Development and Characterization of Electrodes Coated with Plasma-Synthesized Polypyrrole Doped with Iodine, Implanted in the Rat Brain Subthalamic Nucleus
Abstract
1. Introduction
2. Materials and Methods
2.1. Design of the IE
2.2. Abrasion and Synthesis of Polypyrrole Doped with Iodine Coating
2.3. Polymer Characterization
2.3.1. Infrared Spectroscopy by Attenuated Total Reflectance (FTIR-ATR)
2.3.2. X-ray Photoelectron Spectroscopy (XPS)
2.3.3. Raman Spectroscopy
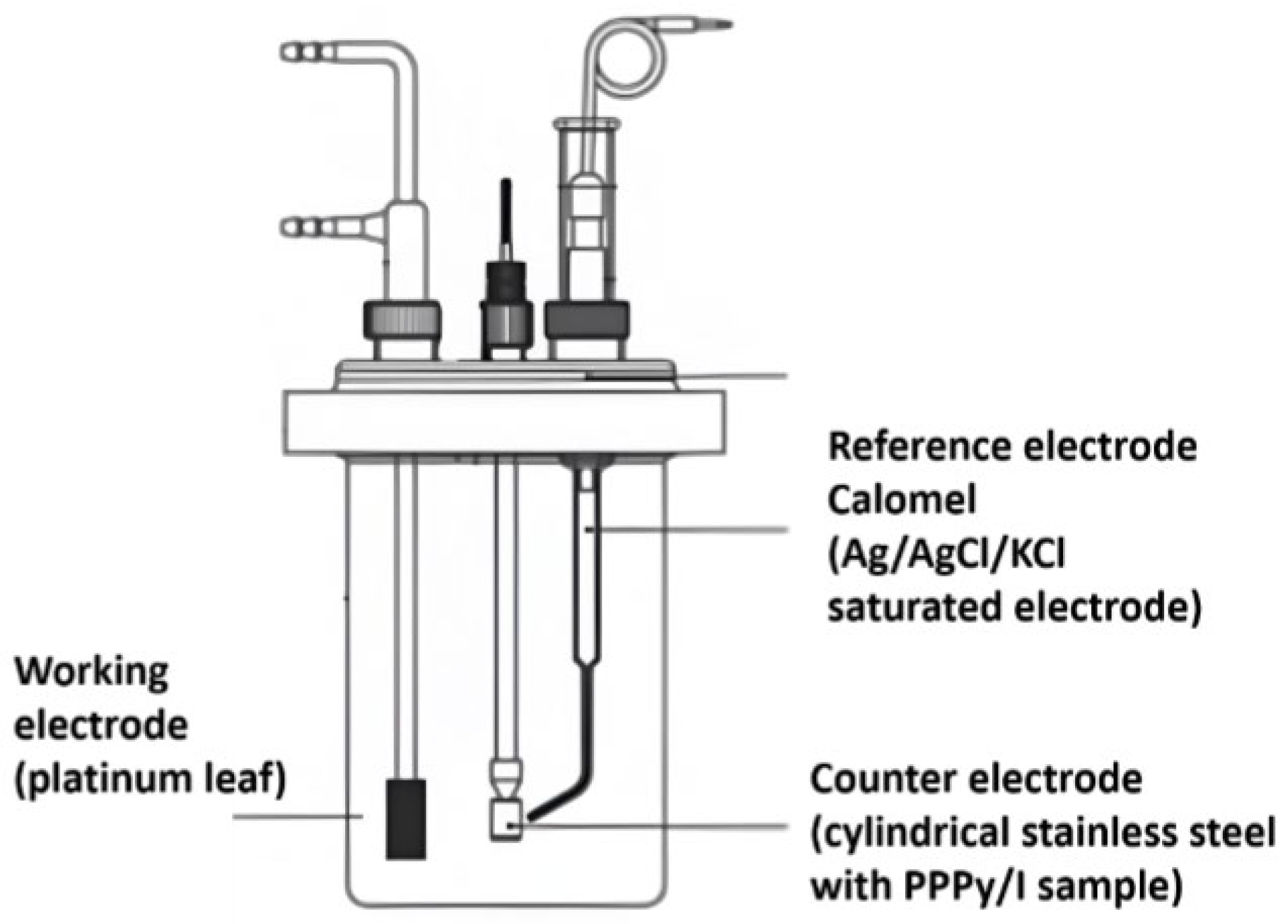
2.3.4. Electrochemical Characterization
2.3.5. Scanning Electron Microscopy
2.4. Biological Characterization
2.4.1. Animals
2.4.2. Implanting of Electrodes in the Subthalamic Nucleus
2.4.3. Electrographic Recordings (EG-R)
2.5. Statistical Analysis
3. Results
3.1. Characterization of the Coating with Plasma-Synthesized Polypyrrole Doped with Iodine
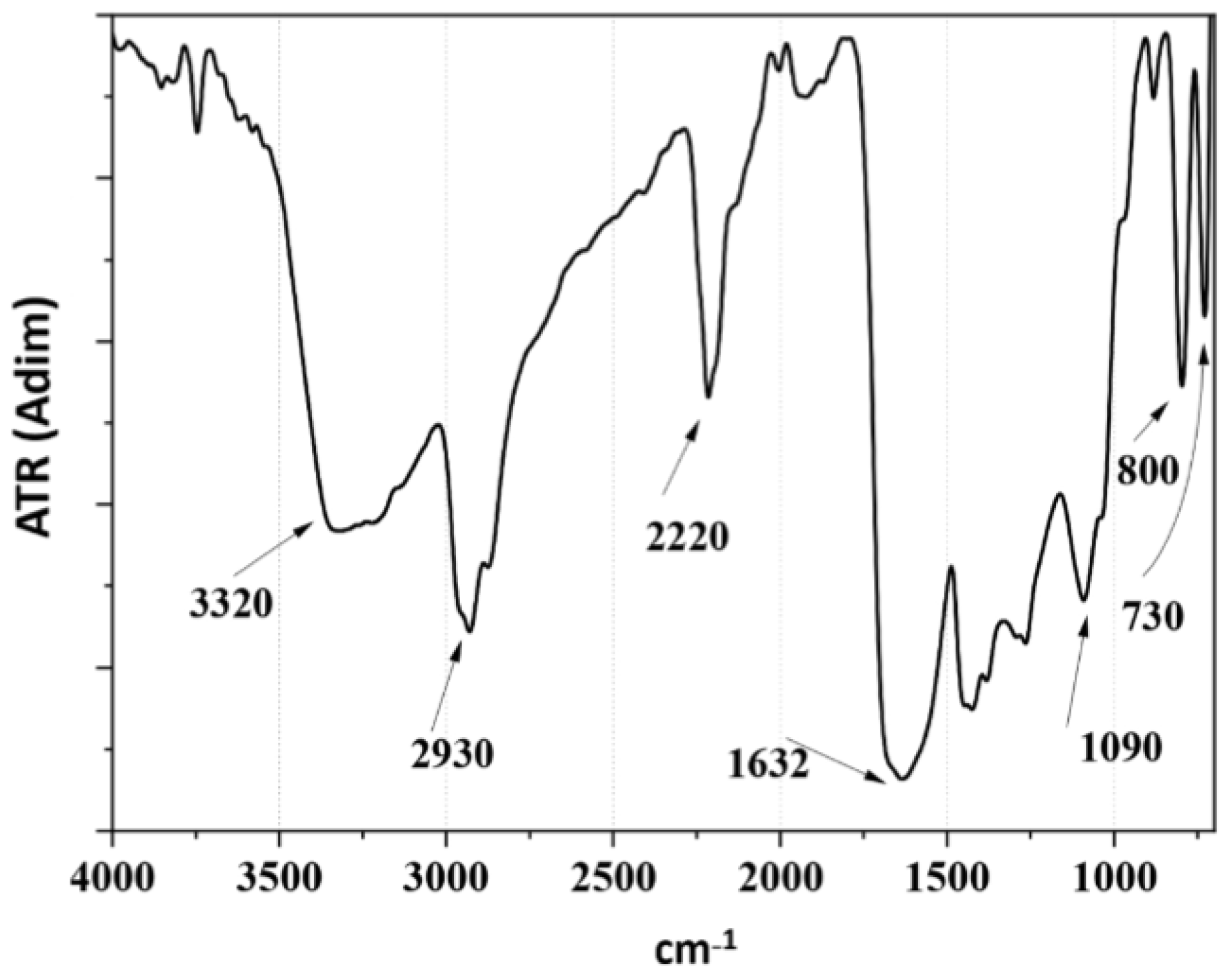
3.2. Structural Analysis by Infrared Spectroscopy (FTIR-ATR)
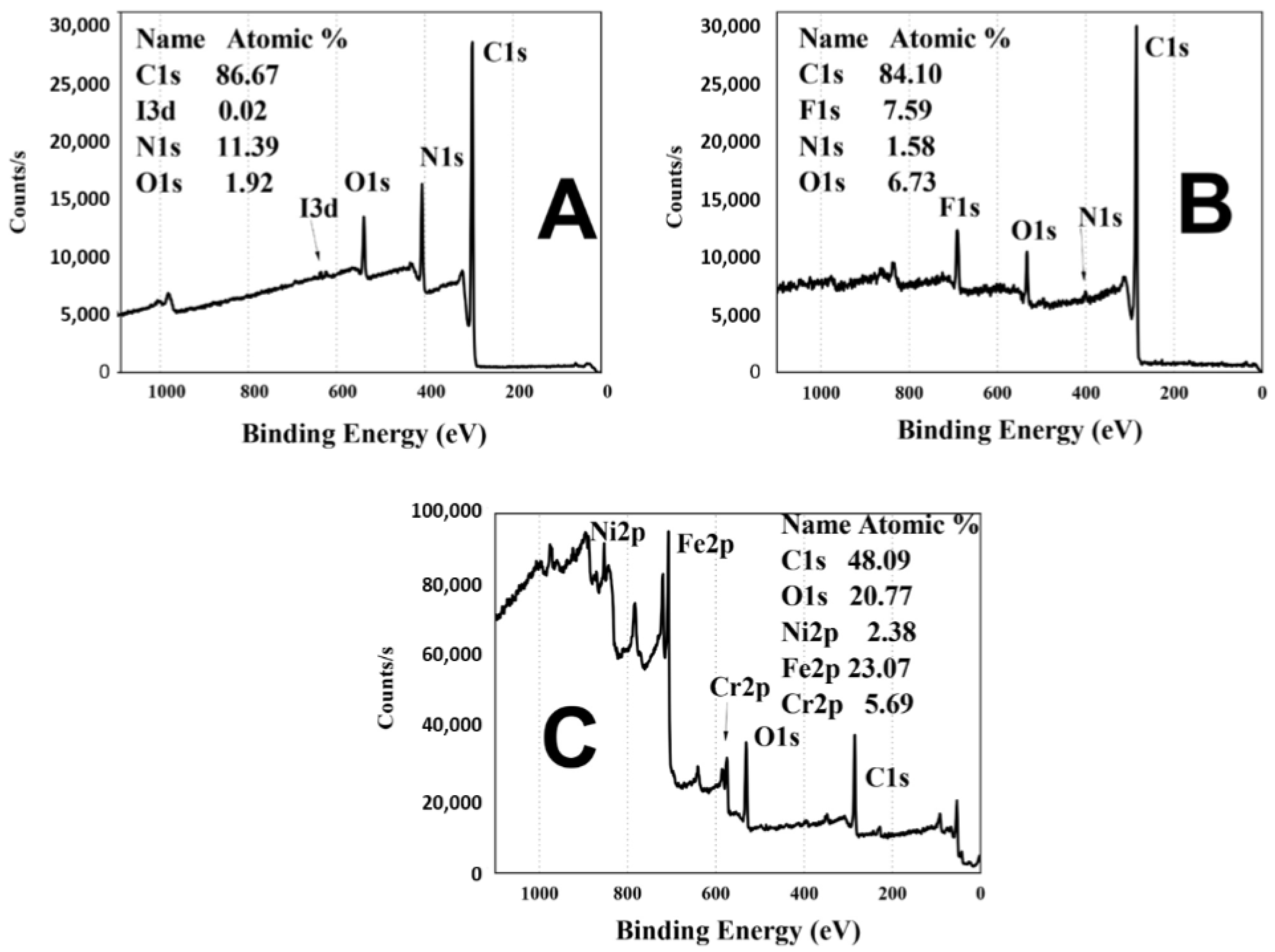
3.3. X-ray Photoelectron Spectroscopy (XPS)
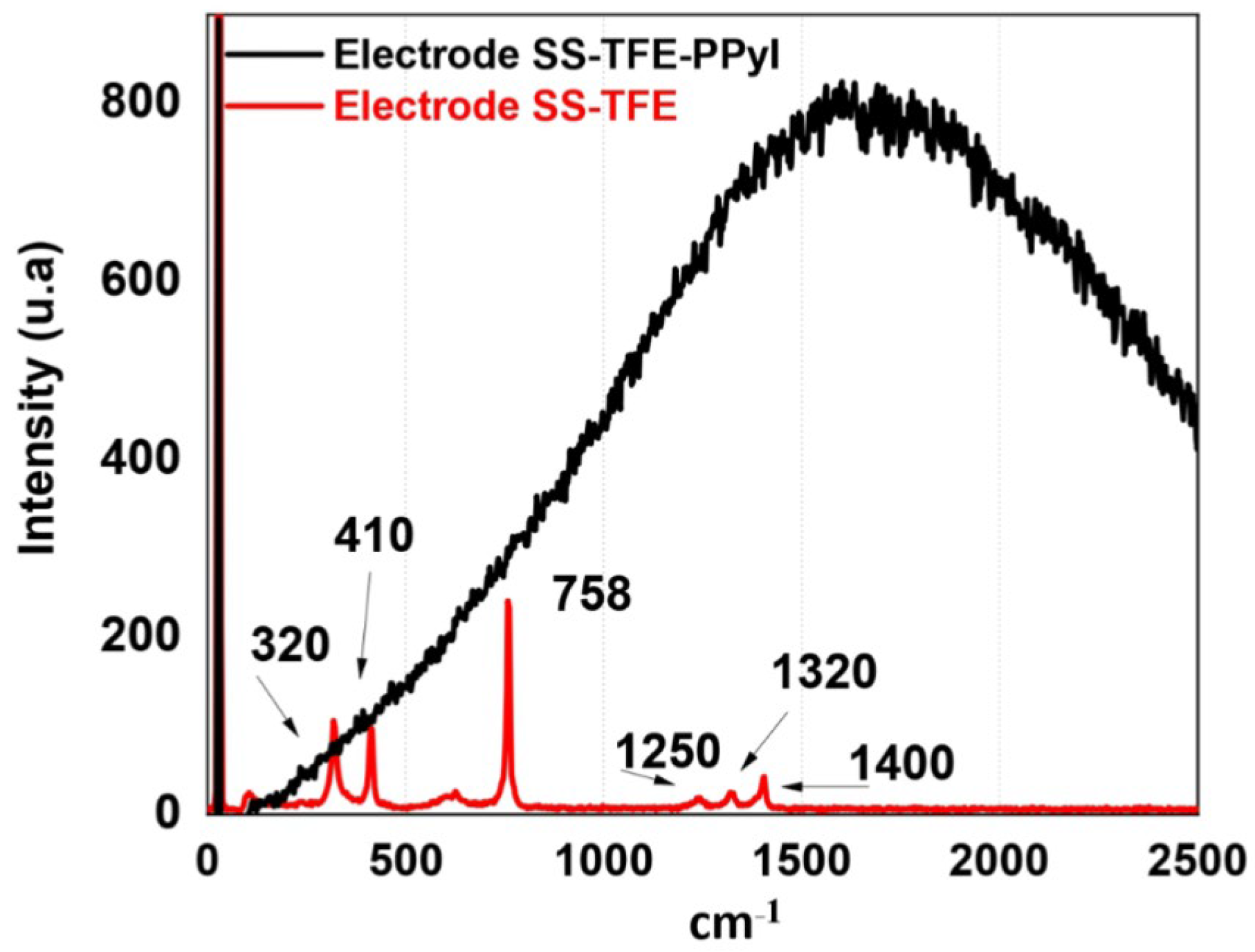
3.4. Raman Spectroscopy
3.5. Electrochemical Characterization
3.6. Scanning Electron Microscopy
3.7. Video-Electrographic Recordings (vEG-Rs) of the Subthalamic Nucleus
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patil, A.C.; Thakor, N.V. Implantable neurotechnologies: A review of micro- and nanoelectrodes for neural recording. Med. Biol. Eng. Comput. 2016, 54, 23–44. [Google Scholar] [CrossRef] [PubMed]
- Saini, M.; Singh, Y.; Arora, P.; Arora, V.; Jain, K. Implant biomaterials: A comprehensive review. World J. Clin. Cases 2015, 3, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Dymond, A.M.; Kaechele, L.E.; Jurist, J.M.; Crandall, P.H. Brain tissue reaction to some chronically implanted metals. J. Neurosurg. 1970, 5, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Merrill, D.R.; Bikson, M.; Jefferys, J.G. Electrical stimulation of excitable tissue: Design of efficacious and safe protocols. J. Neurosci. Methods 2005, 141, 171–198. [Google Scholar] [CrossRef] [PubMed]
- Vernitskaya, T.V.; Efimov, O.N. Polypyrrole: A conducting polymer; its synthesis, properties and applications. Russ. Chem. Rev. 1997, 66, 443–457. [Google Scholar] [CrossRef]
- Alarautalahti, V.; Hiltunen, M.; Onnela, N.; Nymark, S.; Kellomäki, M.; Hyttinen, J. Polypyrrole-coated electrodes show thickness-dependent stability in different conditions during 42-day follow-up in vitro. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 6, 2202–2213. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Jang, L.K.; Park, H.S.; Lee, J.Y. Electrochemical deposition of conductive and adhesive polypyrrole-dopamine films. Sci. Rep. 2016, 6, 30475. [Google Scholar] [CrossRef]
- Bechara, S.; Wadman, L.; Popat, K.C. Electroconductive polymeric nanowire templates facilitates in vitro C17.2 neural stem cell line adhesion, proliferation and differentiation. Acta Biomater. 2011, 7, 2892–2901. [Google Scholar] [CrossRef]
- Kanazawa, K.K.; Diaz, A.F.; Geiss, R.H.; Gill, W.D.; Kwak, J.F.; Logan, J.A.; Rabolt, J.F.; Street, G.B. Organic metals: Polypyrrole, a stable synthetic metallic polymer. J. Chem. Soc. Chem. Commun. 1979, 19, 854–855. [Google Scholar] [CrossRef]
- Martins, J.I.; Bazzaoui, M.; Reis, T.C.; Bazzaoui, E.A.; Martins, L. Electrosynthesis of homogeneous and adherent polypyrrole coatings on iron and steel electrodes by using a new electrochemical procedure. Synth. Met. 2002, 3, 221–228. [Google Scholar] [CrossRef]
- Oliveira, O.N., Jr.; Raposo, M.; Dhanabalan, A. Langmuir-Blodgett and Self-assembled polymeric films. In Handbook of Surfaces and Interfaces of Materials: Solid Thin Films and Layers; Nalwa, H.S., Ed.; Academic Press: Cambridge, MA, USA, 2001; Volume 4, pp. 1–63. [Google Scholar]
- Saleh, T.A. Synthesis of hybrid materials: Methods and classification. In Polymer Hybrid Materials and Nanocomposites: Fundamentals and Applications; Plastics Design Library; Elsevier: Amsterdam, The Netherlands, 2021; pp. 177–212. [Google Scholar]
- Chronakis, I.S.; Grapenson, S.; Jakob, A. Conductive polypyrrole nanofibers via electrospinning: Electrical and morphological properties. Polymer 2006, 47, 1597–1603. [Google Scholar] [CrossRef]
- Cruz, G.J.; Morales, J.; Olayo, R. Films obtained by plasma polymerization of pyrrole. Thin Solid Films 1999, 342, 119–126. [Google Scholar] [CrossRef]
- Rajan, K.; John, D.; Kumar, S. Structural, electrical, and optical studies of plasma-polymerized and iodine-doped poly pyrrole. J. Appl. Polym. Sci. 2002, 83, 1856–1859. [Google Scholar]
- Chung, H.J.; Park, T.G. Surface engineered and drug releasing pre-fabricated scaffolds for tissue engineering. Adv. Drug Deliv. Rev. 2007, 59, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Morales, J.; Olayo, M.G.; Cruz, G.J.; Castillo-Ortega, M.M.; Olayo, R. Electronic conductivity of pyrrole and aniline thin films polymerized by plasma. J. Polym. Sci. Part. B Polym. Phys. 2000, 38, 3247–3255. [Google Scholar] [CrossRef]
- Morales-Guadarrama, A.; Salgado-Ceballos, H.; Grijalva, I.; Morales-Corona, J.; Hernández-Godínez, B.; Ibáñez-Contreras, A.; Ríos, C.; Diaz-Ruiz, A.; Cruz, G.J.; Olayo, M.G.; et al. Evolution of Spinal Cord Transection of Rhesus Monkey Implanted with Polymer Synthesized by Plasma Evaluated by Diffusion Tensor Imaging. Polymers 2022, 14, 962. [Google Scholar] [CrossRef] [PubMed]
- Gómez, L.M.; Olayo, M.G.; Sánchez-Mendieta, V.; González-Torres, M.; González-Salgado, F.; Mondragón-Lozano, R.; Basurto, R.; Cruz, G.J. Plasma polypyrrole micro-coatings on metallic stents. Polym. Bull. 2016, 4, 1253–1266. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates; Academic Press: San Diego, CA, USA, 2004. [Google Scholar]
- Pérez-Barrón, G.; Avila-Acevedo, J.G.; García-Bores, A.M.; Montes, S.; García-Jiménez, S.; León-Rivera, I.; Rubio-Osornio, M.; Monroy-Noyola, A. Neuroprotective effect of Buddleja cordata methanolic extract in the 1-methyl-4-phenylpyridinium Parkinson’s disease rat model. J. Nat. Med. 2015, 69, 86–93. [Google Scholar] [CrossRef]
- Li, X.H.; Wang, J.Y.; Gao, G.; Chang, J.Y.; Woodward, D.J.; Luo, F. High-frequency stimulation of the subthalamic nucleus restores neural and behavioral functions during reaction time task in a rat model of Parkinson’s disease. J. Neurosci. Res. 2010, 88, 21–1510. [Google Scholar] [CrossRef]
- Vasquez-Ortega, M.; Ortega, M.; Morales, J.; Olayo, M.G.; Cruz, G.J.; Olayo, R. Core-shell polypyrrole nanoparticles obtained by atmospheric pressure plasma polymerization. Polym. Int. 2014, 63, 2023–2029. [Google Scholar] [CrossRef]
- Borders, C.; Hsu, F.; Sweidan, A.J.; Matei, E.S.; Bota, R.G. Deep brain stimulation for obsessive compulsive disorder: A review of results by anatomical target. Ment. Illn. 2018, 10, 7900. [Google Scholar] [PubMed]
- Salanova, V. Deep brain stimulation for epilepsy. Epilepsy Behav. 2018, 88, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Boccard, S.G.; Pereira, E.A.; Aziz, T.Z. Deep brain stimulation for chronic pain. J. Clin. Neurosci. 2015, 10, 1537–1543. [Google Scholar] [CrossRef] [PubMed]
- Benabid, A.L. Deep brain stimulation for Parkinson’s disease. Curr. Opin. Neurobiol. 2003, 13, 696–706. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Hetke, J.F.; Wiler, J.A.; Anderson, D.J.; Martin, D.C. Electrochemical deposition and characterization of conducting polymer polypyrrole/PSS on multichannel neural probes. Sens. Actuators 2001, 93, 8–18. [Google Scholar] [CrossRef]
- Mao, J.; Zhang, Z. Polypyrrole as electrically conductive biomaterials: Synthesis, biofunctionalization, potential applications and challenges. In Cutting-Edge Enabling Technologies for Regenerative Medicine; Advances in Experimental Medicine and Biology; Springer: Singapore, 2018; Volume 1078, pp. 347–370. [Google Scholar]
- Ateh, D.D.; Navsaria, H.A.; Vadgama, P. Polypyrrole-based conducting polymers and interactions with biological tissues. J. R. Soc. Interface 2006, 3, 741–752. [Google Scholar] [CrossRef]
- Zhang, J.; Neoh, K.G.; Kang, E.T. Electrical stimulation of adipose-derived mesenchymal stem cells and endothelial cells co-cultured in a conductive scaffold for potential orthopaedic applications. J. Tissue Eng. Regen. Med. 2018, 12, 878–889. [Google Scholar] [CrossRef]
- Liu, C.; Huang, Z.; Pu, X.; Shang, L.; Yin, G.; Chen, X.; Cheng, S. Fabrication of carboxylic graphene oxide-composited polypyrrole film for neurite growth under electrical stimulation. Front. Mater. Sci. 2019, 13, 258–267. [Google Scholar] [CrossRef]
- Uzieliene, I.; Popov, A.; Vaiciuleviciute, R.; Kirdaite, G.; Bernotiene, E.; Ramanaviciene, A. Polypyrrole-based structures for activation of cellular functions under electrical stimulation. Bioelectrochemistry 2024, 155, 108585. [Google Scholar] [CrossRef]
- Lu, Y.; Li, T.; Zhao, X.; Li, M.; Cao, Y.; Yang, H.; Duan, Y.Y. Electrodeposited polypyrrole/carbon nanotubes composite films electrodes for neural interfaces. Biomaterials 2010, 19, 5169–5181. [Google Scholar] [CrossRef]
- Seddon, B.J.; Eddowes, M.J.; Firth, A.; Owen, A.E.; Girault, H.H.J. Thin film electrode: A new method for the fabrication of submicrometer band electrodes. Electrochim. Acta 1991, 36, 763–771. [Google Scholar] [CrossRef]
- Sudwilaia, T.; Ng, J.J.; Boonkrai, C.; Israsena, N.; Chuangchote, S.; Supaphol, P. Polypyrrole-coated electrospun poly(lactic acid) fibrous scaffold: Effects of coating on electrical conductivity and neural cell growth. J. Biomater. Sci. Polym. Ed. 2014, 25, 1240–1252. [Google Scholar] [CrossRef]
- Kolaya, E.; Firestein, B.L. Deep brain stimulation: Challenges at the tissue-electrode interface and current solutions. Biotechnol. Prog. 2021, 37, 3179. [Google Scholar] [CrossRef]
- Sahyouni, R.; Chang, D.T.; Moshtaghi, O.; Mahmoodi, A.; Djalilian, H.R.; Lin, H.W. Functional and Histological Effects of Chronic Neural Electrode Implantation. Laryngoscope Investig. Otolaryngol. 2017, 2, 80–93. [Google Scholar] [CrossRef]
- Polikov, V.S.; Tresco, P.A.; Reichert, W.M. Response of brain tissue to chronically implanted neural electrodes. J. Neurosci. Methods 2005, 148, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Jackson, N.; Anand, S.; Okandan, M.; Muthuswamy, J. Nonhermetic Encapsulation Materials for MEMS-Based Movable Microelectrodes for Long-Term Implantation in the Brain. J. Microelectromech. Syst. 2009, 18, 1234–1245. [Google Scholar] [CrossRef] [PubMed][Green Version]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Diaz, D.; Manjarrez-Marmolejo, J.; Diaz-Ruiz, A.; Ríos, C.; Olayo, M.G.; Olayo, R.; Cruz, G.J.; Salgado-Ceballos, H.; Mendez-Aramenta, M.; Morales-Corona, J. Development and Characterization of Electrodes Coated with Plasma-Synthesized Polypyrrole Doped with Iodine, Implanted in the Rat Brain Subthalamic Nucleus. Polymers 2024, 16, 823. https://doi.org/10.3390/polym16060823
Ruiz-Diaz D, Manjarrez-Marmolejo J, Diaz-Ruiz A, Ríos C, Olayo MG, Olayo R, Cruz GJ, Salgado-Ceballos H, Mendez-Aramenta M, Morales-Corona J. Development and Characterization of Electrodes Coated with Plasma-Synthesized Polypyrrole Doped with Iodine, Implanted in the Rat Brain Subthalamic Nucleus. Polymers. 2024; 16(6):823. https://doi.org/10.3390/polym16060823
Chicago/Turabian StyleRuiz-Diaz, Daniel, Joaquín Manjarrez-Marmolejo, Araceli Diaz-Ruiz, Camilo Ríos, María G. Olayo, Roberto Olayo, Guillermo J. Cruz, Hermelinda Salgado-Ceballos, Marisela Mendez-Aramenta, and Juan Morales-Corona. 2024. "Development and Characterization of Electrodes Coated with Plasma-Synthesized Polypyrrole Doped with Iodine, Implanted in the Rat Brain Subthalamic Nucleus" Polymers 16, no. 6: 823. https://doi.org/10.3390/polym16060823
APA StyleRuiz-Diaz, D., Manjarrez-Marmolejo, J., Diaz-Ruiz, A., Ríos, C., Olayo, M. G., Olayo, R., Cruz, G. J., Salgado-Ceballos, H., Mendez-Aramenta, M., & Morales-Corona, J. (2024). Development and Characterization of Electrodes Coated with Plasma-Synthesized Polypyrrole Doped with Iodine, Implanted in the Rat Brain Subthalamic Nucleus. Polymers, 16(6), 823. https://doi.org/10.3390/polym16060823








