Prospect of Gum Arabic–Cocoliposome Matrix to Encapsulate Curcumin for Oral Administration
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of 0.1 M of Phosphate-Buffered Saline (PBS) Solution
2.2. Preparation of Simulated Intestinal Fluid (SIF) Solution
2.3. Preparation of Simulated Gastric Fluid (SGF) Solution
2.4. Encapsulation of Curcumin in Cocoliposomes with Gum Arabic Matrix
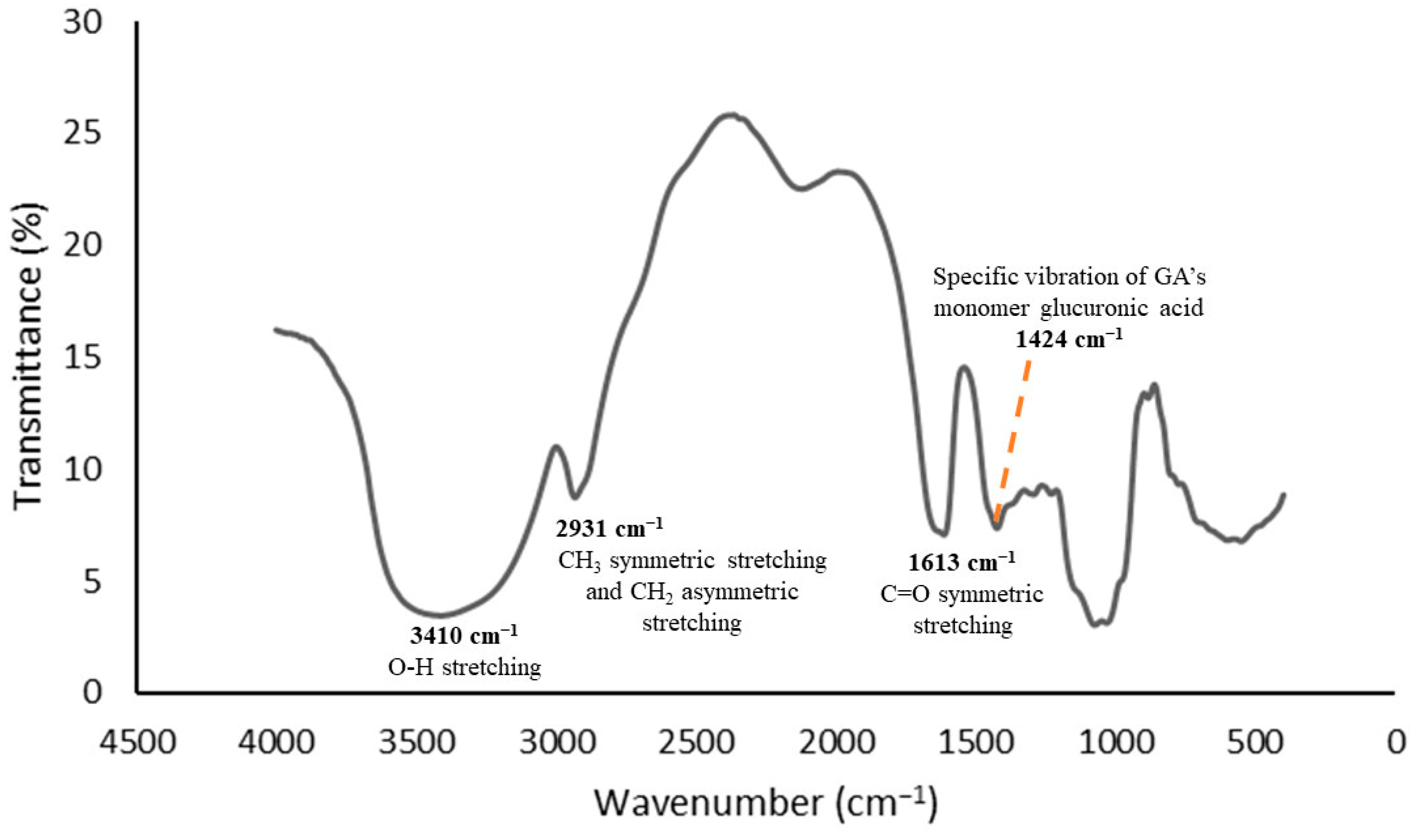
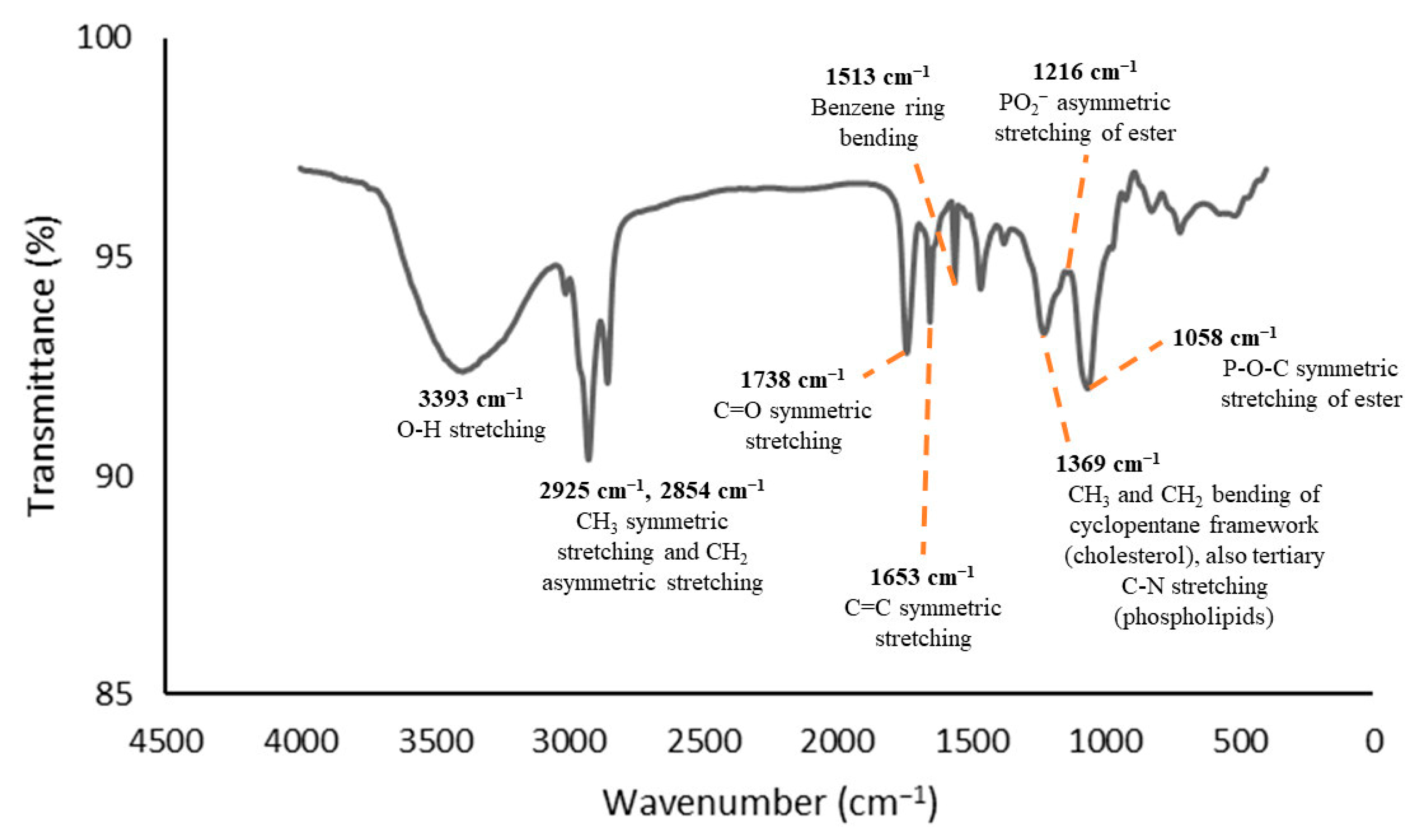
2.5. Determination of the Functional Groups
2.6. Determination of the Encapsulation Efficiency (EE) of the Curcumin
2.7. Determination of the Loading Capacity (LC) of CocoPLs and GA Carriers
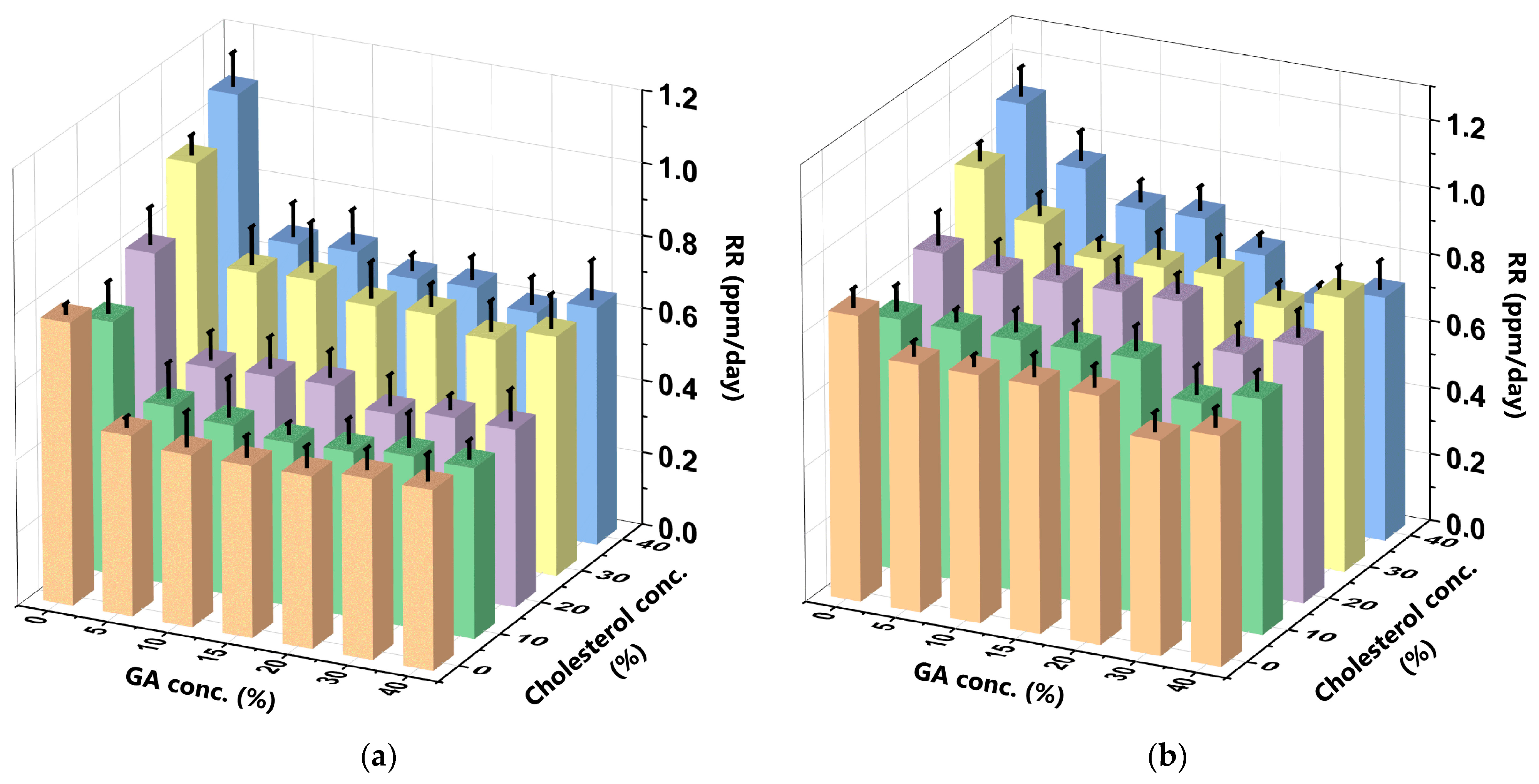
2.8. Determination of the Release Rate (RR) of Curcumin
2.9. Determination of the Particle Size (PS), Polydispersity Index (PDI), and Zeta Potential (ζ-Potential) of the Liposomes
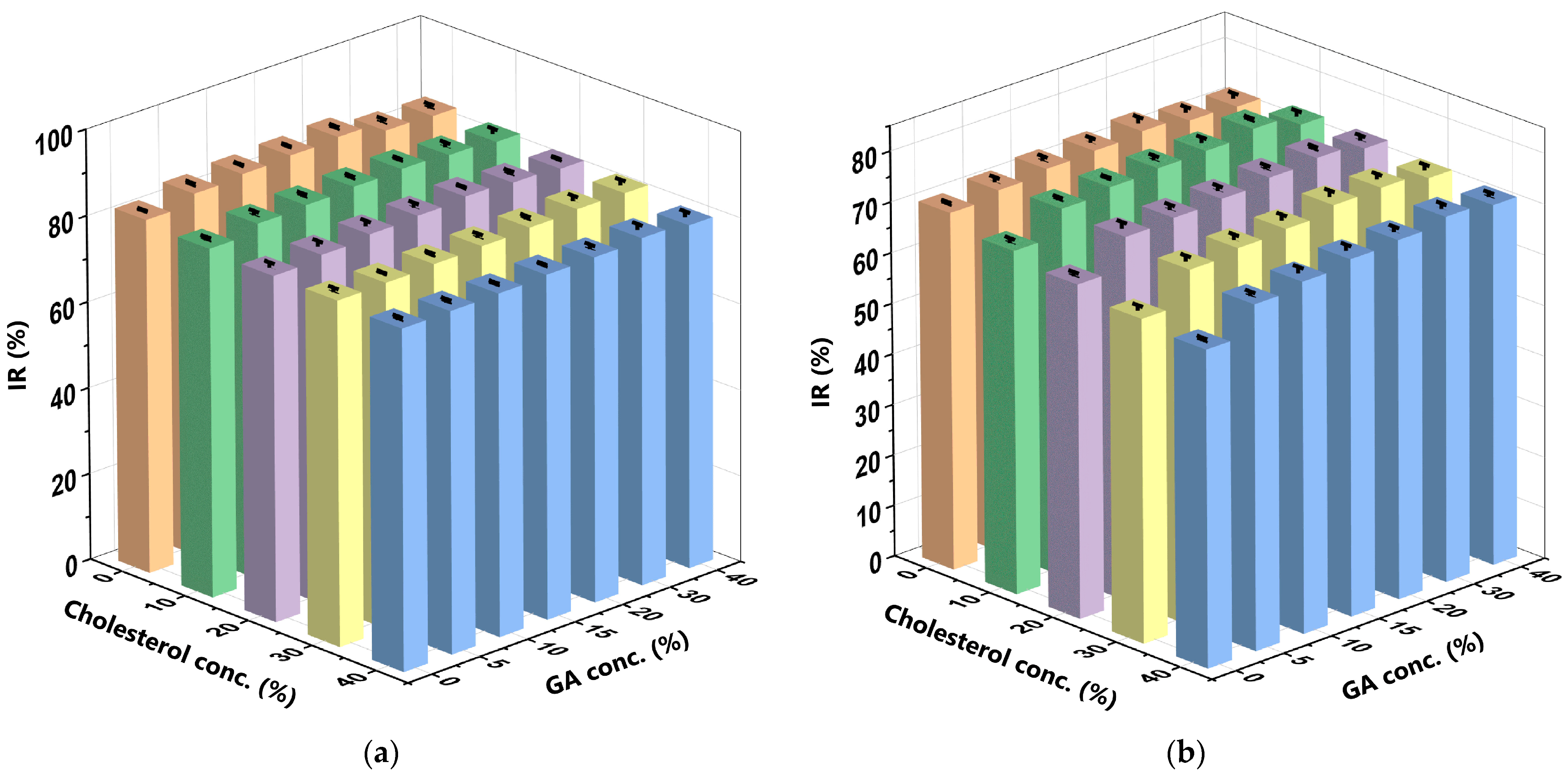
2.10. Determination of the Antioxidant Activity (IR) of Curcumin
2.11. Statistical Analyses
3. Results and Discussion
3.1. Functional Group Analyses
3.2. Encapsulation Efficiency (EE) of Curcumin
3.3. Curcumin Loading Capacity (LC) of the CocoPLs and GA Carriers
3.4. Release Rate (RR) of the Curcumin
3.5. Particle Size (PS), Polydispersity Index (PDI), and Zeta Potential (ζ-Potential) of the Liposomes
3.6. Antioxidant Activity (IR) of the Curcumin
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fang, L.; Gou, S.; Liu, X.; Cao, F.; Cheng, L. Design, synthesis and anti-Alzheimer properties of dimethylaminomethyl-substituted curcumin Derivatives. Bioorg. Med. Chem. Lett. 2014, 24, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhai, Y.; Heng, X.; Che, F.Y.; Chen, W.; Sun, D.; Zhai, G. Oral bioavailability of curcumin: Problems and advancements. J. Drug Target. 2016, 24, 694–702. [Google Scholar] [CrossRef] [PubMed]
- El Khoury, E.; Abiad, M.; Kassaify, Z.G.; Patra, D. Green synthesis of curcumin conjugated nanosilver for the applications in nucleic acid sensing and anti-bacterial activity. Colloids Surf. B Biointerfaces 2015, 127, 274–280. [Google Scholar] [CrossRef]
- Peter, K.V.; Shylaja, M.R. Introduction to herbs and spices: Definitions, trade and applications. In Handbook of Herbs and Spices, 2nd ed.; Peter, K.V., Ed.; Woodhead Publishing: Cambridge, UK, 2012; pp. 1–24. [Google Scholar] [CrossRef]
- Suresh, K.; Nangia, A. Curcumin: Pharmaceutical solids as a platform to improve solubility and bioavailability. CrystEngComm 2018, 20, 3277–3296. [Google Scholar] [CrossRef]
- Mohanty, C.; Das, M.; Sahoo, S.K. Emerging role of nanocarriers to increase the solubility and bioavailability of curcumin. Expert Opin. Drug Deliv. 2012, 9, 1347–1364. [Google Scholar] [CrossRef]
- Farooqui, T.; Farooqui, A.A. Curcumin: Historical background, chemistry, pharmacological action, and potential therapeutic value. Curcumin Neurol. Psychiatr. Disord. 2019, 23–44. [Google Scholar] [CrossRef]
- Sanidad, K.Z.; Sukamtoh, E.; Xiao, H.; McClements, D.J.; Zhang, G. Curcumin: Recent advances in the development of strategies to improve oral bioavailability. Annu. Rev. Food Sci. Technol. 2019, 10, 597–617. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.A.; Tang, Q.D.; Doan, D.C.T.; Dang, M.C. Micro and nano liposome vesicles containing curcumin for a drug delivery system. Adv. Nat. Sci. 2016, 7, 035003. [Google Scholar] [CrossRef]
- Feng, T.; Wei, Y.; Lee, R.J.; Zhao, L. Liposomal curcumin and its application in cancer. Int. J. Nanomed. 2017, 12, 6027–6044. [Google Scholar] [CrossRef]
- Pattni, B.S.; Chupin, V.V.; Torchilin, V.P. New Developments in Liposomal Drug Delivery. Chem. Rev. 2015, 115, 10938–10966. [Google Scholar] [CrossRef]
- Dantas Lopes dos Santos, D.; Besegato, J.F.; de Melo, P.B.G.; Oshiro Junior, J.A.; Chorilli, M.; Deng, D.; Bagnato, V.S.; Rastelli, A.N.d.S. Curcumin-loaded Pluronic® F-127 Micelles as a Drug Delivery System for Curcumin-mediated Photodynamic Therapy for Oral Application. Photochem. Photobiol. 2021, 97, 1072–1088. [Google Scholar] [CrossRef]
- Nayakula, M.; Jeengar, M.K.; Naidu, V.G.M.; Chella, N. Enhanced Pharmacokinetics and Anti-inflammatory Activity of Curcumin Using Dry Emulsion as Drug Delivery Vehicle. Eur. J. Drug Metab. Pharmacokinet. 2023, 48, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Ali, A.; Rahamathulla, M.; Salam, S.; Hani, U.; Wahab, S.; Warsi, M.H.; Yusuf, M.; Ali, A.; Mittal, V.; et al. Fabrication of Sustained Release Curcumin-Loaded Solid Lipid Nanoparticles (Cur-SLNs) as a Potential Drug Delivery System for the Treatment of Lung Cancer: Optimization of Formulation and In Vitro Biological Evaluation. Polymers 2023, 15, 542. [Google Scholar] [CrossRef] [PubMed]
- Santonocito, D.; Sarpietro, M.G.; Carbone, C.; Panico, A.; Campisi, A.; Siciliano, E.A.; Sposito, G.; Castelli, F.; Puglia, C. Curcumin containing PEGylated solid lipid nanoparticles for systemic administration: A preliminary study. Molecules 2020, 25, 2991. [Google Scholar] [CrossRef] [PubMed]
- Ban, C.; Jo, M.; Park, Y.H.; Kim, J.H.; Han, J.Y.; Lee, K.W.; Kweon, D.-H.; Choi, Y.J. Enhancing the oral bioavailability of curcumin using solid lipid nanoparticles. Food Chem. 2020, 302, 125328. [Google Scholar] [CrossRef] [PubMed]
- De Piano, R.; Caccavo, D.; Lamberti, G.; Remaut, K.; Seynaeve, H.; Barba, A.A. A New Productive Approach and Formulative Optimization for Curcumin Nanoliposomal Delivery Systems. Pharmaceutics 2023, 15, 959. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Zong, L.; Zhang, Y.; Zhang, Y.; Guo, X.; Guo, G.; Zhao, L.; Ye, F.; Fu, Y. Antifungal pentachloronitrobenzene/hydroxypropyl-beta-cyclodextrin inclusion complex nanofibers by electrospun with no polymer: Fabrication and characterization. J. Clean. Prod. 2023, 413, 137499. [Google Scholar] [CrossRef]
- Hudiyanti, D.; Al Khafiz, M.F.; Anam, K.; Siahaan, P.; Christa, S.M. In Vitro Evaluation of Curcumin Encapsulation in Gum Arabic Dispersions under Different Environments. Molecules 2022, 27, 3855. [Google Scholar] [CrossRef] [PubMed]
- Al Khafiz, M.F.; Hikmahwati, Y.; Anam, K.; Hudiyanti, D. Key conditions of alpha-tocopherol encapsulation in gum Arabic dispersions. Int. J. Res. Pharm. Sci. 2019, 10, 2622–2627. [Google Scholar] [CrossRef]
- Çağdaş, M.; Sezer, A.D.; Bucak, S. Liposomes as potential drug carrier systems for drug delivery. Appl. Nanotechnol. Drug Deliv. 2014, 1, 1–50. [Google Scholar]
- De Leo, V.; Milano, F.; Mancini, E.; Comparelli, R.; Giotta, L.; Nacci, A.; Longobardi, F.; Garbetta, A.; Agostiano, A.; Catucci, L. Encapsulation of Curcumin-Loaded Liposomes for Colonic Drug Delivery in a pH-Responsive Polymer Cluster Using a pH-Driven and Organic Solvent-Free Process. Molecules 2018, 23, 739. [Google Scholar] [CrossRef] [PubMed]
- Briuglia, M.-L.; Rotella, C.; McFarlane, A.; Lamprou, D.A. Influence of cholesterol on liposome stability and on in vitro drug release. Drug Deliv. Transl. Res. 2015, 5, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lervik, A.; Seddon, J.; Bresme, F. A coarse-grained molecular dynamics investigation of the phase behavior of DPPC/cholesterol mixtures. Chem. Phys. Lipids 2015, 185, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Hu, B.; Ma, R.; Gao, Z.; Nishinari, K.; Phillips, G.O.; Yang, J.; Fang, Y. Effect of arabinogalactan protein complex content on emulsification performance of gum arabic. Carbohydr. Polym. 2019, 224, 115170. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhai, J.; Dyett, B.; Yang, Y.; Drummond, C.J.; Conn, C.E. Effect of gum arabic or sodium alginate incorporation on the physicochemical and curcumin retention properties of liposomes. LWT 2021, 139, 110571. [Google Scholar] [CrossRef]
- Fujimori, S. Gastric acid level of humans must decrease in the future. World J. Gastroenterol. 2020, 26, 6706–6709. [Google Scholar] [CrossRef] [PubMed]
- Hudiyanti, D.; Al Khafiz, M.F.; Anam, K.; Siahaan, P.; Suyati, L. Assessing encapsulation of curcumin in cocoliposome: In vitro study. Open Chem. 2021, 19, 358–366. [Google Scholar] [CrossRef]
- Tiwari, A.; Jain, S.K. Formulation, Optimization, and in vitro Characterization of Curcumin Loaded Liposomes for Colonic Delivery. Int. J. Pharm. Investig. 2021, 11, 408–413. [Google Scholar] [CrossRef]
- Hudiyanti, D.; Jayanti, M.; Al-Khafiz, M.F.; Anam, K. A study of coconut (Cocos nucifera L.) phosphatidylcholine species. Orient. J. Chem. 2018, 34, 2963. [Google Scholar] [CrossRef]
- Hudiyanti, D.; Al Khafiz, M.F.; Anam, K. Coconut (Cocos nucifera L.) lipids: Extraction and characterization. Orient. J. Chem. 2018, 34, 1136. [Google Scholar] [CrossRef]
- Hudiyanti, D.; Sari, R.I.; Arya, A.P.; Siahaan, P. Liposomes from jack beans phospholipid extract for delivering vitamin C. AIP Conf. Proc. 2020, 2237, 020064. [Google Scholar] [CrossRef]
- Taqui, S.N.; Mohan, C.S.; Goodarzi, M.S.; Elkotb, M.A.; Khatoon, B.A.; Soudagar, M.E.M.; Koki, I.B.; Elfasakhany, A.; Khalifa, A.S.; Ali, M.A.; et al. Sustainable Adsorption Method for the Remediation of Crystal Violet Dye Using Nutraceutical Industrial Fenugreek Seed Spent. Appl. Sci. 2021, 11, 7635. [Google Scholar] [CrossRef]
- Ma, Q.; Ren, Y.; Wang, L. Investigation of antioxidant activity and release kinetics of curcumin from tara gum/polyvinyl alcohol active film. Food Hydrocoll. 2017, 70, 286–292. [Google Scholar] [CrossRef]
- Wang, H.; Gong, X.; Guo, X.; Liu, C.; Fan, Y.-Y.; Zhang, J.; Niu, B.; Li, W. Characterization, release, and antioxidant activity of curcumin-loaded sodium alginate/ZnO hydrogel beads. Int. J. Biol. Macromol. 2019, 121, 1118–1125. [Google Scholar] [CrossRef] [PubMed]
- Olusanya, T.O.B.; Haj Ahmad, R.R.; Ibegbu, D.M.; Smith, J.R.; Elkordy, A.A. Liposomal Drug Delivery Systems and Anticancer Drugs. Molecules 2018, 23, 907. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Wu, Z.; McClements, D.J.; Zou, L.; Peng, S.; Zhou, W.; Liu, W. Improvement on stability, loading capacity and sustained release of rhamnolipids modified curcumin liposomes. Colloids Surf. B 2019, 183, 110460. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Ye, A.; Liu, C.; Liu, W.; Singh, H. Structure and integrity of liposomes prepared from milk- or soybean-derived phospholipids during in vitro digestion. Food Res. Int. 2012, 48, 499–506. [Google Scholar] [CrossRef]
- Taylor, T.M.; Weiss, J.; Davidson, P.M.; Bruce, B.D. Liposomal Nanocapsules in Food Science and Agriculture. Crit. Rev. Food Sci. Nutr. 2005, 45, 587–605. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Sun, G.; Wang, Z.; Cheng, X.; Park, H.; Cha, D.; Kong, M.; Chen, X. Transport mechanism of doxorubicin loaded chitosan based nanogels across intestinal epithelium. Eur. J. Pharm. Biopharm. 2014, 87, 197–207. [Google Scholar] [CrossRef]
- Wang, C.; Han, Z.; Wu, Y.; Lu, X.; Tang, X.; Xiao, J.; Li, N. Enhancing stability and anti-inflammatory properties of curcumin in ulcerative colitis therapy using liposomes mediated colon-specific drug delivery system. Food Chem. Toxicol. 2021, 151, 112123. [Google Scholar] [CrossRef]
- Maritim, S.; Boulas, P.; Lin, Y. Comprehensive analysis of liposome formulation parameters and their influence on encapsulation, stability and drug release in glibenclamide liposomes. Int. J. Pharm. 2021, 592, 120051. [Google Scholar] [CrossRef] [PubMed]
- Zatorska, M.; Łazarski, G.; Maziarz, U.; Wilkosz, N.; Honda, T.; Yusa, S.-I.; Bednar, J.; Jamróz, D.; Kepczynski, M. Drug-loading capacity of polylactide-based micro- and nanoparticles—Experimental and molecular modeling study. Int. J. Pharm. 2020, 591, 120031. [Google Scholar] [CrossRef] [PubMed]
- Wilkosz, N.; Łazarski, G.; Kovacik, L.; Gargas, P.; Nowakowska, M.; Jamróz, D.; Kepczynski, M. Molecular insight into drug-loading capacity of PEG–PLGA nanoparticles for itraconazole. J. Phys. Chem. B 2018, 122, 7080–7090. [Google Scholar] [CrossRef]
- Li, Y.; Yang, L. Driving forces for drug loading in drug carriers. J. Microencapsul. 2015, 32, 255–272. [Google Scholar] [CrossRef]
- Dragostin, I.; Dragostin, O.; Pelin, A.-M.; Grigore, C.; Lăcrămioara Zamfir, C. The importance of polymers for encapsulation process and for enhanced cellular functions. J. Macromol. Sci. Part A 2017, 54, 489–493. [Google Scholar] [CrossRef]
- Li, J.; Xiong, S.; Wang, F.; Regenstein, J.M.; Liu, R. Optimization of Microencapsulation of Fish Oil with Gum Arabic/Casein/Beta-Cyclodextrin Mixtures by Spray Drying. J. Food Sci. 2015, 80, C1445–C1452. [Google Scholar] [CrossRef]
- Gébleux, R.; Wulhfard, S.; Casi, G.; Neri, D. Antibody format and drug release rate determine the therapeutic activity of noninternalizing antibody–drug conjugates. Mol. Cancer Ther. 2015, 14, 2606–2612. [Google Scholar] [CrossRef] [PubMed]
- Bhowmik, D.; Gopinath, H.; Kumar, B.P.; Duraivel, S.; Kumar, K.P.S. Controlled release drug delivery systems. Pharma Innov. 2012, 1, 24–32. [Google Scholar]
- Johnston, M.J.W.; Semple, S.C.; Klimuk, S.K.; Edwards, K.; Eisenhardt, M.L.; Leng, E.C.; Karlsson, G.; Yanko, D.; Cullis, P.R. Therapeutically optimized rates of drug release can be achieved by varying the drug-to-lipid ratio in liposomal vincristine formulations. Biochim. Biophys. Acta (BBA) 2006, 1758, 55–64. [Google Scholar] [CrossRef]
- Xiang, S.; Yao, X.; Zhang, W.; Zhang, K.; Fang, Y.; Nishinari, K.; Phillips, G.O.; Jiang, F. Gum Arabic-stabilized conjugated linoleic acid emulsions: Emulsion properties in relation to interfacial adsorption behaviors. Food Hydrocoll. 2015, 48, 110–116. [Google Scholar] [CrossRef]
- Deniz, A.; Sade, A.; Severcan, F.; Keskin, D.; Tezcaner, A.; Banerjee, S. Celecoxib-loaded liposomes: Effect of cholesterol on encapsulation and in vitro release characteristics. Biosci. Rep. 2010, 30, 365–373. [Google Scholar] [CrossRef]
- Peretz Damari, S.; Shamrakov, D.; Varenik, M.; Koren, E.; Nativ-Roth, E.; Barenholz, Y.; Regev, O. Practical aspects in size and morphology characterization of drug-loaded nano-liposomes. Int. J. Pharm. 2018, 547, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Raval, N.; Maheshwari, R.; Kalyane, D.; Youngren-Ortiz, S.R.; Chougule, M.B.; Tekade, R.K. Chapter 10—Importance of Physicochemical Characterization of Nanoparticles in Pharmaceutical Product Development. In Basic Fundamentals of Drug Delivery; Tekade, R.K., Ed.; Academic Press: New York, NY, USA, 2019; pp. 369–400. [Google Scholar] [CrossRef]
- Németh, Z.; Csóka, I.; Semnani Jazani, R.; Sipos, B.; Haspel, H.; Kozma, G.; Kónya, Z.; Dobó, D.G. Quality by Design-Driven Zeta Potential Optimisation Study of Liposomes with Charge Imparting Membrane Additives. Pharmaceutics 2022, 14, 1798. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, D.; Cavaco-Paulo, A.; Nogueira, E. Design of liposomes as drug delivery system for therapeutic applications. Int. J. Pharm. 2021, 601, 120571. [Google Scholar] [CrossRef]
- Fan, Y.; Marioli, M.; Zhang, K. Analytical characterization of liposomes and other lipid nanoparticles for drug delivery. J. Pharm. Biomed. Anal. 2021, 192, 113642. [Google Scholar] [CrossRef] [PubMed]
- Duong, V.A.; Nguyen, T.T.; Maeng, H.J. Recent Advances in Intranasal Liposomes for Drug, Gene, and Vaccine Delivery. Pharmaceutics 2023, 15, 207. [Google Scholar] [CrossRef]
- Okafor, N.I.; Nkanga, C.I.; Walker, R.B.; Noundou, X.S.; Krause, R.W.M. Encapsulation and physicochemical evaluation of efavirenz in liposomes. J. Pharm. Investig. 2020, 50, 201–208. [Google Scholar] [CrossRef]
- Najaf Najafi, M.; Arianmehr, A.; Sani, A.M. Preparation of Barije (Ferula gummosa) Essential Oil–Loaded Liposomes and Evaluation of Physical and Antibacterial Effect on Escherichia coli O157:H7. J. Food Prot. 2020, 83, 511–517. [Google Scholar] [CrossRef]
- Hudiyanti, D.; Putri, V.N.R.; Hikmahwati, Y.; Christa, S.M.; Siahaan, P.; Anugrah, D.S.B. Interaction of Phospholipid, Cholesterol, Beta-Carotene, and Vitamin C Molecules in Liposome-Based Drug Delivery Systems: An In Silico Study. Adv. Pharmacol. Pharm. Sci. 2023, 2023, 4301310. [Google Scholar] [CrossRef]
- Tripathy, S.; Srivastav, P.P. Encapsulation of Centella asiatica leaf extract in liposome: Study on structural stability, degradation kinetics and fate of bioactive compounds during storage. Food Chem. Adv. 2023, 2, 100202. [Google Scholar] [CrossRef]
- Bains, A.; Najda, A.; Chawla, P.; Klepacka, J.; Dhull, S.B.; Sadh, P.K.; Khan, M.A.; Kaushik, R. Formulation and Characterization of Gum Arabic Stabilized Red Rice Extract Nanoemulsion. Polymers 2022, 14, 1938. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, A.V.; Gerasimova, E.L.; Brainina, K.Z. Potentiometric Study of Antioxidant Activity: Development and Prospects. Crit. Rev. Anal. Chem. 2015, 45, 311–322. [Google Scholar] [CrossRef]
- Annisa, R.; Jati Dharma, T.; Mutiah, R.; Nurjannah, S. Antioxidants Activity of Self-Nanoemulsifying Drug Delivery System on Dayak Onions Extract (Eleutherine palmifolia) using DPPH (2,2 difenil-1- picrylhydrazyl) Method. J. Trop. Pharm. Chem. 2021, 5, 396–405. [Google Scholar] [CrossRef]
- Kedare, S.B.; Singh, R.P. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef]
- Warsi, W.; Sardjiman, S.; Riyanto, S. Synthesis and Antioxidant Activity of Curcumin Analogues. J. Chem. Pharm. Res. 2018, 10, 1–9. [Google Scholar]
- Zhao, Z.; Cui, X.; Ma, X.; Wang, Z. Preparation, characterization, and evaluation of antioxidant activity and bioavailability of a self-nanoemulsifying drug delivery system (SNEDDS) for buckwheat flavonoids. Acta Biochim. Biophys. Sin. 2020, 52, 1265–1274. [Google Scholar] [CrossRef]
- Sukati, S.; Khobjai, W. Total Phenolic Content and DPPH Free Radical Scavenging Activity of Young Turmeric Grown in Southern Thailand. Appl. Mech. Mater. 2019, 886, 61–69. [Google Scholar] [CrossRef]
- Mirghani, M.E.S.; Elnour, A.A.M.; Kabbashi, N.A.; Alam, M.Z.; Musa, K.H.; Abdullah, A. Determination of antioxidant activity of gum arabic: An exudation from two different locations. Sci. Asia 2018, 44, 177–186. [Google Scholar] [CrossRef]
- Suhag, Y.; Nayik, G.A.; Nanda, V. Effect of gum arabic concentration and inlet temperature during spray drying on physical and antioxidant properties of honey powder. J. Food Meas. Charact. 2016, 10, 350–356. [Google Scholar] [CrossRef]




| CCL Formulation | Composition (w/w/w; mg) | ||
|---|---|---|---|
| CocoPLs | Cholesterol p.a. (Chol) | * Curcumin p.a. (Cur) | |
| LC0 | 125 | 0 | 1 |
| LC10 | 125 | 12.5 | 1 |
| LC20 | 125 | 25 | 1 |
| LC30 | 125 | 37.5 | 1 |
| LC40 | 125 | 50 | 1 |
| CCL Formulation | CGA | ||||||
|---|---|---|---|---|---|---|---|
| 0% | 5% | 10% | 15% | 20% | 30% | 40% | |
| LC0 | 1 LC0G0 | 3 LC0G5 | LC0G10 | LC0G15 | LC0G20 | LC0G30 | LC0G40 |
| LC10 | LC10G0 | LC10G5 | LC10G10 | LC10G15 | LC10G20 | LC10G30 | LC10G40 |
| LC20 | LC20G0 | LC20G5 | LC20G10 | LC20G15 | LC20G20 | LC20G30 | LC20G40 |
| LC30 | LC30G0 | LC30G5 | LC30G10 | LC30G15 | LC30G20 | LC30G30 | LC30G40 |
| LC40 | LC40G0 | LC40G5 | LC40G10 | LC40G15 | LC40G20 | LC40G30 | 2 LC40G40 |
| LC0 | LC10 | LC20 | LC30 | LC40 | |
|---|---|---|---|---|---|
| PS (nm) | 305.6 | 704.3 | 366.8 | 272.9 | 602.9 |
| PDI | 0.546 | 0.588 | 0.847 | 0.464 | 0.720 |
| ζ-potential (mV) | −30.9 | −34.6 | −31.6 | −31.2 | −34.1 |
| LC10G0 | LC10G5 | LC10G10 | LC10G15 | LC10G20 | LC10G30 | LC10G40 | |
|---|---|---|---|---|---|---|---|
| PS (nm) | 704.3 | 838.1 | 991.4 | 591.4 | 606.9 | 852.5 | 898.4 |
| PDI | 0.588 | 0.574 | 0.640 | 0.647 | 0.494 | 0.404 | 0.571 |
| ζ-potential (mV) | −34.6 | −20.6 | −19.9 | −14.4 | −12.7 | −9.1 | −7.4 |
| LC0 | LC10 | LC20 | LC30 | LC40 | |
|---|---|---|---|---|---|
| SIF | 2.788% | 3.042% | 3.169% | 3.549% | 3.549% |
| SGF | 0.932% | 1.242% | 1.398% | 1.863% | 1.863% |
| G5 | G10 | G15 | G20 | G30 | |
|---|---|---|---|---|---|
| SIF | 1.695% | 2.245% | 3.013% | 3.766% | 4.520% |
| SGF | 1.305% | 1.794% | 2.447% | 2.936% | 3.589% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hudiyanti, D.; Al Khafiz, M.F.; Anam, K.; Siahaan, P.; Suyati, L.; Sunarsih, S.; Christa, S.M. Prospect of Gum Arabic–Cocoliposome Matrix to Encapsulate Curcumin for Oral Administration. Polymers 2024, 16, 944. https://doi.org/10.3390/polym16070944
Hudiyanti D, Al Khafiz MF, Anam K, Siahaan P, Suyati L, Sunarsih S, Christa SM. Prospect of Gum Arabic–Cocoliposome Matrix to Encapsulate Curcumin for Oral Administration. Polymers. 2024; 16(7):944. https://doi.org/10.3390/polym16070944
Chicago/Turabian StyleHudiyanti, Dwi, Muhammad Fuad Al Khafiz, Khairul Anam, Parsaoran Siahaan, Linda Suyati, Sunarsih Sunarsih, and Sherllyn Meida Christa. 2024. "Prospect of Gum Arabic–Cocoliposome Matrix to Encapsulate Curcumin for Oral Administration" Polymers 16, no. 7: 944. https://doi.org/10.3390/polym16070944
APA StyleHudiyanti, D., Al Khafiz, M. F., Anam, K., Siahaan, P., Suyati, L., Sunarsih, S., & Christa, S. M. (2024). Prospect of Gum Arabic–Cocoliposome Matrix to Encapsulate Curcumin for Oral Administration. Polymers, 16(7), 944. https://doi.org/10.3390/polym16070944








