Biobased Composites of Poly(Lactic Acid) Melt Compounded with Bacterial and Vegetal Nanocelluloses Incorporated through Different Strategies
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
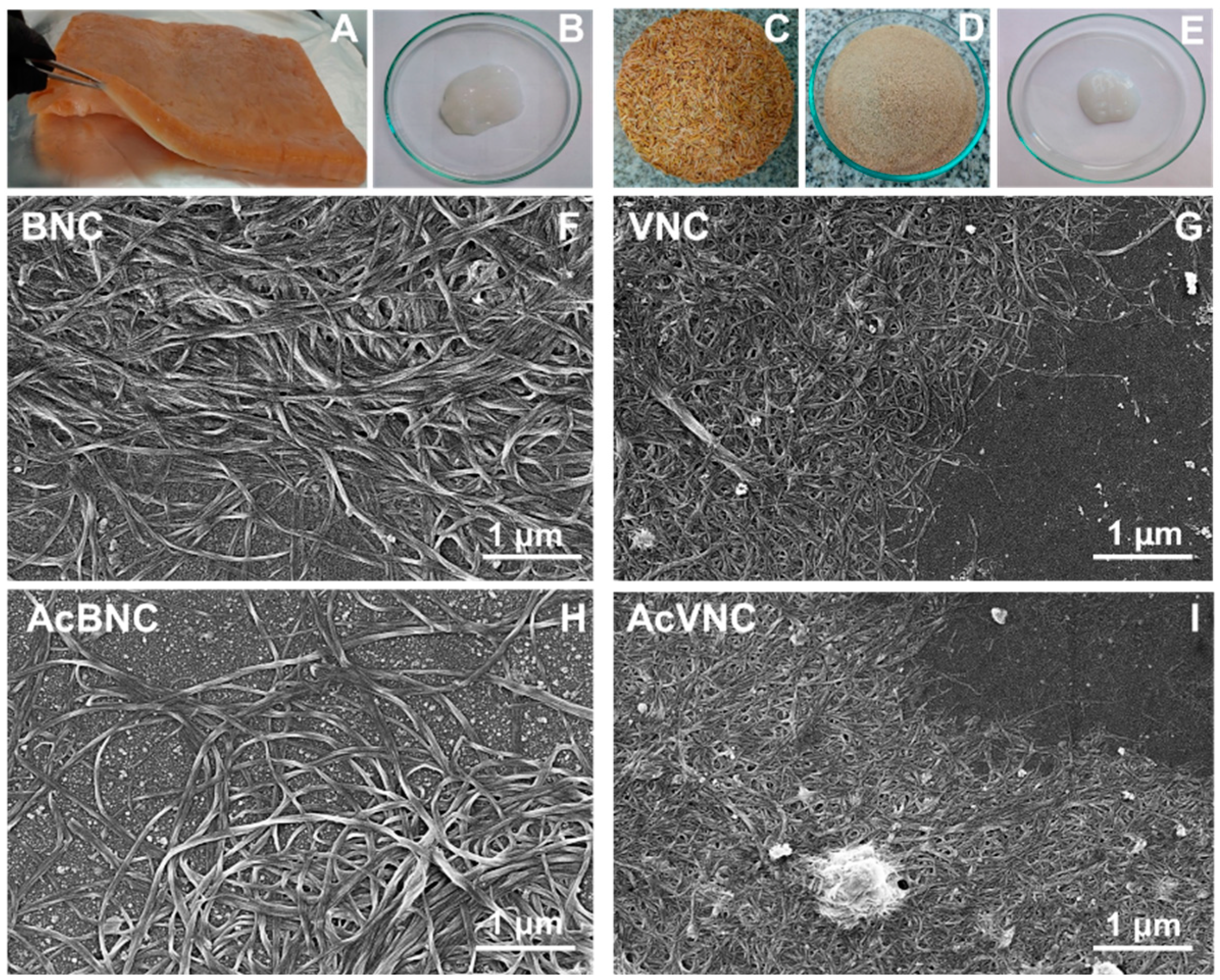
2.2. Bacterial Nanocellulose Production
2.3. Vegetal Nanocellulose Production
2.4. Acetylation of Nanocelluloses
2.5. Masterbatches and Composites Preparation
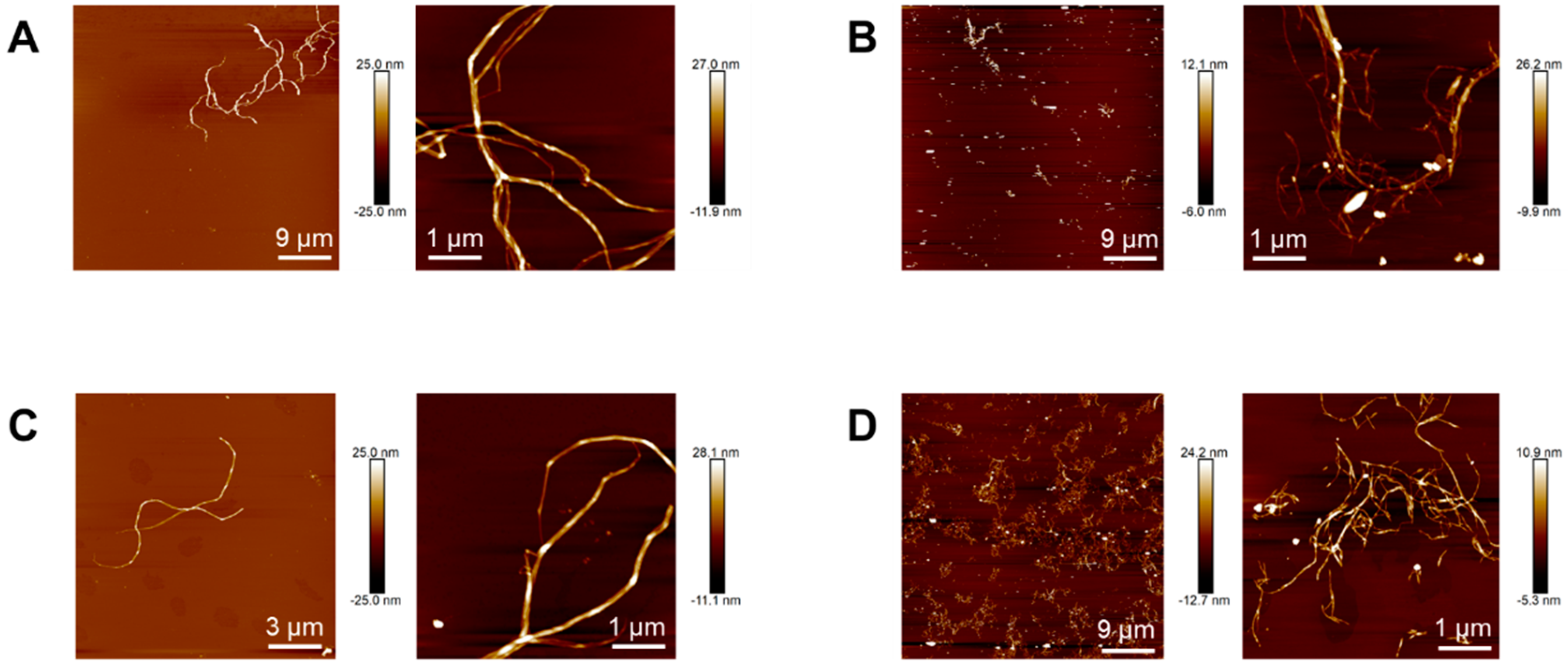
2.6. Characterization of Nanocelluloses
2.6.1. Field Emission Scanning Electron Microscopy (FESEM)
2.6.2. Atomic Force Microscopy (AFM)
2.6.3. Fourier Transform Infrared Spectroscopy (FTIR)
2.6.4. X-ray Diffraction Analysis (XRD)
2.7. Characterization of PLA/Nanocelluloses Composites
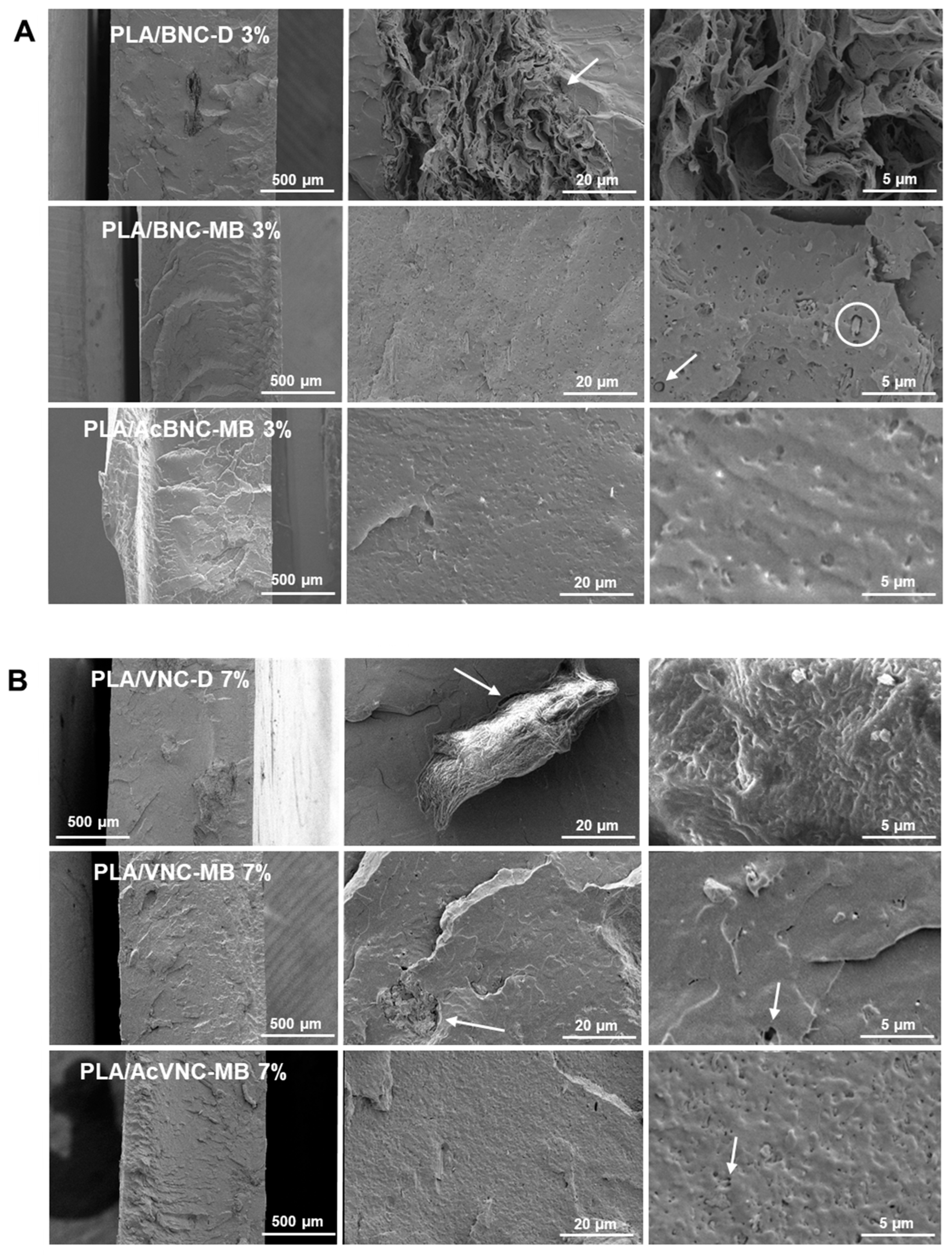
2.7.1. Environmental Scanning Electron Microscopy (ESEM)
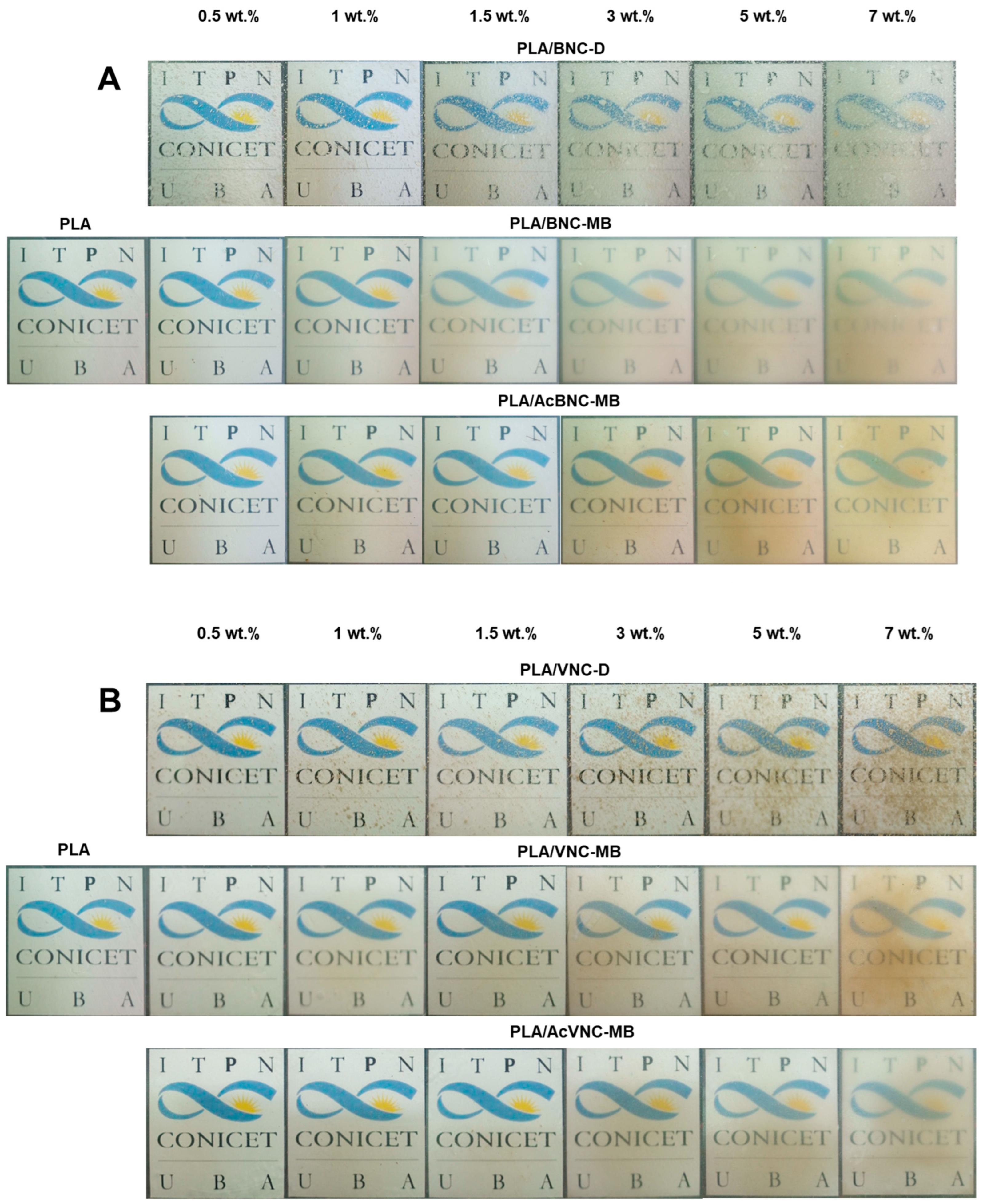
2.7.2. Optical Properties
2.7.3. Mechanical Properties
3. Results and Discussion
3.1. Nanocelluloses
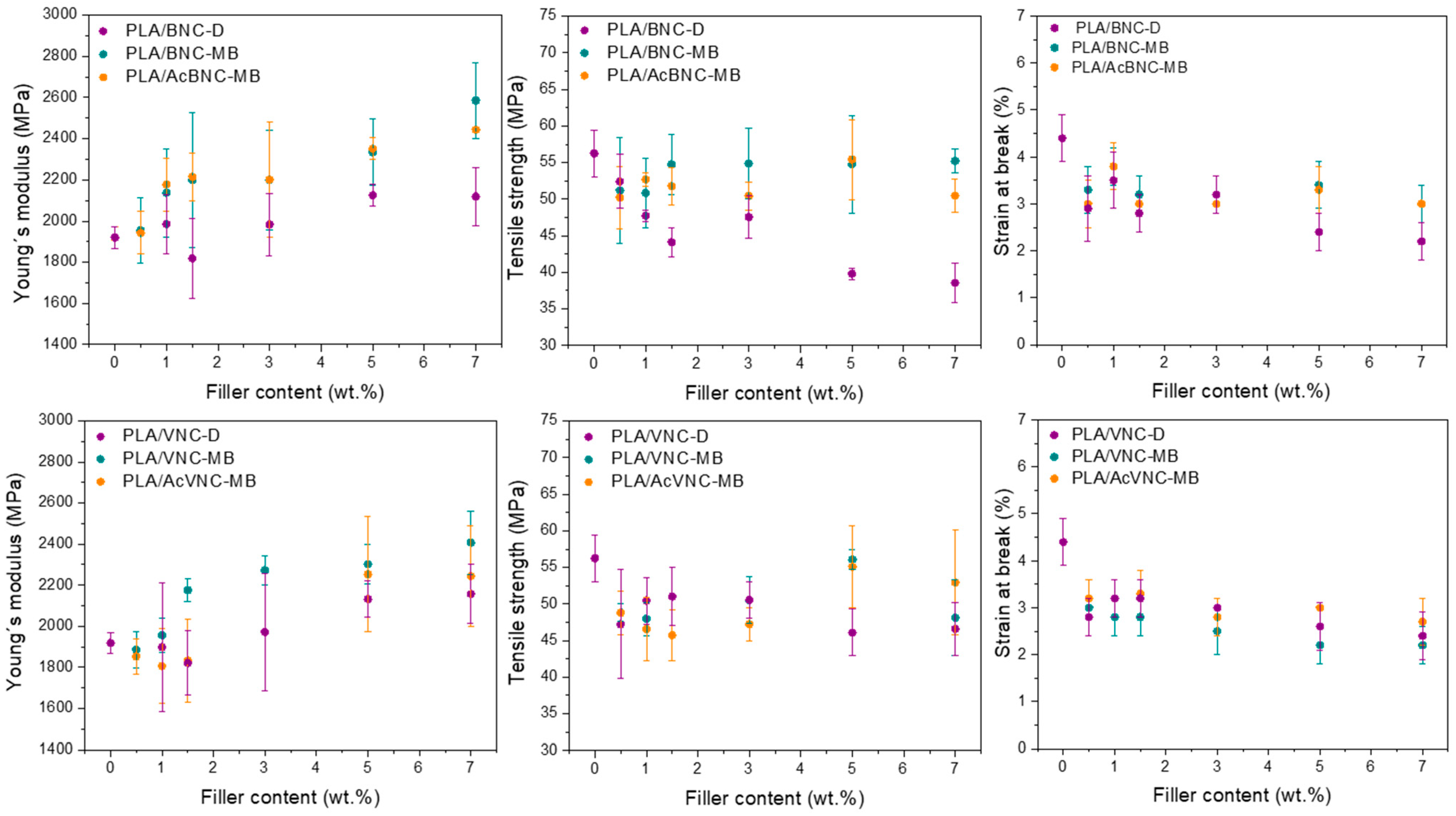
3.2. PLA/Nanocelluloses Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, L.; Ke, K.; Yang, M.B.; Yang, W. Recent Progress on Chemical Modification of Cellulose for High Mechanical-Performance Poly(Lactic Acid)/Cellulose Composite: A Review. Compos. Commun. 2021, 23, 100548. [Google Scholar] [CrossRef]
- Wu, Y.; Gao, X.; Wu, J.; Zhou, T.; Nguyen, T.T.; Wang, Y. Biodegradable Polylactic Acid and Its Composites: Characteristics, Processing, and Sustainable Applications in Sports. Polymers 2023, 15, 3096. [Google Scholar] [CrossRef] [PubMed]
- Rajeshkumar, G.; Arvindh Seshadri, S.; Devnani, G.L.; Sanjay, M.R.; Siengchin, S.; Prakash Maran, J.; Al-Dhabi, N.A.; Karuppiah, P.; Mariadhas, V.A.; Sivarajasekar, N.; et al. Environment Friendly, Renewable and Sustainable Poly Lactic Acid (PLA) Based Natural Fiber Reinforced Composites—A Comprehensive Review. J. Clean. Prod. 2021, 310, 127483. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Meng, L.; Li, C.; Liu, M.; Meng, L. Study of PLA-Based Wood-Plastic Composites. J. Phys. Conf. Ser. 2022, 2437, 012029. [Google Scholar] [CrossRef]
- Ashothaman, A.; Sudha, J.; Senthilkumar, N. A Comprehensive Review on Biodegradable Polylactic Acid Polymer Matrix Composite Material Reinforced with Synthetic and Natural Fibers. Mater. Today Proc. 2023, 80, 2829–2839. [Google Scholar] [CrossRef]
- Vatansever, E.; Arslan, D.; Nofar, M. Polylactide Cellulose-Based Nanocomposites. Int. J. Biol. Macromol. 2019, 137, 912–938. [Google Scholar] [CrossRef] [PubMed]
- Shen, R.; Xue, S.; Xu, Y.; Liu, Q.; Feng, Z.; Ren, H.; Zhai, H. Research Progress and Development Demand of Nanocellulose Reinforced Polymer Composites. Polymers 2020, 12, 2113. [Google Scholar] [CrossRef] [PubMed]
- Bikiaris, N.D.; Koumentakou, I.; Samiotaki, C.; Meimaroglou, D.; Varytimidou, D.; Karatza, A.; Kalantzis, Z.; Roussou, M.; Bikiaris, R.D.; Papageorgiou, G.Z. Recent Advances in the Investigation of Poly(Lactic Acid) (PLA) Nanocomposites: Incorporation of Various Nanofillers and Their Properties and Applications. Polymers 2023, 15, 1196. [Google Scholar] [CrossRef]
- Parvathy, S.U.; Hema, S.; Sajith, M.; Sulthan, R.; Sreelekshmi, C.; Sambhudevan, S.; Shankar, B. A Review on Barrier Properties of Nanocellulose and Polylactic Acid Composites. IOP Conf. Ser. Mater. Sci. Eng. 2022, 1258, 012017. [Google Scholar] [CrossRef]
- Nicharat, A.; Sapkota, J.; Foster, E.J. Pre-mixing and masterbatch approaches for reinforcing poly(vinyl acetate) with cellulose based fillers. Ind. Crop. Prod. 2016, 93, 244–250. [Google Scholar] [CrossRef]
- Fernandes Diniz, J.M.B.; Gil, M.H.; Castro, J.A.A.M. Hornification—Its Origin and Interpretation in Wood Pulps. Wood Sci. Technol. 2004, 37, 489. [Google Scholar] [CrossRef]
- Silva, L.E.; dos Santos, A.d.A.; Torres, L.; McCaffrey, Z.; Klamczynski, A.; Glenn, G.; de Sena Neto, A.R.; Wood, D.; Williams, T.; Orts, W.; et al. Redispersion and structural change evaluation of dried microfibrillated cellulose. Carbohydr. Polym. 2021, 252, 117165. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yao, Q.; Liu, J.; Sun, J.; Zhu, Q.; Chen, H. Processing nanocellulose to bulk materials: A review. Cellulose 2019, 26, 13–14. [Google Scholar] [CrossRef]
- Mokhena, T.C.; Sefadi, J.S.; Sadiku, E.R.; John, M.J.; Mochane, M.J.; Mtibe, A. Thermoplastic Processing of PLA/Cellulose Nanomaterials Composites. Polymers 2018, 10, 1363. [Google Scholar] [CrossRef] [PubMed]
- Aigaje, E.; Riofrio, A.; Baykara, H. Processing, Properties, Modifications, and Environmental Impact of Nanocellulose/Biopolymer Composites: A Review. Polymers 2023, 15, 1219. [Google Scholar] [CrossRef] [PubMed]
- Jonoobi, M.; Harun, J.; Mathew, A.P.; Oksman, K. Mechanical Properties of Cellulose Nanofiber (CNF) Reinforced Polylactic Acid (PLA) Prepared by Twin Screw Extrusion. Compos. Sci. Technol. 2010, 70, 1742–1747. [Google Scholar] [CrossRef]
- Oksman, K.; Aitomäki, Y.; Mathew, A.P.; Siqueira, G.; Zhou, Q.; Butylina, S.; Tanpichai, S.; Zhou, X.; Hooshmand, S. Review of the Recent Developments in Cellulose Nanocomposite Processing. Compos. Part A Appl. Sci. Manuf. 2016, 83, 2–18. [Google Scholar] [CrossRef]
- Olonisakin, K.; Fan, M.; Xin-Xiang, Z.; Ran, L.; Lin, W.S.; Zhang, W.; Wenbin, Y. Key Improvements in Interfacial Adhesion and Dispersion of Fibers/Fillers in Polymer Matrix Composites; Focus on PLA Matrix Composites. Compos. Interfaces 2022, 29, 1071–1120. [Google Scholar] [CrossRef]
- Bovi, J.; Butto, M.; Arroyo, S.; Bernal, C.; Foresti, M.L. Bacterial Cellulose Nanofibrils from a By-Product of the Growing Kombucha Business: Obtention and Use in the Development of Poly (Lactic Acid) Composites. Lat. Am. Appl. Res. 2024, 54, 55–62. [Google Scholar] [CrossRef]
- Delgado, J.F.; de la Osa, O.; Salvay, A.G.; Cavallo, E.; Cerrutti, P.; Foresti, M.L.; Peltzer, M.A. Reinforcement of Yeast Biomass Films with Bacterial Cellulose and Rice Husk Cellulose Nanofibres. J. Polym. Environ. 2021, 29, 3242–3251. [Google Scholar] [CrossRef]
- Ávila Ramírez, J.A.; Juan Suriano, C.; Cerrutti, P.; Foresti, M.L. Surface Esterification of Cellulose Nanofibers by a Simple Organocatalytic Methodology. Carbohydr. Polym. 2014, 114, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Ilharco, L.M.; Garcia, A.R.; Lopes Da Silva, J.; Vieira Ferreira, L.F. Infrared Approach to the Study of Adsorption on Cellulose: Influence of Cellulose Crystallinity on the Adsorption of Benzophenone. Langmuir 1997, 13, 4126–4132. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.; Martin, A.; Conrad, C. An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- ASTM D638-14; Standard Test Method for Tensile Properties of Plastics. West Conshohocken, PA, USA: ASTM International, 2014.
- Marin, D.C.; Vecchio, A.; Ludueña, L.N.; Fasce, D.; Alvarez, V.A.; Stefani, P.M. Revalorization of Rice Husk Waste as a Source of Cellulose and Silica. Fibers Polym. 2015, 16, 285–293. [Google Scholar] [CrossRef]
- Ludueña, L.; Fasce, D.; Alvarez, V.A.; Stefani, P.M. Nanocellulose from Rice Husk Following Alkaline Treatment to Remove Silica. BioResources 2011, 6, 1440–1453. [Google Scholar] [CrossRef]
- Paramasivan, M.; Kumar, T.S.S.; Chandra, T.S. Microbial synthesis of hydroxyapatite-nanocellulose nanocomposites from symbiotic culture of bacteria and yeast pellicle of fermented kombucha tea. Sustainability 2022, 14, 8144. [Google Scholar] [CrossRef]
- Dima, S.O.; Panaitescu, D.M.; Orban, C.; Ghiurea, M.; Doncea, S.M.; Fierascu, R.C.; Nistor, C.L.; Alexandrescu, E.; Nicolae, C.A.; Trica, B.; et al. Bacterial Nanocellulose from Side-Streams of Kombucha Beverages Production: Preparation and Physical-Chemical Properties. Polymers 2017, 9, 374. [Google Scholar] [CrossRef]
- Johar, N.; Ahmad, I.; Dufresne, A. Extraction, Preparation and Characterization of Cellulose Fibres and Nanocrystals from Rice Husk. Ind. Crop. Prod. 2012, 37, 93–99. [Google Scholar] [CrossRef]
- Zuluaga, R.; Putaux, J.L.; Cruz, J.; Vélez, J.; Mondragon, I.; Gañán, P. Cellulose Microfibrils from Banana Rachis: Effect of Alkaline Treatments on Structural and Morphological Features. Carbohydr. Polym. 2009, 76, 51–59. [Google Scholar] [CrossRef]
- Delgado, J.F. Desarrollo de Películas Multicomponentes a Base de Levadura. Evaluación de Sus Propiedades Físicas y Aplicaciones Potenciales. Ph.D. Thesis, Universidad Nacional de Quilmes, Buenos Aires, Argentina, 2021. [Google Scholar]
- Ramírez Tapias, Y.A.; Peltzer, M.A.; Delgado, J.F.; Salvay, A.G. Kombucha Tea By-Product as Source of Novel Materials: Formulation and Characterization of Films. Food Bioprocess. Technol. 2020, 13, 1166. [Google Scholar] [CrossRef]
- Castro-Guerrero, C.F.; Díaz-Guillén, M.R.; Delgado-Arroyo, F.; Rodas-Grapain, A.; Godavarthi, S. Purification of Cellulose from Rice Husk for the Synthesis of Nanocellulose. In Proceedings of the 16th International Conference of Nanotechnology, IEEE NANO 2016, Sendai, Japan, 22–25 August 2016. [Google Scholar]
- Jonoobi, M.; Mathew, A.P.; Abdi, M.M.; Makinejad, M.D.; Oksman, K. A Comparison of Modified and Unmodified Cellulose Nanofiber Reinforced Polylactic Acid (PLA) Prepared by Twin Screw Extrusion. J. Polym. Environ. 2012, 20, 991–997. [Google Scholar] [CrossRef]
- Ávila Ramírez, J.A.; Bovi, J.; Bernal, C.; Errea, M.I.; Foresti, M.L. Development of Poly(Lactic Acid) Nanocomposites Reinforced with Hydrophobized Bacterial Cellulose. J. Polym. Environ. 2020, 28, 61–73. [Google Scholar] [CrossRef]
- Park, M.E.; Baker, S.; Himmel, J.O.; Parilla, P.A.; Johnson, D.K. Cellulose crystallinity index: Measurement techniques and their impact on interpreting cellulase performance. Biotechnol. Biofuels 2010, 3, 10. [Google Scholar] [CrossRef] [PubMed]
- Arteaga-Ballesteros, B.E.; Guevara-Morales, A.; Martín-Martínez, E.S.; Figueroa-López, U.; Vieyra, H. Composite of Polylactic Acid and Microcellulose from Kombucha Membranes. E-Polymers 2021, 20, 15–26. [Google Scholar] [CrossRef]
- Goh, W.N.; Rosma, A.; Kaur, B.; Fazilah, A.; Karim, A.A.; Bhat, R. Microstructure and Physical Properties of Microbial Cellulose Produced during Fermentation of Black Tea Broth (Kombucha). II. Int. Food Res. J. 2012, 19, 153–158. [Google Scholar]
- Oliver-Ortega, H.; Geng, S.; Espinach, F.X.; Oksman, K.; Vilaseca, F. Bacterial Cellulose Network from Kombucha Fermentation Impregnated with Emulsion-Polymerized Poly(Methyl Methacrylate) to Form Nanocomposite. Polymers 2021, 13, 664. [Google Scholar] [CrossRef]
- Tomé, L.C.; Pinto, R.J.B.; Trovatti, E.; Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P.; Gandini, A. Transparent Bionanocomposites with Improved Properties Prepared from Acetylated Bacterial Cellulose and Poly(Lactic Acid) through a Simple Approach. Green Chem. 2011, 13, 419–427. [Google Scholar] [CrossRef]
- Cunha, A.G.; Zhou, Q.; Larsson, P.T.; Berglund, L.A. Topochemical Acetylation of Cellulose Nanopaper Structures for Biocomposites: Mechanisms for Reduced Water Vapour Sorption. Cellulose 2014, 21, 2773–2787. [Google Scholar] [CrossRef]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindström, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A New Family of Nature-Based Materials. Angew. Chem.-Int. Ed. 2011, 50, 5438–5466. [Google Scholar] [CrossRef]
- Tingaut, P.; Zimmermann, T.; Lopez-Suevos, F. Synthesis and characterization of bionanocomposites with tunable properties from poly(lactic acid) and acetylated microfibrillated cellulose. Biomacromolecules 2010, 11, 454–464. [Google Scholar] [CrossRef]
- Yang, W.; Weng, Y.; Puglia, D.; Qi, G.; Dong, W.; Kenny, J.M.; Ma, P. Poly(lactic acid)/lignin films with enhanced toughness and anti-oxidation performance for active food packaging. Int. J. Biol. Macromol. 2020, 144, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Hervy, M.; Blaker, J.J.; Braz, A.L.; Lee, K.Y. Mechanical response of multi-layer bacterial cellulose nanopaper reinforced polylactide laminated composites. Compos. Part A 2018, 107, 155–163. [Google Scholar] [CrossRef]
- Quero, F.; Nogi, M.; Yano, H.; Abdulsalami, K.; Holmes, S.M.; Sakakini, B.H.; Eichhorn, S.J. Optimization of the Mechanical Performance of Bacterial Cellulose/Poly(L-Lactic) Acid Composites. ACS Appl. Mater. Interfaces 2010, 2, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, W.; Ye, B.; Lin, Z.; Rong, J. Studies on Confined Crystallization Behavior of Nanobiocomposites Consisting of Acetylated Bacterial Cellulose and Poly (Lactic Acid). J. Thermoplast. Compos. Mater. 2013, 26, 346–361. [Google Scholar] [CrossRef]
- Fortunati, E.; Armentano, I.; Iannoni, A.; Barbale, M.; Zaccheo, S.; Scavone, M.; Visai, L.; Kenny, J.M. New multifunctional poly (lactide acid) composites: Mechanical, antibacterial, and degradation properties. J. Appl. Polym. Sci. 2012, 124, 87–98. [Google Scholar] [CrossRef]
- Mathew, A.P.; Oksman, K.; Sain, M. Mechanical properties of biodegradable composites from poly lactic acid (PLA) and microcrystalline cellulose (MCC). J. Appl. Polym. Sci. 2005, 97, 2014–2025. [Google Scholar] [CrossRef]
- Bagheriasl, D.; Safdari, F.; Carreau, P.J.; Dubois, C.; Riedl, B. Development of Cellulose Nanocrystal-Reinforced Polylactide: A Comparative Study on Different Preparation Methods. Polym. Compos. 2017, 40, 342–350. [Google Scholar] [CrossRef]
- Patwa, R.; Saha, N.; Sáha, P.; Katiyar, V. Biocomposites of Poly(Lactic Acid) and Lactic Acid Oligomer-Grafted Bacterial Cellulose: It’s Preparation and Characterization. J. Appl. Polym. Sci. 2019, 136, 47903. [Google Scholar] [CrossRef]
- Oliveira, A.C.S.D.; Ribeiro, M.N.; Ugucioni, J.C.; Rocha, R.A.D.; Borges, S.V. Consumer perception of biodegradable packaging for food. Polímeros 2024, 33, e20230045. [Google Scholar] [CrossRef]
- Móczó, J.; Pukánszky, B. Polymer micro and nanocomposites: Structure, interactions, properties. J. Ind. Eng. Chem. 2008, 14, 535–563. [Google Scholar] [CrossRef]
- Kale, R.D.; Gorade, V.G.; Madye, N.; Chaudhary, B.; Bangde, P.S.; Dandekar, P.P. Preparation and Characterization of Biocomposite Packaging Film from Poly(Lactic Acid) and Acylated Microcrystalline Cellulose Using Rice Bran Oil. Int. J. Biol. Macromol. 2018, 118, 1090–1102. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Li, S.; Gao, M.; Xing, X.; Tian, Y.; Ling, Z.; Yang, W.; Pan, L.; Fan, W.; Zheng, Y. Preparation and Characterization of Microcrystalline Cellulose/Polylactic Acid Biocomposite Films and Its Application in Lanzhou Lily (Lilium davidii var. unicolor) Bulbs Preservation. Sustainability 2023, 15, 13770. [Google Scholar] [CrossRef]
- Johari, A.P.; Kurmvanshi, S.K.; Mohanty, S.; Nayak, S.K. Influence of Surface Modified Cellulose Microfibrils on the Improved Mechanical Properties of Poly (Lactic Acid). Int. J. Biol. Macromol. 2016, 84, 329–339. [Google Scholar] [CrossRef]
- Sucinda, E.F.; Abdul Majid, M.S.; Ridzuan, M.J.M.; Cheng, E.M.; Alshahrani, H.A.; Mamat, N. Development and characterisation of packaging film from Napier cellulose nanowhisker reinforced polylactic acid (PLA) bionanocomposites. Int. J. Biol. Macromol. 2021, 187, 43–53. [Google Scholar] [CrossRef]
- Fortunati, E.; Armentano, I.; Zhou, Q.; Iannoni, A.; Saino, E.; Visai, L.; Berglund, L.A.; Kenny, J.M. Multifunctional bionanocomposite films of poly(lactic acid), cellulose nanocrystals and silver nanoparticles. Carbohydr. Polym. 2012, 87, 1596–1605. [Google Scholar] [CrossRef]



| Composite | PLA (wt.%) | BNC (wt.%) | VNC (wt.%) | AcBNC (wt.%) | AcVNC (wt.%) |
|---|---|---|---|---|---|
| PLA | 100 | - | - | - | - |
| PLA/BNC-D 0.5% | 99.5 | 0.5 | - | - | - |
| PLA/BNC-D 1% | 99 | 1 | - | - | - |
| PLA/BNC-D 1.5% | 98.5 | 1.5 | - | - | - |
| PLA/BNC-D 3% | 97 | 3 | - | - | - |
| PLA/BNC-D 5% | 95 | 5 | - | - | - |
| PLA/BNC-D 7% | 93 | 7 | - | - | - |
| PLA/VNC-D 0.5% | 99.5 | - | 0.5 | - | - |
| PLA/VNC-D 1% | 99 | - | 1 | - | - |
| PLA/VNC-D 1.5% | 98.5 | - | 1.5 | - | - |
| PLA/VNC-D 3% | 97 | - | 3 | - | - |
| PLA/VNC-D 5% | 95 | - | 5 | - | - |
| PLA/VNC-D 7% | 93 | - | 7 | - | - |
| PLA/BNC-MB 0.5% | 99.5 | 0.5 | - | - | - |
| PLA/BNC-MB 1% | 99 | 1 | - | - | - |
| PLA/BNC-MB 1.5% | 98.5 | 1.5 | - | - | - |
| PLA/BNC-MB 3% | 97 | 3 | - | - | - |
| PLA/BNC-MB 5% | 95 | 5 | - | - | - |
| PLA/BNC-MB 7% | 93 | 7 | - | - | - |
| PLA/VNC-MB 0.5% | 99.5 | - | 0.5 | - | - |
| PLA/VNC-MB 1% | 99 | - | 1 | - | - |
| PLA/VNC-MB 1.5% | 98.5 | - | 1.5 | - | - |
| PLA/VNC-MB 3% | 97 | - | 3 | - | - |
| PLA/VNC-MB 5% | 95 | - | 5 | - | - |
| PLA/VNC-MB 7% | 93 | - | 7 | - | - |
| PLA/AcBNC-MB 0.5% | 99.5 | - | - | 0.5 | - |
| PLA/AcBNC-MB 1% | 99 | - | - | 1 | - |
| PLA/AcBNC-MB 1.5% | 98.5 | - | - | 1.5 | - |
| PLA/AcBNC-MB 3% | 97 | - | - | 3 | - |
| PLA/AcBNC-MB 5% | 95 | - | - | 5 | - |
| PLA/AcBNC-MB 7% | 93 | - | - | 7 | - |
| PLA/AcVNC-MB 0.5% | 99.5 | - | - | - | 0.5 |
| PLA/AcVNC-MB 1% | 99 | - | - | - | 1 |
| PLA/AcVNC-MB 1.5% | 98.5 | - | - | - | 1.5 |
| PLA/AcVNC-MB 3% | 97 | - | - | - | 3 |
| PLA/AcVNC-MB 5% | 95 | - | - | - | 5 |
| PLA/AcVNC-MB 7% | 93 | - | - | - | 7 |
| Composite | L* | a* | b* | ΔE | Composite | L* | a* | b* | ΔE |
|---|---|---|---|---|---|---|---|---|---|
| PLA | 80.8 ± 0.9 | −0.2 ± 0.1 | −1.5 ± 0.6 | - | PLA | 80.8 ± 0.9 | −0.2 ± 0.1 | −1.5 ± 0.6 | - |
| PLA/BNC-D 0.5% | 78.6 ± 1.5 | −0.6 ± 0.5 | −0.5 ± 0.4 | 2.5 | PLA/VNC-D 0.5% | 80.6 ± 0.5 | −0.2 ± 0.0 | −1.9 ± 0.3 | 0.0 |
| PLA/BNC-D 1% | 79.3 ± 0.6 | −0.5 ± 0.0 | −0.8 ± 0.3 | 1.7 | PLA/VNC-D 1% | 79.7 ± 0.5 | −0.2 ± 0.0 | −1.2 ± 0.3 | 1.1 |
| PLA/BNC-D 1.5% | 78.7 ± 1.4 | −0.5 ± 0.0 | 1.9 ± 1.0 | 4.0 | PLA/VNC-D 1.5% | 78.5 ± 0.9 | −0.1 ± 0.1 | −0.7 ± 0.4 | 2.4 |
| PLA/BNC-D 3% | 77.9 ± 1.1 | −0.6 ± 0.0 | 1.9 ± 1.1 | 4.5 | PLA/VNC-D 3% | 76.4 ± 0.8 | 0.0 ± 0.0 | 0.8 ± 0.5 | 5.0 |
| PLA/BNC-D 5% | 79.9 ± 0.5 | −0.5 ± 0.0 | 3.4 ± 0.4 | 5.0 | PLA/VNC-D 5% | 75.5 ± 1.2 | 0.2 ± 0.1 | 2.5 ± 0.8 | 6.7 |
| PLA/BNC-D 7% | 79.5 ± 0.5 | −0.2 ± 0.2 | 6.2 ± 0.9 | 7.8 | PLA/VNC-D 7% | 73.9 ± 1.6 | 0.5 ± 0.2 | 4.4 ± 0.1 | 9.1 |
| PLA/BNC-MB 0.5% | 79.3 ± 1.1 | −0.2 ± 0.0 | −0.2 ± 0.5 | 2.0 | PLA/VNC-MB 0.5% | 80.7 ± 0.8 | −0.2 ± 0.1 | −1.8 ± 0.6 | 0.3 |
| PLA/BNC-MB 1% | 78.1 ± 0.5 | −0.5 ± 0.0 | 1.4 ± 0.4 | 4.0 | PLA/VNC-MB 1% | 79.2 ± 0.8 | −0.3 ± 0.0 | 0.4 ± 0.2 | 2.5 |
| PLA/BNC-MB 1.5% | 75.4 ± 1.5 | −0.5 ± 0.0 | 3.4 ± 0.4 | 7.3 | PLA/VNC-MB 1.5% | 78.9 ± 1.2 | −0.3 ± 0.0 | 0.4 ± 0.8 | 2.7 |
| PLA/BNC-MB 3% | 77.2 ± 0.9 | −0.2 ± 0.2 | 7.3 ± 0.5 | 9.5 | PLA/VNC-MB 3% | 75.6 ± 1.3 | 0.1 ± 0.1 | 4.4 ± 0.8 | 7.9 |
| PLA/BNC-MB 5% | 78.1 ± 1.1 | −0.2 ± 0.1 | 8.2 ± 0.9 | 10.1 | PLA/VNC-MB 5% | 69.3 ± 0.7 | 1.2 ± 0.0 | 10.2 ± 0.3 | 16.5 |
| PLA/BNC-MB 7% | 72.5 ± 1.8 | 2.0 ± 0.4 | 14.5 ± 0.8 | 18.2 | PLA/VNC-MB 7% | 68.8 ± 1.0 | 1.7 ± 0.3 | 11.6 ± 1.0 | 17.9 |
| PLA/AcBNC-MB 0.5% | 79.3 ± 0.5 | −0.3 ± 0.4 | −0.5 ± 0.5 | 1.8 | PLA/AcVNC-MB 0.5% | 81.7 ± 0.5 | −0.3 ± 0.0 | −1.4 ± 0.3 | 0.9 |
| PLA/AcBNC-MB 1% | 77.4 ± 1.2 | 0.2 ± 0.1 | 2.4 ± 1.0 | 5.2 | PLA/AcVNC-MB 1% | 81.1 ± 0.5 | −0.4 ± 0.0 | −1.4 ± 0.3 | 0.4 |
| PLA/AcBNC-MB 1.5% | 78.2 ± 0.9 | −0.2 ± 0.0 | 0.6 ± 0.6 | 3.4 | PLA/AcVNC-MB 1.5% | 80.7 ± 0.7 | −0.3 ± 0.0 | −1.2 ± 0.3 | 0.3 |
| PLA/AcBNC-MB 3% | 73.6 ± 0.5 | 1.0 ± 0.1 | 7.7 ± 0.4 | 11.8 | PLA/AcVNC-MB 3% | 78.9 ± 0.5 | −0.4 ± 0.0 | 0.5 ± 0.4 | 2.8 |
| PLA/AcBNC-MB 5% | 68.6 ± 1.6 | 1.8 ± 0.3 | 12 ± 0.8 | 18.3 | PLA/AcVNC-MB 5% | 77.2 ± 0.5 | −0.3 ± 0.1 | 2.2 ± 0.4 | 5.2 |
| PLA/AcBNC-MB 7% | 66.0 ± 0.7 | 3.0 ± 0.1 | 15.3 ± 0.3 | 27.4 | PLA/AcVNC-MB 7% | 72.8 ± 0.3 | 0.0 ± 0.1 | 6.1 ± 0.3 | 11.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bovi, J.; Delgado, J.F.; de la Osa, O.; Peltzer, M.A.; Bernal, C.R.; Foresti, M.L. Biobased Composites of Poly(Lactic Acid) Melt Compounded with Bacterial and Vegetal Nanocelluloses Incorporated through Different Strategies. Polymers 2024, 16, 898. https://doi.org/10.3390/polym16070898
Bovi J, Delgado JF, de la Osa O, Peltzer MA, Bernal CR, Foresti ML. Biobased Composites of Poly(Lactic Acid) Melt Compounded with Bacterial and Vegetal Nanocelluloses Incorporated through Different Strategies. Polymers. 2024; 16(7):898. https://doi.org/10.3390/polym16070898
Chicago/Turabian StyleBovi, Jimena, Juan Francisco Delgado, Orlando de la Osa, Mercedes Ana Peltzer, Celina Raquel Bernal, and María Laura Foresti. 2024. "Biobased Composites of Poly(Lactic Acid) Melt Compounded with Bacterial and Vegetal Nanocelluloses Incorporated through Different Strategies" Polymers 16, no. 7: 898. https://doi.org/10.3390/polym16070898
APA StyleBovi, J., Delgado, J. F., de la Osa, O., Peltzer, M. A., Bernal, C. R., & Foresti, M. L. (2024). Biobased Composites of Poly(Lactic Acid) Melt Compounded with Bacterial and Vegetal Nanocelluloses Incorporated through Different Strategies. Polymers, 16(7), 898. https://doi.org/10.3390/polym16070898








