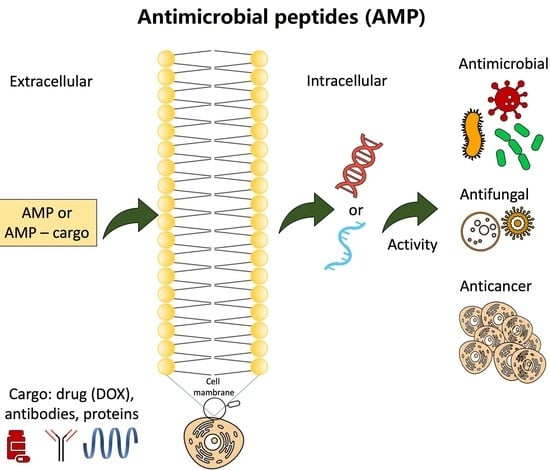
Anticancer Potential of Antimicrobial Peptides: Focus on Buforins
Abstract
1. Introduction
2. Antimicrobial Peptides (AMPs) with Anticancer Potential
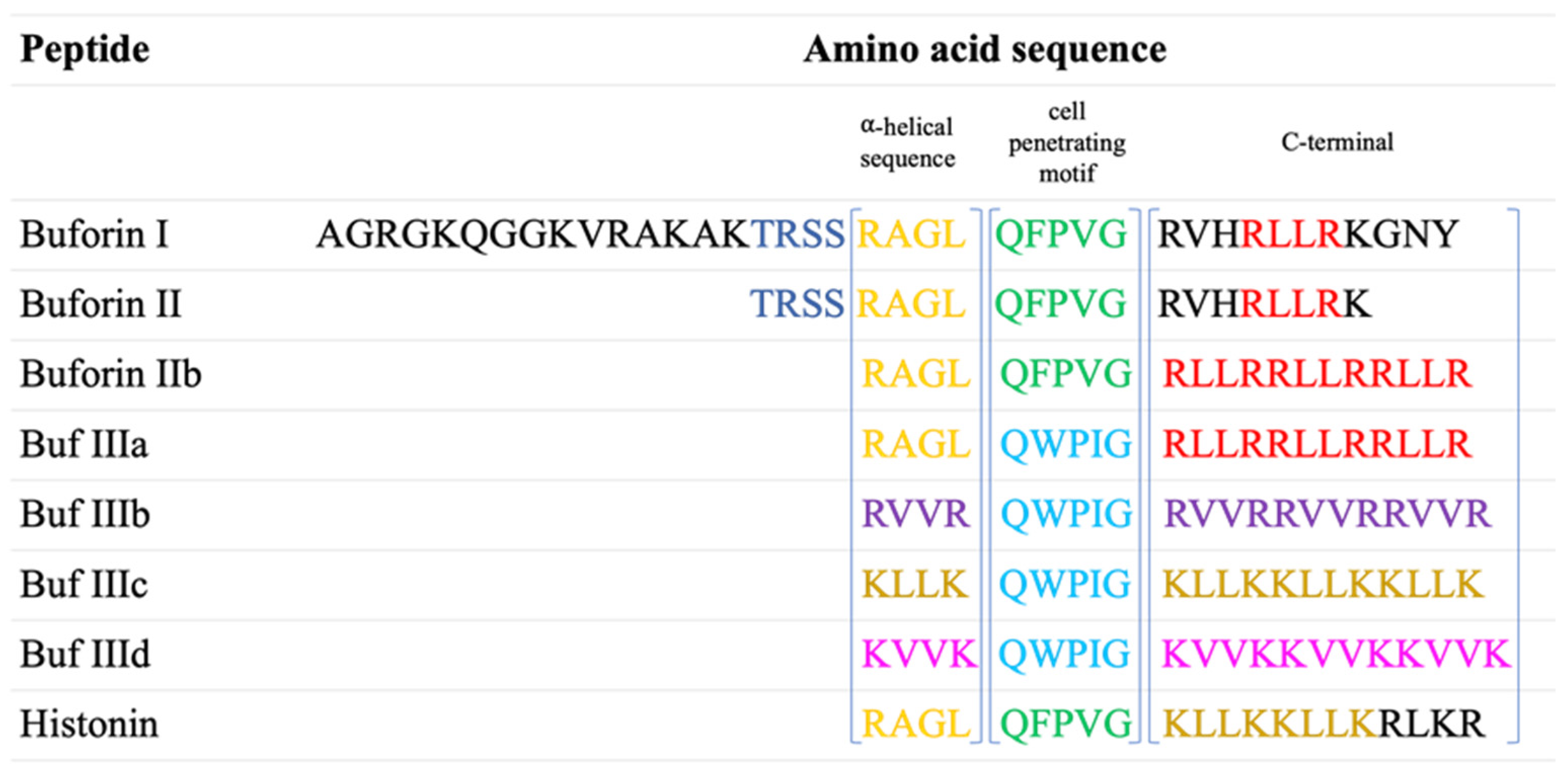
2.1. Buforin Peptides Identification
2.2. Buforins’ Preparation and Characterization
2.3. Buforins’ Mechanisms of Action
3. Anticancer Activity of Buforins
3.1. Buforins Used for HeLa Cells
3.2. Buforins Used for Breast Cancer
3.3. Buforins Used for Lung Cancer
3.4. Buforins for Ovarian Cancer
3.5. Buforins for Prostate Cancer
3.6. Buforins for Liver Cancer
4. Anticancer Activity of Buforin-Containing Bioconjugates
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmed, S.; Rehman, S.U.; Tabish, M. Cancer nanomedicine: A step towards improving the drug delivery and enhanced efficacy of chemotherapeutic drugs. OpenNano 2022, 7, 100051. [Google Scholar] [CrossRef]
- Zorko, M.; Jones, S.; Langel, Ü. Cell-penetrating peptides in protein mimicry and cancer therapeutics. Adv. Drug Deliv. Rev. 2022, 180, 114044. [Google Scholar] [CrossRef]
- Abbasi, M.; Ghoran, S.H.; Niakan, M.H.; Jamali, K.; Moeini, Z.; Jangjou, A.; Izadpanah, P.; Amani, A.M. Mesoporous silica nanoparticle: Heralding a brighter future in cancer nanomedicine. Microporous Mesoporous Mater. 2021, 319, 110967. [Google Scholar] [CrossRef]
- Akhtarkhavari, T.; Bahrami, A.R.; Matin, M.M. Downregulation of miR-21 as a promising strategy to overcome drug resistance in cancer. Eur. J. Pharmacol. 2022, 932, 175233. [Google Scholar] [CrossRef] [PubMed]
- Al-mansoori, L.; Elsinga, P.; Goda, S.K. Bio-vehicles of cytotoxic drugs for delivery to tumor specific targets for cancer precision therapy. Biomed. Pharmacother. 2021, 144, 112260. [Google Scholar] [CrossRef] [PubMed]
- Askari Rizvi, S.F.; Zhang, H. Emerging trends of receptor-mediated tumor targeting peptides: A review with perspective from molecular imaging modalities. Eur. J. Med. Chem. 2021, 221, 113538. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, E.; Ali, A.; Fatima, M.T.; Nimisha; Apurva; Kumar, A.; Sumi, M.P.; Sattar, R.S.A.; Mahajan, B.; Saluja, S.S. Ligand decorated biodegradable nanomedicine in the treatment of cancer. Pharmacol. Res. 2021, 167, 105544. [Google Scholar] [CrossRef] [PubMed]
- Afshari, A.R.; Sanati, M.; Mollazadeh, H.; Kesharwani, P.; Johnston, T.P.; Sahebkar, A. Nanoparticle-based drug delivery systems in cancer: A focus on inflammatory pathways. Semin. Cancer Biol. 2022, 86, 860–872. [Google Scholar] [CrossRef] [PubMed]
- Abuhelwa, Z.; Alloghbi, A.; Nagasaka, M. A comprehensive review on antibody-drug conjugates (ADCs) in the treatment landscape of non-small cell lung cancer (NSCLC). Cancer Treat. Rev. 2022, 106, 102393. [Google Scholar] [CrossRef]
- Ang, M.J.Y.; Chan, S.Y.; Goh, Y.-Y.; Luo, Z.; Lau, J.W.; Liu, X. Emerging strategies in developing multifunctional nanomaterials for cancer nanotheranostics. Adv. Drug Deliv. Rev. 2021, 178, 113907. [Google Scholar] [CrossRef]
- Ranjitha, V.R.; Muddegowda, U.; Ravishankar Rai, V. Potent activity of bioconjugated peptide and selenium nanoparticles against colorectal adenocarcinoma cells. Drug Dev. Ind. Pharm. 2019, 45, 1496–1505. [Google Scholar] [CrossRef] [PubMed]
- Khandelwal, S.; Boylan, M.; Spallholz, J.E.; Gollahon, L. Cytotoxicity of Selenium Immunoconjugates against Triple Negative Breast Cancer Cells. Int. J. Mol. Sci. 2018, 19, 3352. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Dong, X.; Wang, L.; Ji, H.; Liu, A. Antitumor effects of seleno-beta-lactoglobulin (Se-beta-Lg) against human gastric cancer MGC-803 cells. Eur. J. Pharmacol. 2018, 833, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Zeng, D.; Deng, S.; Sang, C.; Zhao, J.; Chen, T. Rational Design of Cancer-Targeted Selenadiazole Derivative as Efficient Radiosensitizer for Precise Cancer Therapy. Bioconjugate Chem. 2018, 29, 2039–2049. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.; Kopel, J.; Ristic, B.; Marsh, H.; Fralick, J.; Reid, T. Antimicrobial seleno-organic coatings and compounds acting primarily on the plasma membrane: A review. Adv. Redox Res. 2022, 4, 100031. [Google Scholar] [CrossRef]
- Ahmed, S.; Mirzaei, H.; Aschner, M.; Khan, A.; Al-Harrasi, A.; Khan, H. Marine peptides in breast cancer: Therapeutic and mechanistic understanding. Biomed. Pharmacother. 2021, 142, 112038. [Google Scholar] [CrossRef] [PubMed]
- de la Torre, B.G.; Albericio, F. Peptide Therapeutics 2.0. Molecules 2020, 25, 2293. [Google Scholar] [CrossRef]
- Hetz, C.A. ER stress signaling and the BCL-2 family of proteins: From adaptation to irreversible cellular damage. Antioxid. Redox Signal. 2007, 9, 2345–2355. [Google Scholar] [CrossRef]
- Bhatia, S.; Frangioni, J.V.; Hoffman, R.M.; Iafrate, A.J.; Polyak, K. The challenges posed by cancer heterogeneity. Nat. Biotechnol. 2012, 30, 604–610. [Google Scholar] [CrossRef]
- Shore, G.C.; Papa, F.R.; Oakes, S.A. Signaling cell death from the endoplasmic reticulum stress response. Curr. Opin. Cell Biol. 2011, 23, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Jafari, A.; Babajani, A.; Sarrami Forooshani, R.; Yazdani, M.; Rezaei-Tavirani, M. Clinical Applications and Anticancer Effects of Antimicrobial Peptides: From Bench to Bedside. Front. Oncol. 2022, 12, 819563. [Google Scholar] [CrossRef]
- Kordi, M.; Borzouyi, Z.; Chitsaz, S.; Asmaei, M.h.; Salami, R.; Tabarzad, M. Antimicrobial peptides with anticancer activity: Today status, trends and their computational design. Arch. Biochem. Biophys. 2023, 733, 109484. [Google Scholar] [CrossRef]
- Arias, M.; Hilchie, A.L.; Haney, E.F.; Bolscher, J.G.M.; Hyndman, M.E.; Hancock, R.E.W.; Vogel, H.J. Anticancer activities of bovine and human lactoferricin-derived peptides. Biochem. Cell Biol. 2016, 95, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, A.C. Antimicrobial peptides from amphibian skin: An expanding scenario. Curr. Opin. Chem. Biol. 2002, 6, 799–804. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shen, B.; Chen, Q.; Zhang, X.; Ye, Y.; Wang, F.; Zhang, X. Antitumor effects of cecropin B-LHRH’on drug-resistant ovarian and endometrial cancer cells. BMC Cancer 2016, 16, 1–9. [Google Scholar]
- Kuroda, K.; Okumura, K.; Isogai, H.; Isogai, E. The Human Cathelicidin Antimicrobial Peptide LL-37 and Mimics are Potential Anticancer Drugs. Front. Oncol. 2015, 5, 144. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Y.; Han, H.; Miller, D.W.; Wang, G. Solution Structures of Human LL-37 Fragments and NMR-Based Identification of a Minimal Membrane-Targeting Antimicrobial and Anticancer Region. J. Am. Chem. Soc. 2006, 128, 5776–5785. [Google Scholar] [CrossRef]
- Wu, W.K.K.; Wang, G.; Coffelt, S.B.; Betancourt, A.M.; Lee, C.W.; Fan, D.; Wu, K.; Yu, J.; Sung, J.J.Y.; Cho, C.H. Emerging roles of the host defense peptide LL-37 in human cancer and its potential therapeutic applications. Int. J. Cancer 2010, 127, 1741–1747. [Google Scholar] [CrossRef]
- Panjeta, A.; Preet, S. Anticancer potential of human intestinal defensin 5 against 1, 2-dimethylhydrazine dihydrochloride induced colon cancer: A therapeutic approach. Peptides 2020, 126, 170263. [Google Scholar] [CrossRef] [PubMed]
- Baindara, P.; Gautam, A.; Raghava, G.P.S.; Korpole, S. Anticancer properties of a defensin like class IId bacteriocin Laterosporulin10. Sci. Rep. 2017, 7, 46541. [Google Scholar] [CrossRef]
- Baker, M.A.; Maloy, W.L.; Zasloff, M.; Jacob, L. Anticancer efficacy of Magainin2 and analogue peptides. Cancer Res. 1993, 53, 3052–3057. [Google Scholar]
- Lehmann, J.; Retz, M.; Sidhu, S.S.; Suttmann, H.; Sell, M.; Paulsen, F.; Harder, J.; Unteregger, G.; Stöckle, M. Antitumor Activity of the Antimicrobial Peptide Magainin II against Bladder Cancer Cell Lines. Eur. Urol. 2006, 50, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Anghel, R.; Jitaru, D.; Bădescu, L.; Bădescu, M.; Ciocoiu, M. The Cytotoxic Effect of Magainin II on the MDA-MB-231 and M14K Tumour Cell Lines. BioMed Res. Int. 2013, 2013, 831709. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Camargo, C.; Salazar, V.A.; Barrero-Guevara, L.; Camargo, S.; Mosquera, A.; Groot, H.; Boix, E. Unveiling the Multifaceted Mechanisms of Antibacterial Activity of Buforin II and Frenatin 2.3S Peptides from Skin Micro-Organs of the Orinoco Lime Treefrog (Sphaenorhynchus lacteus). Int. J. Mol. Sci. 2018, 19, 2170. [Google Scholar] [CrossRef] [PubMed]
- Roshanak, S.; Shahidi, F.; Yazdi, F.T.; Javadmanesh, A.; Movaffagh, J. Evaluation of Antimicrobial Activity of Buforin I and Nisin and the Synergistic Effect of Their Combination as a Novel Antimicrobial Preservative. J. Food Prot. 2020, 83, 2018–2025. [Google Scholar] [CrossRef] [PubMed]
- Wanyan, Y.; Xu, X.; Liu, K.; Zhang, H.; Zhen, J.; Zhang, R.; Wen, J.; Liu, P.; Chen, Y. 2-Deoxy-d-glucose Promotes Buforin IIb-Induced Cytotoxicity in Prostate Cancer DU145 Cells and Xenograft Tumors. Molecules 2020, 25, 5778. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, M.H.; Meneguetti, B.T.; Costa, B.O.; Buccini, D.F.; Oshiro, K.G.N.; Preza, S.L.E.; Carvalho, C.M.E.; Migliolo, L.; Franco, O.L. Non-Lytic Antibacterial Peptides That Translocate Through Bacterial Membranes to Act on Intracellular Targets. Int. J. Mol. Sci. 2019, 20, 4877. [Google Scholar] [CrossRef]
- Li, D.; Xu, Y. Buforin IIb induced cell cycle arrest in liver cancer. Anim. Cells Syst. 2019, 23, 176–183. [Google Scholar] [CrossRef]
- Giuliani, A.; Pirri, G.; Nicoletto, S.F. Antimicrobial peptides: An overview of a promising class of therapeutics. Cent. Eur. J. Biol. 2007, 2, 1–33. [Google Scholar] [CrossRef]
- Cho, J.H.; Sung, B.H.; Kim, S.C. Buforins: Histone H2A-derived antimicrobial peptides from toad stomach. Biochim. Biophys. Acta (BBA)-Biomembr. 2009, 1788, 1564–1569. [Google Scholar] [CrossRef]
- Kim, H.S.; Yoon, H.; Minn, I.; Park, C.B.; Lee, W.T.; Zasloff, M.; Kim, S.C. Pepsin-Mediated Processing of the Cytoplasmic Histone H2A to Strong Antimicrobial Peptide Buforin I1. J. Immunol. 2000, 165, 3268–3274. [Google Scholar] [CrossRef]
- Chen, B.; Fan, D.-Q.; Zhu, K.-X.; Shan, Z.-G.; Chen, F.-Y.; Hou, L.; Cai, L.; Wang, K.-J. Mechanism study on a new antimicrobial peptide Sphistin derived from the N-terminus of crab histone H2A identified in haemolymphs of Scylla paramamosain. Fish Shellfish Immunol. 2015, 47, 833–846. [Google Scholar] [CrossRef] [PubMed]
- Haney, E.F.; Hunter, H.N.; Matsuzaki, K.; Vogel, H.J. Solution NMR studies of amphibian antimicrobial peptides: Linking structure to function? Biochim. Biophys. Acta (BBA)-Biomembr. 2009, 1788, 1639–1655. [Google Scholar] [CrossRef] [PubMed]
- Roshanak, S.; Shahidi, F.; Tabatabaei Yazdi, F.; Javadmanesh, A.; Movaffagh, J. Buforin I an alternative to conventional antibiotics: Evaluation of the antimicrobial properties, stability, and safety. Microb. Pathog. 2021, 161, 105301. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.M.; Azevedo, M.I.G.; Sousa, L.M.; Oliveira, N.S.; Andrade, C.R.; Freitas, C.D.T.; Souza, P.F.N. Plant antimicrobial peptides: An overview about classification, toxicity and clinical applications. Int. J. Biol. Macromol. 2022, 214, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.-Y.; Chowdhury, M.; Huang, Y.-D.; Yu, X.-Q. Insect antimicrobial peptides and their applications. Appl. Microbiol. Biotechnol. 2014, 98, 5807–5822. [Google Scholar] [CrossRef] [PubMed]
- Ghanbarzadeh, Z.; Hemmati, S.; Mohagheghzadeh, A. Humanizing plant-derived snakins and their encrypted antimicrobial peptides. Biochimie 2022, 199, 92–111. [Google Scholar] [CrossRef]
- Jallouk, A.P.; Palekar, R.U.; Pan, H.; Schlesinger, P.H.; Wickline, S.A. Chapter Two—Modifications of Natural Peptides for Nanoparticle and Drug Design. In Advances in Protein Chemistry and Structural Biology; Donev, R., Ed.; Academic Press: Amsterdam, The Netherlands, 2015; Volume 98, pp. 57–91. [Google Scholar]
- Park, C.B.; Yi, K.-S.; Matsuzaki, K.; Kim, M.S.; Kim, S.C. Structure–activity analysis of buforin II, a histone H2A-derived antimicrobial peptide: The proline hinge is responsible for the cell-penetrating ability of buforin II. Proc. Natl. Acad. Sci. USA 2000, 97, 8245–8250. [Google Scholar] [CrossRef]
- Shafiee, F.; Rabbani, M.; Jahanian-Najafabadi, A. Production and evaluation of cytotoxic effects of DT386-BR2 fusion protein as a novel anti-cancer agent. J. Microbiol. Methods 2016, 130, 100–105. [Google Scholar] [CrossRef]
- Han, Y.; Lu, M.; Zhou, J. Buforin IIb induces androgen-independent prostate cancer cells apoptosis though p53 pathway in vitro. Toxicon Off. J. Int. Soc. Toxinology 2019, 168, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.H.; Kim, Y.J.; Kim, H.; Kim, S.C.; Cho, J.H. Buforin IIb induces endoplasmic reticulum stress-mediated apoptosis in HeLa cells. Peptides 2015, 69, 144–149. [Google Scholar] [CrossRef]
- Sruthy, K.S.; Nair, A.; Antony, S.P.; Puthumana, J.; Singh, I.S.B.; Philip, R. A histone H2A derived antimicrobial peptide, Fi-Histin from the Indian White shrimp, Fenneropenaeus indicus: Molecular and functional characterization. Fish Shellfish Immunol. 2019, 92, 667–679. [Google Scholar] [CrossRef]
- Verdurmen, W.P.R.; Brock, R. Biological responses towards cationic peptides and drug carriers. Trends Pharmacol. Sci. 2011, 32, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.H.; Kim, M.Y.; Lee, J.-W.; Kim, S.C.; Cho, J.H. Enhancement of the cancer targeting specificity of buforin IIb by fusion with an anionic peptide via a matrix metalloproteinases-cleavable linker. Peptides 2011, 32, 895–899. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.J.; Sung, B.H.; Shin, J.R.; Lee, Y.W.; Kim, D.J.; Yang, K.S.; Kim, S.C. A Cancer Specific Cell-Penetrating Peptide, BR2, for the Efficient Delivery of an scFv into Cancer Cells. PLoS ONE 2013, 8, e66084. [Google Scholar] [CrossRef]
- Schweizer, F. Cationic amphiphilic peptides with cancer-selective toxicity. Eur. J. Pharmacol. 2009, 625, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.A.; Kim, H.; Lee, J.Y.; Shin, J.R.; Kim, D.J.; Cho, J.H.; Kim, S.C. Mechanism of action and specificity of antimicrobial peptides designed based on buforin IIb. Peptides 2012, 34, 283–289. [Google Scholar] [CrossRef]
- Bolhassani, A.; Jafarzade, B.S.; Mardani, G. In vitro and in vivo delivery of therapeutic proteins using cell penetrating peptides. Peptides 2017, 87, 50–63. [Google Scholar] [CrossRef] [PubMed]
- Vale, N.; Duarte, D.; Silva, S.; Correia, A.S.; Costa, B.; Gouveia, M.J.; Ferreira, A. Cell-penetrating peptides in oncologic pharmacotherapy: A review. Pharmacol. Res. 2020, 162, 105231. [Google Scholar] [CrossRef] [PubMed]
- Kunda, N.K. Antimicrobial peptides as novel therapeutics for non-small cell lung cancer. Drug Discov. Today 2020, 25, 238–247. [Google Scholar] [CrossRef]
- Francis, F.; Chaudhary, N. 3—Antimicrobial peptides: Features and modes of action. In Antimicrobial Peptides; Ajesh, K., Sreejith, K., Eds.; Academic Press: Amsterdam, The Netherlands, 2023; pp. 33–65. [Google Scholar]
- Saha, R.; Bhattacharya, D.; Mukhopadhyay, M. Advances in modified antimicrobial peptides as marine antifouling material. Colloids Surf. B Biointerfaces 2022, 220, 112900. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Jin, Y.; Qiao, Y.; Zhong, S.; Xing, Y.; Chen, X. Antimicrobial activity of histone1-derived peptides from large yellow croaker Larimichthys crocea. Aquaculture 2023, 570, 739430. [Google Scholar] [CrossRef]
- Zhu, M.; Liu, P.; Niu, Z.-W. A perspective on general direction and challenges facing antimicrobial peptides. Chin. Chem. Lett. 2017, 28, 703–708. [Google Scholar] [CrossRef]
- Arias, M.; Haney, E.F.; Hilchie, A.L.; Corcoran, J.A.; Hyndman, M.E.; Hancock, R.E.W.; Vogel, H.J. Selective anticancer activity of synthetic peptides derived from the host defence peptide tritrpticin. Biochim. Biophys. Acta (BBA)-Biomembr. 2020, 1862, 183228. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, Y.; Song, Z.; Tan, Z.; Cheng, J. Recent advances in design of antimicrobial peptides and polypeptides toward clinical translation. Adv. Drug Deliv. Rev. 2021, 170, 261–280. [Google Scholar] [CrossRef]
- Nogrado, K.; Adisakwattana, P.; Reamtong, O. Antimicrobial peptides: On future antiprotozoal and anthelminthic applications. Acta Trop. 2022, 235, 106665. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xiang, Q.; Zhang, Q.; Huang, Y.; Su, Z. Overview on the recent study of antimicrobial peptides: Origins, functions, relative mechanisms and application. Peptides 2012, 37, 207–215. [Google Scholar] [CrossRef]
- Neshani, A.; Sedighian, H.; Mirhosseini, S.A.; Ghazvini, K.; Zare, H.; Jahangiri, A. Antimicrobial peptides as a promising treatment option against Acinetobacter baumannii infections. Microb. Pathog. 2020, 146, 104238. [Google Scholar] [CrossRef]
- E-kobon, T.; Thongararm, P.; Roytrakul, S.; Meesuk, L.; Chumnanpuen, P. Prediction of anticancer peptides against MCF-7 breast cancer cells from the peptidomes of Achatina fulica mucus fractions. Comput. Struct. Biotechnol. J. 2016, 14, 49–57. [Google Scholar] [CrossRef]
- Lee, H.; Yang, S. Dimerization of cell-penetrating buforin II enhances antimicrobial properties. J. Anal. Sci. Technol. 2021, 12, 9. [Google Scholar] [CrossRef]
- Giacometti, A.; Cirioni, O.; Del Prete, M.S.; Barchiesi, F.; Fineo, A.; Scalise, G. Activity of buforin II alone and in combination with azithromycin and minocycline against Cryptosporidium parvum in cell culture. J. Antimicrob. Chemother. 2001, 47, 97–99. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kobayashi, S.; Chikushi, A.; Tougu, S.; Imura, Y.; Nishida, M.; Yano, Y.; Matsuzaki, K. Membrane translocation mechanism of the antimicrobial peptide buforin 2. Biochemistry 2004, 43, 15610–15616. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Takeshima, K.; Park, C.B.; Kim, S.C.; Matsuzaki, K. Interactions of the novel antimicrobial peptide buforin 2 with lipid bilayers: Proline as a translocation promoting factor. Biochemistry 2000, 39, 8648–8654. [Google Scholar] [CrossRef] [PubMed]
- Vermeer, L.S.; Lan, Y.; Abbate, V.; Ruh, E.; Bui, T.T.; Wilkinson, L.J.; Kanno, T.; Jumagulova, E.; Kozlowska, J.; Patel, J.; et al. Conformational Flexibility Determines Selectivity and Antibacterial, Antiplasmodial, and Anticancer Potency of Cationic α-Helical Peptides*. J. Biol. Chem. 2012, 287, 34120–34133. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Ji, S.; Peng, J.; Han, P.; An, X.; Wang, S.; Cao, B. Isolation and characterisation of a novel antibacterial peptide from a native swine intestinal tract-derived bacterium. Int. J. Antimicrob. Agents 2017, 49, 427–436. [Google Scholar] [CrossRef]
- Pardhi, D.M.; Şen Karaman, D.; Timonen, J.; Wu, W.; Zhang, Q.; Satija, S.; Mehta, M.; Charbe, N.; McCarron, P.A.; Tambuwala, M.M.; et al. Anti-bacterial activity of inorganic nanomaterials and their antimicrobial peptide conjugates against resistant and non-resistant pathogens. Int. J. Pharm. 2020, 586, 119531. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Park, C.B.; Kim, J.M.; Jang, S.A.; Park, I.Y.; Kim, M.S.; Cho, J.H.; Kim, S.C. Mechanism of anticancer activity of buforin IIb, a histone H2A-derived peptide. Cancer Lett. 2008, 271, 47–55. [Google Scholar] [CrossRef]
- Moretta, A.; Scieuzo, C.; Petrone, A.M.; Salvia, R.; Manniello, M.D.; Franco, A.; Lucchetti, D.; Vassallo, A.; Vogel, H.; Sgambato, A.; et al. Antimicrobial Peptides: A New Hope in Biomedical and Pharmaceutical Fields. Front. Cell. Infect. Microbiol. 2021, 11, 668632. [Google Scholar] [CrossRef]
- Rutkowski, M. CHAPTER 4—Cancer and the science of innate immunity. In Clinical Immuno-Oncology; Niederhuber, J.E., Ed.; Elsevier: New Delhi, India, 2024; pp. 61–90.e11. [Google Scholar]
- Parker, J.P.; Devocelle, M.; Morgan, M.P.; Marmion, C.J. Derivatisation of buforin IIb, a cationic henicosapeptide, to afford its complexation to platinum(ii) resulting in a novel platinum(ii)–buforin IIb conjugate with anti-cancer activity. Dalton Trans. 2016, 45, 13038–13041. [Google Scholar] [CrossRef]
- Tornesello, A.L.; Borrelli, A.; Buonaguro, L.; Buonaguro, F.M.; Tornesello, M.L. Antimicrobial Peptides as Anticancer Agents: Functional Properties and Biological Activities. Molecules 2020, 25, 2850. [Google Scholar] [CrossRef]
- Kohno, M.; Horibe, T.; Ohara, K.; Ito, S.; Kawakami, K. The Membrane-Lytic Peptides K8L9 and Melittin Enter Cancer Cells via Receptor Endocytosis following Subcytotoxic Exposure. Chem. Biol. 2014, 21, 1522–1532. [Google Scholar] [CrossRef]
- Liscano, Y.; Oñate-Garzón, J.; Delgado, J.P. Peptides with Dual Antimicrobial-Anticancer Activity: Strategies to Overcome Peptide Limitations and Rational Design of Anticancer Peptides. Molecules 2020, 25, 4245. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Guo, H.; Xu, D.; Yu, C.; Xv, R.; Wu, Q.; Di, L.; Cheng, H.; Duan, J.; Zhou, J.; et al. Cell affinity screening combined with nanoLC-MS/MS based peptidomics for identifying cancer cell binding peptides from Bufo Bufo gargarizans. J. Pharm. Biomed. Anal. 2021, 206, 114354. [Google Scholar] [CrossRef]
- Takeshima, K.; Chikushi, A.; Lee, K.K.; Yonehara, S.; Matsuzaki, K. Translocation of analogues of the antimicrobial peptides magainin and buforin across human cell membranes. J. Biol. Chem. 2003, 278, 1310–1315. [Google Scholar] [CrossRef]
- Roudi, R.; Syn, N.L.; Roudbary, M. Antimicrobial Peptides As Biologic and Immunotherapeutic Agents against Cancer: A Comprehensive Overview. Front. Immunol. 2017, 8, 1320. [Google Scholar] [CrossRef]
- Aghamiri, S.; Zandsalimi, F.; Raee, P.; Abdollahifar, M.-A.; Tan, S.C.; Low, T.Y.; Najafi, S.; Ashrafizadeh, M.; Zarrabi, A.; Ghanbarian, H.; et al. Antimicrobial peptides as potential therapeutics for breast cancer. Pharmacol. Res. 2021, 171, 105777. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, S.; Szwej, E.; Nikodinovic-Runic, J.; O’Connor, A.; Byrne, A.T.; Devocelle, M.; O’Donovan, N.; Gallagher, W.M.; Babu, R.; Kenny, S.T.; et al. The anti-cancer activity of a cationic anti-microbial peptide derived from monomers of polyhydroxyalkanoate. Biomaterials 2013, 34, 2710–2718. [Google Scholar] [CrossRef]
- Anjomshoa, M.; Amirheidari, B. Nuclease-like metalloscissors: Biomimetic candidates for cancer and bacterial and viral infections therapy. Coord. Chem. Rev. 2022, 458, 214417. [Google Scholar] [CrossRef] [PubMed]
- Perez, J.; Cifuentes, J.; Cuellar, M.; Suarez-Arnedo, A.; Cruz, J.C.; Muñoz-Camargo, C. Cell-Penetrating And Antibacterial BUF-II Nanobioconjugates: Enhanced Potency Via Immobilization On Polyetheramine-Modified Magnetite Nanoparticles. Int. J. Nanomed. 2019, 14, 8483–8497. [Google Scholar] [CrossRef] [PubMed]
- Alas, M.; Saghaeidehkordi, A.; Kaur, K. Peptide-Drug Conjugates with Different Linkers for Cancer Therapy. J. Med. Chem. 2021, 64, 216–232. [Google Scholar] [CrossRef]
- Szumilak, M.; Wiktorowska-Owczarek, A.; Stanczak, A. Hybrid Drugs-A Strategy for Overcoming Anticancer Drug Resistance? Molecules 2021, 26, 2601. [Google Scholar] [CrossRef]
- Yang, S.B.; Banik, N.; Han, B.; Lee, D.N.; Park, J. Peptide-Based Bioconjugates and Therapeutics for Targeted Anticancer Therapy. Pharmaceutics 2022, 14, 1378. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, A.; Neundorf, I. Design and Application of Antimicrobial Peptide Conjugates. Int. J. Mol. Sci. 2016, 17, 701. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, P.P.; Biswas, S.; Torchilin, V.P. Current trends in the use of liposomes for tumor targeting. Nanomedicine 2013, 8, 1509–1528. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhou, Y.; Liu, L.; Xu, Y.; Chen, Q.; Wang, Y.; Wu, S.; Deng, Y.; Zhang, J.; Shao, A. Nanoparticle-Based Drug Delivery in Cancer Therapy and Its Role in Overcoming Drug Resistance. Front. Mol. Biosci. 2020, 7, 193. [Google Scholar] [CrossRef]
- Libardo, M.D.; Paul, T.J.; Prabhakar, R.; Angeles-Boza, A.M. Hybrid peptide ATCUN-sh-Buforin: Influence of the ATCUN charge and stereochemistry on antimicrobial activity. Biochimie 2015, 113, 143–155. [Google Scholar] [CrossRef]
- Cuellar, M.; Cifuentes, J.; Perez, J.; Suarez-Arnedo, A.; Serna, J.A.; Groot, H.; Muñoz-Camargo, C.; Cruz, J.C. Novel BUF2-magnetite nanobioconjugates with cell-penetrating abilities. Int. J. Nanomed. 2018, 13, 8087–8094. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tolos, A.M.; Moisa, C.; Dochia, M.; Popa, C.; Copolovici, L.; Copolovici, D.M. Anticancer Potential of Antimicrobial Peptides: Focus on Buforins. Polymers 2024, 16, 728. https://doi.org/10.3390/polym16060728
Tolos AM, Moisa C, Dochia M, Popa C, Copolovici L, Copolovici DM. Anticancer Potential of Antimicrobial Peptides: Focus on Buforins. Polymers. 2024; 16(6):728. https://doi.org/10.3390/polym16060728
Chicago/Turabian StyleTolos (Vasii), Ana Maria, Cristian Moisa, Mihaela Dochia, Carmen Popa, Lucian Copolovici, and Dana Maria Copolovici. 2024. "Anticancer Potential of Antimicrobial Peptides: Focus on Buforins" Polymers 16, no. 6: 728. https://doi.org/10.3390/polym16060728
APA StyleTolos, A. M., Moisa, C., Dochia, M., Popa, C., Copolovici, L., & Copolovici, D. M. (2024). Anticancer Potential of Antimicrobial Peptides: Focus on Buforins. Polymers, 16(6), 728. https://doi.org/10.3390/polym16060728