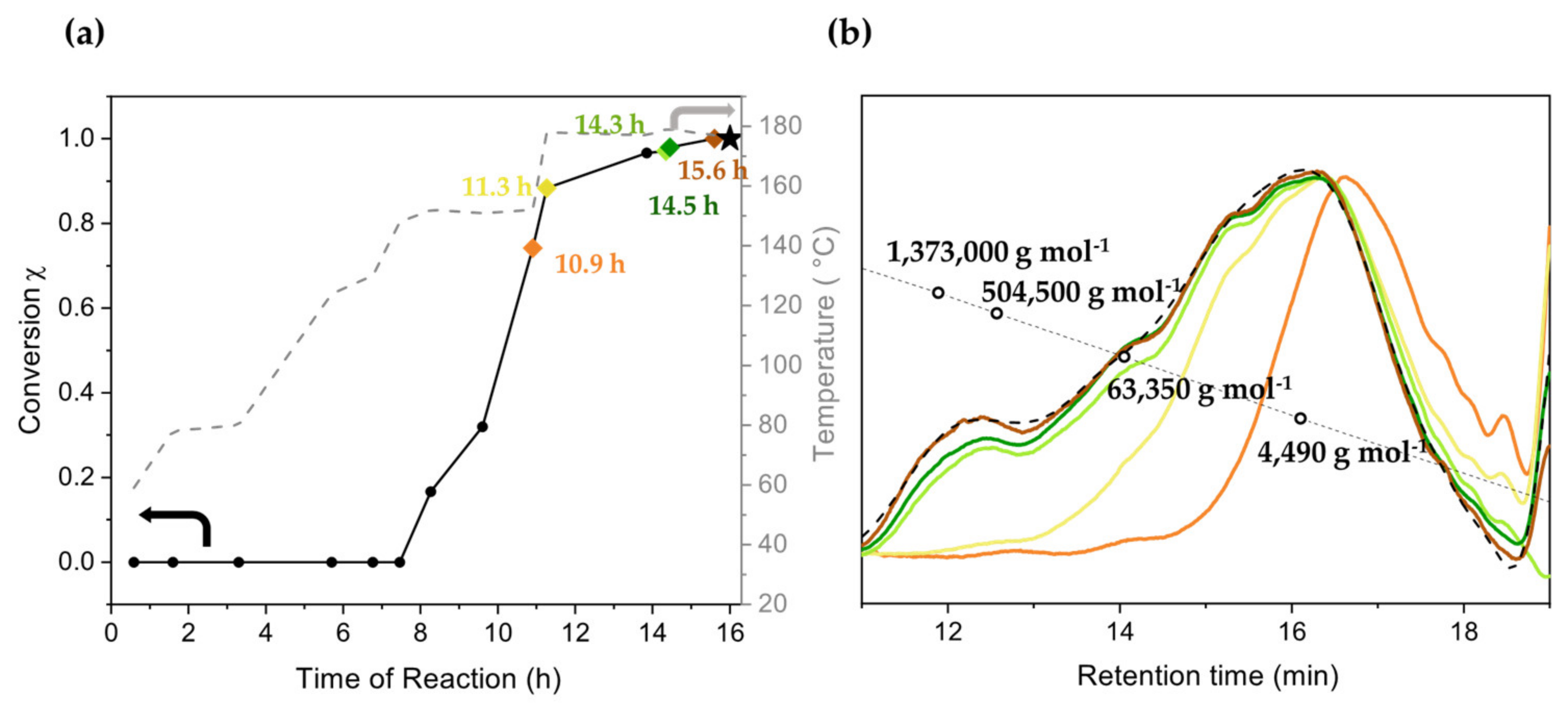
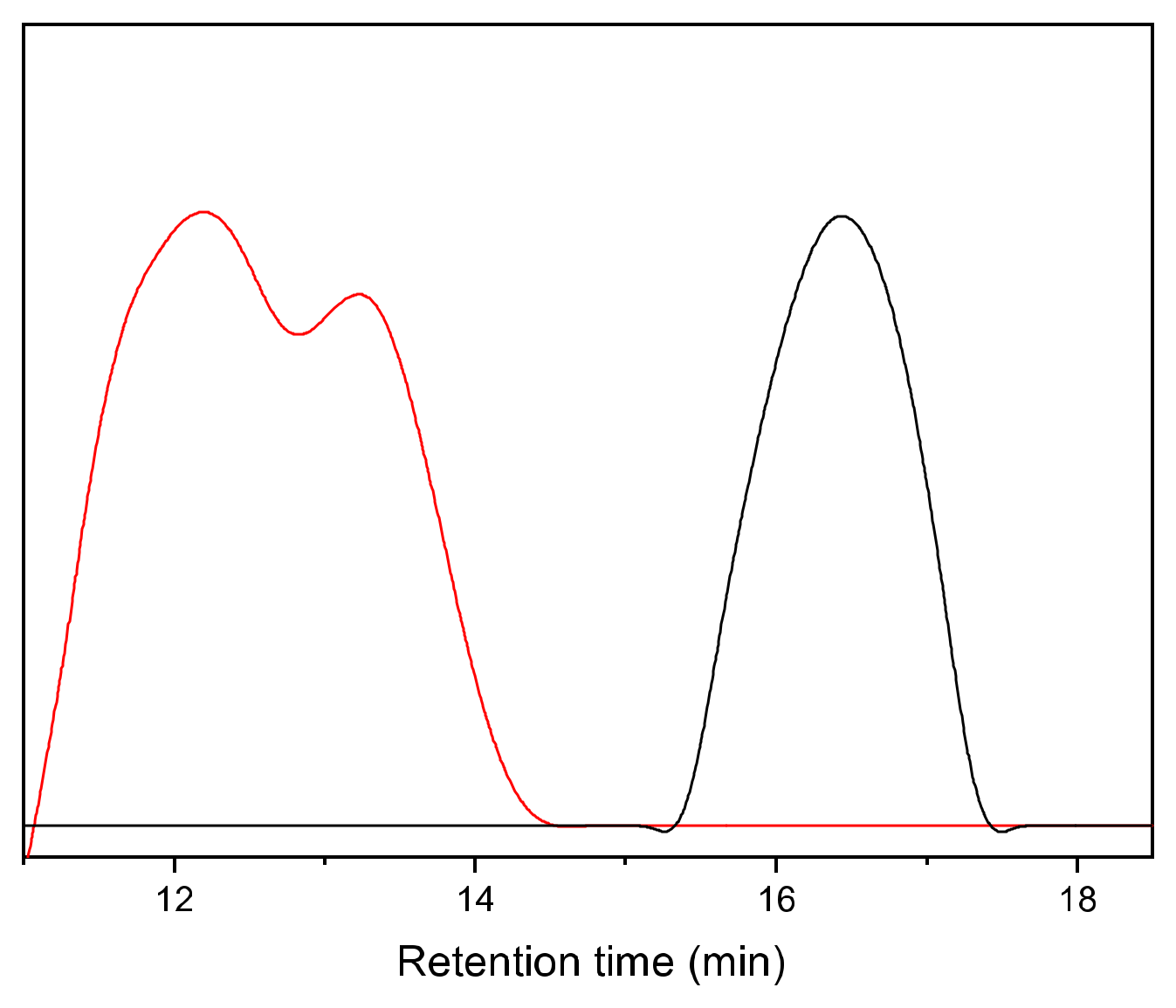
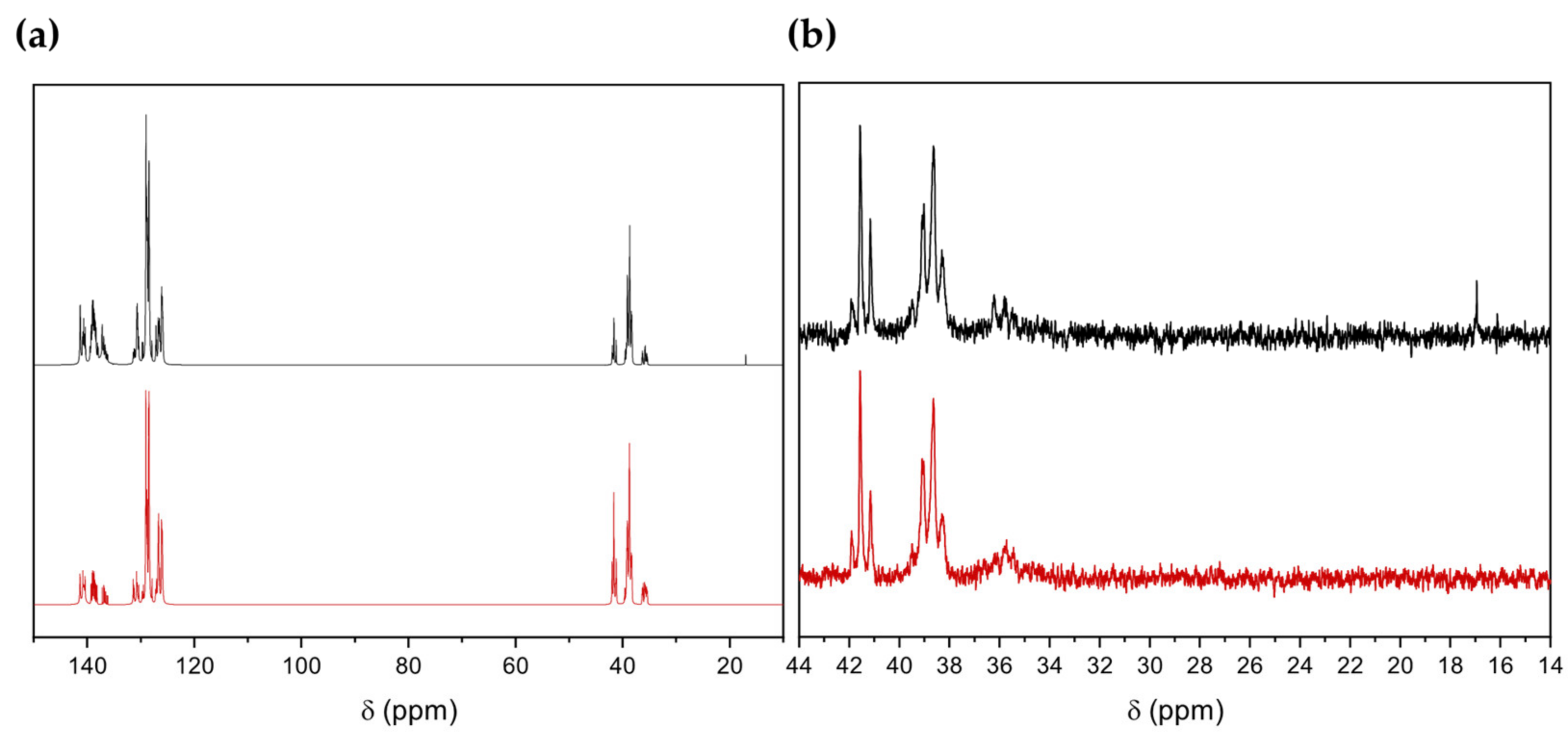



| Fraction | Mn (g mol−1) | Mw (g mol−1) | PDI | Yield of First Fractionation (%) | Yield of Second Fractionation (%) |
|---|---|---|---|---|---|
| Flow | 1821 | 3794 | 2.1 | 55 | |
| Fmedium | 33,520 | 322,100 | 9.6 | 45 | 54 |
| Fhigh | 205,300 | 772,900 | 3.7 | 46 |
| Product | Mn (g mol−1) | Mw (g mol−1) | PDI |
|---|---|---|---|
| PPM | 3244 | 12,100 | 3.7 |
| PPM-D | 3772 | 35,440 | 9.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Elia, M.F.; Yu, Y.; Renggli, M.; Ehweiner, M.A.; Vidovic, C.; Mösch-Zanetti, N.C.; Niederberger, M.; Caseri, W. Synthesis of Soluble High Molar Mass Poly(Phenylene Methylene)-Based Polymers. Polymers 2024, 16, 967. https://doi.org/10.3390/polym16070967
D’Elia MF, Yu Y, Renggli M, Ehweiner MA, Vidovic C, Mösch-Zanetti NC, Niederberger M, Caseri W. Synthesis of Soluble High Molar Mass Poly(Phenylene Methylene)-Based Polymers. Polymers. 2024; 16(7):967. https://doi.org/10.3390/polym16070967
Chicago/Turabian StyleD’Elia, Marco F., Yingying Yu, Melvin Renggli, Madeleine A. Ehweiner, Carina Vidovic, Nadia C. Mösch-Zanetti, Markus Niederberger, and Walter Caseri. 2024. "Synthesis of Soluble High Molar Mass Poly(Phenylene Methylene)-Based Polymers" Polymers 16, no. 7: 967. https://doi.org/10.3390/polym16070967
APA StyleD’Elia, M. F., Yu, Y., Renggli, M., Ehweiner, M. A., Vidovic, C., Mösch-Zanetti, N. C., Niederberger, M., & Caseri, W. (2024). Synthesis of Soluble High Molar Mass Poly(Phenylene Methylene)-Based Polymers. Polymers, 16(7), 967. https://doi.org/10.3390/polym16070967











