Cr-Free Anticorrosive Primers for Marine Propeller Applications
Abstract
1. Introduction
2. Experimental Section
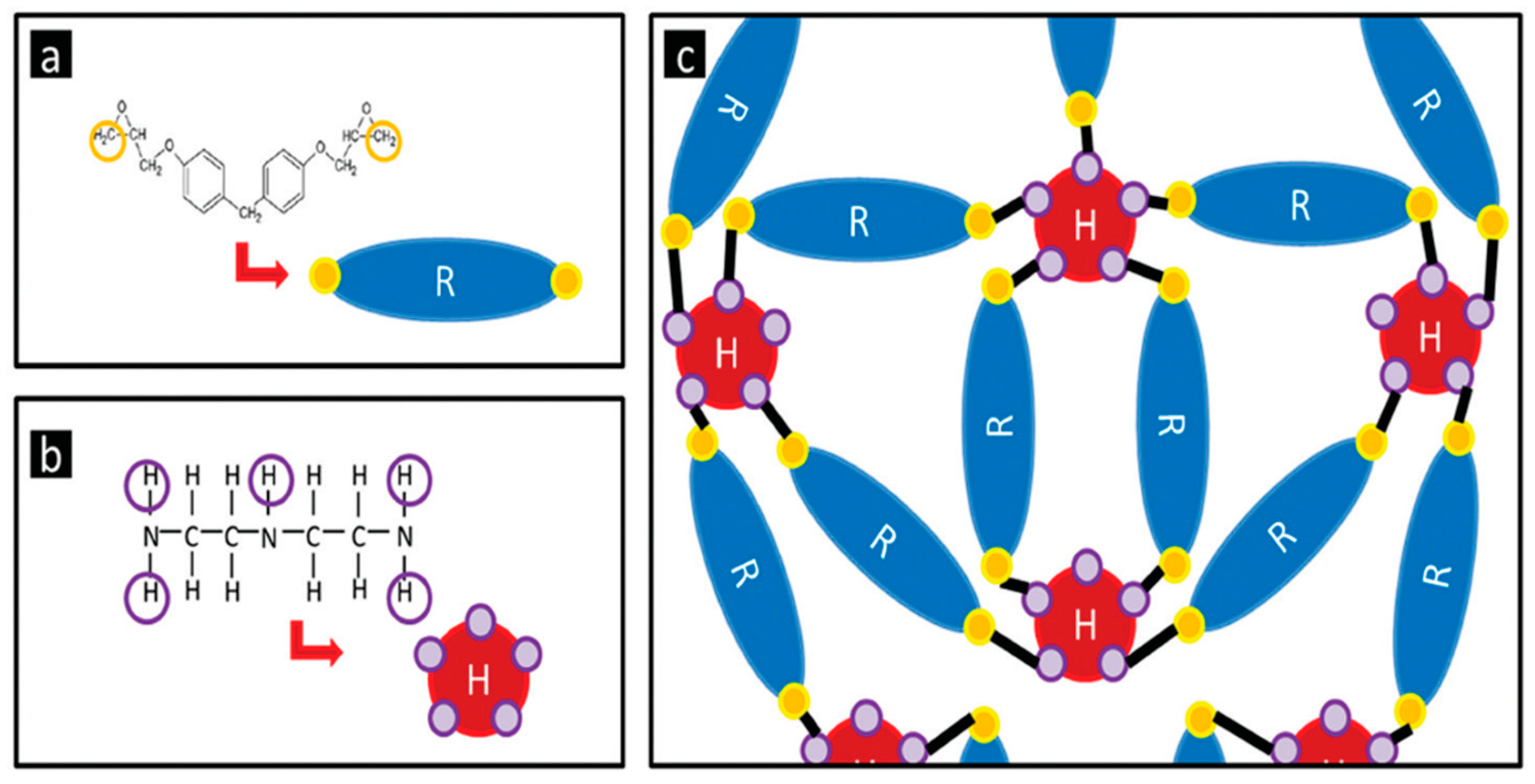
2.1. Materials
2.2. Sample Preparation
2.3. Electrochemical Characterisation
2.4. Mechanical Characterisation
3. Results
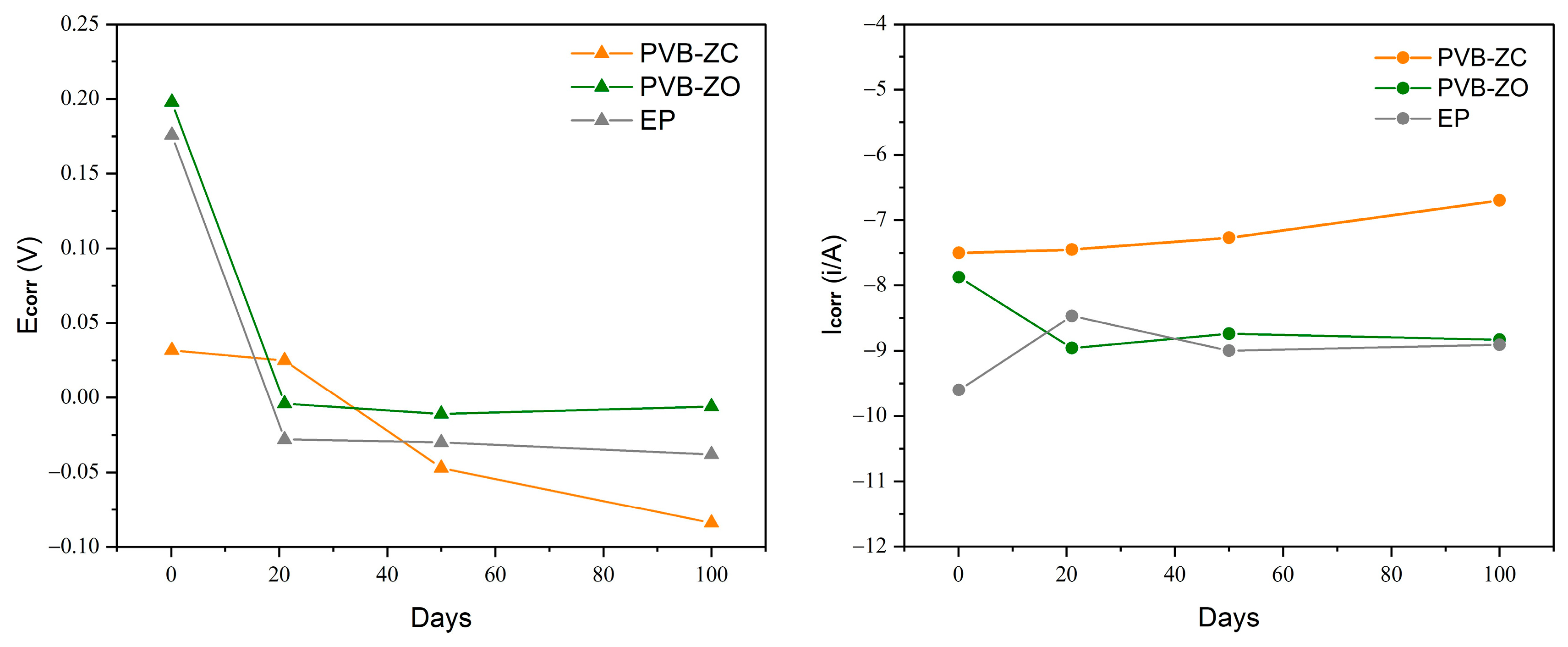
3.1. Electrochemical Impedance Spectroscopy (EIS)
3.2. Artificially Scratched EIS
3.3. Potentiodynamic Polarization Test (PDP)
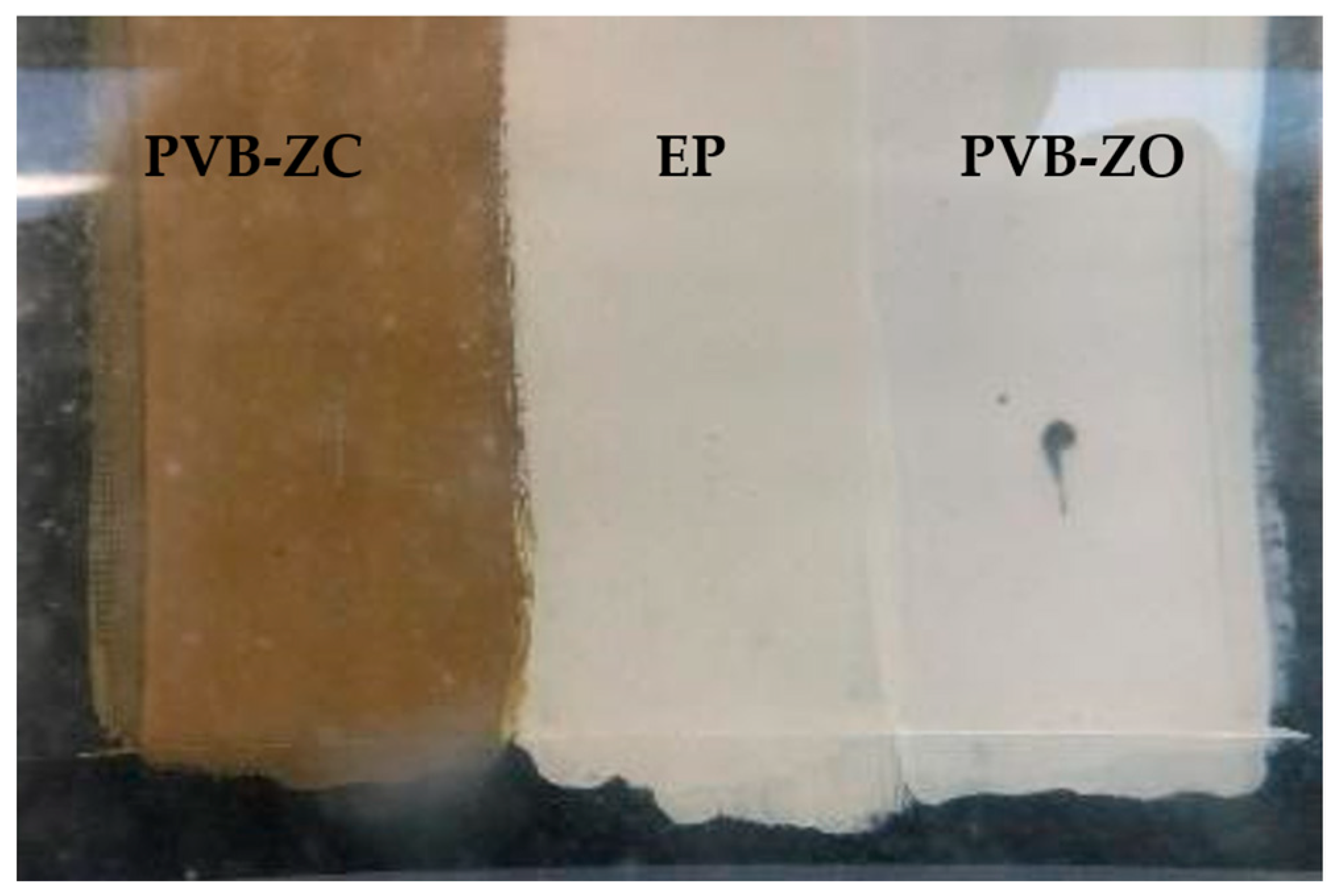
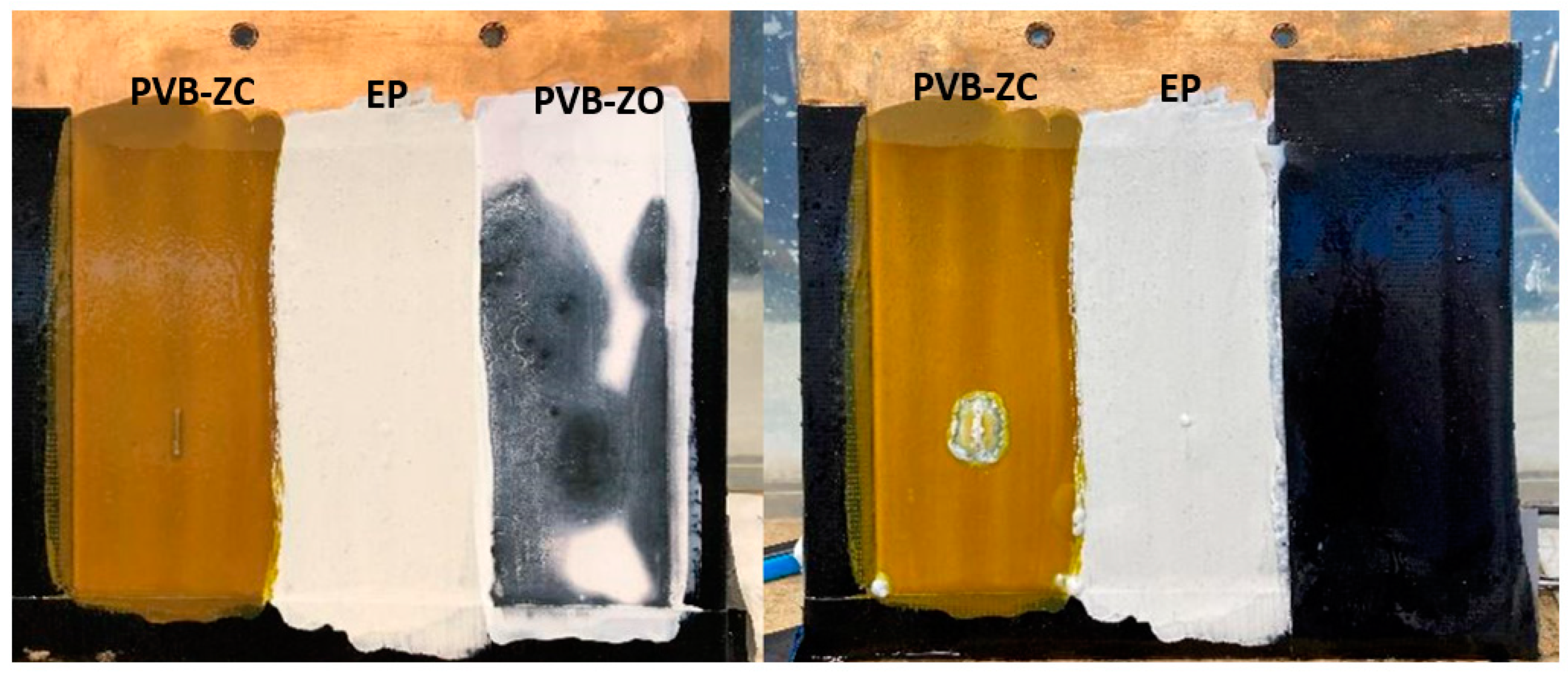
3.4. Electrolysis Corrosion
3.5. Microhardness Test
3.6. Conical Mandrel Bend Tests
3.7. Pull-Off Adhesion
4. Discussion: Primer Failure Mechanisms and Required Properties of Propeller Coatings
4.1. Electrochemical Properties
4.1.1. EIS and PDP Test
4.1.2. Artificially Scratched EIS Test
4.1.3. Electrolysis Test
4.2. Mechanical Properties
4.2.1. Microhardness Test
4.2.2. Conical Mandrel Bending Test
4.2.3. Pull-Off Adhesion Test
4.3. Analysis Conclusion
5. Conclusions
- (1)
- Excellent adhesion between the primer and the substrate to ensure the primer is intact on the substrate even when water is absorbed into the coating;
- (2)
- Flexibility to minimize cracking and to avoid continuous flaking upon a coating damage;
- (3)
- Resistance to electrolysis/cathodic disbondment and high alkalinity in the cathodic area;
- (4)
- Self-healing after the coating is damaged. This is often achieved by controllable release of a corrosion inhibitive to slow down the corrosion when the coating is damaged.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marine Propeller Market Size, Share, Industry Analysis, and Regional Forecast, 2022–2029, FBI103074. 2022. Available online: https://www.fortunebusinessinsights.com/marine-propeller-market-103074 (accessed on 4 July 2022).
- Birn, J.; Skalski, I. Corrosion behaviour of non-ferrous alloys in seawater in the polish marine industry. In Corrosion Behaviour and Protection of Copper and Aluminium Alloys in Seawater; Elsevier: Amsterdam, The Netherlands, 2007; pp. 3–18. [Google Scholar]
- Wang, A.; De Silva, K.; Jones, M.; Robinson, P.; Larribe, G.; Gao, W. Anticorrosive coating systems for marine propellers. Prog. Org. Coat. 2023, 183, 107768. [Google Scholar] [CrossRef]
- Sørensen, P.A.; Kiil, S.; Dam-Johansen, K.; Weinell, C.E. Anticorrosive coatings: A review. J. Coat. Technol. Res. 2009, 6, 135–176. [Google Scholar] [CrossRef]
- Pokorny, P.; Tej, P.; Szelag, P. Chromate conversion coatings and their current application. Metalurgija 2016, 55, 253–256. [Google Scholar]
- Puthran, D.; Patil, D. Usage of heavy metal-free compounds in surface coatings. J. Coat. Technol. Res. 2023, 20, 87–112. [Google Scholar] [CrossRef]
- Lakshmi, R.V.; Sampath, S.; Aruna, S.T. Silica-alumina based sol-gel coating containing cerium oxide nanofibers as a potent alternative to conversion coating for AA2024 alloy. Surf. Coat. Technol. 2021, 411, 127007. [Google Scholar] [CrossRef]
- Ma, L.; Wang, X.; Wang, J.; Zhang, J.; Yin, C.; Fan, L.; Zhang, D. Graphene oxide–cerium oxide hybrids for enhancement of mechanical properties and corrosion resistance of epoxy coatings. J. Mater. Sci. 2021, 56, 10108–10123. [Google Scholar] [CrossRef]
- Niratiwongkorn, T.; Luckachan, G.E.; Mittal, V. Self-healing protective coatings of polyvinyl butyral/polypyrrole-carbon black composite on carbon steel. RSC Adv. 2016, 6, 43237–43249. [Google Scholar] [CrossRef]
- Jia, Y.; Qiu, T.; Guo, L.; Ye, J.; He, L.; Li, X. Preparation of pH responsive smart nanocontainer via inclusion of inhibitor in graphene/halloysite nanotubes and its application in intelligent anticorrosion protection. Appl. Surf. Sci. 2020, 504, 144496. [Google Scholar] [CrossRef]
- Ou, B.; Wang, Y.; Lu, Y. A review on fundamentals and strategy of epoxy-resin-based anticorrosive coating materials. Polym.-Plast. Technol. Mater. 2021, 60, 601–625. [Google Scholar] [CrossRef]
- Zhang, S.; He, Y.; Zhang, T.; Wang, G.; Du, X. Long-Term Atmospheric Corrosion Behavior of Epoxy Prime Coated Aluminum Alloy 7075-T6 in Coastal Environment. Materials 2018, 11, 965. [Google Scholar] [CrossRef]
- Khodaei, P.; Shabani-Nooshabadi, M.; Behpour, M. Epoxy-Based nanocomposite coating reinforced by a zeolite complex: Its anticorrosion properties on mild steel in 3.5 wt% NaCl media. Prog. Org. Coat. 2019, 136, 105254. [Google Scholar] [CrossRef]
- Carrot, C.; Bendaoud, A.; Pillon, C. Handbook of Thermoplastics; Routledge Handbooks Online: London, UK, 2015. [Google Scholar]
- Jing, L.-C.; Wang, T.; Rhen, D.S.; Yuan, X.-T.; Tian, Y.; Xie, Q.; Geng, H.-Z. Wrinkled p-phenylenediamine grafted graphene oxide as reinforcement for polyvinyl butyral anti-corrosive coating. J. Mater. Sci. 2021, 56, 12686–12699. [Google Scholar] [CrossRef]
- Zhu, G.; Cui, X.; Zhang, Y.; Chen, S.; Dong, M.; Liu, H.; Shao, Q.; Ding, T.; Wu, S.; Guo, Z. Poly (vinyl butyral)/Graphene oxide/poly (methylhydrosiloxane) nanocomposite coating for improved aluminum alloy anticorrosion. Polymer 2019, 172, 415–422. [Google Scholar] [CrossRef]
- Mahdavi, F.; Forsyth, M.; Tan, M.Y.J. Techniques for testing and monitoring the cathodic disbondment of organic coatings: An overview of major obstacles and innovations. Prog. Org. Coat. 2017, 105, 163–175. [Google Scholar] [CrossRef]
- Caldona, E.B.; Smith, D.W., Jr.; Wipf, D.O. Surface electroanalytical approaches to organic polymeric coatings. Soc. Ind. Chem. 2020, 70, 927–937. [Google Scholar] [CrossRef]
- Trentin, A.; Pakseresht, A.; Duran, A.; Castro, Y.; Galusek, D. Electrochemical Characterization of Polymeric Coatings for Corrosion Protection: A Review of Advances and Perspectives. Polymers 2022, 14, 2306. [Google Scholar] [CrossRef] [PubMed]
- Olivier, M.-G.; Poelman, M. Use of Electrochemical Impedance Spectroscopy (EIS) for the Evaluation of Electrocoatings Performances. In Recent Researches in Corrosion Evaluation and Protection; Razavi, R.S., Ed.; IntechOpen: Rijeka, Croatia, 2012; Chapter 1. [Google Scholar]
- Zinc Oxide Is in Your Paint. Citra Cakralogram. Available online: https://www.citracakralogam.com/zinc-oxide-is-in-your-paint/ (accessed on 4 July 2023).
- ACS. Zinc Oxide. Available online: https://www.acs.org/molecule-of-the-week/archive/z/zinc-oxide.html#:~:text=It%20is%20insoluble%20in%20water,%C2%BAC%2C%20where%20it%20also%20decomposes (accessed on 4 July 2023).
- Fink-Jensen, P. Hardness Testing of Organic Coatings. Pure Appl. Chem. 1965, 10, 239–292. [Google Scholar] [CrossRef]
- Zhang, J.; Koo, B.; Subramanian, N.; Liu, Y.; Chattopadhyay, A. An optimized cross-linked network model to simulate the linear elastic material response of a smart polymer. J. Intell. Mater. Syst. Struct. 2016, 27, 1461–1475. [Google Scholar] [CrossRef]
- Pélissier, K.; Thierry, D. Powder and High-Solid Coatings as Anticorrosive Solutions for Marine and Offshore Applications? A Review. Coatings 2020, 10, 916. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.; Chen, Y.; Song, X.; Yang, Y. Effect of a DC Stray Current on the Corrosion of X80 Pipeline Steel and the Cathodic Disbondment Behavior of the Protective 3PE Coating in 3.5% NaCl Solution. Coatings 2019, 9, 29. [Google Scholar] [CrossRef]
- Zarras, P.; Miller, C.; Webber, C.; Anderson, N.; Stenger-Smith, J. Laboratory and Field Studies of Poly(2,5-bis(N-methyl-N-hexylamino)phenylene vinylene) (BAM-PPV): A Potential Wash Primer Replacement for Army Military Vehicles. Coatings 2014, 4, 687–700. [Google Scholar] [CrossRef]
- Joseph, R. Zinc Chromate Primers, Wash Primers and Epoxy Primers. Paints and Coatings Resouce Center. Available online: https://www.paintcenter.org/rj/jul02k.php (accessed on 4 July 2023).













| Primer | Coating System | Main Active Component |
|---|---|---|
| PVB-ZC | 2K PVB |
|
| PVB-ZO | 1K PVB |
|
| EP | 2K Epoxy |
|
| Test | Substrate | Dimensions (mm) |
|---|---|---|
| Electrochemical Impedance Spectroscopy | C95800 Al Bronze | 100 × 100 × 3.0 |
| Potentiodynamic Polarization Test | C95800 Al Bronze | 100 × 100 × 3.0 |
| Microhardness | Al 5005 | 100 × 150 × 1.5 |
| Mandrel Bend | Al 1100 | 100 × 150 × 0.8 |
| Pull-off Adhesion | C95800 Al Bronze | 100 × 100 × 3.0 |
| Electrolysis | C95800 Al Bronze | 200 × 200 × 3.0 |
| Test | DFT (µm) ± STDEV |
|---|---|
| Electrochemical Impedance Spectroscopy | PVB-ZC: 102.7 ± 3.6 |
| PVB-ZO: 107.3 ± 3.0 | |
| EP: 99.3 ± 6.1 | |
| Potentiodynamic Polarization Test | PVB-ZC: 116.3 ± 8.0 |
| PVB-ZO: 111.3 ± 14.7 | |
| EP: 157.6 ± 13.5 | |
| Microhardness | PVB-ZC: 325.9 ± 16.4 |
| PVB-ZO: 309.0 ± 14.4 | |
| EP: 314.7 ± 19.8 | |
| Mandrel Bend | PVB-ZC: 94.4 ± 4.2 |
| PVB-ZO: 96.0 ± 6.6 | |
| EP: 107.6 ± 10.9 | |
| Pull-off Adhesion | PVB-ZC: 141.9 ± 8.6 |
| PVB-ZO: 150.7 ± 9.2 | |
| EP: 141.9 ± 12.7 | |
| Electrolysis | PVB-ZC: 36.4 ± 4.4 |
| PVB-ZO: 43.5 ± 7.1 | |
| EP: 240.0 ± 47.9 |
| Primers | Average Hardness (HV0.1) | STDEV |
|---|---|---|
| PVB-ZC | 3.90 | 0.20 |
| PVB-ZO | 1.60 | 0.10 |
| EP | 17.76 | 2.61 |
| Primers | Average MPa ± STDV |
|---|---|
| PVB-ZC | 4.79 ± 0.28 |
| PVB-ZO | 5.93 ± 0.26 |
| EP | 3.20 ± 0.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, A.; De Silva, K.; Jones, M.; Gao, W. Cr-Free Anticorrosive Primers for Marine Propeller Applications. Polymers 2024, 16, 408. https://doi.org/10.3390/polym16030408
Wang A, De Silva K, Jones M, Gao W. Cr-Free Anticorrosive Primers for Marine Propeller Applications. Polymers. 2024; 16(3):408. https://doi.org/10.3390/polym16030408
Chicago/Turabian StyleWang, Annie, Karnika De Silva, Mark Jones, and Wei Gao. 2024. "Cr-Free Anticorrosive Primers for Marine Propeller Applications" Polymers 16, no. 3: 408. https://doi.org/10.3390/polym16030408
APA StyleWang, A., De Silva, K., Jones, M., & Gao, W. (2024). Cr-Free Anticorrosive Primers for Marine Propeller Applications. Polymers, 16(3), 408. https://doi.org/10.3390/polym16030408






