On Antimicrobial Polymers: Development, Mechanism of Action, International Testing Procedures, and Applications
Abstract
1. Introduction
2. Polymers
2.1. Biocidal-Releasing Polymers
2.2. Nanoparticles (NPs) of Special Polymers
2.3. Polymer Micelles
2.4. Polymer Vesicles
2.5. Dendrimers
2.6. Polymeric Hydrogels
2.7. Thermoplastics
3. Antimicrobial Surface Modification
3.1. Design of Surfaces to Prevent Adhesion of Microbes
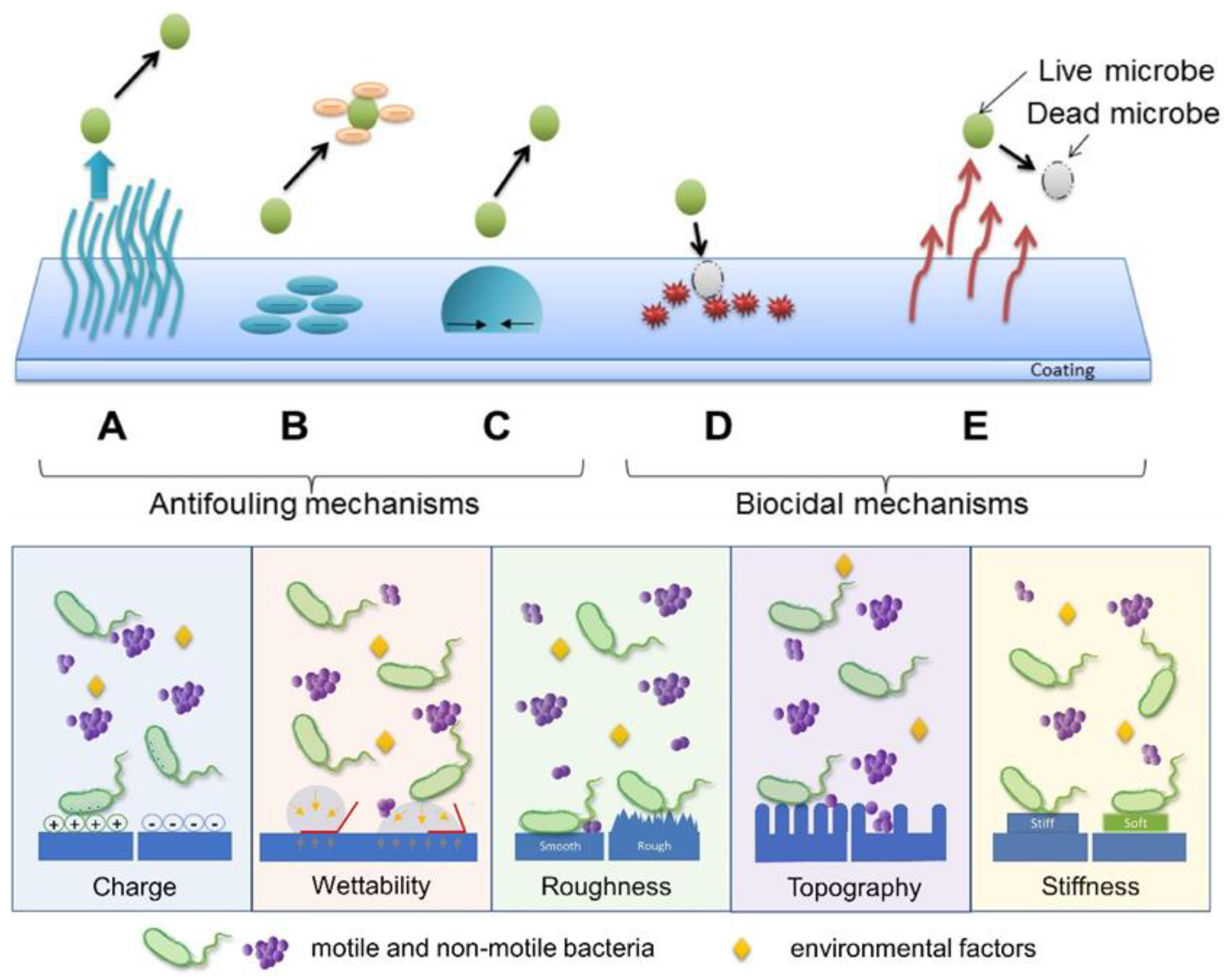
3.2. Killing Microbes by Making Biocidal Active Surfaces
3.3. Polymeric Surface Structures Combining Antiadhesive and Biocidal Properties towards Microbes
3.4. Coating
4. Antimicrobial Polymers
4.1. Antimicrobial Polymer Blends
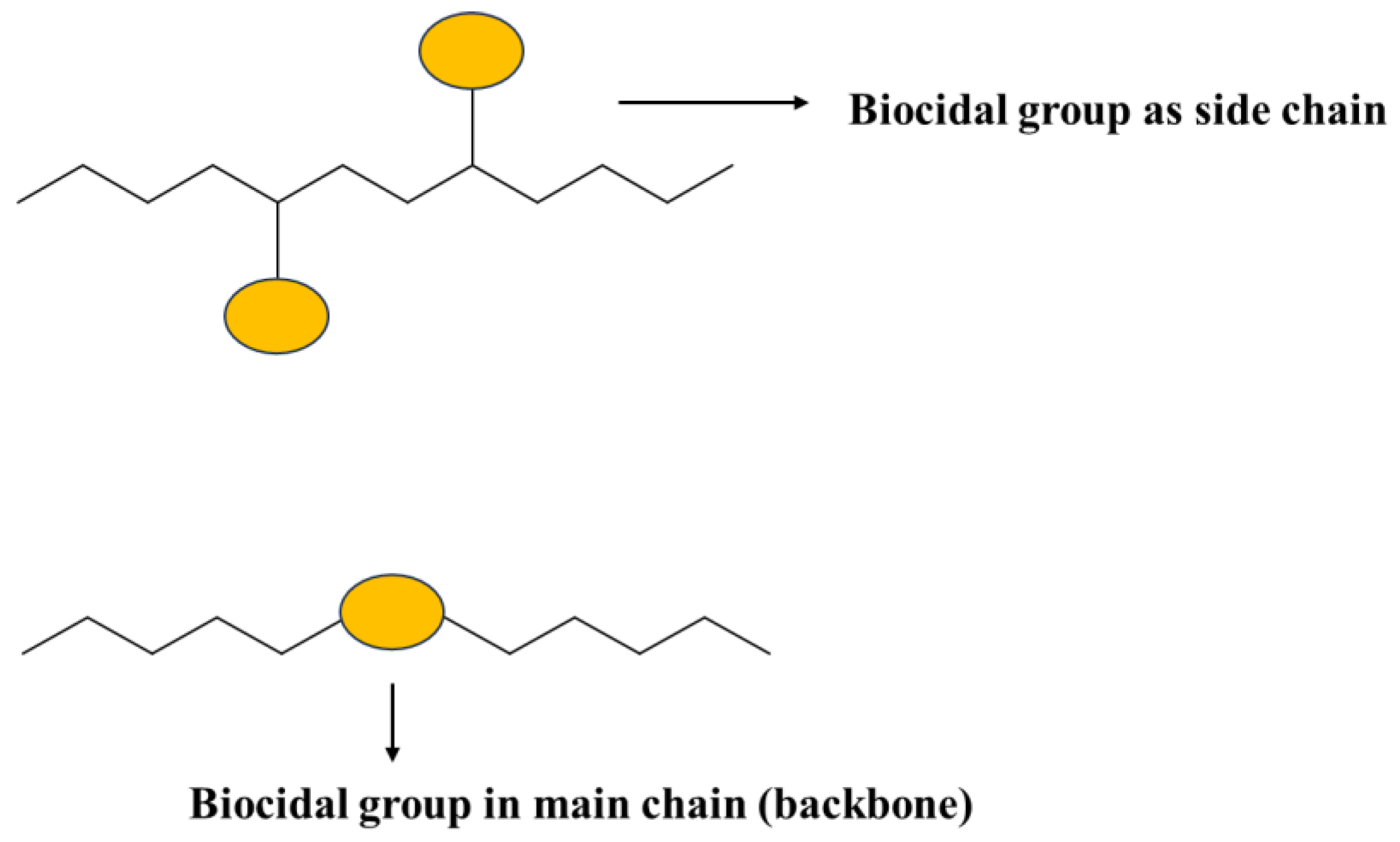
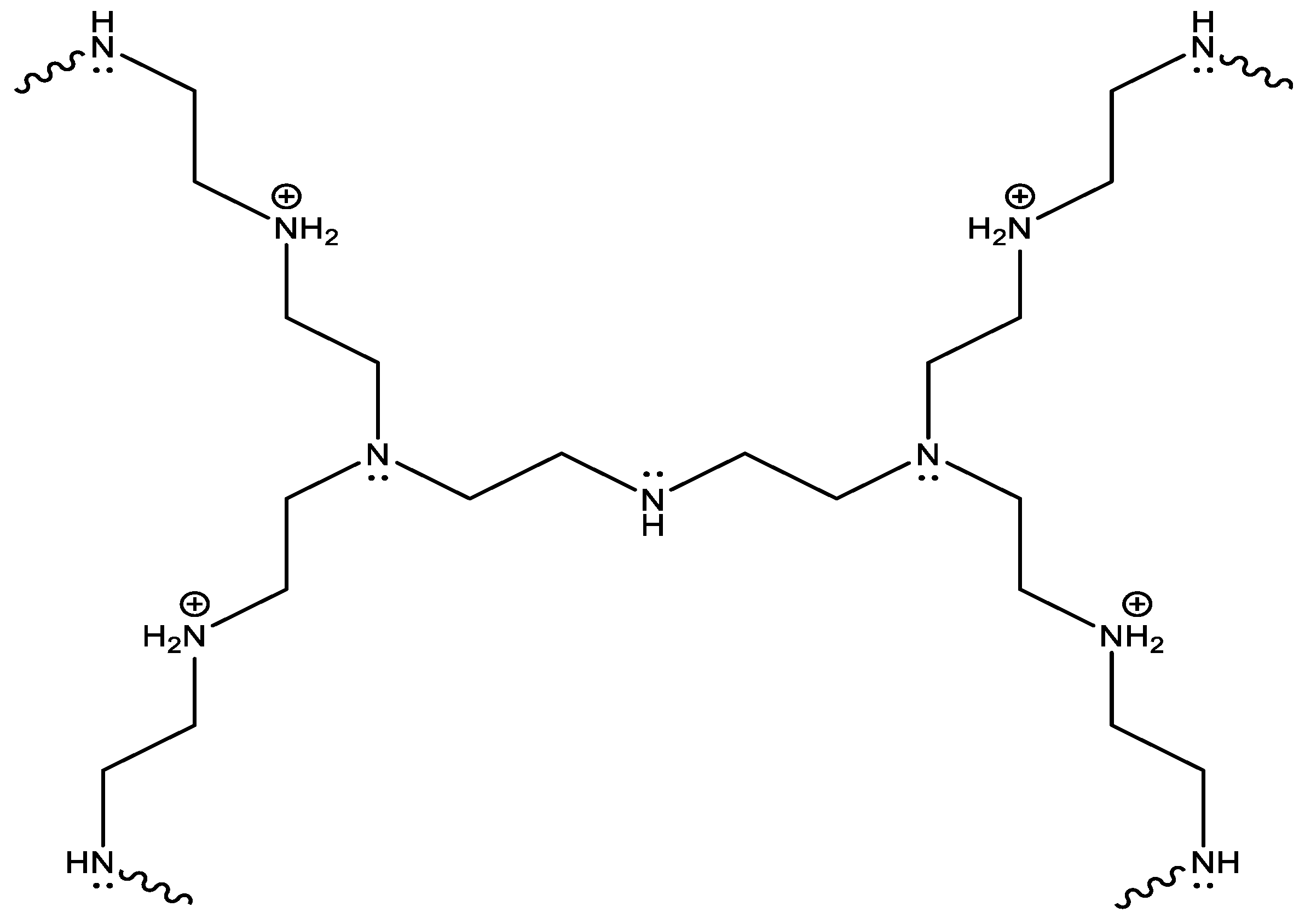
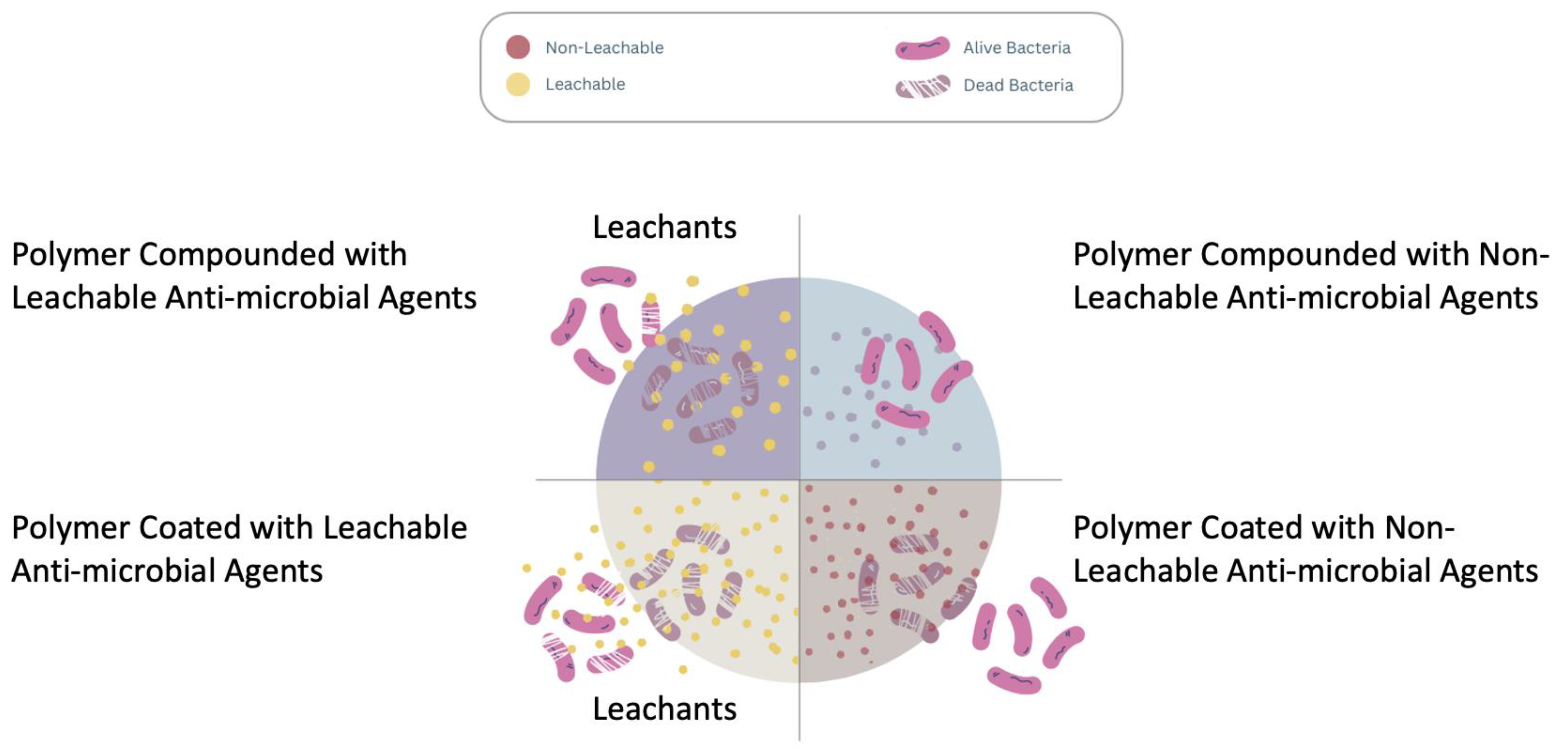
4.2. Leachable and Nonleachable Antimicrobial Agents
5. Polymer Nanocomposites with Biocidal Metals and Inorganic Nanocrystals
6. Attraction between NPs and Microorganisms due to Electrostatic Charges
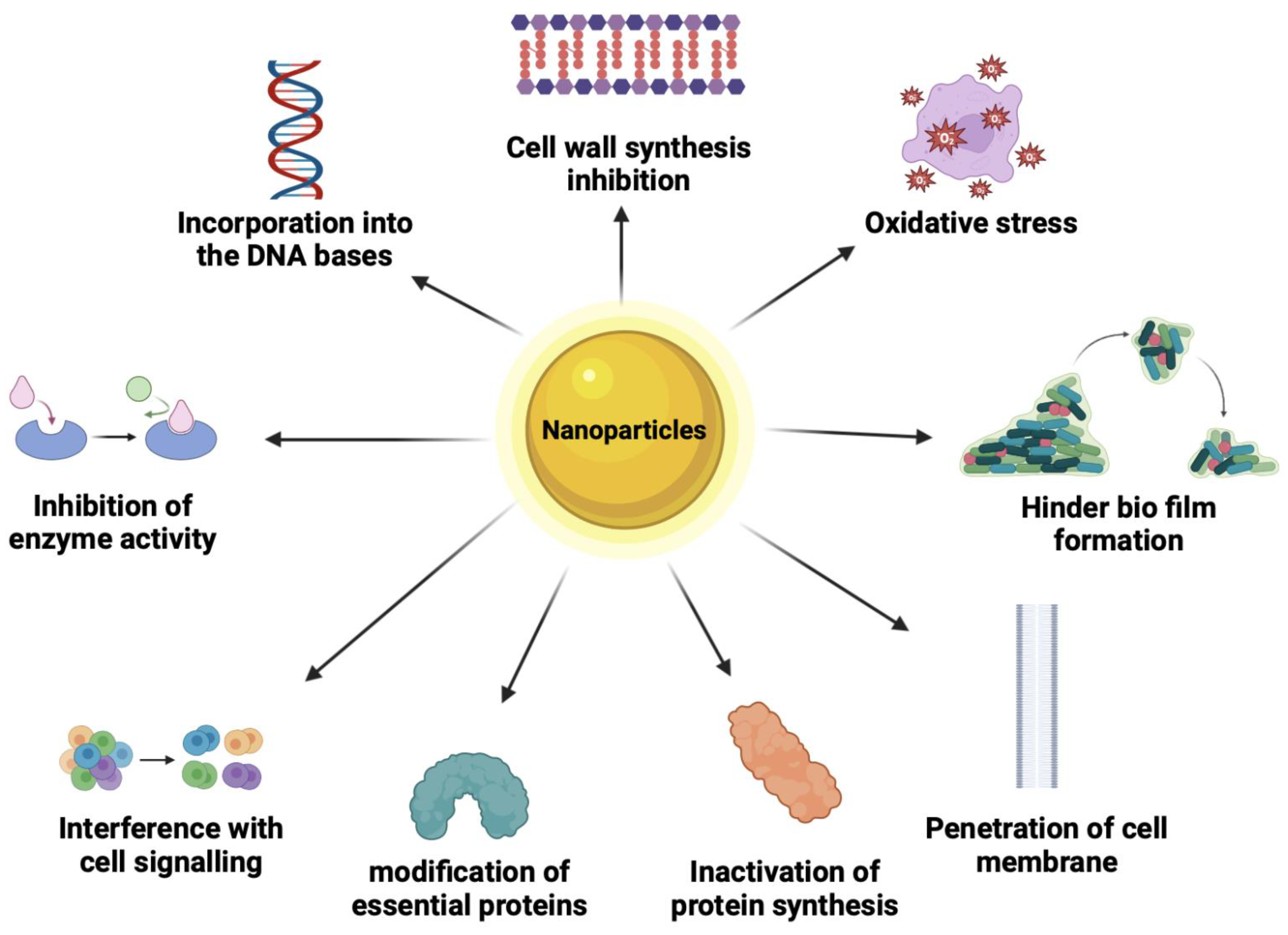
7. Mode of Mechanisms of Antimicrobial NPs
7.1. Cell Wall Synthesis Inhibition
7.2. Oxidative Stress
7.3. Protein Synthesis Inhibition
7.4. Inhibition of Enzyme Activity
7.5. Hinder Biofilm Formation
7.6. Interference with Cell Signaling
7.7. Modification of Essential Proteins
7.8. Penetration of Cell Membrane
7.9. Incorporation into the Nucleic Bases
7.10. Mode of Action for Inorganic NPs

8. Broad-Spectrum or Narrow-Spectrum Microbials and the Shape of Micro-Organisms
8.1. Bacteria
8.2. Fungi
8.3. Viruses
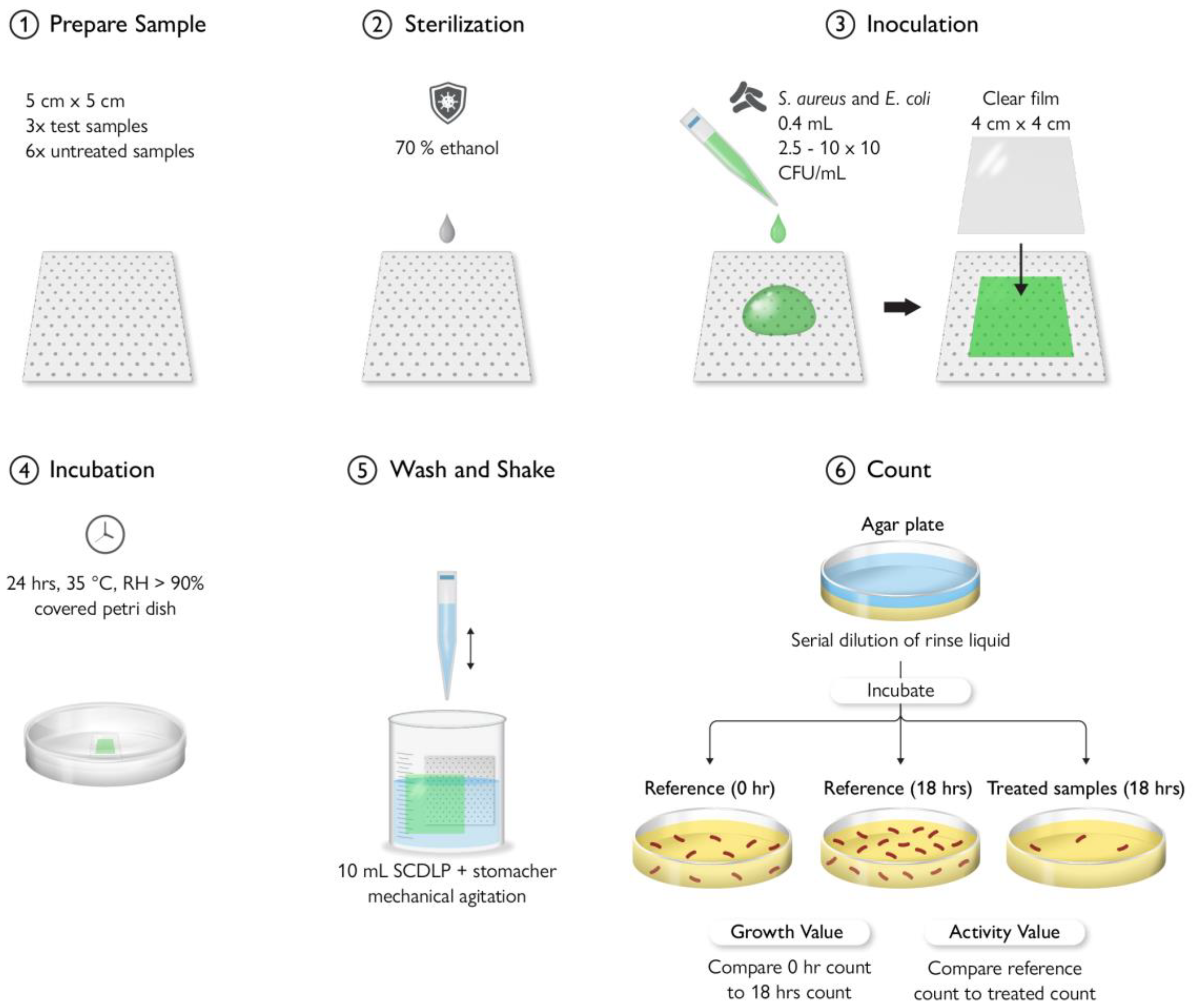
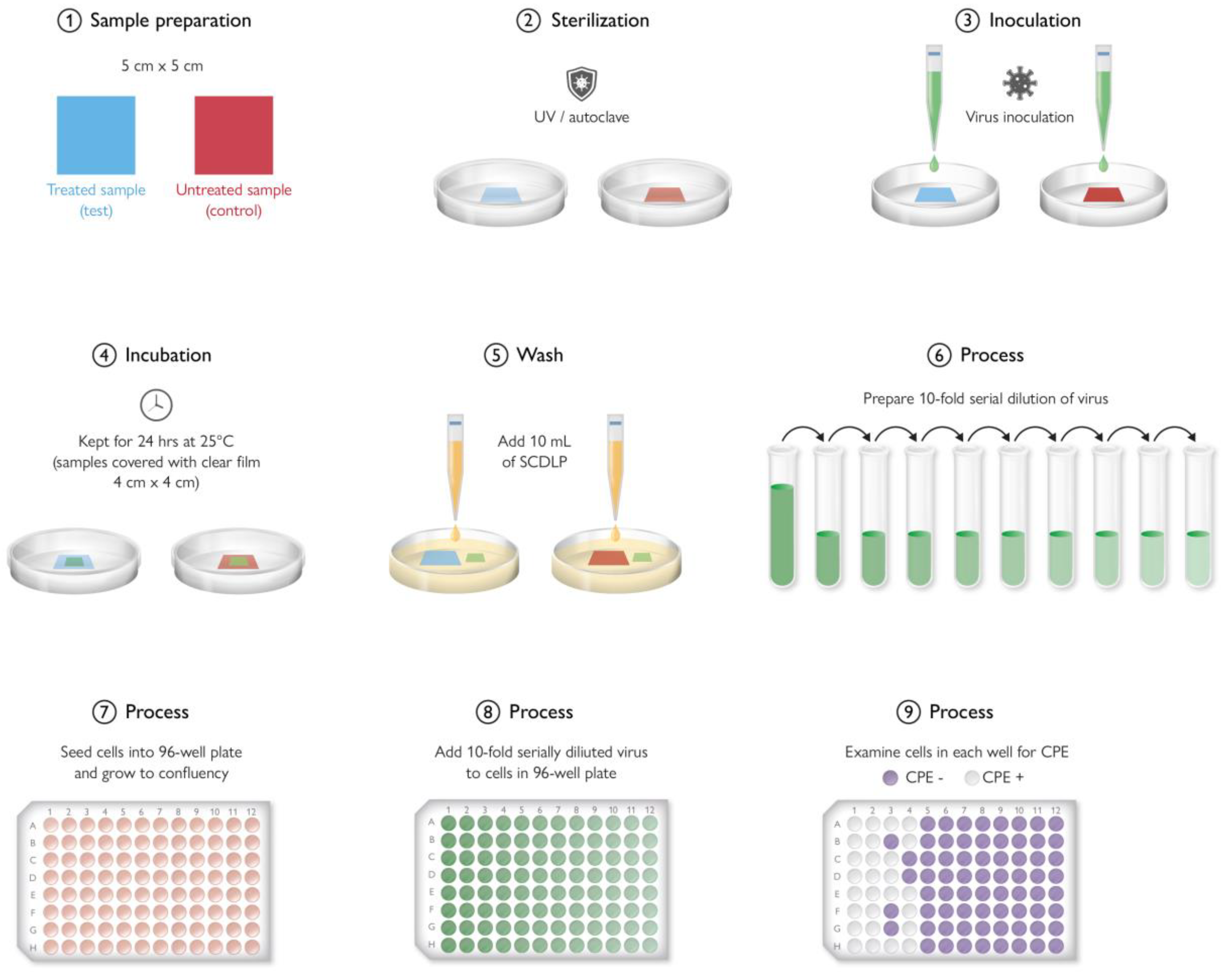
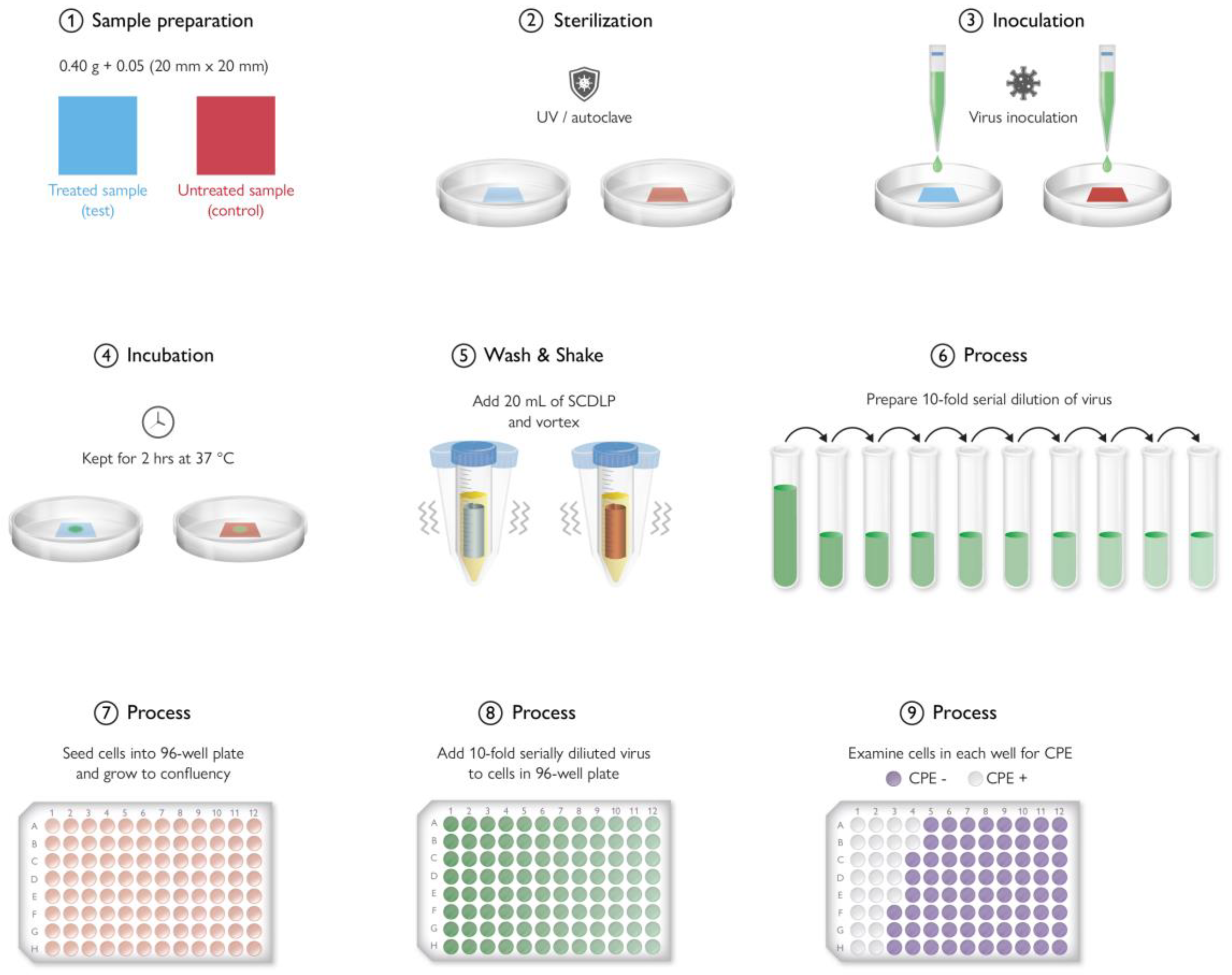
9. Antimicrobial Testing Protocols
9.1. In Vivo Testing Method
9.2. In Vitro Testing Method
9.3. Antimicrobial Plastic Results Variation
10. Toxicity
11. Antimicrobial Market Size
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Microbiology by numbers. Nat. Rev. Microbiol. 2011, 9, 628. [CrossRef]
- Sugden, R.; Kelly, R.; Davies, S. Combatting antimicrobial resistance globally. Nat. Microbiol. 2016, 1, 16187. [Google Scholar] [CrossRef]
- Morales, E.; Cots, F.; Sala, M.; Comas, M.; Belvis, F.; Riu, M.; Salvadó, M.; Grau, S.; Horcajada, J.P.; Montero, M.M.; et al. Hospital costs of nosocomial multi-drug resistant Pseudomonas aersuginosa acquisition. BMC Health Serv. Res. 2012, 12, 122. [Google Scholar] [CrossRef]
- Nathwani, D.; Raman, G.; Sulham, K.; Gavaghan, M.; Menon, V. Clinical and economic consequences of hospital-acquired resistant and multidrug-resistant Pseudomonas aeruginosa infections: A systematic review and meta-analysis. Antimicrob. Resist. Infect. Control 2014, 3, 32. [Google Scholar] [CrossRef]
- Levy, S.B.; Marshall, B. Antibacterial resistance worldwide: Causes, challenges and responses. Nat. Med. 2004, 10 (Suppl. S12), S122–S129. [Google Scholar] [CrossRef]
- Antibiotic Chemical Compound, Britiannica. Available online: https://www.britannica.com/science/antibiotic/Antibiotic-resistance (accessed on 21 May 2023).
- Silhavy, T.J.; Kahne, D.; Walker, S. The bacterial cell envelope. Cold Spring Harb. Perspect. Biol. 2010, 2, a000414. [Google Scholar] [CrossRef]
- Cetin-Karaca, H.; Newman, M.C. Antimicrobial efficacy of natural phenolic compounds against gram positive foodborne pathogens. J. Food Res. 2015, 4, 14. [Google Scholar] [CrossRef]
- Shlaes, D.M. Research and Development of Antibiotics: The Next Battleground. ACS Infect. Dis. 2015, 1, 232–233. [Google Scholar] [CrossRef]
- Ghani, S. Why is the Pharmaceutical Industry Not Developing New Antibiotics? HealthManagement 2021, 21. Available online: https://healthmanagement.org/uploads/article_attachment/why-is-the-pharmaceutical-industry.pdf (accessed on 5 March 2024).
- Darouiche, R.O. Treatment of infections associated with surgical implants. N. Engl. J. Med. 2004, 350, 1422–1429. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, X.; Jia, W.; Jin, T.; Xiao, S.; Chen, W.; Hang, J.; Ou, C.; Lei, H.; Qian, H.; et al. Evidence for lack of transmission by close contact and surface touch in a restaurant outbreak of COVID-19. J. Infect. 2021, 83, 207–216. [Google Scholar] [CrossRef]
- Lemiech-Mirowska, E.; Kiersnowska, Z.M.; Michałkiewicz, M.; Depta, A.; Marczak, M. Nosocomial infections as one of the most important problems of healthcare system. Ann. Agric. Environ. Med. AAEM 2021, 28, 361–366. [Google Scholar] [CrossRef]
- Chua, M.H.; Cheng, W.; Goh, S.S.; Kong, J.; Li, B.; Lim, J.Y.C.; Mao, L.; Wang, S.; Xue, K.; Yang, L.; et al. Face Masks in the New COVID-19 Normal: Materials, Testing, and Perspectives. Research 2020, 2020, 7286735. [Google Scholar] [CrossRef]
- Liu, M.; Bauman, L.; Nogueira, C.L.; Aucoin, M.G.; Anderson, W.A.; Zhao, B. Antimicrobial polymeric composites for high-touch surfaces in healthcare applications. Curr. Opin. Biomed. Eng. 2022, 22, 100395. [Google Scholar] [CrossRef]
- Muñoz-Bonilla, A.; Fernández-García, M. Polymeric materials with antimicrobial activity. Prog. Polym. Sci. 2012, 37, 281–339. [Google Scholar] [CrossRef]
- Olatunji, O. Natural Polymers: Industry Techniques and Applications; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Wypych, G. PDMS Polydimethylsiloxane, Handbook of Polymers; ChemTec Publishing: Toronto, ON, Canada, 2012. [Google Scholar]
- Shakiba, M.; Rezvani Ghomi, E.; Khosravi, F.; Jouybar, S.; Bigham, A.; Zare, M.; Abdouss, M.; Moaref, R.; Ramakrishna, S. Nylon—A material introduction and overview for biomedical applications. Polym. Adv. Technol. 2021, 32, 3368–3383. [Google Scholar] [CrossRef]
- Ghosh, P. Polymer Science and Technology: Plastics, Rubbers, Blends and Composites; McGraw-Hill Education: New York, NY, USA, 2011. [Google Scholar]
- Bhat, S.V. Synthetic polymers. In Biomaterials; Springer: Dordrecht, The Netherlands, 2002; pp. 51–71. [Google Scholar]
- Liechty, W.B.; Kryscio, D.R.; Slaughter, B.V.; Peppas, N.A. Polymers for drug delivery systems. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 149–173. [Google Scholar] [CrossRef]
- Gagliardi, A.; Giuliano, E.; Venkateswararao, E.; Fresta, M.; Bulotta, S.; Awasthi, V.; Cosco, D. Biodegradable polymeric nanoparticles for drug delivery to solid tumors. Front. Pharmacol. 2021, 12, 601626. [Google Scholar] [CrossRef]
- Begines, B.; Ortiz, T.; Pérez-Aranda, M.; Martínez, G.; Merinero, M.; Argüelles-Arias, F.; Alcudia, A. Polymeric nanoparticles for drug delivery: Recent developments and future prospects. Nanomaterials 2020, 10, 1403. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef]
- Lam, S.J.; Wong, E.H.; Boyer, C.; Qiao, G.G. Antimicrobial polymeric nanoparticles. Prog. Polym. Sci. 2018, 76, 40–64. [Google Scholar] [CrossRef]
- Lam, S.J.; O’Brien-Simpson, N.M.; Pantarat, N.; Sulistio, A.; Wong, E.H.; Chen, Y.-Y.; Lenzo, J.C.; Holden, J.A.; Blencowe, A.; Reynolds, E.C.; et al. Combating multidrug-resistant Gram-negative bacteria with structurally nanoengineered antimicrobial peptide polymers. Nat. Microbiol. 2016, 1, 16162. [Google Scholar] [CrossRef]
- Aguilar, Z. Nanomaterials for Medical Applications; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Nederberg, F.; Zhang, Y.; Tan, J.P.; Xu, K.; Wang, H.; Yang, C.; Gao, S.; Guo, X.D.; Fukushima, K.; Li, L.; et al. Biodegradable nanostructures with selective lysis of microbial membranes. Nat. Chem. 2011, 3, 409–414. [Google Scholar] [CrossRef]
- Qiao, Y.; Yang, C.; Coady, D.J.; Ong, Z.Y.; Hedrick, J.L.; Yang, Y.-Y. Highly dynamic biodegradable micelles capable of lysing Gram-positive and Gram-negative bacterial membrane. Biomaterials 2012, 33, 1146–1153. [Google Scholar] [CrossRef]
- Fukushima, K.; Tan, J.P.; Korevaar, P.A.; Yang, Y.Y.; Pitera, J.; Nelson, A.; Maune, H.; Coady, D.J.; Frommer, J.E.; Engler, A.C.; et al. Broad-spectrum antimicrobial supramolecular assemblies with distinctive size and shape. ACS Nano 2012, 6, 9191–9199. [Google Scholar] [CrossRef]
- Fukushima, K.; Liu, S.; Wu, H.; Engler, A.C.; Coady, D.J.; Maune, H.; Pitera, J.; Nelson, A.; Wiradharma, N.; Venkataraman, S.; et al. Supramolecular high-aspect ratio assemblies with strong antifungal activity. Nat. Commun. 2013, 4, 2861. [Google Scholar] [CrossRef]
- Waschinski, C.J.; Tiller, J.C. Poly (oxazoline) s with telechelic antimicrobial functions. Biomacromolecules 2005, 6, 235–243. [Google Scholar] [CrossRef]
- Kiss, É.; Heine, E.T.; Hill, K.; He, Y.C.; Keusgen, N.; Pénzes, C.B.; Schnöller, D.; Gyulai, G.; Mendrek, A.; Keul, H.; et al. Membrane affinity and antibacterial properties of cationic polyelectrolytes with different hydrophobicity. Macromol. Biosci. 2012, 12, 1181–1189. [Google Scholar] [CrossRef]
- Antonietti, M.; Förster, S. Vesicles and liposomes: A self-assembly principle beyond lipids. Adv. Mater. 2003, 15, 1323–1333. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, Y.; Zhou, C.; Yuan, W.; Du, J. Antibacterial vesicles by direct dissolution of a block copolymer in water. Polym. Chem. 2013, 4, 255–259. [Google Scholar] [CrossRef]
- Zhu, H.; Geng, Q.; Chen, W.; Zhu, Y.; Chen, J.; Du, J. Antibacterial high-genus polymer vesicle as an “armed” drug carrier. J. Mater. Chem. B 2013, 1, 5496–5504. [Google Scholar] [CrossRef]
- Grayson, S.M.; Frechet, J.M. Convergent dendrons and dendrimers: From synthesis to applications. Chem. Rev. 2001, 101, 3819–3868. [Google Scholar] [CrossRef]
- Gupta, U.; Perumal, O. Dendrimers and its biomedical applications. In Natural and Synthetic Biomedical Polymers; Elsevier: Amsterdam, The Netherlands, 2014; pp. 243–257. [Google Scholar]
- Ganewatta, M.S.; Tang, C. Controlling macromolecular structures towards effective antimicrobial polymers. Polymer 2015, 63, A1–A29. [Google Scholar] [CrossRef]
- Chen, C.Z.; Beck-Tan, N.C.; Dhurjati, P.; van Dyk, T.K.; LaRossa, R.A.; Cooper, S.L. Quaternary ammonium functionalized poly (propylene imine) dendrimers as effective antimicrobials: Structure–activity studies. Biomacromolecules 2000, 1, 473–480. [Google Scholar] [CrossRef]
- Jain, A.; Duvvuri, L.; Farah, S.; Beyth, N.; Domb, A.; Khan, W. Antimicrobial polymers. Adv. Healthc Mater. 2014, 3, 1969–1985. [Google Scholar] [CrossRef]
- Chen, C.Z.; Cooper, S.L. Recent advances in antimicrobial dendrimers. Adv. Mater. 2000, 12, 843–846. [Google Scholar] [CrossRef]
- Gillies, E.R.; Frechet, J.M. Dendrimers and dendritic polymers in drug delivery. Drug Discov. Today 2005, 10, 35–43. [Google Scholar] [CrossRef]
- Yang, K.; Han, Q.; Chen, B.; Zheng, Y.; Zhang, K.; Li, Q.; Wang, J. Antimicrobial hydrogels: Promising materials for medical application. Int. J. Nanomed. 2018, 13, 2217. [Google Scholar] [CrossRef]
- Salomé Veiga, A.; Schneider, J.P. Antimicrobial hydrogels for the treatment of infection. Pept. Sci. 2013, 100, 637–644. [Google Scholar] [CrossRef]
- Vranic, E. Amorphous pharmaceutical solids. Bosn. J. Basic Med. Sci. 2004, 4, 35. [Google Scholar] [CrossRef]
- Paul, D.R. Polymer Blends Volume 1; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Lu, Z.; Tan, H.; Ng, S.; Li, Y. The correlation between reduced glass transition temperature and glass forming ability of bulk metallic glasses. Scr. Mater. 2000, 42, 667–673. [Google Scholar] [CrossRef]
- Wypych, G. SBC styrene-butadiene block copolymer. In Handbook of Polymers; ChemTec Publishing: Toronto, ON, Canada, 2012; pp. 637–640. [Google Scholar]
- Greene, J.P. Automotive Plastics and Composites: Materials and Processing; William Andrew: Norwich, NY, USA, 2021. [Google Scholar]
- Wagner, J.R., Jr. Multilayer Flexible Packaging; William Andrew: Norwich, NY, USA, 2016. [Google Scholar]
- Polypropylene Market Size, Trends and Global Forecast to 2032. 2023. Available online: https://www.precedenceresearch.com/polypropylene-market#:~:text=The%20global%20polypropylene%20market%20size,5.3%25%20from%202023%20to%202032 (accessed on 6 July 2023).
- Shan, C.L.P.; Soares, J.B.; Penlidis, A. HDPE/LLDPE reactor blends with bimodal microstructures—Part I: Mechanical properties. Polymer 2002, 43, 7345–7365. [Google Scholar] [CrossRef]
- Gilbert, M. Brydson’s Plastics Materials; William Andrew: Norwich, NY, USA, 2016. [Google Scholar]
- Polyethylene Market to Worth USD 140.21 Billion by 2029. Fortune Bus. Insights 2023. Available online: https://www.globenewswire.com/en/news-release/2023/05/10/2665280/0/en/Polyethylene-Market-to-Worth-USD-140-21-Billion-by-2029-Fortune-Business-Insights.html (accessed on 6 July 2023).
- Sin, L.T.; Tueen, B.S. Plastics and Sustainability: Practical Approaches; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Zohuri, G. Polymer Science: A Comprehensive Reference; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Al-Haydari, I.S.; Al-Haidari, H.S. Mechanical properties of polyethylene terephthalate-modified pavement mixture. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2020; Volume 870, p. 012073. [Google Scholar]
- Filella, M. Antimony and PET bottles: Checking facts. Chemosphere 2020, 261, 127732. [Google Scholar] [CrossRef]
- Weller, J.E. Synthesis of a Manufacturing Process for Making Microcellular Polycarbonate Parts. Master’s Thesis, University of Washington, Washington, DC, USA, 1991. [Google Scholar]
- Kausar, A.; Zulfiqar, S.; Sarwar, M.I. Recent developments in sulfur-containing polymers. Polym. Rev. 2014, 54, 185–267. [Google Scholar] [CrossRef]
- Park, S.-J.; Seo, M.-K. Interface Science and Composites; Academic Press: Cambridge, MA, USA, 2011. [Google Scholar]
- Global Polycarbonate Market Size, Share, Growth Analysis by 2030. Spherical Insights 2023. Available online: https://www.sphericalinsights.com/reports/polycarbonate-market#:~:text=What%20is%20the%20market%20size,5.7%25%20from%202022%20to%202030 (accessed on 8 July 2023).
- Li, L.; Chen, S.; Zheng, J.; Ratner, B.D.; Jiang, S. Protein adsorption on oligo (ethylene glycol)-terminated alkanethiolate self-assembled monolayers: The molecular basis for nonfouling behavior. J. Phys. Chem. B 2005, 109, 2934–2941. [Google Scholar] [CrossRef]
- Hamilton-Brown, P.; Gengenbach, T.; Griesser, H.J.; Meagher, L. End terminal, poly (ethylene oxide) graft layers: Surface forces and protein adsorption. Langmuir 2009, 25, 9149–9156. [Google Scholar] [CrossRef]
- Barbey, R.; Lavanant, L.; Paripovic, D.; Schuwer, N.; Sugnaux, C.; Tugulu, S.; Klok, H.-A. Polymer brushes via surface-initiated controlled radical polymerization: Synthesis, characterization, properties, and applications. Chem. Rev. 2009, 109, 5437–5527. [Google Scholar] [CrossRef]
- Feng, W.; Brash, J.L.; Zhu, S. Non-biofouling materials prepared by atom transfer radical polymerization grafting of 2-methacryloloxyethyl phosphorylcholine: Separate effects of graft density and chain length on protein repulsion. Biomaterials 2006, 27, 847–855. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, M.; Chen, S.; Horbett, T.A.; Ratner, B.D.; Jiang, S. Blood compatibility of surfaces with superlow protein adsorption. Biomaterials 2008, 29, 4285–4291. [Google Scholar] [CrossRef]
- Kuang, J.; Messersmith, P.B. Universal surface-initiated polymerization of antifouling zwitterionic brushes using a mussel-mimetic peptide initiator. Langmuir 2012, 28, 7258–7266. [Google Scholar] [CrossRef]
- Harding, J.L.; Reynolds, M.M. Combating medical device fouling. Trends Biotechnol. 2014, 32, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Freschauf, L.R.; McLane, J.; Sharma, H.; Khine, M. Shrink-induced superhydrophobic and antibacterial surfaces in consumer plastics. PLoS ONE 2012, 7, e40987. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, L.; Levänen, E. Superhydrophobic surfaces for the reduction of bacterial adhesion. RSC Adv. 2013, 3, 12003–12020. [Google Scholar] [CrossRef]
- Singha, P.; Locklin, J.; Handa, H. A review of the recent advances in antimicrobial coatings for urinary catheters. Acta Biomater. 2017, 50, 20–40. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Bawazir, M.; Dhall, A.; Kim, H.-E.; He, L.; Heo, J.; Hwang, G. Implication of Surface Properties, Bacterial Motility, and Hydrodynamic Conditions on Bacterial Surface Sensing and Their Initial Adhesion. Front. Bioeng. Biotechnol. 2021, 9, 643722. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Yu, K.; Kindrachuk, J.; Brooks, D.E.; Hancock, R.E.; Kizhakkedathu, J.N. Antibacterial surfaces based on polymer brushes: Investigation on the influence of brush properties on antimicrobial peptide immobilization and antimicrobial activity. Biomacromolecules 2011, 12, 3715–3727. [Google Scholar] [CrossRef]
- Gao, G.; Lange, D.; Hilpert, K.; Kindrachuk, J.; Zou, Y.; Cheng, J.T.; Kazemzadeh-Narbat, M.; Yu, K.; Wang, R.; Straus, S.K.; et al. The biocompatibility and biofilm resistance of implant coatings based on hydrophilic polymer brushes conjugated with antimicrobial peptides. Biomaterials 2011, 32, 3899–3909. [Google Scholar] [CrossRef]
- Bakhshi, H.; Yeganeh, H.; Mehdipour-Ataei, S.; Shokrgozar, M.A.; Yari, A.; Saeedi-Eslami, S.N. Synthesis and characterization of antibacterial polyurethane coatings from quaternary ammonium salts functionalized soybean oil based polyols. Mater. Sci. Eng. C 2013, 33, 153–164. [Google Scholar] [CrossRef]
- Majumdar, P.; Lee, E.; Patel, N.; Stafslien, S.J.; Daniels, J.; Chisholm, B.J. Development of environmentally friendly, antifouling coatings based on tethered quaternary ammonium salts in a crosslinked polydimethylsiloxane matrix. J. Coat. Technol. Res. 2008, 5, 405–417. [Google Scholar] [CrossRef]
- Lungu, C.N.; Diudea, M.V.; Putz, M.V.; Grudziński, I.P. Linear and Branched PEIs (Polyethylenimines) and Their Property Space. Int. J. Mol. Sci. 2016, 17, 555. [Google Scholar] [CrossRef]
- Yemul, O.; Imae, T. Synthesis and characterization of poly (ethyleneimine) dendrimers. Colloid Polym. Sci. 2008, 286, 747–752. [Google Scholar] [CrossRef]
- Hui, F.; Debiemme-Chouvy, C. Antimicrobial N-halamine polymers and coatings: A review of their synthesis, characterization, and applications. Biomacromolecules 2013, 14, 585–601. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Kim, Y. Fabrication of monodisperse silica–polymer core–shell nanoparticles with excellent antimicrobial efficacy. Chem. Commun. 2008, 4016–4018. [Google Scholar] [CrossRef] [PubMed]
- Dong, A.; Huang, J.; Lan, S.; Wang, T.; Xiao, L.; Wang, W.; Zhao, T.; Zheng, X.; Liu, F.; Gao, G.; et al. Synthesis of N-halamine-functionalized silica–polymer core–shell nanoparticles and their enhanced antibacterial activity. Nanotechnology 2011, 22, 295602. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Han, Q. Designing N-halamine based antibacterial surface on polymers: Fabrication, characterization, and biocidal functions. Appl. Surf. Sci. 2011, 257, 6034–6039. [Google Scholar] [CrossRef]
- Petrie, E.M. Methods of Joining Plastics Other Than with Adhesives; McGraw Hill: New York, NY, USA, 2021. [Google Scholar]
- Lowe, S.; O’Brien-Simpson, N.M.; Connal, L.A. Antibiofouling polymer interfaces: Poly (ethylene glycol) and other promising candidates. Polym. Chem. 2015, 6, 198–212. [Google Scholar] [CrossRef]
- Yu, Q.; Zhang, Y.; Wang, H.; Brash, J.; Chen, H. Anti-fouling bioactive surfaces. Acta Biomater. 2011, 7, 1550–1557. [Google Scholar] [CrossRef]
- Kokubo, T.; Matsushita, T.; Takadama, H.; Kizuki, T. Development of bioactive materials based on surface chemistry. J. Eur. Ceram. Soc. 2009, 29, 1267–1274. [Google Scholar] [CrossRef]
- Muszanska, A.K.; Busscher, H.J.; Herrmann, A.; van der Mei, H.C.; Norde, W. Pluronic–lysozyme conjugates as anti-adhesive and antibacterial bifunctional polymers for surface coating. Biomaterials 2011, 32, 6333–6341. [Google Scholar] [CrossRef]
- Voo, Z.X.; Khan, M.; Narayanan, K.; Seah, D.; Hedrick, J.L.; Yang, Y.Y. Antimicrobial/antifouling polycarbonate coatings: Role of block copolymer architecture. Macromolecules 2015, 48, 1055–1064. [Google Scholar] [CrossRef]
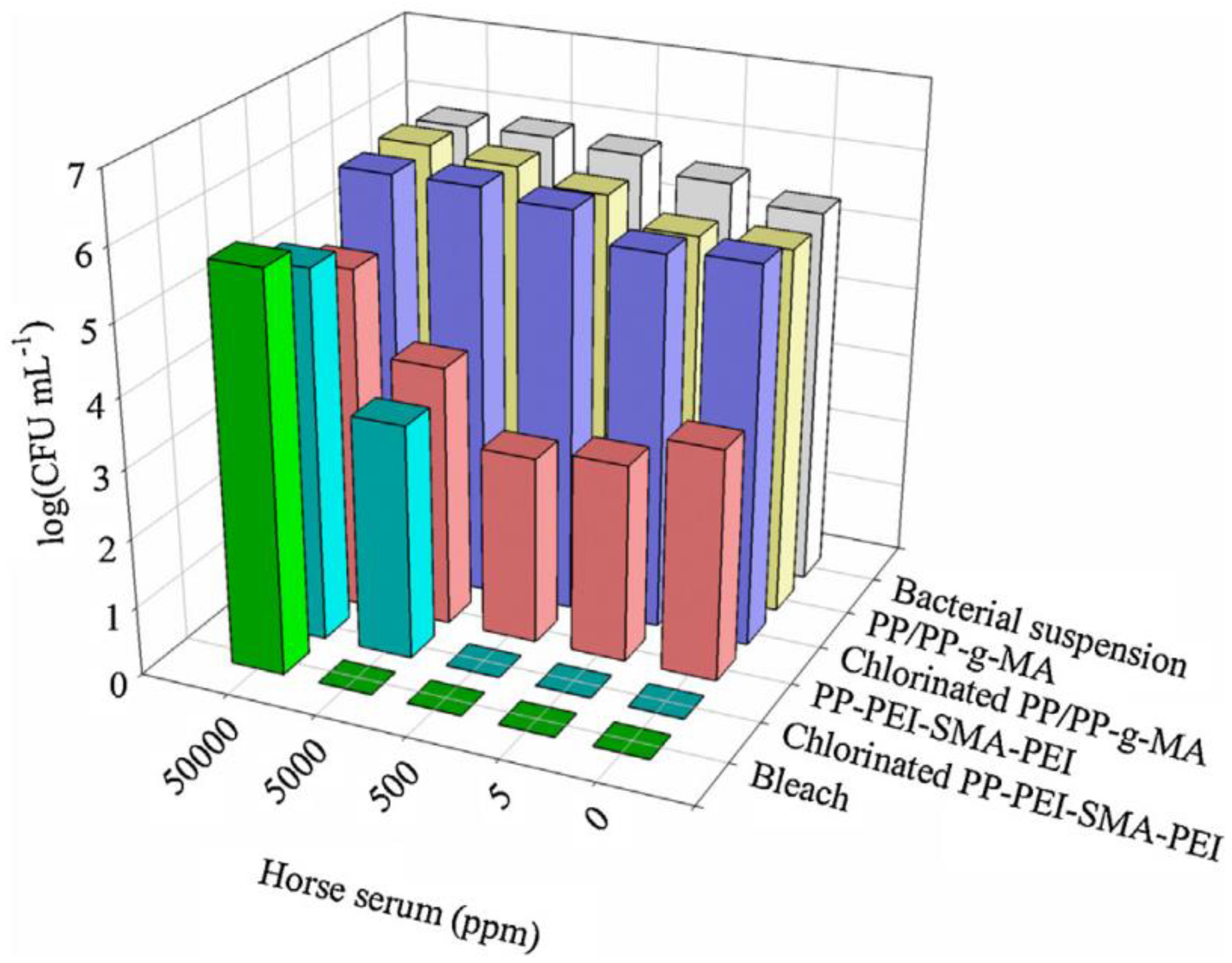
- Bastarrachea, L.J.; Goddard, J.M. Self-healing antimicrobial polymer coating with efficacy in the presence of organic matter. Appl. Surf. Sci. 2016, 378, 479–488. [Google Scholar] [CrossRef]
- Irshad, K.; Rehman, K.; Sharif, H.; Akash, M.S.H.J. Antimicrobial polymer coating. In Polymer Coatings; John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 347–358. [Google Scholar]
- Salwiczek, M.; Qu, Y.; Gardiner, J.; Strugnell, R.A.; Lithgow, T.; McLean, K.M.; Thissen, H. Emerging rules for effective antimicrobial coatings. Trends Biotechnol. 2014, 32, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, H.; Shirai, T.; Nishida, H.; Murakami, H.; Kabata, T.; Yamamoto, N.; Watanabe, K.; Nakase, J. Innovative antimicrobial coating of titanium implants with iodine. J. Orthop. Sci. 2012, 17, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Wu, Z.; Li, Y.; Wang, Y. Graphene family nanomaterials (GFNs)—Promising materials for antimicrobial coating and film: A review. Chem. Eng. J. 2019, 358, 1022–1037. [Google Scholar] [CrossRef]
- Chidanand, B.; Nikhil, M.; Eswara, P. Antimicrobial Coatings Market Trends. 2022. Available online: https://www.alliedmarketresearch.com/antimicrobial-coatings-market-A07268 (accessed on 6 July 2023).
- Alkarri, S.; Sharma, D.; Bergholz, T.M.; Rabnawaz, M. Fabrication methodologies for antimicrobial polypropylene surfaces with leachable and nonleachable antimicrobial agents. J. Appl. Polym. Sci. 2023, 141, e54757. [Google Scholar] [CrossRef]
- Avinash Patil, N.; Macchindra Gore, P.; Shanmugrajan, D.; Patil, H.; Kudav, M.; Kandasubramanian, B. Functionalized non-woven surfaces for combating the spread of the COVID-19 pandemic. Interface Focus 2022, 12, 20210040. [Google Scholar] [CrossRef] [PubMed]
- Hiragond, C.B.; Kshirsagar, A.S.; Dhapte, V.V.; Khanna, T.; Joshi, P.; More, P.V. Enhanced anti-microbial response of commercial face mask using colloidal silver nanoparticles. Vacuum 2018, 156, 475–482. [Google Scholar] [CrossRef]
- Struszczyk, M.H.; Brzoza-Malczewska, K.; Szalczyńska, M. A Nonwovens Coated by Chitosan with Potential Anti-microbial Behaviour–Preliminary Results. Fibres Text. East. Eur. 2007, 15, 5–6. [Google Scholar]
- Novikova, E.D.; Pronina, E.V.; Vorotnikov, Y.A.; Adamenko, L.S.; Alekseev, A.Y.; Shestopalov, A.M.; Tsygankova, A.R.; Gusel’nikova, T.Y.; Kubát, P.; Kirakci, K. Cotton fabrics modified with molybdenum nanoclusters for photodynamic inactivation of bacteria and viruses. J. Environ. Chem. Eng. 2023, 11, 110796. [Google Scholar] [CrossRef]
- Tejero, R.; Gutiérrez, B.; López, D.; López-Fabal, F.; Gómez-Garcés, J.L.; Muñoz-Bonilla, A.; Fernández-García, M. Tailoring Macromolecular Structure of Cationic Polymers towards Efficient Contact Active Antimicrobial Surfaces. Polymers 2018, 10, 241. [Google Scholar] [CrossRef]
- Bratek-Skicki, A.; Eloy, P.; Morga, M.; Dupont-Gillain, C. Reversible Protein adsorption on mixed PEO/PAA Polymer brushes: Role of Ionic strength and PEO content. Langmuir 2018, 34, 3037–3048. [Google Scholar] [CrossRef]
- Muszanska, A.K.; Rochford, E.T.; Gruszka, A.; Bastian, A.A.; Busscher, H.J.; Norde, W.; van der Mei, H.C.; Herrmann, A. Antiadhesive polymer brush coating functionalized with antimicrobial and RGD peptides to reduce biofilm formation and enhance tissue integration. Biomacromolecules 2014, 15, 2019–2026. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.; Laquerre, J.-É.; Forcier, P.; Deregnaucourt, V.; Decaens, J.; Vermeersch, O. Antimicrobial agents for textiles: Types, mechanisms and analysis standards. Text. Funct. Appl. 2021, 13, 261–293. [Google Scholar]
- Bruenke, J.; Roschke, I.; Agarwal, S.; Riemann, T.; Greiner, A. Quantitative comparison of the antimicrobial efficiency of leaching versus nonleaching polymer materials. Macromol. Biosci. 2016, 16, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Brunke, S.; Mogavero, S.; Kasper, L.; Hube, B. Virulence factors in fungal pathogens of man. Curr. Opin. Microbiol. 2016, 32, 89–95. [Google Scholar] [CrossRef]
- Raloff, J. Nanosilver Disinfects, But at What Price? ScienceNews. 2008. Available online: https://www.sciencenews.org/blog/science-the-public/nanosilver-disinfects-what-price (accessed on 5 March 2024).
- Prabhu, S.; Poulose, E.K. Silver nanoparticles: Mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett. 2012, 2, 1–10. [Google Scholar] [CrossRef]
- Alkarri, S.; Sharma, D.; Bergholz, T.M.; Rabnawaz, M. P22 Fabrication methodologies for antimicrobial polypropylene surfaces with leachable and non-leachable antimicrobial agents. JAC-Antimicrob. Resist. 2023, 5 (Suppl. S2), dlad066-026. [Google Scholar] [CrossRef]
- Kregiel, D. Advances in biofilm control for food and beverage industry using organo-silane technology: A review. Food Control 2014, 40, 32–40. [Google Scholar] [CrossRef]
- Thamilselvi, V.; Radha, K.V. A review on the diverse application of silver nanoparticle. IOSR J. Pharm. 2017, 7, 21–27. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Liu, Z.-G.; Shen, W.; Gurunathan, S. Silver nanoparticles: Synthesis, characterization, properties, applications, and therapeutic approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef]
- Loza, K.; Diendorf, J.; Sengstock, C.; Ruiz-Gonzalez, L.; Gonzalez-Calbet, J.; Vallet-Regi, M.; Köller, M.; Epple, M. The dissolution and biological effects of silver nanoparticles in biological media. J. Mater. Chem. B 2014, 2, 1634–1643. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, C.-y.; Rong, Q.-h.; Zhang, Z. Characteristics of the silver-doped TiO2 nanoparticles. Appl. Surf. Sci. 2003, 220, 7–11. [Google Scholar] [CrossRef]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The antibacterial mechanism of silver nanoparticles and its application in dentistry. Int. J. Nanomed. 2020, 2020, 2555–2562. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, F.; Yalamarty, S.S.K.; Filipczak, N.; Jin, Y.; Li, X. Nano silver-induced toxicity and associated mechanisms. Int. J. Nanomed. 2022, 17, 1851–1864. [Google Scholar] [CrossRef]
- Paladini, F.; Pollini, M. Antimicrobial silver nanoparticles for wound healing application: Progress and future trends. Materials 2019, 12, 2540. [Google Scholar] [CrossRef]
- Nirmala, T.S.; Iyandurai, N.; Yuvaraj, S.; Sundararajan, M. Effect of Cu2+ ions on structural, morphological, optical and magnetic behaviors of ZnAl2O4 spinel. Mater. Res. Express 2020, 7, 046104. [Google Scholar] [CrossRef]
- Hejazy, M.; Koohi, M.K.; Bassiri Mohamad Pour, A.; Najafi, D. Toxicity of manufactured copper nanoparticles—A review. Nanomed. Res. J. 2018, 3, 1–9. [Google Scholar]
- Ma, X.; Zhou, S.; Xu, X.; Du, Q. Copper-containing nanoparticles: Mechanism of antimicrobial effect and application in dentistry—A narrative review. Front. Surg. 2022, 9, 905892. [Google Scholar] [CrossRef]
- Grigore, M.E.; Biscu, E.R.; Holban, A.M.; Gestal, M.C.; Grumezescu, A.M. Methods of synthesis, properties and biomedical applications of CuO nanoparticles. Pharmaceuticals 2016, 9, 75. [Google Scholar] [CrossRef]
- Asemani, M.; Anarjan, N. Green synthesis of copper oxide nanoparticles using Juglans regia leaf extract and assessment of their physico-chemical and biological properties. Green Process. Synth. 2019, 8, 557–567. [Google Scholar] [CrossRef]
- Yang, Q.; Liu, Y.; Qiu, Y.; Wang, Z.; Li, H. Dissolution kinetics and solubility of copper oxide nanoparticles as affected by soil properties and aging time. Environ. Sci. Pollut. Res. 2022, 29, 40674–40685. [Google Scholar] [CrossRef] [PubMed]
- Kayestha, R.; Hajela, K. ESR studies on the effect of ionic radii on displacement of Mn2+ bound to a soluble β-galactoside binding hepatic lectin. FEBS Lett. 1995, 368, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Angelé-Martínez, C.; Nguyen, K.V.; Ameer, F.S.; Anker, J.N.; Brumaghim, J.L. Reactive oxygen species generation by copper(II) oxide nanoparticles determined by DNA damage assays and EPR spectroscopy. Nanotoxicology 2017, 11, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Naz, S.; Gul, A.; Zia, M. Toxicity of copper oxide nanoparticles: A review study. IET Nanobiotechnol. 2020, 14, 1–13. [Google Scholar] [CrossRef]
- Bharathiraja, D.B.; Krishnamoorthy, S.; Masi, C.; Dinakarkumar, Y.; Ramanujam, P.K.; Sutha, Á. Calcium Oxide Nanoparticles as an Effective Filtration Aid for Purification of Vehicle Gas Exhaust. Adv. Intern. Combust. Engine Res. 2018, 181–192. [Google Scholar] [CrossRef]
- Khine, E.E.; Koncz-Horvath, D.; Kristaly, F.; Ferenczi, T.; Karacs, G.; Baumli, P.; Kaptay, G. Synthesis and characterization of calcium oxide nanoparticles for CO2 capture. J. Nanopart. Res. 2022, 24, 139. [Google Scholar] [CrossRef]
- Sato, Y.; Ishihara, M.; Nakamura, S.; Fukuda, K.; Takayama, T.; Hiruma, S.; Murakami, K.; Fujita, M.; Yokoe, H. Preparation and Application of Bioshell Calcium Oxide (BiSCaO) Nanoparticle-Dispersions with Bactericidal Activity. Molecules 2019, 24, 3415. [Google Scholar] [CrossRef]
- Han, C.; Li, H.; Li, Y.; Zhu, J.; Zhi, C. Proton-assisted calcium-ion storage in aromatic organic molecular crystal with coplanar stacked structure. Nat. Commun. 2021, 12, 2400. [Google Scholar] [CrossRef]
- Liang, X.; Dai, R.; Chang, S.; Wei, Y.; Zhang, B. Antibacterial mechanism of biogenic calcium oxide and antibacterial activity of calcium oxide/polypropylene composites. Colloids Surf. A Physicochem. Eng. Asp. 2022, 650, 129446. [Google Scholar] [CrossRef]
- Calcium Oxide: Crops. Agricultural Marketing Service. USDA 2002. Available online: https://www.ams.usda.gov/rules-regulations/organic/petitioned-substances/calcium-oxide (accessed on 8 July 2023).
- Hornak, J. Synthesis, Properties, and Selected Technical Applications of Magnesium Oxide Nanoparticles: A Review. Int. J. Mol. Sci. 2021, 22, 12752. [Google Scholar] [CrossRef]
- Leitner, J.; Sedmidubský, D.; Jankovský, O. Size and shape-dependent solubility of CuO nanostructures. Materials 2019, 12, 3355. [Google Scholar] [CrossRef] [PubMed]
- Makshakova, O.N.; Gafurov, M.R.; Goldberg, M.A. The Mutual Incorporation of Mg2+ and CO32− into Hydroxyapatite: A DFT Study. Materials 2022, 15, 9046. [Google Scholar] [CrossRef]
- Lu, X.; Zhu, T.; Chen, C.; Liu, Y. Right or Left: The Role of Nanoparticles in Pulmonary Diseases. Int. J. Mol. Sci. 2014, 15, 17577–17600. [Google Scholar] [CrossRef] [PubMed]
- Fouda, A.; Eid, A.M.; Abdel-Rahman, M.A.; El-Belely, E.F.; Awad, M.A.; Hassan, S.E.; Al-Faifi, Z.E.; Hamza, M.F. Enhanced Antimicrobial, Cytotoxicity, Larvicidal, and Repellence Activities of Brown Algae, Cystoseira crinita-Mediated Green Synthesis of Magnesium Oxide Nanoparticles. Front. Bioeng. Biotechnol. 2022, 10, 849921. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Xie, R.; Ding, P.; Qu, B. Preparation and Characterization of Mg(OH)2 Nanoparticles and Flame-Retardant Property of Its Nanocomposites with EVA. Compos. Struct. 2003, 62, 391–395. [Google Scholar] [CrossRef]
- Zhu, Y.; Tang, Y.; Ruan, Z.; Dai, Y.; Li, Z.; Lin, Z.; Zhao, S.; Cheng, L.; Sun, B.; Zeng, M.; et al. Mg(OH)2 nanoparticles enhance the antibacterial activities of macrophages by activating the reactive oxygen species. J. Biomed. Mater. Res. Part A 2021, 109, 2369–2380. [Google Scholar] [CrossRef] [PubMed]
- Ropp, R.C. Encyclopedia of the Alkaline Earth Compounds; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Sakar, M.; Balakrishna, R.G.; Do, T.-O. Photocatalytic Systems by Design: Materials, Mechanisms and Applications; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Gaetke, L.M.; Chow-Johnson, H.S.; Chow, C.K. Copper: Toxicological relevance and mechanisms. Arch. Toxicol. 2014, 88, 1929–1938. [Google Scholar] [CrossRef]
- Ameh, T.; Gibb, M.; Stevens, D.; Pradhan, S.H.; Braswell, E.; Sayes, C.M. Silver and Copper Nanoparticles Induce Oxidative Stress in Bacteria and Mammalian Cells. Nanomaterials 2022, 12, 2402. [Google Scholar] [CrossRef]
- Okamoto, K.; Kudo, D.; Phuong, D.N.D.; Iwamoto, Y.; Watanabe, K.; Yoshioka, Y.; Ariyoshi, W.; Yamasaki, R. Magnesium Hydroxide Nanoparticles Inhibit the Biofilm Formation of Cariogenic Microorganisms. Nanomaterials 2023, 13, 864. [Google Scholar] [CrossRef]
- PubChem. Cupric Chloride. 2023. Available online: https://pubchem.ncbi.nlm.nih.gov/substance/481107052 (accessed on 7 July 2023).
- Ma, Z.; Ren, F.; Ming, X.; Long, Y.; Volinsky, A.A. Cu-Doped ZnO Electronic Structure and Optical Properties Studied by First-Principles Calculations and Experiments. Materials 2019, 12, 196. [Google Scholar] [CrossRef]
- Bayade, G.; Rong Wu, M.; Massicotte, R.; Gennad’evich Deryabin, D.; Yahia, L.H. Biocidal properties of copper nanoparticles. Eng. Biomater. 2021, 24, 2–17. [Google Scholar] [CrossRef]
- Longano, D.; Ditaranto, N.; Sabbatini, L.; Torsi, L.; Cioffi, N. Synthesis and Antimicrobial Activity of Copper Nanomaterials. In Nano-Antimicrobials: Progress and Prospects; Cioffi, N., Rai, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 85–117. [Google Scholar]
- Hyewon, Y. Extraordinary Enhancement of UV Absorption in TiO2 Nanoparticles Enabled by Low-Oxidized Graphene Nanodots. J. Phys. Chem. 2018, 122, 12114–12121. [Google Scholar] [CrossRef]
- Waghmode, M.S.; Gunjal, A.B.; Mulla, J.A.; Patil, N.N.; Nawani, N.N. Studies on the titanium dioxide nanoparticles: Biosynthesis, applications and remediation. SN Appl. Sci. 2019, 1, 310. [Google Scholar] [CrossRef]
- Shi, H.; Magaye, R.; Castranova, V.; Zhao, J. Titanium dioxide nanoparticles: A review of current toxicological data. Part. Fibre Toxicol. 2013, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Wang, L.; Wang, C.; Zhang, S.; Liu, H.; Li, S.; Wang, X. Toxicity and mechanisms of action of titanium dioxide nanoparticles in living organisms. J. Environ. Sci. 2019, 75, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Talam, S.; Karumuri, S.R.; Gunnam, N. Synthesis, Characterization, and Spectroscopic Properties of ZnO Nanoparticles. Int. Sch. Res. Not. 2012, 2012, 1–6. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Ur Rahman, A.; Tajuddin; Husen, A. Properties of Zinc Oxide Nanoparticles and Their Activity Against Microbes. Nanoscale Res. Lett. 2018, 13, 141. [Google Scholar] [CrossRef]
- Ohashi, H. Effective ionic radii of Ni and Zn in orthopyroxenes. J. Mineral. Petrol. Econ. Geol. 1989, 84, 329–334. [Google Scholar] [CrossRef]
- Jin, M.; Li, N.; Sheng, W.; Ji, X.; Liang, X.; Kong, B.; Yin, P.; Li, Y.; Zhang, X.; Liu, K. Toxicity of different zinc oxide nanomaterials and dose-dependent onset and development of Parkinson’s disease-like symptoms induced by zinc oxide nanorods. Environ. Int. 2021, 146, 106179. [Google Scholar] [CrossRef]
- Mendes, C.R.; Dilarri, G.; Forsan, C.F.; Sapata, V.d.M.R.; Lopes, P.R.M.; de Moraes, P.B.; Montagnolli, R.N.; Ferreira, H.; Bidoia, E.D. Antibacterial action and target mechanisms of zinc oxide nanoparticles against bacterial pathogens. Sci. Rep. 2022, 12, 2658. [Google Scholar] [CrossRef]
- Tiwari, V.; Mishra, N.; Gadani, K.; Solanki, P.S.; Shah, N.A.; Tiwari, M. Mechanism of Anti-bacterial Activity of Zinc Oxide Nanoparticle Against Carbapenem-Resistant Acinetobacter baumannii. Front. Microbiol. 2018, 9, 1218. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Pi, J.; Cai, J. The Advancing of Zinc Oxide Nanoparticles for Biomedical Applications. Bioinorg. Chem. Appl. 2018, 2018, 1062562. [Google Scholar] [CrossRef] [PubMed]
- Cotton, G.; Gee, C.; Jude, A.; Duncan, W.; Abdelmoneim, D.; Coates, D. Efficacy and safety of alpha lipoic acid-capped silver nanoparticles for oral applications. RSC Adv. 2019, 9, 6973–6985. [Google Scholar] [CrossRef]
- Idisi, D.O.; Oke, J.A.; Bello, I.T. Graphene oxide/Au nanoparticles: Synthesis, properties, and application: A mini-review. Int. J. Energy Res. 2021, 45, 19772–19788. [Google Scholar] [CrossRef]
- Rhazouani, A.; Gamrani, H.; El Achaby, M.; Aziz, K.; Gebrati, L.; Uddin, M.S.; Aziz, F. Synthesis and Toxicity of Graphene Oxide Nanoparticles: A Literature Review of In Vitro and In Vivo Studies. BioMed Res. Int. 2021, 2021, 5518999. [Google Scholar] [CrossRef]
- Johnson, D.W.; Dobson, B.P.; Coleman, K.S. A manufacturing perspective on graphene dispersions. Curr. Opin. Colloid Interface Sci. 2015, 20, 367–382. [Google Scholar] [CrossRef]
- Sánchez-López, L.; Chico, B.; Llorente, I.; Escudero, M.L.; Lozano, R.M.; García-Alonso, M.C. Covalent immobilization of graphene oxide on biomedical grade CoCr alloy by an improved multilayer system assembly via Silane/GO bonding. Mater. Chem. Phys. 2022, 287, 126296. [Google Scholar] [CrossRef]
- Yadav, S.; Singh Raman, A.P.; Meena, H.; Goswami, A.G.; Bhawna; Kumar, V.; Jain, P.; Kumar, G.; Sagar, M.; Rana, D.K.; et al. An Update on Graphene Oxide: Applications and Toxicity. ACS Omega 2022, 7, 35387–35445. [Google Scholar] [CrossRef]
- Dimiev, A.M.; Tour, J.M. Mechanism of Graphene Oxide Formation. ACS Nano 2014, 8, 3060–3068. [Google Scholar] [CrossRef]
- Sharma, H.; Mondal, S. Functionalized Graphene Oxide for Chemotherapeutic Drug Delivery and Cancer Treatment: A Promising Material in Nanomedicine. Int. J. Mol. Sci. 2020, 21, 6280. [Google Scholar] [CrossRef]
- Wani, I.; Ganguly, A.; Ahmed, J.; Ahmad, T. Silver nanoparticles: Ultrasonic wave assisted synthesis, optical characterization and surface area studies. Mater. Lett. 2011, 65, 520–522. [Google Scholar] [CrossRef]
- Kailasa, S.; Park, T.-J.; Rohit, J.; Koduru, J. Antimicrobial Activity of Silver Nanoparticles; William Andrew: Norwich, NY, USA, 2019; pp. 461–484. [Google Scholar]
- Cheon, J.Y.; Kim, S.J.; Rhee, Y.H.; Kwon, O.H.; Park, W.H. Shape-dependent antimicrobial activities of silver nanoparticles. Int. J. Nanomed. 2019, 14, 2773–2780. [Google Scholar] [CrossRef]
- Di Martino, P. Antimicrobial agents and microbial ecology. AIMS Microbiol. 2022, 8, 1–4. [Google Scholar] [CrossRef]
- Antiseptics. 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK507853/ (accessed on 7 July 2023).
- Ahmed, S.; Ahmad, M.; Ikram, S. Chitosan: A Natural Antimicrobial Agent-A Review. J. Appl. Chem. 2014, 3, 493–503. [Google Scholar]
- Babutan, I.; Lucaci, A.-D.; Botiz, I. Antimicrobial polymeric structures assembled on surfaces. Polymers 2021, 13, 1552. [Google Scholar] [CrossRef]
- Song, J.; Kang, H.; Lee, C.; Hwang, S.H.; Jang, J. Aqueous synthesis of silver nanoparticle embedded cationic polymer nanofibers and their antibacterial activity. ACS Appl. Mater. Interfaces 2012, 4, 460–465. [Google Scholar] [CrossRef]
- Dacarro, G.; Cucca, L.; Grisoli, P.; Pallavicini, P.; Patrini, M.; Taglietti, A. Monolayers of polyethilenimine on flat glass: A versatile platform for cations coordination and nanoparticles grafting in the preparation of antibacterial surfaces. Dalton Trans. 2012, 41, 2456–2463. [Google Scholar] [CrossRef]
- Li, J.-h.; Zhang, D.-b.; Ni, X.-x.; Zheng, H.; Zhang, Q.-q. Excellent hydrophilic and anti-bacterial fouling PVDF membrane based on ag nanoparticle self-assembled PCBMA polymer brush. Chin. J. Polym. Sci. 2017, 35, 809–822. [Google Scholar] [CrossRef]
- Turalija, M.; Bischof, S.; Budimir, A.; Gaan, S. Antimicrobial PLA films from environment friendly additives. Compos. Part B Eng. 2016, 102, 94–99. [Google Scholar] [CrossRef]
- Iurciuc-Tincu, C.E.; Cretan, M.S.; Purcar, V.; Popa, M.; Daraba, O.M.; Atanase, L.I.; Ochiuz, L. Drug Delivery System Based on pH-Sensitive Biocompatible Poly(2-vinyl pyridine)-b-poly(ethylene oxide) Nanomicelles Loaded with Curcumin and 5-Fluorouracil. Polymers 2020, 12, 1450. [Google Scholar] [CrossRef]
- Ben-Sasson, M.; Zodrow, K.R.; Genggeng, Q.; Kang, Y.; Giannelis, E.P.; Elimelech, M. Surface functionalization of thin-film composite membranes with copper nanoparticles for antimicrobial surface properties. Environ. Sci. Technol. 2014, 48, 384–393. [Google Scholar] [CrossRef]
- Demchenko, V.; Riabov, S.; Rybalchenko, N.; Goncharenko, L.; Kobylinskyi, S.; Shtompel, V. X-ray study of structural formation, thermomechanical and antimicrobial properties of copper-containing polymer nanocomposites obtained by the thermal reduction method. Eur. Polym. J. 2017, 96, 326–336. [Google Scholar] [CrossRef]
- Joo, Y.T.; Jung, K.h.; Kim, M.J.; Kim, Y. Preparation of antibacterial PDMAEMA-functionalized multiwalled carbon nanotube via atom transfer radical polymerization. J. Appl. Polym. Sci. 2013, 127, 1508–1518. [Google Scholar] [CrossRef]
- Murugan, E.; Vimala, G. Effective functionalization of multiwalled carbon nanotube with amphiphilic poly(propyleneimine) dendrimer carrying silver nanoparticles for better dispersability and antimicrobial activity. J. Colloid Interface Sci. 2011, 357, 354–365. [Google Scholar] [CrossRef]
- Mural, P.K.S.; Banerjee, A.; Rana, M.S.; Shukla, A.; Padmanabhan, B.; Bhadra, S.; Madras, G.; Bose, S. Polyolefin based antibacterial membranes derived from PE/PEO blends compatibilized with amine terminated graphene oxide and maleated PE. J. Mater. Chem. A 2014, 2, 17635–17648. [Google Scholar] [CrossRef]
- Ozdal, M.; Gurkok, S. Recent advances in nanoparticles as antibacterial agent. ADMET DMPK 2022, 10, 115–129. [Google Scholar] [CrossRef]
- Jindal, H.; Le, C.F.; Yusof, M.Y.; Sekaran, S. Net charge, hydrophobicity and specific amino acids contribute to the activity of antimicrobial peptides. J. Health Transl. Med. 2014, 17, 1–7. [Google Scholar] [CrossRef]
- Singh, R.; Smitha, M.S.; Singh, S.P. The role of nanotechnology in combating multi-drug resistant bacteria. J. Nanosci. Nanotechnol. 2014, 14, 4745–4756. [Google Scholar] [CrossRef]
- Kapoor, G.; Saigal, S.; Elongavan, A. Action and resistance mechanisms of antibiotics: A guide for clinicians. J. Anaesthesiol. Clin. Pharmacol. 2017, 33, 300. [Google Scholar] [CrossRef]
- Yu, Z.; Li, Q.; Wang, J.; Yu, Y.; Wang, Y.; Zhou, Q.; Li, P. Reactive oxygen species-related nanoparticle toxicity in the biomedical field. Nanoscale Res. Lett. 2020, 15, 1–14. [Google Scholar] [CrossRef]
- Park, J.T.; Strominger, J.L. Mode of action of penicillin: Biochemical basis for the mechanism of action of penicillin and for its selective toxicity. Science 1957, 125, 99–101. [Google Scholar] [CrossRef]
- Di Somma, A.; Moretta, A.; Canè, C.; Cirillo, A.; Duilio, A. Inhibition of bacterial biofilm formation. In Bacterial Biofilms; IntechOpen: London, UK, 2020. [Google Scholar]
- Asfahl, K.L.; Schuster, M. Social interactions in bacterial cell–cell signaling. FEMS Microbiol. Rev. 2017, 41, 92–107. [Google Scholar] [CrossRef] [PubMed]
- Aliashkevich, A.; Alvarez, L.; Cava, F. New insights into the mechanisms and biological roles of D-amino acids in complex eco-systems. Front. Microbiol. 2018, 9, 683. [Google Scholar] [CrossRef]
- Beyth, N.; Houri-Haddad, Y.; Domb, A.; Khan, W.; Hazan, R. Alternative antimicrobial approach: Nano-antimicrobial materials. Evid.-Based Complement. Altern. Med. 2015, 2015, 246012. [Google Scholar] [CrossRef]
- Kohanski, M.A.; Dwyer, D.J.; Collins, J.J. How antibiotics kill bacteria: From targets to networks. Nat. Rev. Microbiol. 2010, 8, 423–435. [Google Scholar] [CrossRef]
- Guo, L.; Yuan, W.; Lu, Z.; Li, C. Polymer/nanosilver composite coatings for antibacterial applications. Colloids Surf. A Physicochem. Eng. Asp. 2013, 439, 69–83. [Google Scholar] [CrossRef]
- Kamaruzzaman, N.F.; Tan, L.P.; Hamdan, R.H.; Choong, S.S.; Wong, W.K.; Gibson, A.J.; Chivu, A.; Pina, M.F. Antimicrobial Polymers: The Potential Replacement of Existing Antibiotics? Int. J. Mol. Sci. 2019, 20, 2747. [Google Scholar] [CrossRef]
- Tamayo, L.; Azócar, M.; Kogan, M.; Riveros, A.; Páez, M. Copper-polymer nanocomposites: An excellent and cost-effective biocide for use on antibacterial surfaces. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 69, 1391–1409. [Google Scholar] [CrossRef]
- Thampi, V.A.; Thanka Rajan, S.; Anupriya, K.; Subramanian, B. Functionalization of fabrics with PANI/CuO nanoparticles by precipitation route for anti-bacterial applications. J. Nanoparticle Res. 2015, 17, 1–12. [Google Scholar] [CrossRef]
- Chapman, J.; Nor, L.; Brown, R.; Kitteringham, E.; Russell, S.; Sullivan, T.; Regan, F. Antifouling performances of macro- to micro- to nano-copper materials for the inhibition of biofouling in its early stages. J. Mater. Chem. B 2013, 1, 6194–6200. [Google Scholar] [CrossRef] [PubMed]
- Motoike, K.; Hirano, S.; Yamana, H.; Onda, T.; Maeda, T.; Ito, T.; Hayakawa, M. Antiviral activities of heated dolomite powder. Biocontrol Sci. 2008, 13, 131–138. [Google Scholar] [CrossRef]
- Leung, Y.H.; Ng, A.M.; Xu, X.; Shen, Z.; Gethings, L.A.; Wong, M.T.; Chan, C.M.; Guo, M.Y.; Ng, Y.H.; Djurišić, A.B.; et al. Mechanisms of antibacterial activity of MgO: Non-ROS mediated toxicity of MgO nanoparticles towards Escherichia coli. Small 2014, 10, 1171–1183. [Google Scholar] [CrossRef] [PubMed]
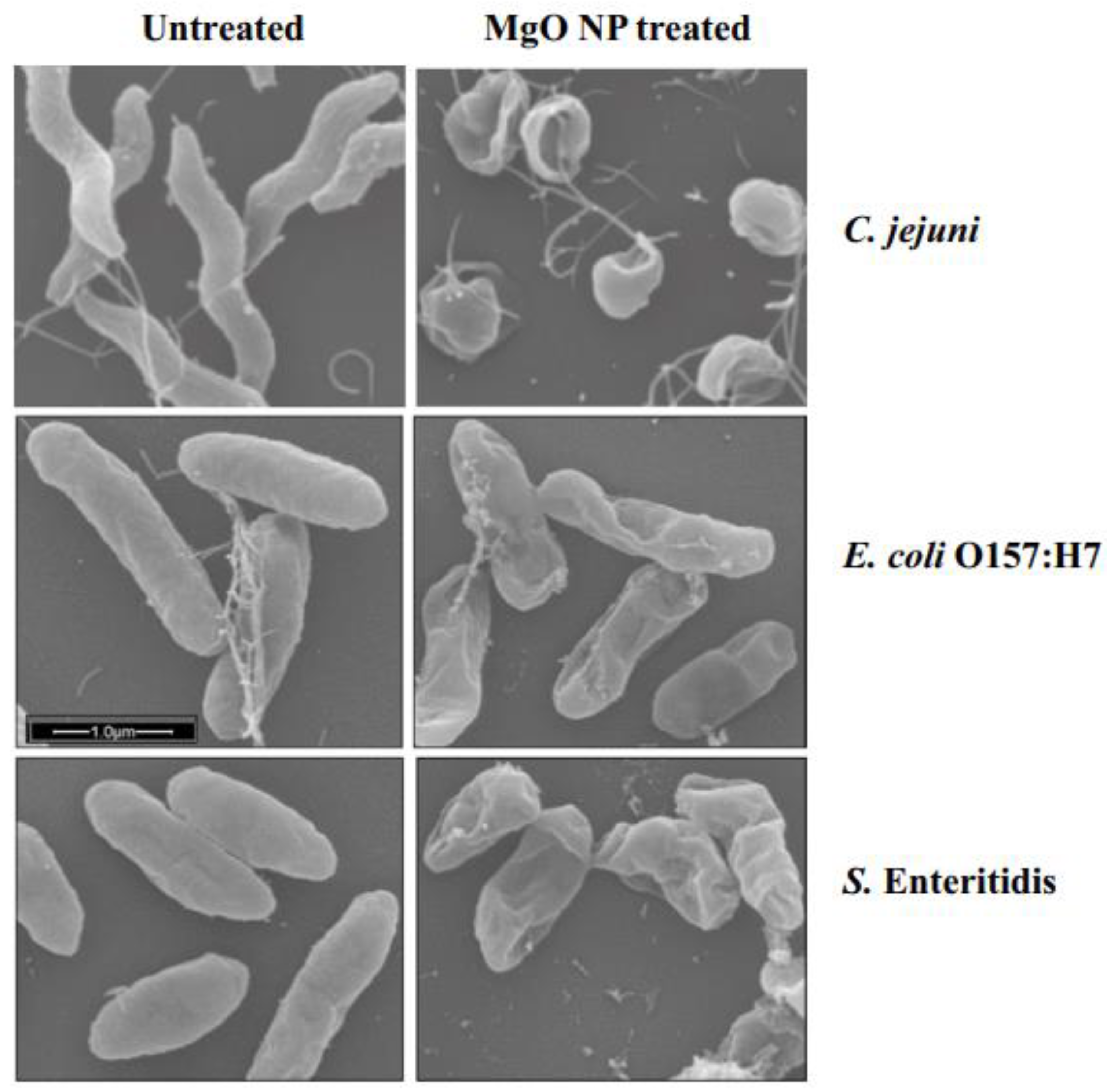
- He, Y.; Ingudam, S.; Reed, S.; Gehring, A.; Strobaugh, T.P.; Irwin, P. Study on the mechanism of antibacterial action of magnesium oxide nanoparticles against foodborne pathogens. J. Nanobiotechnol. 2016, 14, 54. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.Y.; Tatsumura, Y. Alexander Fleming (1881–1955): Discoverer of penicillin. Singap. Med. J. 2015, 56, 366–367. [Google Scholar] [CrossRef]
- Dong, C.; Cairney, J.; Sun, Q.; Maddan, O.; He, G.; Deng, Y. Investigation of Mg(OH)2 nanoparticles as an antibacterial agent. J. Nanoparticle Res. 2010, 12, 2101–2109. [Google Scholar] [CrossRef]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef]
- Sajjad, S.; Khan Leghari, S.A.; Iqbal, A. Study of Graphene Oxide Structural Features for Catalytic, Antibacterial, Gas Sensing, and Metals Decontamination Environmental Applications. ACS Appl. Mater. Interfaces 2017, 9, 43393–43414. [Google Scholar] [CrossRef]
- Tarcan, R.; Handrea-Dragan, M.; Todor-Boer, O.; Petrovai, I.; Farcau, C.; Rusu, M.; Vulpoi, A.; Todea, M.; Astilean, S.; Botiz, I. A new, fast and facile synthesis method for reduced graphene oxide in N,N-dimethylformamide. Synth. Met. 2020, 269, 116576. [Google Scholar] [CrossRef]
- Kang, S.; Pinault, M.; Pfefferle, L.D.; Elimelech, M. Single-Walled Carbon Nanotubes Exhibit Strong Antimicrobial Activity. Langmuir 2007, 23, 8670–8673. [Google Scholar] [CrossRef]
- Zou, P.; Hartleb, W.; Lienkamp, K. It takes walls and knights to defend a castle—Synthesis of surface coatings from antimicrobial and antibiofouling polymers. J. Mater. Chem. 2012, 22, 19579–19589. [Google Scholar] [CrossRef]
- Mangadlao, J.D.; Santos, C.M.; Felipe, M.J.L.; de Leon, A.C.C.; Rodrigues, D.F.; Advincula, R.C. On the antibacterial mechanism of graphene oxide (GO) Langmuir–Blodgett films. Chem. Commun. 2015, 51, 2886–2889. [Google Scholar] [CrossRef]
- Zou, X.; Zhang, L.; Wang, Z.; Luo, Y. Mechanisms of the Antimicrobial Activities of Graphene Materials. J. Am. Chem. Soc. 2016, 138, 2064–2077. [Google Scholar] [CrossRef]
- National Research Council (US) Steering Group for the Workshop on Size Limits of Very Small Microorganisms. Size Limits of Very Small Microorganisms: Proceedings of a Workshop; National Academies Press: Washington, DC, USA, 1999. [Google Scholar]
- Baron, E.J. Medical Microbiology; Baron, S., Ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996. [Google Scholar]
- Lorimer, J.; Hodgetts, T. Good germs, bad germs: Citizen science and microbiology. Biochemist 2017, 39, 35–37. [Google Scholar] [CrossRef]
- Webster, J.; Weber, R. Introduction to Fungi; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- McCoy, C.W.; Samson, R.A.; Boucias, D.G. Entomogenous fungi. In Handbook of Natural Pesticides, Vol. V:“Microbial Insecticides, Part A: Entomogenous Protozoa and Fungi”; Ignoffo, C.M., Mandava, N.B., Eds.; CRC Press: Boca Raton, FL, USA, 1988; Volume 2, pp. 157–172. [Google Scholar]
- Zarandi, M.M.; Bonakdar, A.; Stiharu, I. Investigations on natural frequencies of individual spherical and ellipsoidal bakery yeast cells. In Proceedings of the COMSOL Conference; 2010; pp. 5–6. Available online: https://cn.comsol.com/paper/download/114889/molavi2_paper.pdf (accessed on 5 March 2023).
- Louten, J. Chapter 2—Virus Structure and Classification. In Essential Human Virology; Louten, J., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 19–29. [Google Scholar]
- Wiegand, C.; Völpel, A.; Ewald, A.; Remesch, M.; Kuever, J.; Bauer, J.; Griesheim, S.; Hauser, C.; Thielmann, J.; Tonndorf-Martini, S.; et al. Critical physiological factors influencing the outcome of antimicrobial testing according to ISO 22196/JIS Z 2801. PLoS ONE 2018, 13, e0194339. [Google Scholar] [CrossRef]
- Perez-Gavilan, A.; de Castro, J.V.; Arana, A.; Merino, S.; Retolaza, A.; Alves, S.A.; Francone, A.; Kehagias, N.; Sotomayor-Torres, C.M.; Cocina, D.; et al. Antibacterial activity testing methods for hydrophobic patterned surfaces. Sci. Rep. 2021, 11, 6675. [Google Scholar] [CrossRef]
- ASTM D7907-14; Test Methods for Determination of Bactericidal Efficacy on the Surface of Medical Examination Gloves. ASTM International: West Conshohocken, PA, USA, 2019.
- ISO 20743/2021; Determination of Antibacterial Activity of Textile Products. ISO: Geneva, Switzerland, 2021.
- JIS L 1902:2015; Testing for Antimicrobial Activity and Efficacy on Textile Products. JAFET/JSA from Japanese Industrial Standards. Available online: https://webdesk.jsa.or.jp/preview/pre_jis_l_01902_000_000_2015_e_ed10_i4.pdf (accessed on 5 March 2023).
- ISO/TS 16782:2016; Clinical Laboratory Testing, Criteria for Acceptable lots of Dehydrated Mueller-Hinton Agar and Broth for Antimicrobial Susceptibility Testing. ISO: Geneva, Switzerland, 2016.
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- ISO 21702; Measurement of Antiviral Activity on Plastics and other Non-Porous Surfaces. ISO: Geneva, Switzerland, 2019.
- Furer, L.A.; Clement, P.; Herwig, G.; Rossi, R.M.; Bhoelan, F.; Amacker, M.; Stegmann, T.; Buerki-Thurnherr, T.; Wick, P. A novel inactivated virus system (InViS) for a fast and inexpensive assessment of viral disintegration. Sci. Rep. 2022, 12, 11583. [Google Scholar] [CrossRef]
- ISO 18184:2019; Textiles, Determination of Antiviral Activity of Textile Products. ISO: Geneva, Switzerland, 2019.
- ASTM G21; Standard Practice for Determining Resistance of Synthetic Polymeric Materials to Fungi. ASTM International: West Conshohocken, PA, USA, 2021.
- AATCC TM30; Test Method for Antifungal Activity, Assessment on Textile Materials: Mildew and Rot Resistance of Textile Materials. American Association of Textile Chemists and Colorists: Durham, NC, USA, 2017.
- ISO 846:2019; Plastics, Evaluation of the action of microorganisms. ISO: Geneva, Switzerland, 2019.
- ISO 16256:2021; Clinical Laboratory Testing and In Vitro Diagnostic Test Systems, Broth Micro-Dilution Reference Method for Testing the In Vitro Activity of Antimicrobial Agents against Yeast Fungi Involved in Infectious Diseases. ISO: Geneva, Switzerland, 2021.
- Berkow, E.L.; Lockhart, S.R.; Ostrosky-Zeichner, L. Antifungal Susceptibility Testing: Current Approaches. Clin. Microbiol. Rev. 2020, 33, 10–1128. [Google Scholar] [CrossRef]
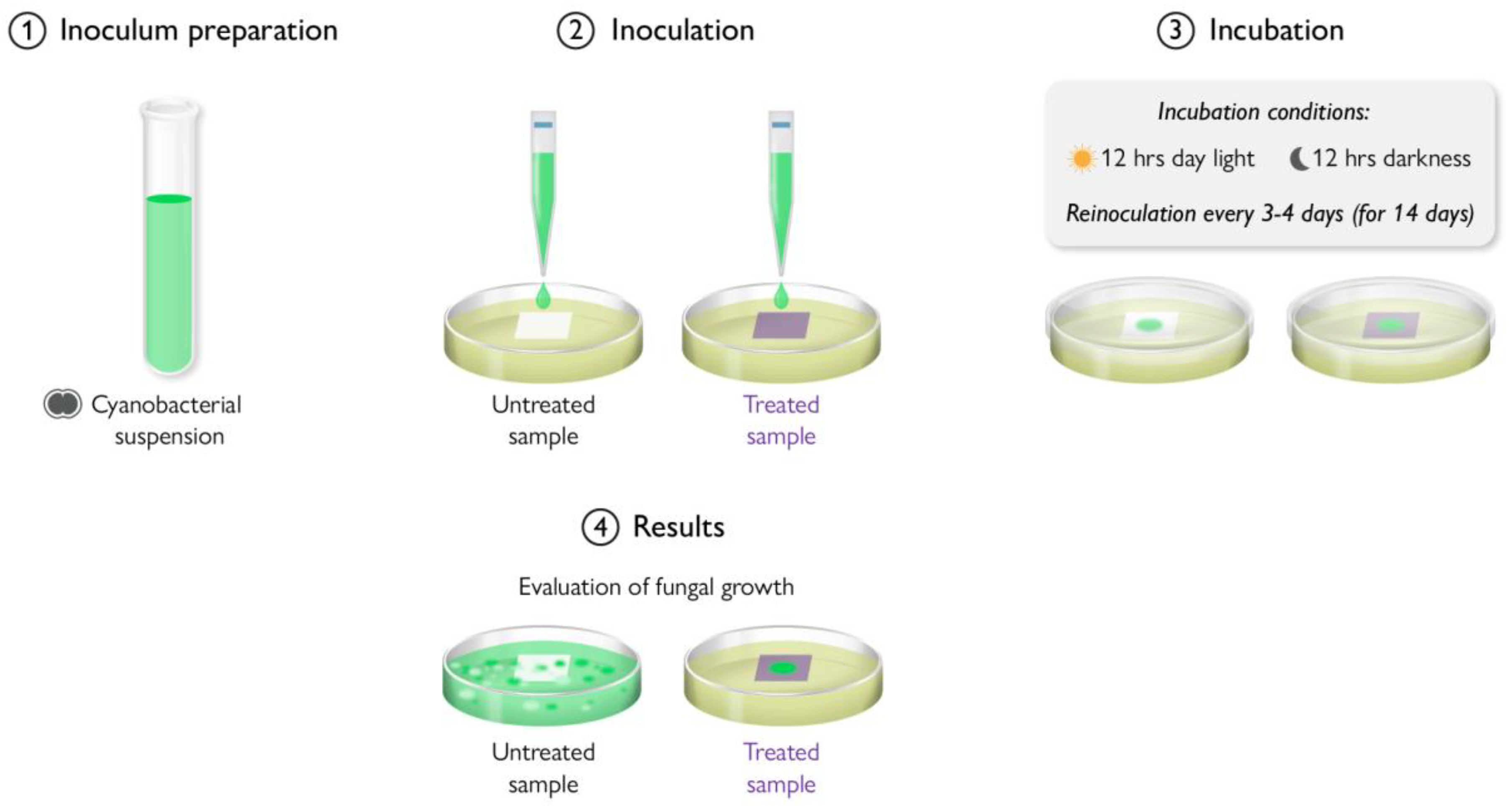
- ASTM G29-21; Standard Practice for Determining Cyanobacterial Resistance of Polymeric Films. ASTM International: West Conshohocken, PA, USA, 2022.
- Saeidnia, S.; Manayi, A.; Abdollahi, M. From in vitro Experiments to in vivo and Clinical Studies; Pros and Cons. Curr. Drug Discov. Technol. 2015, 12, 218–224. [Google Scholar] [CrossRef]
- Fini, M.; Giardino, R. In vitro and in vivo tests for the biological evaluation of candidate orthopedic materials: Benefits and limits. J. Appl. Biomater. Biomech. JABB 2003, 1, 155–163. [Google Scholar]
- Ghallab, A.; Bolt, H.M. In vitro systems: Current limitations and future perspectives. Arch. Toxicol. 2014, 88, 2085–2087. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Cormican, M.; Flamm, R.K.; Mendes, R.E.; Jones, R.N. Temporal and Geographic Variation in Antimicrobial Susceptibility and Resistance Patterns of Enterococci: Results From the SENTRY Antimicrobial Surveillance Program, 1997–2016. Open Forum Infect. Dis. 2019, 6 (Suppl. S1), S54–S62. [Google Scholar] [CrossRef]
- Campos, M.D.; Zucchi, P.C.; Phung, A.; Leonard, S.N.; Hirsch, E.B. The Activity of Antimicrobial Surfaces Varies by Testing Protocol Utilized. PLoS ONE 2016, 11, e0160728. [Google Scholar] [CrossRef]
- Dahlia, F.; Barouagui, S.; Hemida, H.; Bousaadia, D.; Rahmoune, B. Influence of environment variations on anti-glycaemic, anti-cholesterolemic, antioxidant and antimicrobial activities of natural wild fruits of Ziziphus lotus (L.). S. Afr. J. Bot. 2020, 132, 215–225. [Google Scholar] [CrossRef]
- Boks, N.P.; Norde, W.; van der Mei, H.C.; Busscher, H.J. Forces involved in bacterial adhesion to hydrophilic and hydrophobic surfaces. Microbiology 2008, 154 Pt 10, 3122–3133. [Google Scholar] [CrossRef]
- Zhou, C.; Wu, Y.; Thappeta, K.R.V.; Subramanian, J.T.L.; Pranantyo, D.; Kang, E.T.; Duan, H.; Kline, K.; Chan-Park, M.B. In Vivo Anti-Biofilm and Anti-Bacterial Non-Leachable Coating Thermally Polymerized on Cylindrical Catheter. ACS Appl. Mater. Interfaces 2017, 9, 36269–36280. [Google Scholar] [CrossRef] [PubMed]
- Huh, A.J.; Kwon, Y.J. “Nanoantibiotics”: A new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. J. Control. Release 2011, 156, 128–145. [Google Scholar] [CrossRef] [PubMed]
- Viitanen, H.; Ojanen, T. Improved model to predict mold growth in building materials. In Proceedings of the 10th Thermal Exterior Envelopes of Whole Buildings Conference, Atlanta, GA, USA, 2–7 December 2007; pp. 2–7. [Google Scholar]
- Savage, D.T.; Hilt, J.Z.; Dziubla, T.D. In Vitro Methods for Assessing Nanoparticle Toxicity. In Nanotoxicity: Methods and Protocols; Zhang, Q., Ed.; Springer: New York, NY, USA, 2019; pp. 1–29. [Google Scholar]
- Antimicrobial Plastics Market Size. Growth Report [2023–2030]. 2023. Available online: https://www.fortunebusinessinsights.com/antimicrobial-plastics-market-107635 (accessed on 7 July 2023).











| Blend/Composite | Configuration/ Nanostructure | Dimension | Antimicrobial Mechanism | Efficacy | Microbe of Interest | Ref. |
|---|---|---|---|---|---|---|
| PMMA/Ag, PTBAM/Ag | Nanofibers | 40 nm diameter, 10 µm long | Bioactive reinforced | - | E. coli, S. aureus | [177] |
| PEI/Ag | NPs grafted on SAM | 10–14 nm thick (total) | Bioactive reinforced | ~6 log 0.86 log | E. coli S. aureus | [178] |
| PVDF-g-PCBMA/Ag | Pores/brushes | - | Bioactive reinforced | - | E. coli, S. aureus | [179] |
| PLA/PEG, PLA/PEG/Ag | Films; NPs | ~40 µm thick 25 nm thick | Biopassive + bioactive | - | E. coli, S. aureus | [180] |
| P2VP-b-PEG | Smart micelles | 60 nm (unloaded) | Bioactive | - | [181] | |
| PEI/Cu | Positively charged NPs | 34 nm radius | Bioactive reinforced | 87% 96% 80% | E. coli P. aeruginosa S. aureus | [182] |
| Pectin-PEI-Cu | Films with Cu NPs | 100 µm thick | Bioactive reinforced | - | S. aureus, E. coli | [183] |
| PDMEMA-MWCNTs | Nanotubes | 26 nm diameter | Bioactive reinforced | 42% - | E. coli S. aureus E. coli | [184] |
| MWCNTs-APPI/MWCNTs-APPI-Ag Nps | Nanotubes Ag NPs | 15 nm diameter | Bioactive reinforced | 96%/99% 96/99% 87%/83% | B. subtilis S. aureus E. coli | [185] |
| PE/PEG/GO-NH2 | Films | - | Bioactive reinforced | 90% | E. coli | [186] |
| The Radicals (Not Stable) | The Nonradicals (Stable) |
|---|---|
| Superoxide (O2∙−) | Hydrogen peroxide (H2O2) |
| Hydroxyl (OH∙) | Hypochlorous acid |
| Peroxyl, alkoxyl (RO2∙,RO∙) | Ozone (O3) |
| Oxides of nitrogen (NO∙,NO2∙) | Singlet oxygen (1O2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkarri, S.; Bin Saad, H.; Soliman, M. On Antimicrobial Polymers: Development, Mechanism of Action, International Testing Procedures, and Applications. Polymers 2024, 16, 771. https://doi.org/10.3390/polym16060771
Alkarri S, Bin Saad H, Soliman M. On Antimicrobial Polymers: Development, Mechanism of Action, International Testing Procedures, and Applications. Polymers. 2024; 16(6):771. https://doi.org/10.3390/polym16060771
Chicago/Turabian StyleAlkarri, Saleh, Hawra Bin Saad, and Maria Soliman. 2024. "On Antimicrobial Polymers: Development, Mechanism of Action, International Testing Procedures, and Applications" Polymers 16, no. 6: 771. https://doi.org/10.3390/polym16060771
APA StyleAlkarri, S., Bin Saad, H., & Soliman, M. (2024). On Antimicrobial Polymers: Development, Mechanism of Action, International Testing Procedures, and Applications. Polymers, 16(6), 771. https://doi.org/10.3390/polym16060771





