Ligand-Induced Synthesis of Highly Stable NM88(DB)@COF-JLU19 Composite: Accelerating Electron Flow for Visible-Light-Efficient Degradation of Tetracycline Hydrochloride
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
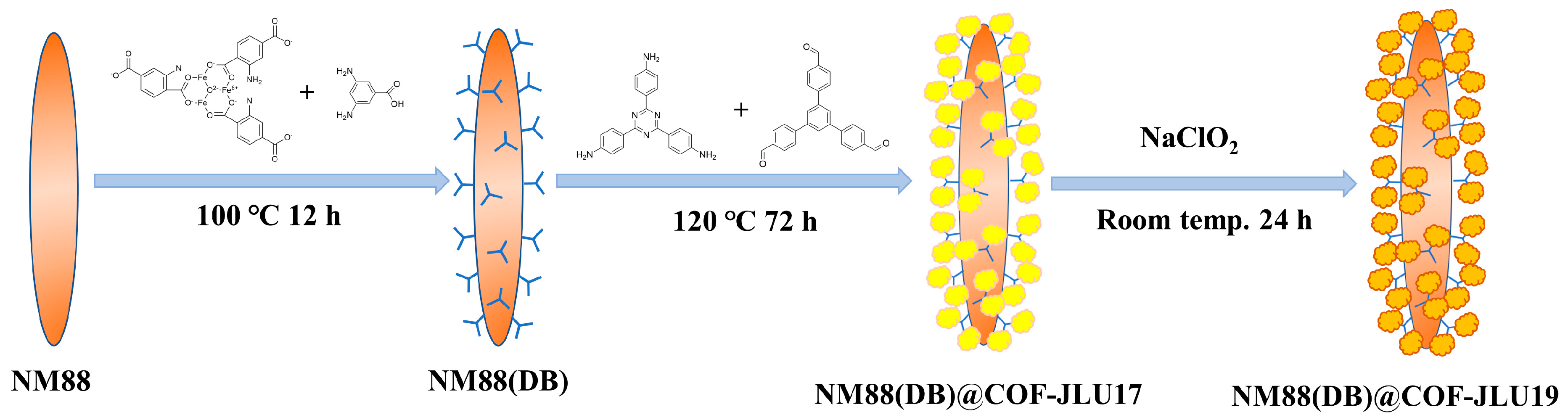
2.2. Sample Preparation
2.2.1. Synthesis of NH2-MIL-88B(Fe)
2.2.2. Synthesis of DB Anchored NH2-MIL-88B(Fe)
2.2.3. Synthesis of COF-JLU19
2.2.4. Synthesis of NM88(DB)@COF-JLU19
2.3. Characterization
2.4. Evaluation of Photocatalytic Activity
3. Results and Discussion
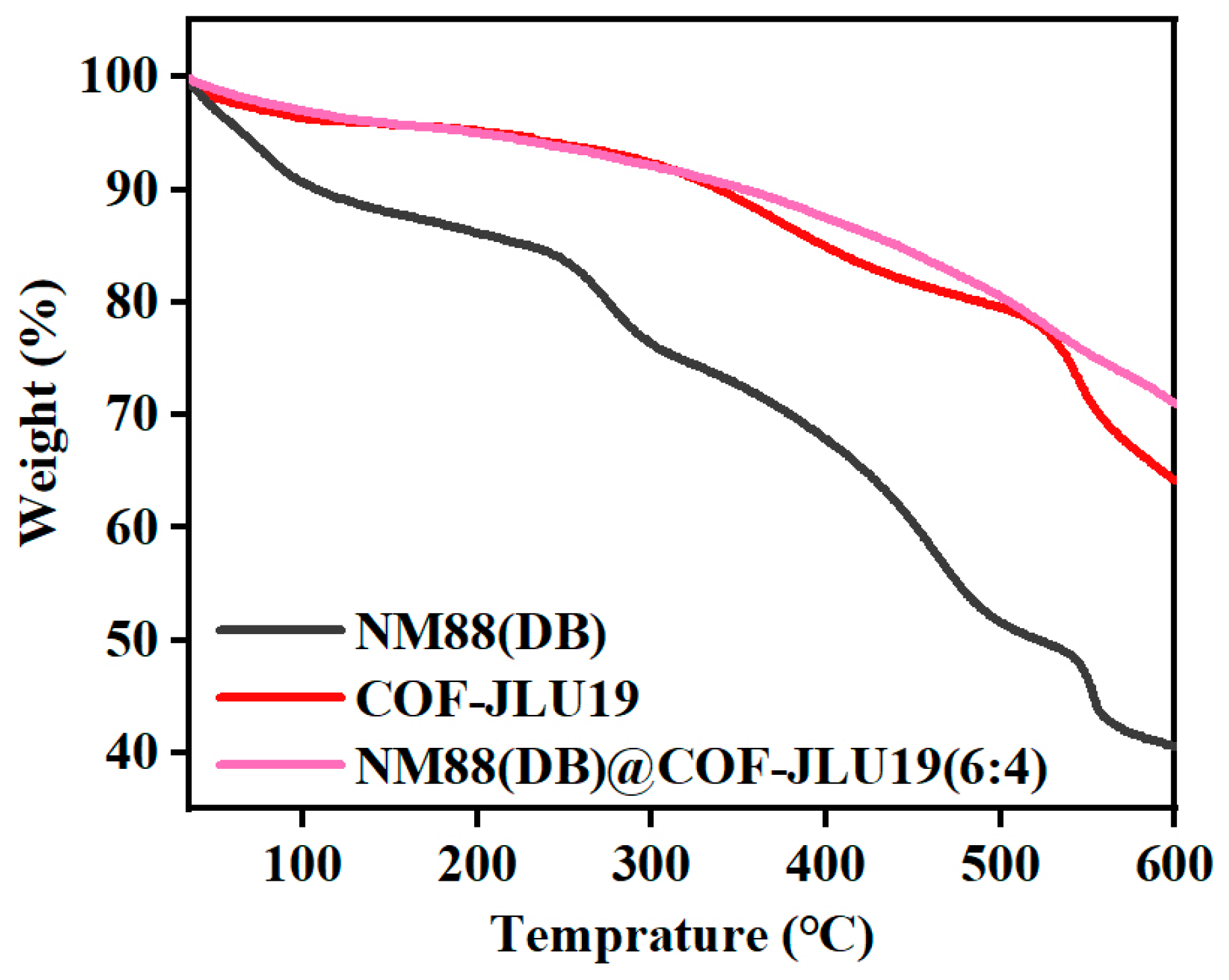
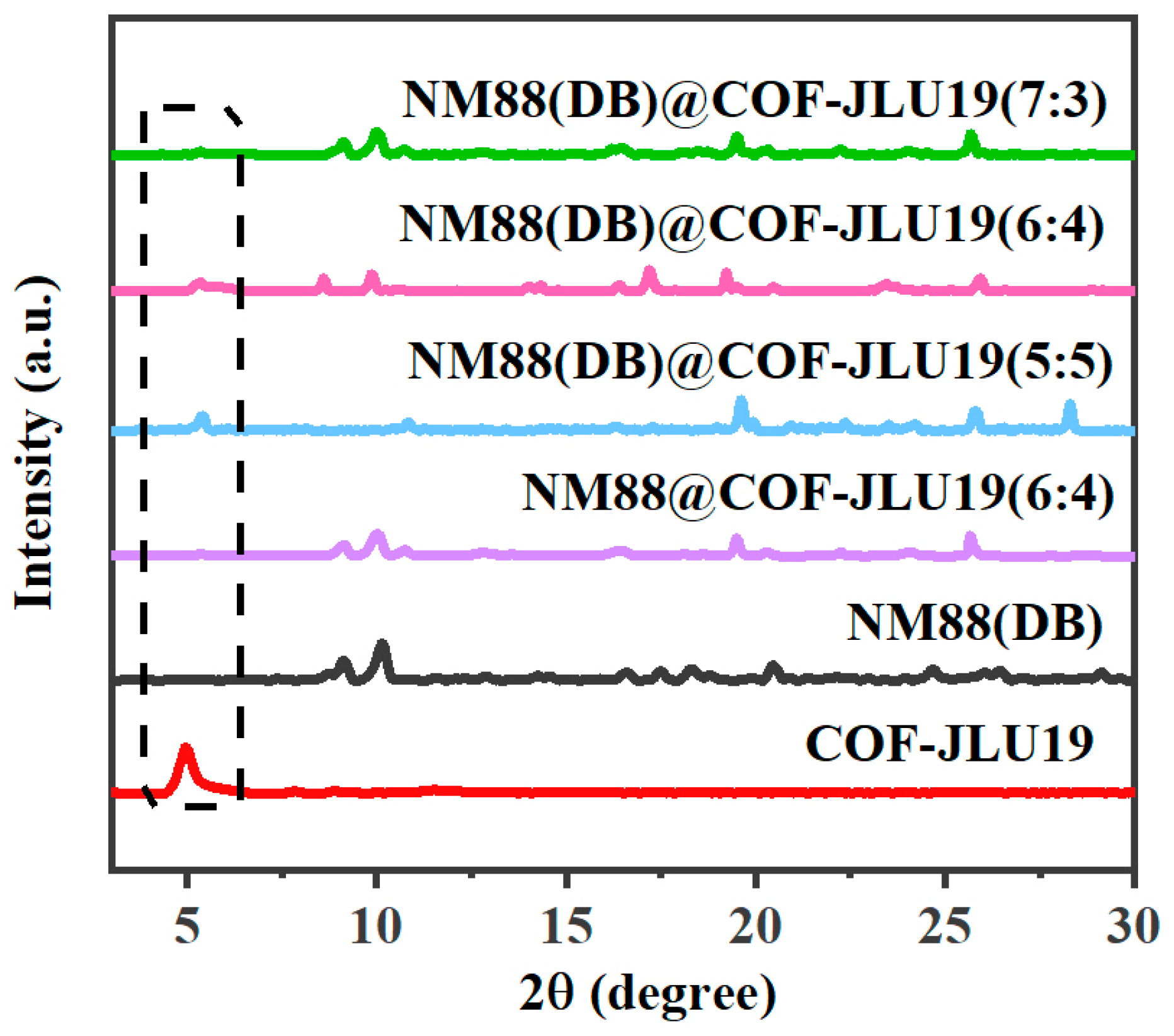
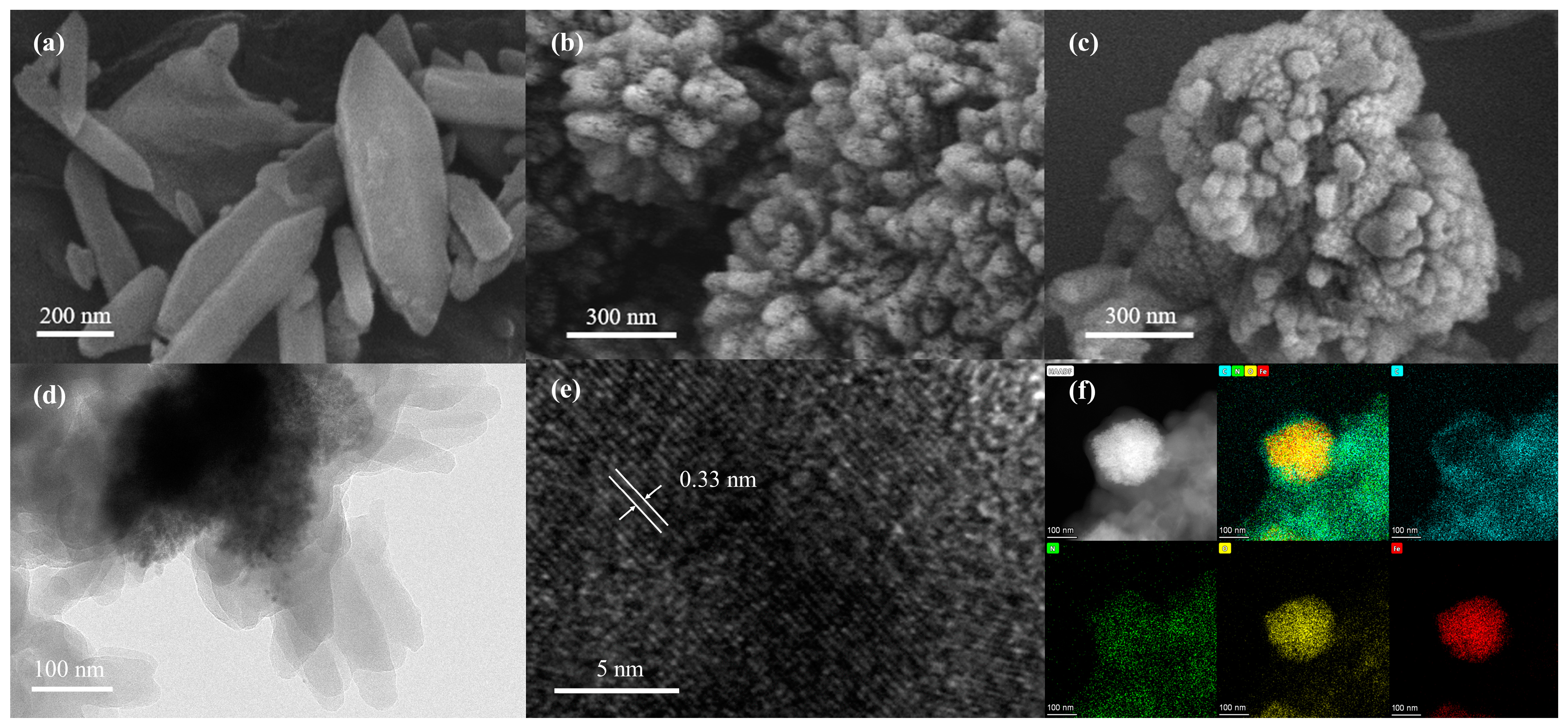
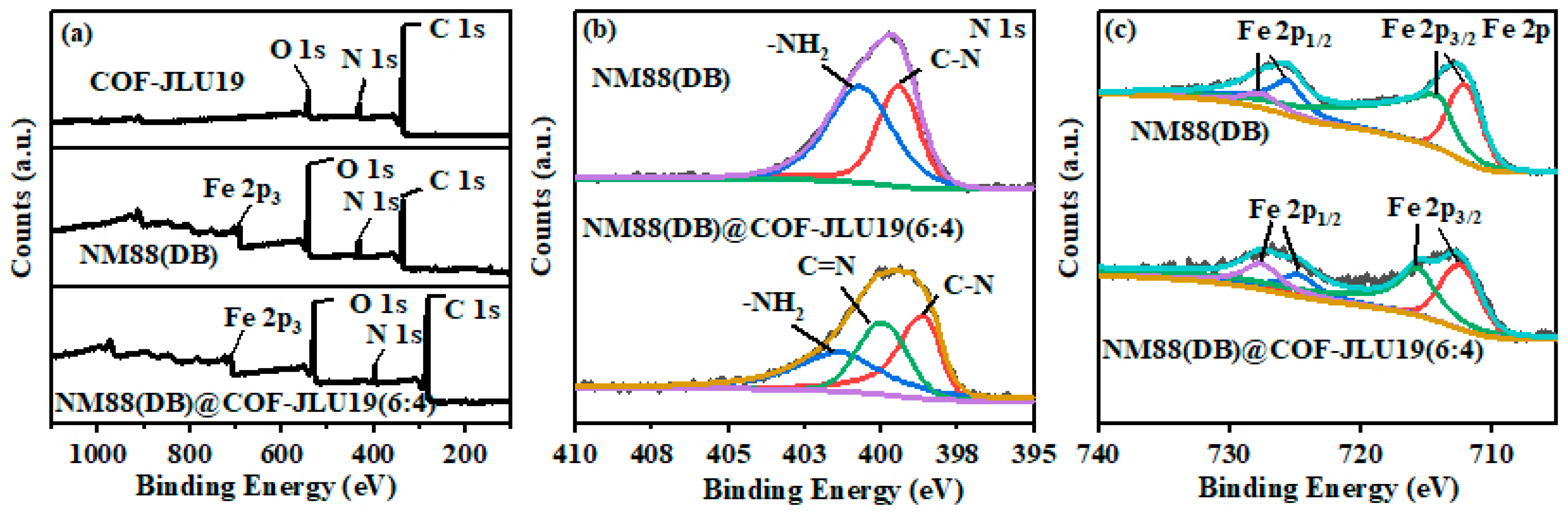
3.1. Morphological and Structural Characterization
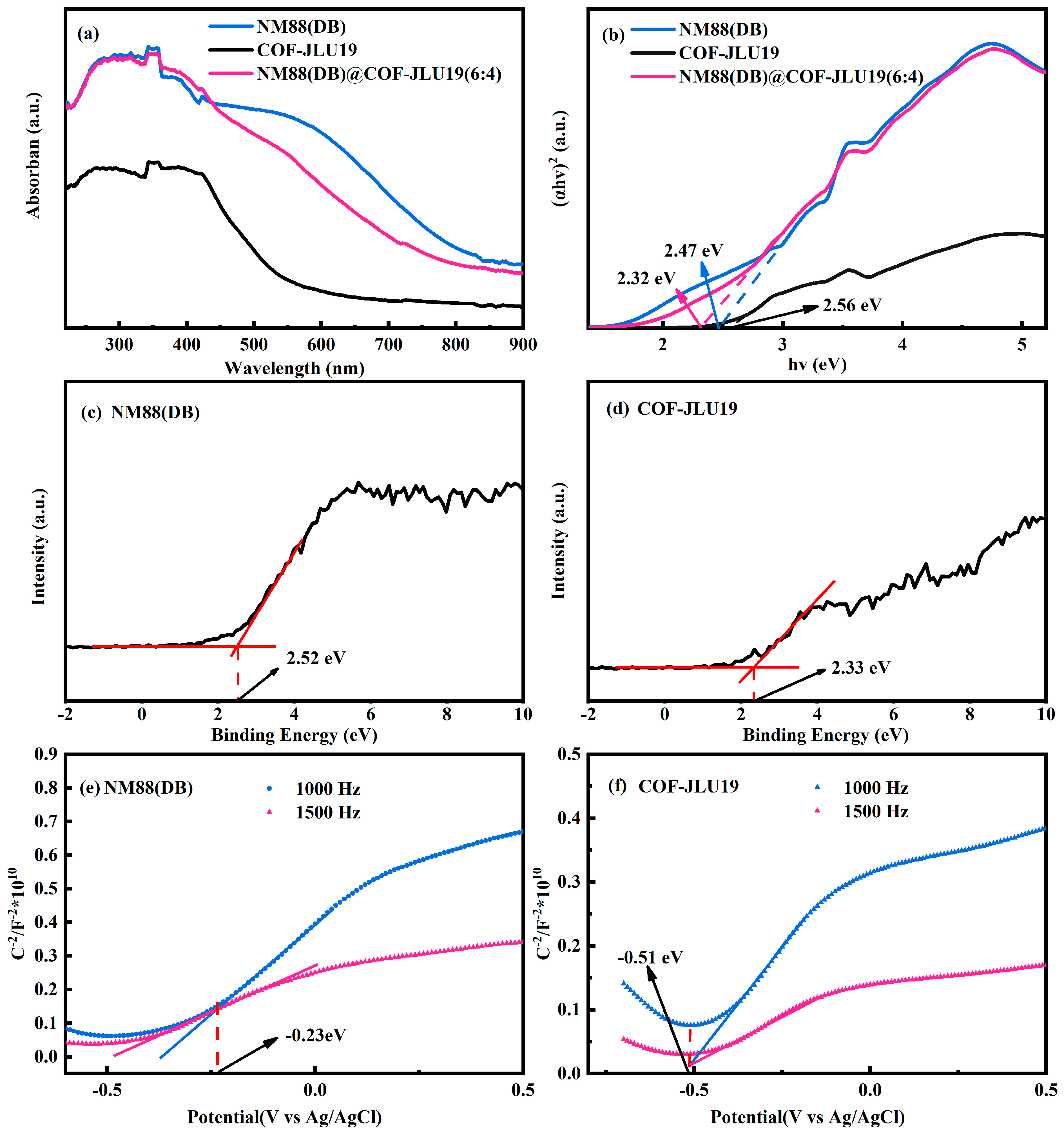
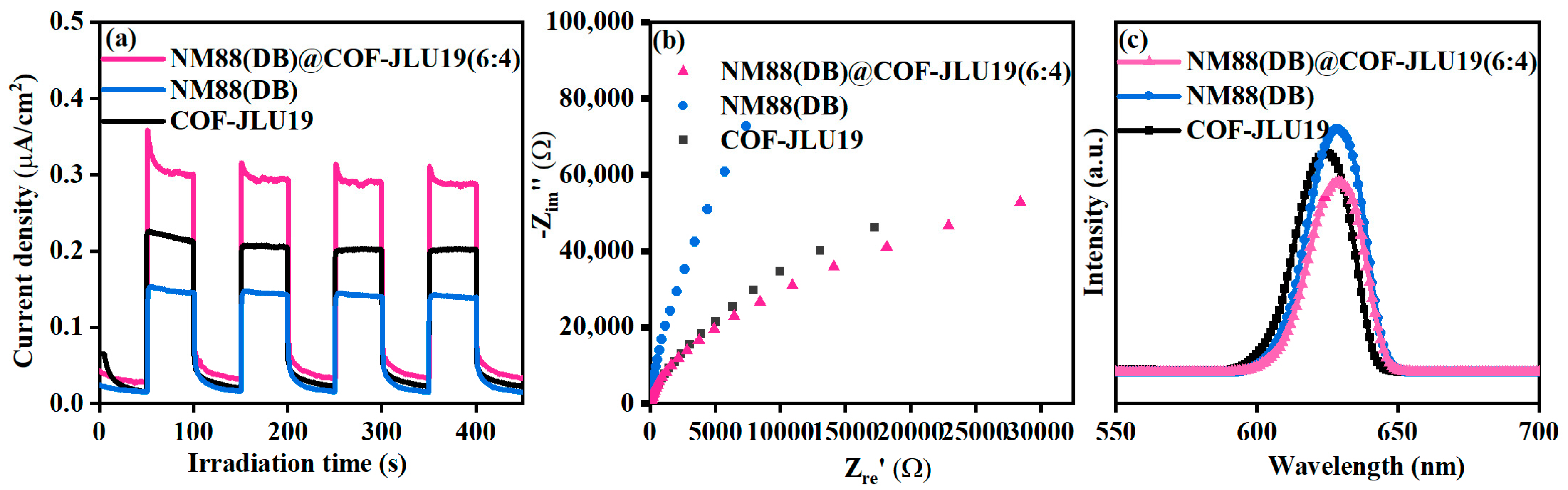
3.2. Enhanced Photocatalytic Activity Mechanism
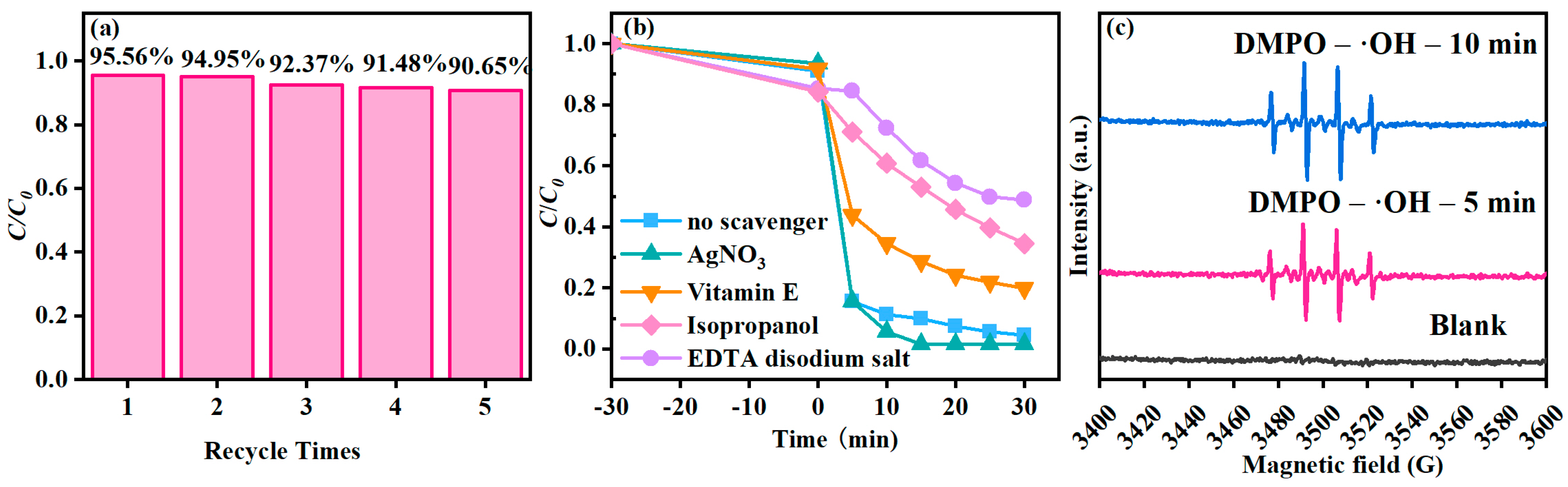
3.3. Photocatalytic Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


References
- She, W.-Z.; Li, C.-H.; Li, R.S.; Ling, J.; Cao, Q. Construction of two-dimensional fluorescent covalent organic framework nanospheres for the detection and removal of tetracycline. Sep. Purif. Technol. 2024, 330, 125294. [Google Scholar] [CrossRef]
- Nehra, M.; Kumar, R.; Dilbaghi, N.; Kumar, S. Controlled synthesis of Cu-MOF possessing peroxidase-mimetic activity for the colorimetric detection of tetracycline in aqueous solution. New J. Chem. 2023, 47, 7595–7603. [Google Scholar] [CrossRef]
- Lu, T.; Gao, Y.; Yang, Y.; Ming, H.; Huang, Z.; Liu, G.; Zheng, D.; Zhang, J.; Hou, Y. Efficient degradation of tetracycline hydrochloride by photocatalytic ozonation over Bi2WO6. Chemosphere 2021, 283, 131256. [Google Scholar] [CrossRef]
- Gaffar, S.; Kumar, A.; Alam, J.; Riaz, U. Efficient visible light–induced photocatalytic degradation of tetracycline hydrochloride using CuFe2O4 and PANI/CuFe2O4 nanohybrids. Environ. Sci. Pollut. Res. 2023, 30, 108878–108888. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Sun, C.; Jian, J.; Xue, X.; Shi, J.; Zhou, T.; Xu, Z.; Che, G.; Zhao, L. Enhanced photocatalytic degradation performance of new and stable 2D/3D Ln-MOFs for tetracycline under visible light. J. Mol. Struct. 2023, 1293, 136235. [Google Scholar] [CrossRef]
- Gang, R.; Xu, L.; Xia, Y.; Zhang, L.; Wang, S.; Li, R. Facile One-step Production of 2D/2D ZnO/rGO Nanocomposites under Microwave Irradiation for Photocatalytic Removal of Tetracycline. ACS Omega 2021, 6, 3831–3839. [Google Scholar] [CrossRef] [PubMed]
- Bi, R.; Liu, J.; Zhou, C.; Shen, Y.; Liu, Z.; Wang, Z. In situ synthesis of g-C3N4/TiO2 heterojunction by a concentrated absorption process for efficient photocatalytic degradation of tetracycline hydrochloride. Environ. Sci. Pollut. Res. 2023, 30, 55044–55056. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Z.; Jia, J.; Liu, X. Covalent Organic Frameworks for Photocatalytic Organic Transformation. Chem. Res. Chin. Univ. 2022, 38, 275–289. [Google Scholar] [CrossRef]
- Zhao, B.; Fu, J.; Zhou, C.; Yu, L.; Qiu, M. Emerging Porous Two-Dimensional Materials: Current Status, Existing Challenges, and Applications. Small 2023, 19, 2301917. [Google Scholar] [CrossRef]
- Lin, H.; Chen, C.; Zhou, T.; Zhang, J. Two-Dimensional Covalent-Organic Frameworks for Photocatalysis: The Critical Roles of Building Block and Linkage. Sol. RRL 2021, 5, 2170061. [Google Scholar] [CrossRef]
- Bhunia, S.; Deo, K.A.; Gaharwar, A.K. 2D Covalent Organic Frameworks for Biomedical Applications. Adv. Funct. Mater. 2020, 30, 2002046. [Google Scholar] [CrossRef]
- Chen, B.; Xie, H.; Shen, L.; Xu, Y.; Zhang, M.; Zhou, M.; Li, B.; Li, R.; Lin, H. Covalent Organic Frameworks: The Rising-Star Platforms for the Design of CO2 Separation Membranes. Small 2023, 19, 2207313. [Google Scholar] [CrossRef]
- Zhang, F.; Sheng, J.; Yang, Z.; Sun, X.; Tang, H.; Lu, M.; Dong, H.; Shen, F.; Liu, J.; Lan, Y. Rational Design of MOF/COF Hybrid Materials for Photocatalytic H2 Evolution in the Presence of Sacrificial Electron Donors. Angew. Chem. Int. Ed. 2018, 57, 12106–12110. [Google Scholar] [CrossRef]
- Bagheri, A.R.; Aramesh, N.; Liu, Z.; Chen, C.; Shen, W.; Tang, S. Recent Advances in the Application of Covalent Organic Frameworks in Extraction: A Review. Crit. Rev. Anal. Chem. 2022, 52, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Prakash, K.; Mishra, B.; Díaz, D.D.; Nagaraja, C.M.; Pachfule, P. Strategic design of covalent organic frameworks (COFs) for photocatalytic hydrogen generation. J. Mater. Chem. A 2023, 11, 14489–14538. [Google Scholar] [CrossRef]
- Zhu, L.; Zhu, H.; Wang, L.; Lei, J.; Liu, J. Efficient proton conduction in porous and crystalline covalent-organic frameworks (COFs). J. Energy Chem. 2023, 82, 198–218. [Google Scholar] [CrossRef]
- Zhai, L.; Xie, Z.; Cui, C.-X.; Yang, X.; Xu, Q.; Ke, X.; Liu, M.; Qu, L.-B.; Chen, X.; Mi, L. Constructing Synergistic Triazine and Acetylene Cores in Fully Conjugated Covalent Organic Frameworks for Cascade Photocatalytic H2O2 Production. Chem. Mater. 2022, 34, 5232–5240. [Google Scholar] [CrossRef]
- Qin, N.; Mao, A.; Li, L.; Yang, X.; Liu, J.; Chen, K.; Zhai, L.; Liang, R.; Mi, L. Construction of benzothiadiazole-based D-A covalent organic frameworks for photocatalytic reduction of Cr (VI) and synergistic elimination of organic pollutants. Polymer 2022, 262, 125483. [Google Scholar] [CrossRef]
- Si, W.; Yang, J.; Cao, Y.; Qin, W. Construction of rGO/BP/COF high-low heterojunction for efficient photocatalytic hydrogen evolution. J. Alloys Compd. 2023, 968, 172218. [Google Scholar] [CrossRef]
- He, H.; Chen, S.; Bi, W.; Wen, X.; Sun, S.; Zhang, P.; Shen, R.; Li, X. Assembling Ti3C2 MXene on a S-Scheme heterojunction with oxidative covalent organic frameworks for photocatalytic hydrogen evolution. Sol. RRL 2023, 7, 2300532. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Li, Z.; Garcia, H. Catalysis and photocatalysis by metal organic frameworks. Chem. Soc. Rev. 2018, 47, 8134–8172. [Google Scholar] [CrossRef] [PubMed]
- Jeoung, S.; Kim, S.; Kim, M.; Moon, H.R. Pore engineering of metal-organic frameworks with coordinating functionalities. Coord. Chem. Rev. 2020, 420, 213377. [Google Scholar] [CrossRef]
- Zhao, D.; Li, X.; Zhang, K.; Guo, J.; Huang, X.; Wang, G. Recent advances in thermocatalytic hydrogenation of unsaturated organic compounds with Metal-Organic Frameworks-based materials: Construction strategies and related mechanisms. Coord. Chem. Rev. 2023, 487, 215159. [Google Scholar] [CrossRef]
- Shao, L.-H.; Huang, A.-X.; Yan, X.-C.; Liu, Y.-H.; Wang, Y.; Jin, X.; Zhang, F.-M. Constructing tightly integrated conductive metal-organic framework/covalent triazine framework heterostructure by coordination bonds for photocatalytic hydrogen evolution. J. Colloid Interface Sci. 2023, 633, 233–242. [Google Scholar] [CrossRef]
- Chen, J.; Yuan, D.; Wang, Y. Covalent Organic Frameworks Based Heterostructure in Solar-to-Fuel Conversion. Adv. Funct. Mater. 2023, 33, 2304071. [Google Scholar] [CrossRef]
- Sun, D.; Kim, D.-P. Hydrophobic MOFs@Metal Nanoparticles@COFs for Interfacially Confined Photocatalysis with High Efficiency. ACS Appl. Mater. Interfaces 2020, 12, 20589–20595. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Zhao, M.; Chen, B.; Zhang, Z.; Huang, Y.; Dai, F.; Lai, Z.; Cui, X.; Tan, C.; Zhang, H. Hybridization of MOFs and COFs: A New Strategy for Construction of MOF@COF Core–Shell Hybrid Materials. Adv. Mater. 2018, 30, 1705454. [Google Scholar] [CrossRef]
- Sun, D.; Jang, S.; Yim, S.; Ye, L.; Kim, D. Metal Doped Core–Shell Metal-Organic Frameworks@Covalent Organic Frameworks (MOFs@COFs) Hybrids as a Novel Photocatalytic Platform. Adv. Funct. Mater. 2018, 28, 1707110. [Google Scholar] [CrossRef]
- Li, F.; Wang, D.; Xing, Q.-J.; Zhou, G.; Liu, S.-S.; Li, Y.; Zheng, L.-L.; Ye, P.; Zou, J.-P. Design and syntheses of MOF/COF hybrid materials via postsynthetic covalent modification: An efficient strategy to boost the visible-light-driven photocatalytic performance. Appl. Catal. B Environ. 2019, 243, 621–628. [Google Scholar] [CrossRef]
- Lu, G.; Huang, X.; Li, Y.; Zhao, G.; Pang, G.; Wang, G. Covalently integrated core-shell MOF@COF hybrids as efficient visible-light-driven photocatalysts for selective oxidation of alcohols. J. Energy Chem. 2020, 43, 8–15. [Google Scholar] [CrossRef]
- Wang, H.; Qian, C.; Liu, J.; Zeng, Y.; Wang, D.; Zhou, W.; Gu, L.; Wu, H.; Liu, G.; Zhao, Y. Integrating Suitable Linkage of Covalent Organic Frameworks into Covalently Bridged Inorganic/Organic Hybrids toward Efficient Photocatalysis. J. Am. Chem. Soc. 2020, 142, 4862–4871. [Google Scholar] [CrossRef]
- Ma, S.; Li, Z.; Jia, J.; Zhang, Z.; Xia, H.; Li, H.; Chen, X.; Xu, Y.; Liu, X. Amide-linked covalent organic frameworks as efficient heterogeneous photocatalysts in water. Chin. J. Catal. 2021, 42, 2010–2019. [Google Scholar] [CrossRef]
- Yuan, H.; Ren, H.; Li, M.; Li, Z.; Liu, M.; Dong, W.; Wang, G.; Zhuang, T. Synthesis of N-TiO2@NH2-MIL-88(Fe) Core-shell Structure for Efficient Fenton Effect Assisted Methylene Blue Degradation Under Visible Light. Chem. Res. Chin. Univ. 2020, 36, 1068–1075. [Google Scholar] [CrossRef]
- Hu, X.; Bao, J.; Chen, D.; Shah, S.J.; Subhan, S.; Gong, W.; Li, W.; Luan, X.; Zhao, Z.; Zhao, Z. Accelerating the Fe(III)/Fe(II) cycle via enhanced electronic effect in NH2-MIL-88B(Fe)/TPB-DMTP-COF composite for boosting photo-Fenton degradation of sulfamerazine. J. Colloid Interface Sci. 2022, 624, 121–136. [Google Scholar] [CrossRef]
- Hassan, N.; Shahat, A.; El-Deen, I.; El-Afify, M.; El-Bindary, M. Synthesis and characterization of NH2-MIL-88(Fe) for efficient adsorption of dyes. J. Mol. Struct. 2022, 1258, 132662. [Google Scholar] [CrossRef]
- Liu, B.; Wu, Y.; Han, X.; Lv, J.; Zhang, J.; Shi, H. Facile synthesis of g-C3N4/amine-functionalized MIL-101(Fe) composites with efficient photocatalytic activities under visible light irradiation. J. Mater. Sci. Mater. Electron. 2018, 29, 17591–17601. [Google Scholar] [CrossRef]
- Xie, D.; Ma, Y.; Gu, Y.; Zhou, H.; Zhang, H.; Wang, G.; Zhang, Y.; Zhao, H. Bifunctional NH2-MIL-88(Fe) metal–organic framework nanooctahedra for highly sensitive detection and efficient removal of arsenate in aqueous media. J. Mater. Chem. A 2017, 5, 23794–23804. [Google Scholar] [CrossRef]
- Yuan, R.; Yue, C.; Qiu, J.; Liu, F.; Li, A. Highly efficient sunlight-driven reduction of Cr(VI) by TiO2@NH2-MIL-88B(Fe) heterostructures under neutral conditions. Appl. Catal. B Environ. 2019, 251, 229–239. [Google Scholar] [CrossRef]
- He, J.; Xu, H.; Wang, T.; Zhang, Y.; Dong, J.; Wei, J.; Dai, C. Simultaneous reconstruction of columella and philtrum using prolabial flap combined with Abbe flap in secondary bilateral cleft lip and nasal deformity. J. Cranio-Maxillofac. Surg. 2018, 46, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Huang, X.; Zheng, H.; Wang, P.; Hai, G.; Dong, W.; Wang, G. Aromatic heterocycle-grafted NH2-MIL-125(Ti) via conjugated linker with enhanced photocatalytic activity for selective oxidation of alcohols under visible light. Appl. Catal. B Environ. 2018, 224, 479–487. [Google Scholar] [CrossRef]
- Ye, Z.; Oriol, R.; Yang, C.; Sirés, I.; Li, X.-Y. A novel NH2-MIL-88B(Fe)-modified ceramic membrane for the integration of electro-Fenton and filtration processes: A case study on naproxen degradation. Chem. Eng. J. 2022, 433, 133547. [Google Scholar] [CrossRef]
- Qiao, X.; Liu, X.; Zhang, W.; Cai, Y.; Zhong, Z.; Li, Y.; Lü, J. Superior photo–Fenton activity towards chlortetracycline degradation over novel g–C3N4 nanosheets/schwertmannite nanocomposites with accelerated Fe(III)/Fe(II) cycling. Sep. Purif. Technol. 2021, 279, 119760. [Google Scholar] [CrossRef]
- Yang, Z.; Xia, X.; Shao, L.; Wang, L.; Liu, Y. Efficient photocatalytic degradation of tetracycline under visible light by Z-scheme Ag3PO4/mixed-valence MIL-88A(Fe) heterojunctions: Mechanism insight, degradation pathways and DFT calculation. Chem. Eng. J. 2021, 410, 128454. [Google Scholar] [CrossRef]
- Shao, L.; Petersen, K.; Shiri, F.; Feng, H.; Gale, B. Characteristics of electrical field flow fractionation with chronoamperometry and electrochemical impedance. Micro Nano Lett. 2020, 15, 13–17. [Google Scholar] [CrossRef]
- Tian, J.; Sang, Y.; Yu, G.; Jiang, H.; Mu, X.; Liu, H. A Bi2WO6-Based Hybrid Photocatalyst with Broad Spectrum Photocatalytic Properties under UV, Visible, and Near-Infrared Irradiation. Adv. Mater. 2013, 25, 5075–5080. [Google Scholar] [CrossRef]
- He, W.; Li, Z.; Lv, S.; Niu, M.; Zhou, W.; Li, J.; Lu, R.; Gao, H.; Pan, C.; Zhang, S. Facile synthesis of Fe3O4@MIL-100(Fe) towards enhancing photo-Fenton like degradation of levofloxacin via a synergistic effect between Fe3O4 and MIL-100(Fe). Chem. Eng. J. 2021, 409, 128274. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, D.; Shi, B.; Dai, W.; Ren, H.; An, K.; Zhou, Z.; Zhao, Z.; Wang, W.; Jiang, Z. In situ construction of hydrazone-linked COF-based core–shell hetero-frameworks for enhanced photocatalytic hydrogen evolution. J. Mater. Chem. A 2020, 8, 7724–7732. [Google Scholar] [CrossRef]
- Cui, K.-P.; He, Y.-Y.; Xu, K.-J.; Zhang, Y.; Chen, C.-B.; Xu, Z.-J.; Chen, X. Degradation of Tetracycline Hydrochloride by Cu-Doped MIL-101(Fe) Loaded Diatomite Heterogeneous Fenton Catalyst. Nanomaterials 2022, 12, 811. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xu, Y.; Zhang, Y.; Feng, J.; Li, Y.; Lan, J.; Cheng, X. Fabrication of NiCoP decorated TiO2/polypyrrole nanocomposites for the effective photocatalytic degradation of tetracycline. Chin. Chem. Lett. 2022, 33, 2741–2746. [Google Scholar] [CrossRef]
- Liang, H.; Zhu, C.; Wang, A.; Palanisamy, K.; Chen, F. Facile synthesis of NiAl2O4/g-C3N4 composite for efficient photocatalytic degradation of tetracycline. J. Environ. Sci. 2023, 127, 700–713. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Mo, G.; Luo, J. Metal–organic frameworks-derived TiO2 for photocatalytic degradation of tetracycline hydrochloride. Can. J. Chem. Eng. 2023, 101, 1358–1370. [Google Scholar] [CrossRef]
- Jin, J.; Hu, H.; Xu, M.; Yang, Y.; Jin, W.; Zhang, Z.; Dong, F.; Shao, M.; Wan, Y. Z-type Bi2O2CO3/NH2-MIL-125(Ti) heterojunction for efficient photocatalytic degradation of tetracycline. J. Mater. Sci. Mater. Electron. 2024, 35, 295. [Google Scholar] [CrossRef]
- Yu, F.; Gong, F.; Yang, Q.; Wang, Y. Fabrication of a magnetic retrievable dual Z-scheme g-C3N4/BiVO4/CoFe2O4 composite photocatalyst with significantly enhanced activity for the degradation of rhodamine B and hydrogen evolution under visible light. Diam. Relat. Mater. 2022, 125, 109004. [Google Scholar] [CrossRef]
- Hsu, H.-L.; Roselin, L.S.; Savidha, R.; Selvin, R. Enhanced photocatalytic performance of magnetite/TS-1 thin film for phenol degradation. J. Saudi Chem. Soc. 2022, 26, 101538. [Google Scholar] [CrossRef]
- Zeng, T.; Shi, D.; Cheng, Q.; Liao, G.; Zhou, H.; Pan, Z. Construction of novel phosphonate-based MOF/P–TiO2 heterojunction photocatalysts: Enhanced photocatalytic performance and mechanistic insight. Environ. Sci. Nano 2020, 7, 861–879. [Google Scholar] [CrossRef]



| Catalyst | C (mg/L) | Catalyst Dosage 1 | k × 10−3 (min−1) | Ref. |
|---|---|---|---|---|
| MIL-125 (Ti)-derived TiO2 | 300 | 15 | 1.03 | [48] |
| NiAl2O4/g-C3N4 | 300 | 15 | 0.50 | [49] |
| TiO2/PPy/NiCoP | 400 | 40 | 0.12 | [50] |
| BOC/NMLT-5 | 600 | 24 | 0.40 | [51] |
| Fe0.25Cu0.75(BDC)@DE | 500 | 25 | 0.31 | [52] |
| NM88(DB)@COF-JLU19 | 100 | 10 | 3.17 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Liu, J.; Li, Z.; Yin, Y. Ligand-Induced Synthesis of Highly Stable NM88(DB)@COF-JLU19 Composite: Accelerating Electron Flow for Visible-Light-Efficient Degradation of Tetracycline Hydrochloride. Polymers 2024, 16, 539. https://doi.org/10.3390/polym16040539
Zhao J, Liu J, Li Z, Yin Y. Ligand-Induced Synthesis of Highly Stable NM88(DB)@COF-JLU19 Composite: Accelerating Electron Flow for Visible-Light-Efficient Degradation of Tetracycline Hydrochloride. Polymers. 2024; 16(4):539. https://doi.org/10.3390/polym16040539
Chicago/Turabian StyleZhao, Jinxia, Jingchao Liu, Zenghe Li, and Yilin Yin. 2024. "Ligand-Induced Synthesis of Highly Stable NM88(DB)@COF-JLU19 Composite: Accelerating Electron Flow for Visible-Light-Efficient Degradation of Tetracycline Hydrochloride" Polymers 16, no. 4: 539. https://doi.org/10.3390/polym16040539
APA StyleZhao, J., Liu, J., Li, Z., & Yin, Y. (2024). Ligand-Induced Synthesis of Highly Stable NM88(DB)@COF-JLU19 Composite: Accelerating Electron Flow for Visible-Light-Efficient Degradation of Tetracycline Hydrochloride. Polymers, 16(4), 539. https://doi.org/10.3390/polym16040539







