Microbially Mediated Rubber Recycling to Facilitate the Valorization of Scrap Tires
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experiment Setup for the Microbial Etching of Crumb Rubber
2.3. Chemical Analysis
2.4. Microbial Community DNA Amplicon Sequencing
2.5. Physical Analysis
2.6. Modification of Bitumen
2.7. Fourier Transform Infrared Spectroscopy (FT-IR)
2.8. Dynamic Shear Rheometer
2.9. Phase Separation Analysis
2.10. Low-Temperature Cracking
3. Results and Discussion
3.1. Microbial Community Analysis
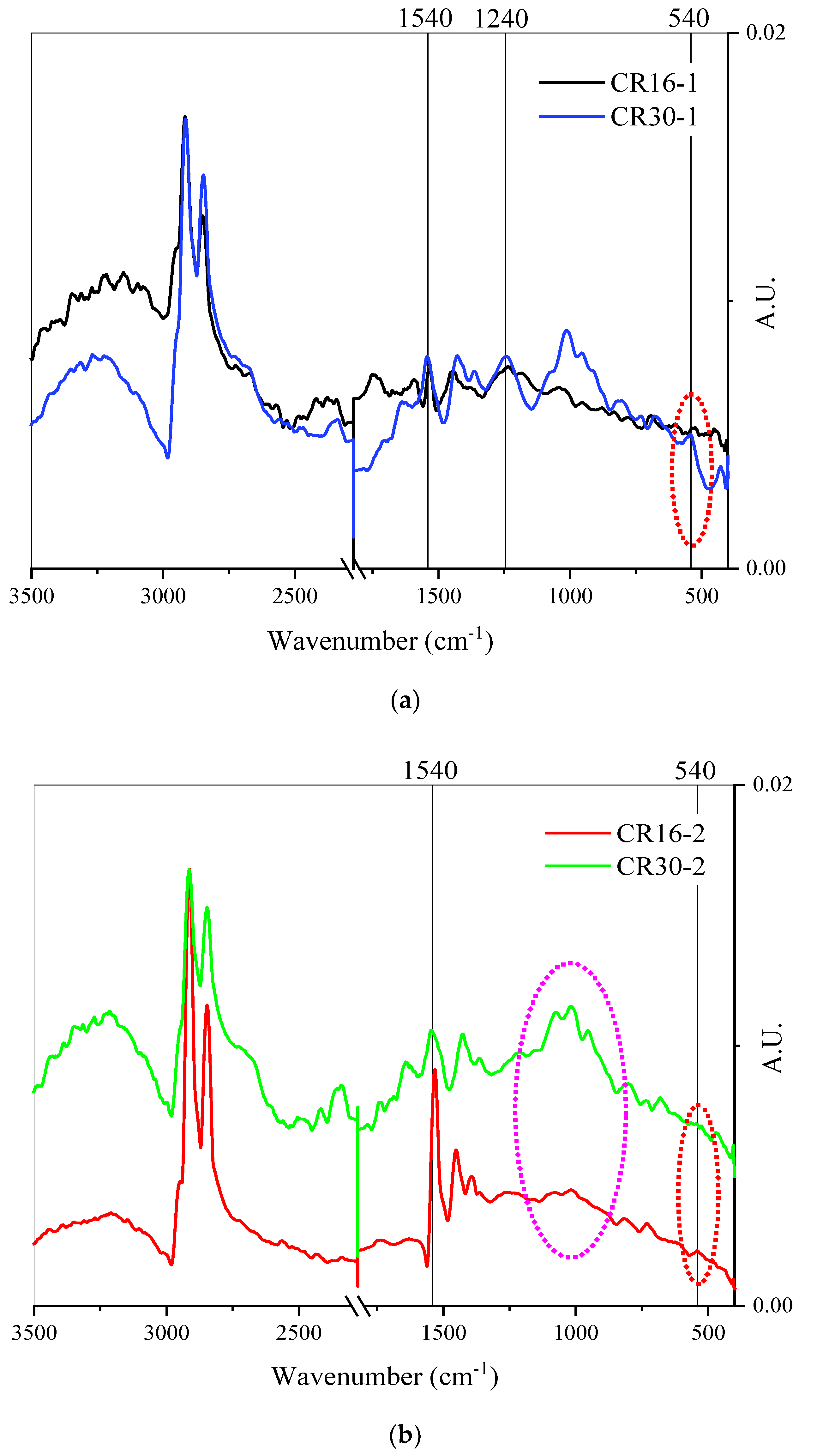
3.2. Chemical Analysis
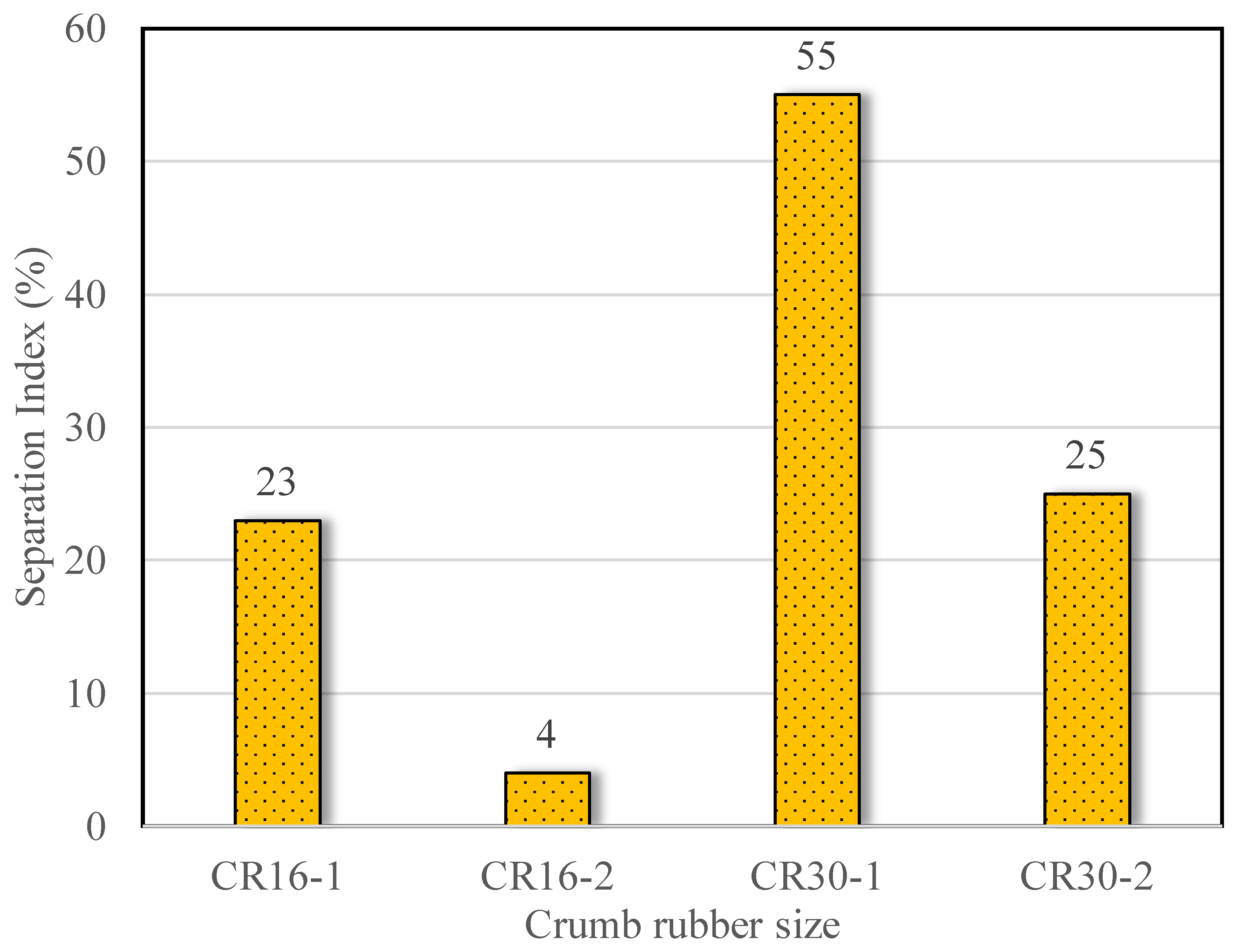
3.3. Phase Separation
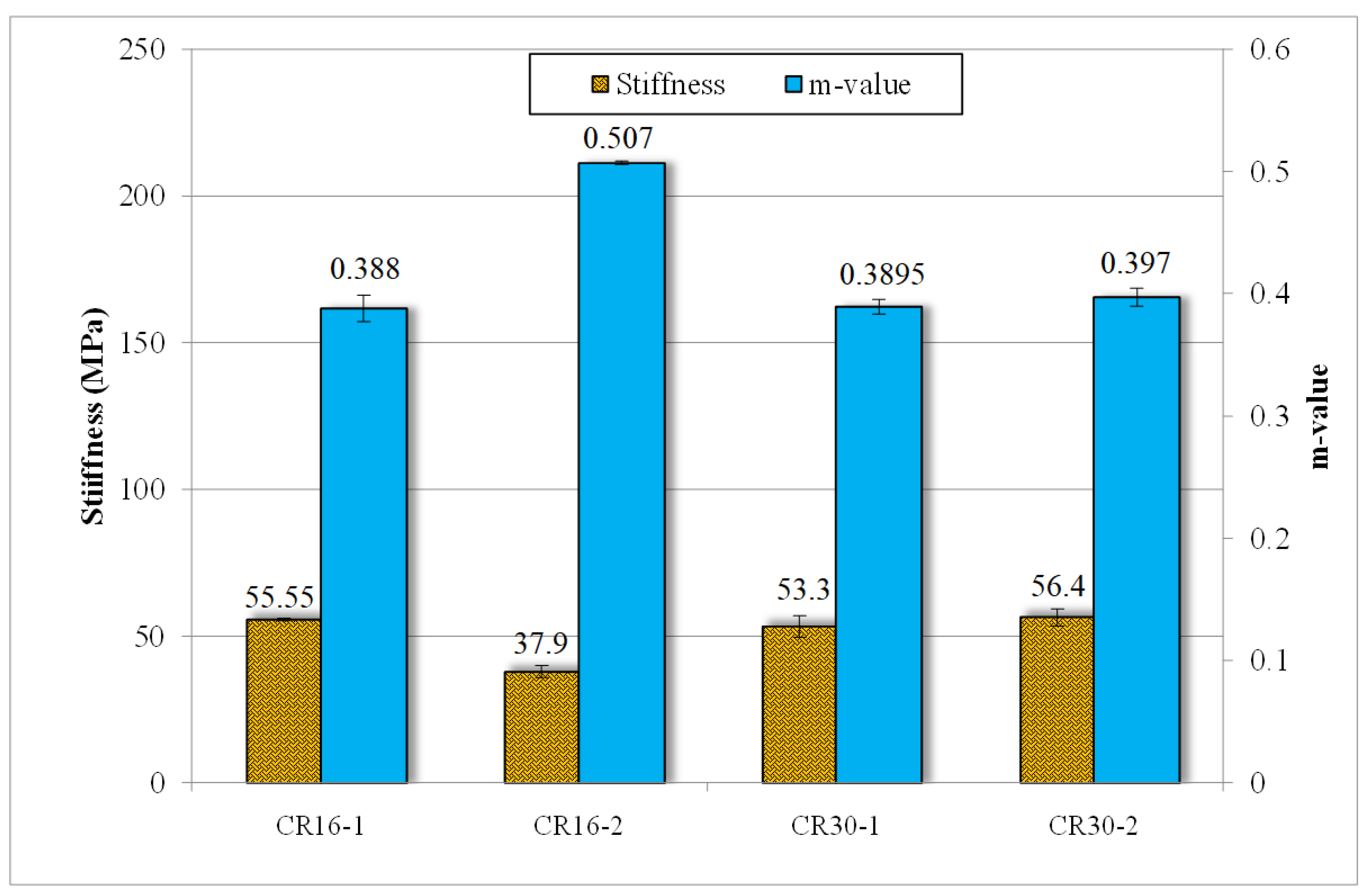
3.4. Rheological Analysis
3.5. Low-Temperature Performance
3.6. Industry Implications and Carbon Management Perspective
4. Conclusions
- The surface chemistry of rubber particles changed after microbial treatment, as shown by the reduction in peak intensity at 500–540 cm−1 in FTIR spectra.
- The extent of rubber–bitumen separation was greatly reduced after microbial treatment, as deduced from the 55 to 82% reductions in the Segregation Index in the studied particle sizes (0.6 and 1.18 mm).
- Bitumen containing treated rubber (CR16-2) showed a reduction in percentage recovery, indicating reduced elasticity of rubber particles; this can be attributed to the breakage of the sulfur crosslinks of the rubber after microbial treatment. This was evidenced by the high release of sulfur in the process.
- The enrichment of the known rubber degraders Gordonia and Nocardia, and other microorganisms containing rubber oxygenases, supported chemical and physical evidence of microbial treatment.
- Bitumen containing treated rubber (CR16-2) showed an increased resistance to fatigue cracking, indicating the contribution of rubber polymers to bitumen’s mechanical properties.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Biligiri, K.P. Effect of pavement materials’ damping properties on tyre/road noise characteristics. Constr. Build. Mater. 2013, 49, 223–232. [Google Scholar] [CrossRef]
- Liang, M.; Xin, X.; Fan, W.; Ren, S.; Shi, J.; Luo, H. Thermo-stability and aging performance of modified asphalt with crumb rubber activated by microwave and TOR. Mater. Des. 2017, 127, 84–96. [Google Scholar] [CrossRef]
- Rath, P.; Gettu, N.; Chen, S.; Buttlar, W.G. Investigation of cracking mechanisms in rubber-modified asphalt through fracture testing of mastic specimens. Road Mater. Pavement Des. 2021, 23, 1544–1563. [Google Scholar] [CrossRef]
- Hosseinnezhad, S.; Kabir, S.F.; Oldham, D.; Mousavi, M.; Fini, E.H. Surface functionalization of rubber particles to reduce phase separation in rubberized asphalt for sustainable construction. J. Clean. Prod. 2019, 225, 82–89. [Google Scholar] [CrossRef]
- Rasool, R.T.; Wang, S.; Zhang, Y.; Li, Y.; Zhang, G. Improving the aging resistance of SBS modified asphalt with the addition of highly reclaimed rubber. Constr. Build. Mater. 2017, 145, 126–134. [Google Scholar] [CrossRef]
- Yu, G.-X.; Li, Z.-M.; Zhou, X.-L.; Li, C.-L. Crumb rubber–modified asphalt: Microwave treatment effects. Pet. Sci. Technol. 2011, 29, 411–417. [Google Scholar] [CrossRef]
- Shu, X.; Huang, B. Recycling of waste tire rubber in asphalt and portland cement concrete: An overview. Constr. Build. Mater. 2014, 67, 217–224. [Google Scholar] [CrossRef]
- Xiang, Y.; Xie, Y.; Long, G.; Zeng, L. Ultraviolet irradiation of crumb rubber on mechanical performance and mechanism of rubberised asphalt. Road Mater. Pavement Des. 2019, 20, 1624–1637. [Google Scholar] [CrossRef]
- Li, J.; Xiao, F.; Amirkhanian, S.N. High temperature rheological characteristics of plasma-treated crumb rubber modified binders. Constr. Build. Mater. 2020, 236, 117614. [Google Scholar] [CrossRef]
- Xiao, F.; Yao, S.; Wang, J.; Wei, J.; Amirkhanian, S. Physical and chemical properties of plasma treated crumb rubbers and high temperature characteristics of their rubberised asphalt binders. Road Mater. Pavement Des. 2020, 21, 587–606. [Google Scholar] [CrossRef]
- Yin, L.; Yang, X.; Shen, A.; Wu, H.; Lyu, Z.; Li, B. Mechanical properties and reaction mechanism of microwave-activated crumb rubber-modified asphalt before and after thermal aging. Constr. Build. Mater. 2021, 267, 120773. [Google Scholar] [CrossRef]
- Kabir, S.F.; Mousavi, M.; Fini, E.H. Selective adsorption of bio-oils’ molecules onto rubber surface and its effects on stability of rubberized asphalt. J. Clean. Prod. 2020, 252, 119856. [Google Scholar] [CrossRef]
- Kocevski, S.; Yagneswaran, S.; Xiao, F.; Punith, V.; Smith, D.W., Jr.; Amirkhanian, S. Surface modified ground rubber tire by grafting acrylic acid for paving applications. Constr. Build. Mater. 2012, 34, 83–90. [Google Scholar] [CrossRef]
- Liu, B.; Li, J.; Han, M.; Zhang, Z.; Jiang, X. Properties of polystyrene grafted activated waste rubber powder (PS-ARP) composite SBS modified asphalt. Constr. Build. Mater. 2020, 238, 117737. [Google Scholar] [CrossRef]
- Kabir, S.F.; Zheng, R.; Delgado, A.G.; Fini, E.H. Use of microbially desulfurized rubber to produce sustainable rubberized bitumen. Resour. Conserv. Recycl. 2021, 164, 105144. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, S.; Wang, Y. Microbial desulfurization of ground tire rubber by Thiobacillus ferrooxidans. Polym. Degrad. Stab. 2011, 96, 1662–1668. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, S.; Wang, Y. Microbial desulfurization of ground tire rubber by Sphingomonas sp.: A novel technology for crumb rubber composites. J. Polym. Environ. 2012, 20, 372–380. [Google Scholar] [CrossRef]
- Allan, K.M.; Bedzo, O.K.K.; van Rensburg, E.; Görgens, J.F. The Microbial Devulcanisation of Waste Ground Tyre Rubber Using At. ferrooxidans DSMZ 14,882 and an Unclassified Sulphur-Oxidising Consortium. Waste Biomass Valorization 2021, 12, 6659–6670. [Google Scholar] [CrossRef]
- Cui, X.; Zhao, S.; Wang, B. Microbial desulfurization for ground tire rubber by mixed consortium-Sphingomonas sp. and Gordonia sp. Polym. Degrad. Stab. 2016, 128, 165–171. [Google Scholar] [CrossRef]
- Ghavipanjeh, F.; Ziaei Rad, Z.; Pazouki, M. Devulcanization of ground tires by different strains of bacteria: Optimization of culture condition by taguchi method. J. Polym. Environ. 2018, 26, 3168–3175. [Google Scholar] [CrossRef]
- Kaewpetch, B.; Prasongsuk, S.; Poompradub, S. Devulcanization of natural rubber vulcanizates by Bacillus cereus TISTR 2651. Express Polym. Lett. 2019, 13, 877–888. [Google Scholar] [CrossRef]
- Kim, J.K.; Park, J.W. The biological and chemical desulfurization of crumb rubber for the rubber compounding. J. Appl. Polym. Sci. 1999, 72, 1543–1549. [Google Scholar] [CrossRef]
- Tatangelo, V.; Mangili, I.; Caracino, P.; Bestetti, G.; Collina, E.; Anzano, M.; Branduardi, P.; Posteri, R.; Porro, D.; Lasagni, M.; et al. Microbial desulfurization of ground tire rubber (GTR): Characterization of microbial communities and rheological and mechanical properties of GTR and natural rubber composites (GTR/NR). Polym. Degrad. Stab. 2019, 160, 102–109. [Google Scholar] [CrossRef]
- Jiang, G.; Zhao, S.; Li, W.; Luo, J.; Wang, Y.; Zhou, Q.; Zhang, C. Microbial desulfurization of SBR ground rubber by Sphingomonas sp. and its utilization as filler in NR compounds. Polym. Adv. Technol. 2011, 22, 2344–2351. [Google Scholar] [CrossRef]
- Yao, C.; Zhao, S.; Wang, Y.; Wang, B.; Wei, M.; Hu, M. Microbial desulfurization of waste latex rubber with Alicyclobacillus sp. Polym. Degrad. Stab. 2013, 98, 1724–1730. [Google Scholar] [CrossRef]
- Lei, Y.; Wei, Z.; Wang, H.; You, Z.; Yang, X.; Chen, Y. Effect of crumb rubber size on the performance of rubberized asphalt with bio-oil pretreatment. Constr. Build. Mater. 2021, 285, 122864. [Google Scholar] [CrossRef]
- Qian, C.; Fan, W.; Yang, G.; Han, L.; Xing, B.; Lv, X. Influence of crumb rubber particle size and SBS structure on properties of CR/SBS composite modified asphalt. Constr. Build. Mater. 2020, 235, 117517. [Google Scholar] [CrossRef]
- ASTM-D70; Standard Test Method for Density of Semi-Solid Asphalt Binder (Pycnometer Method). ASTM International: West Conshohocken, PA, USA, 2018. Available online: www.astm.org (accessed on 20 August 2023).
- ASTM-D5; Standard Test Method for Penetration of Bituminous Materials. ASTM International: West Conshohocken, PA, USA, 2018. Available online: www.astm.org (accessed on 20 August 2023).
- ASTM-36; Standard Test Method for Softening Point of Bitumen (Ring-and-Ball Apparatus). ASTM International: West Conshohocken, PA, USA, 2018. Available online: www.astm.org (accessed on 20 August 2023).
- ASTM-D113; Standard Test Method for Ductility of Asphalt Materials. ASTM International: West Conshohocken, PA, USA, 2023. Available online: www.astm.org (accessed on 26 December 2023).
- ASTM-D92; Standard Test Method for Flash and Fire Points by Cleveland Open Cup Tester. ASTM International: West Conshohocken, PA, USA, 2018. Available online: www.astm.org (accessed on 20 August 2023).
- ASTM-D6; Standard Test Method for Loss on Heating of Oil and Asphaltic Compounds. ASTM International: West Conshohocken, PA, USA, 2018. Available online: www.astm.org (accessed on 20 August 2023).
- ASTM-D2171; Standard Test Method for Viscosity of Asphalts by Vacuum Capillary Viscometer. ASTM International: West Conshohocken, PA, USA, 2022. Available online: www.astm.org (accessed on 20 August 2023).
- ASTM-D6648; Standard Test Method for Determining the Flexural Creep Stiffness of Asphalt Binder Using the Bending Beam Rheometer (BBR). ASTM International: West Conshohocken, PA, USA, 2018. Available online: www.astm.org (accessed on 20 August 2023).
- Miranda, E.M.; Severson, C.; Reep, J.K.; Hood, D.; Hansen, S.; Santisteban, L.; Hamdan, N.; Delgado, A.G. Continuous-mode acclimation and operation of lignocellulosic sulfate-reducing bioreactors for enhanced metal immobilization from acidic mining-influenced water. J. Hazard. Mater. 2022, 425, 128054. [Google Scholar] [CrossRef] [PubMed]
- Robles, A.; Yellowman, T.L.; Joshi, S.; Rangan, S.M.; Delgado, A.G. Microbial chain elongation and subsequent fermentation of elongated carboxylates as H2-producing processes for sustained reductive dechlorination of chlorinated ethenes. Environ. Sci. Technol. 2021, 55, 10398–10410. [Google Scholar] [CrossRef]
- Joshi, S.; Robles, A.; Aguiar, S.; Delgado, A.G. The occurrence and ecology of microbial chain elongation of carboxylates in soils. ISME J. 2021, 15, 1907–1918. [Google Scholar] [CrossRef]
- Rangan, S.M.; Mouti, A.; LaPat-Polasko, L.; Lowry, G.V.; Krajmalnik-Brown, R.; Delgado, A.G. Synergistic zerovalent Iron (Fe0) and microbiological trichloroethene and perchlorate reductions are determined by the concentration and speciation of Fe. Environ. Sci. Technol. 2020, 54, 14422–14431. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M.; et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012, 6, 1621–1624. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4516–4522. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; Mcmurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Yilmaz, P.; Parfrey, L.W.; Yarza, P.; Gerken, J.; Pruesse, E.; Quast, C.; Schweer, T.; Peplies, J.; Ludwig, W.; Glöckner, F.O. The SILVA and “All-species Living Tree Project (LTP)” taxonomic frameworks. Nucleic Acids Res. 2014, 42, D643–D648. [Google Scholar] [CrossRef] [PubMed]
- ASTM-D7173; Standard Practice for Determining the Separation Tendency of Polymer from Polymer-Modified Asphalt. ASTM International: West Conshohocken, PA, USA, 2020. Available online: www.astm.org (accessed on 20 August 2023).
- AASHTO-M332; Standard Specification for Performance-Graded Asphalt Binder Using Multiple Stress Creep Recovery (MSCR) Test. American Association of State Highway and Transportation Officials (AASHTO): Washington, DC, USA, 2023.
- Shatanawi, K.; Biro, S.; Thodesen, C.; Amirkhanian, S. Effects of water activation of crumb rubber on the properties of crumb rubber-modified binders. Int. J. Pavement Eng. 2009, 10, 289–297. [Google Scholar] [CrossRef]
- Jones, Z.; Romero, P.; VanFrank, K. Development of low-temperature performance specifications for asphalt mixtures using the bending beam rheometer. Road Mater. Pavement Des. 2014, 15, 574–587. [Google Scholar] [CrossRef]
- AASHTO-T-313; Standard Method of Test for Determining the Flexural Creep Stiffness of Asphalt Binder Using the Bending Beam Rheometer (BBR). American Association of State Highway and Transportation Officials (AASHTO): Washington, DC, USA, 2022.
- Andler, R. Bacterial and enzymatic degradation of poly(cis-1,4-isoprene) rubber: Novel biotechnological applications. Biotechnol. Adv. 2020, 44, 107606. [Google Scholar] [CrossRef]
- Basik, A.A.; Sanglier, J.-J.; Yeo, C.T.; Sudesh, K. Microbial degradation of rubber: Actinobacteria. Polymers 2021, 13, 1989. [Google Scholar] [CrossRef] [PubMed]
- Hiessl, S.; Schuldes, J.; Thürmer, A.; Halbsguth, T.; Bröker, D.; Angelov, A.; Liebl, W.; Daniel, R.; Steinbüchel, A. Involvement of two latex-clearing proteins during rubber degradation and insights into the subsequent degradation pathway revealed by the genome sequence of Gordonia polyisoprenivorans strain VH2. Appl. Environ. Microbiol. 2012, 78, 2874–2887. [Google Scholar] [CrossRef] [PubMed]
- Yikmis, M.; Steinbüchel, A. Historical and recent achievements in the field of microbial degradation of natural and synthetic rubber. Appl. Environ. Microbiol. 2012, 78, 4543–4551. [Google Scholar] [CrossRef] [PubMed]
- Jendrossek, D.; Birke, J. Rubber oxygenases. Appl. Microbiol. Biotechnol. 2019, 103, 125–142. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Li, W.; Guo, X.; Qu, Z.; Zhu, X.; Wang, X. Isolation and identification of a carbazole degradation gene cluster from Sphingomonas sp. JS1. World J. Microbiol. Biotechnol. 2009, 25, 1625–1631. [Google Scholar] [CrossRef]
- Friedrich, C.G.; Bardischewsky, F.; Rother, D.; Quentmeier, A.; Fischer, J. Prokaryotic sulfur oxidation. Curr. Opin. Microbiol. 2005, 8, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Bressi, S.; Fiorentini, N.; Huang, J.; Losa, M. Crumb rubber modifier in road asphalt pavements: State of the art and statistics. Coatings 2019, 9, 384. [Google Scholar] [CrossRef]
- Wen, Y.; Liu, Q.; Chen, L.; Pei, J.; Zhang, J.; Li, R. Review and comparison of methods to assess the storage stability of terminal blend rubberized asphalt binders. Constr. Build. Mater. 2020, 258, 119586. [Google Scholar] [CrossRef]
- Asphalt Institute. 2019 Report, LCA of Asphalt Binder, Life Cycle Assessment of Asphalt Binder; Asphalt Institute: Lexington, KY, USA, 2019. [Google Scholar]
- Zhou, T.; Kabir, S.F.; Cao, L.; Luan, H.; Dong, Z.; Fini, E.H. Comparing effects of physisorption and chemisorption of bio-oil onto rubber particles in asphalt. J. Clean. Prod. 2020, 273, 123112. [Google Scholar] [CrossRef]
- Institute of Scrap Recycling Industries. Carbon Footprint of USA Rubber Tire Recycling. 2007. Available online: https://cmshredders.com/wp-content/uploads/2017/03/FinalRubberTireRecyclingCarbonFootprint.pdf (accessed on 20 August 2023).




| Property | Value | Testing Method |
|---|---|---|
| Specific gravity @ 15.6 °C | 1.041 | ASTM D70 [28] |
| Penetration @ 25 °C | 700.1 mm | ASTM D5 [29] |
| Softening point | 46.0 °C | ASTM D36 [30] |
| Ductility @ 15 °C | >100 cm | ASTM D113 [31] |
| Cleveland open-cup method flash point | 335 °C | ASTM D92 [32] |
| Mass change after rolling thin-film oven test | −0.013% | ASTM D6 [33] |
| Absolute viscosity @ 60 °C | 179 Pa·s | ASTM D2171 [34] |
| Stiffness @ −12 °C, 60 s | 85.8 MPa | ASTM D6648 [35] |
| Label | Rubber (g) | DI Water (mL) | Mineral Medium (mL) | WAS (mL) | Sulfate (mM) | Thiosulfate (mM) | Acetate (mM) |
|---|---|---|---|---|---|---|---|
| CR30-1 | 50 | 200 | 0 | 0 | 0 | 0 | 0 |
| CR30-2 | 50 | 0 | 200 | 10 | 0.2 | 6.3 | 25 |
| CR30-3 | 50 | 0 | 200 | 10 | 0.2 | 0 | 25 |
| CR16-1 | 50 | 200 | 0 | 0 | 0 | 0 | 0 |
| CR16-2 | 50 | 0 | 200 | 10 | 0.2 | 6.3 | 25 |
| SC | 0 | 0 | 200 | 10 | 0.2 | 6.3 | 25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kabir, S.F.; Sundar, S.V.; Robles, A.; Miranda, E.M.; Delgado, A.G.; Fini, E.H. Microbially Mediated Rubber Recycling to Facilitate the Valorization of Scrap Tires. Polymers 2024, 16, 1017. https://doi.org/10.3390/polym16071017
Kabir SF, Sundar SV, Robles A, Miranda EM, Delgado AG, Fini EH. Microbially Mediated Rubber Recycling to Facilitate the Valorization of Scrap Tires. Polymers. 2024; 16(7):1017. https://doi.org/10.3390/polym16071017
Chicago/Turabian StyleKabir, Sk Faisal, Skanda Vishnu Sundar, Aide Robles, Evelyn M. Miranda, Anca G. Delgado, and Elham H. Fini. 2024. "Microbially Mediated Rubber Recycling to Facilitate the Valorization of Scrap Tires" Polymers 16, no. 7: 1017. https://doi.org/10.3390/polym16071017
APA StyleKabir, S. F., Sundar, S. V., Robles, A., Miranda, E. M., Delgado, A. G., & Fini, E. H. (2024). Microbially Mediated Rubber Recycling to Facilitate the Valorization of Scrap Tires. Polymers, 16(7), 1017. https://doi.org/10.3390/polym16071017







