Preparation and Properties of Atomic-Oxygen Resistant Polyimide Films Based on Multi-Ring Fluoro-Containing Dianhydride and Phosphorus-Containing Diamine
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization Methods
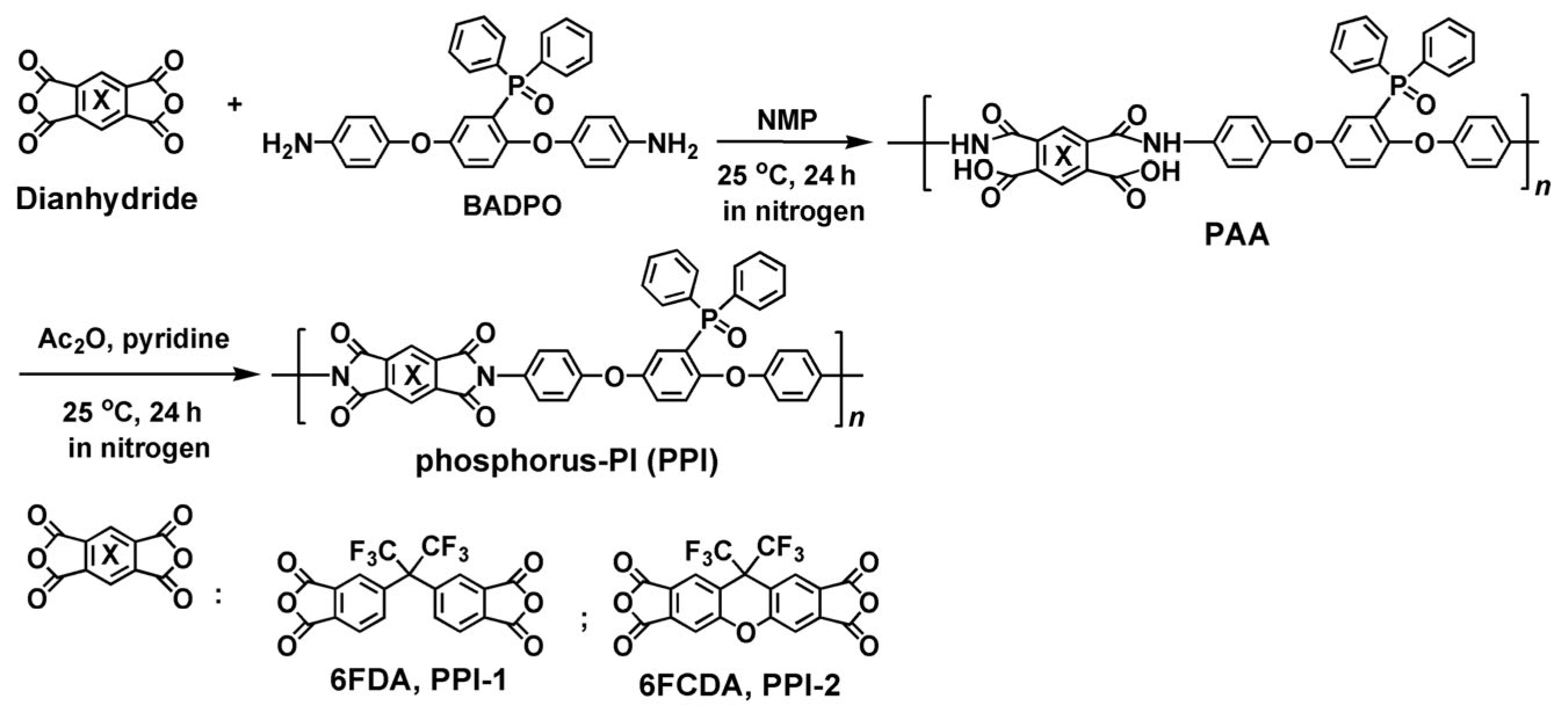
2.3. PPI Resins Synthesis and Films Preparation
3. Results and Discussion
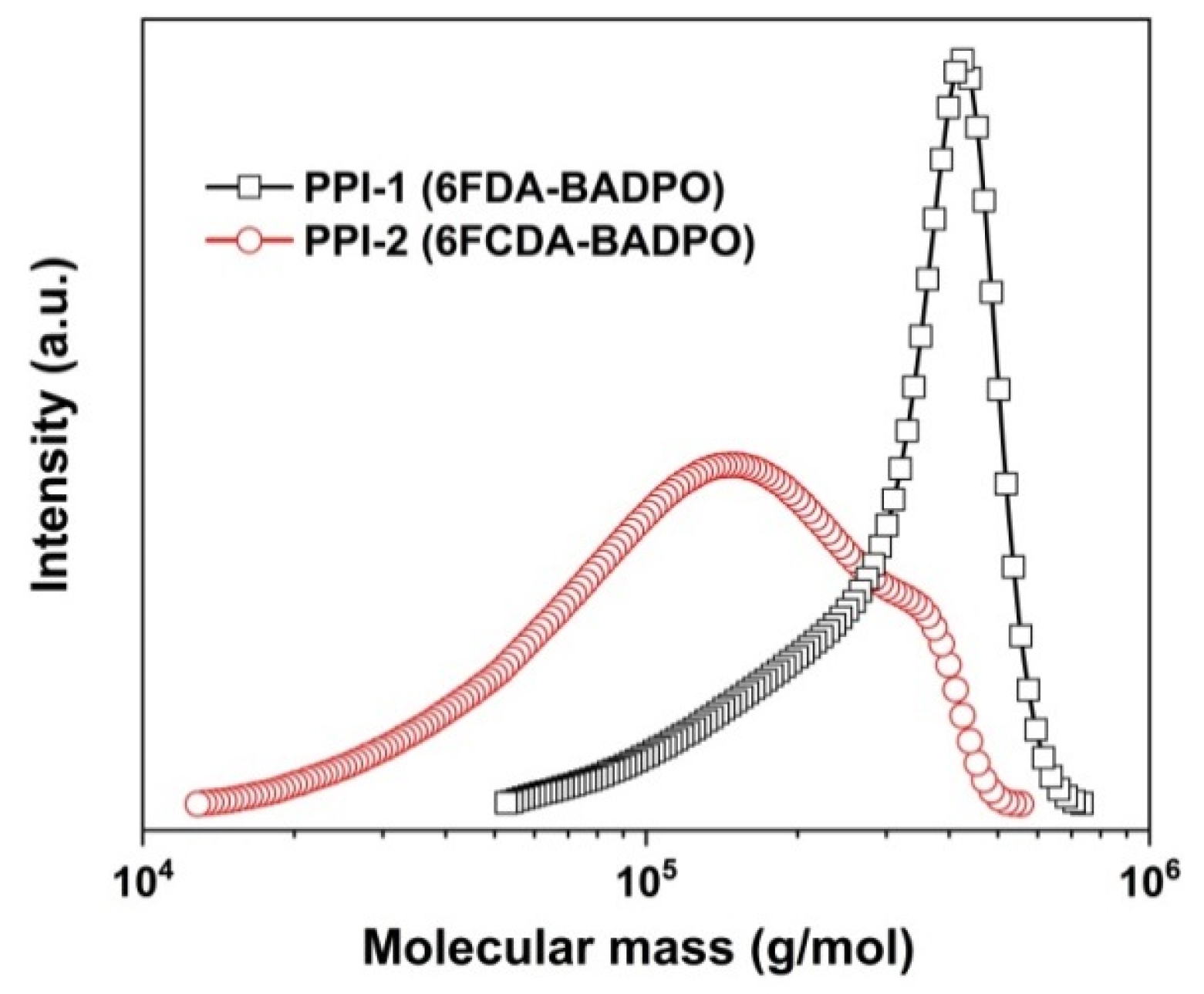
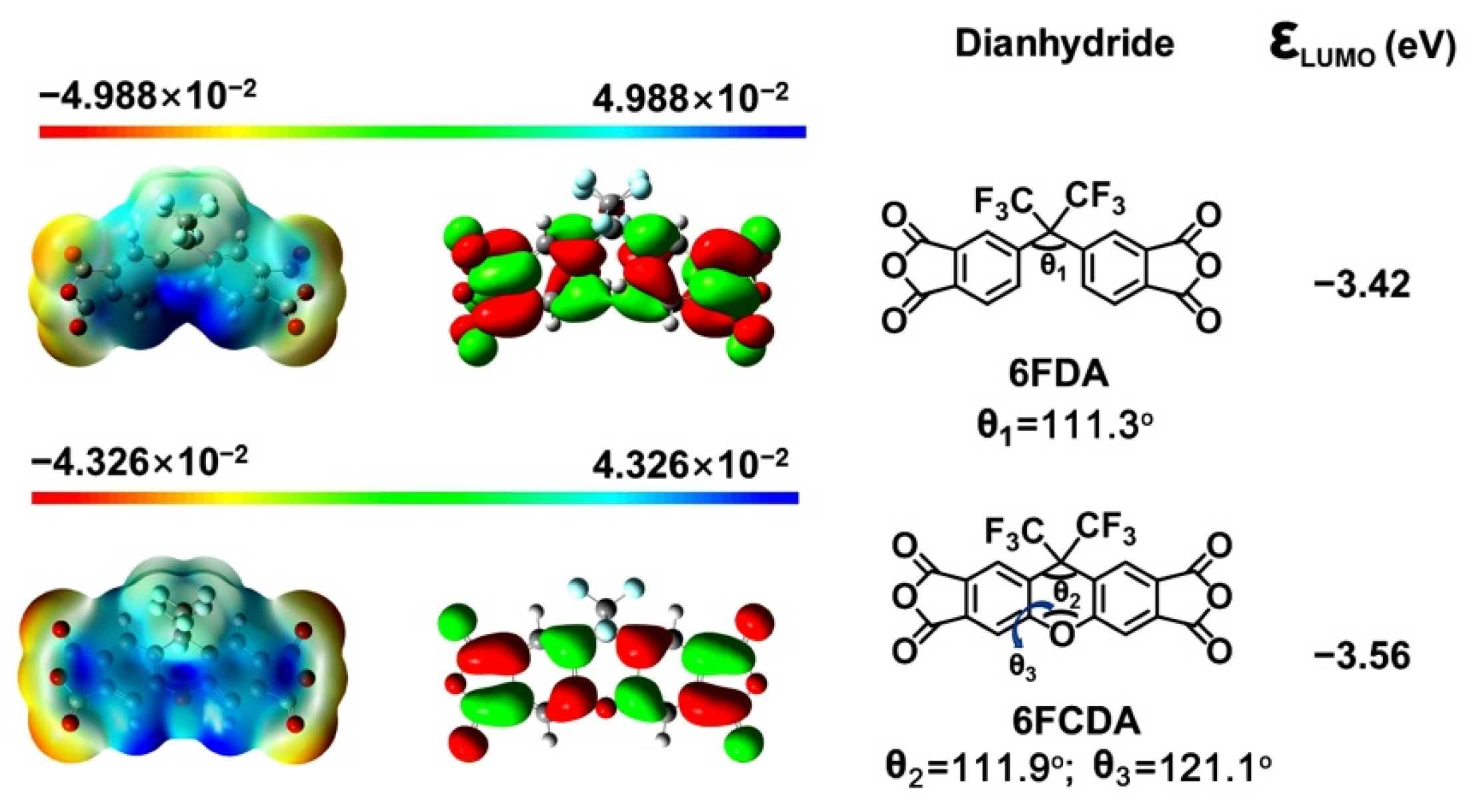
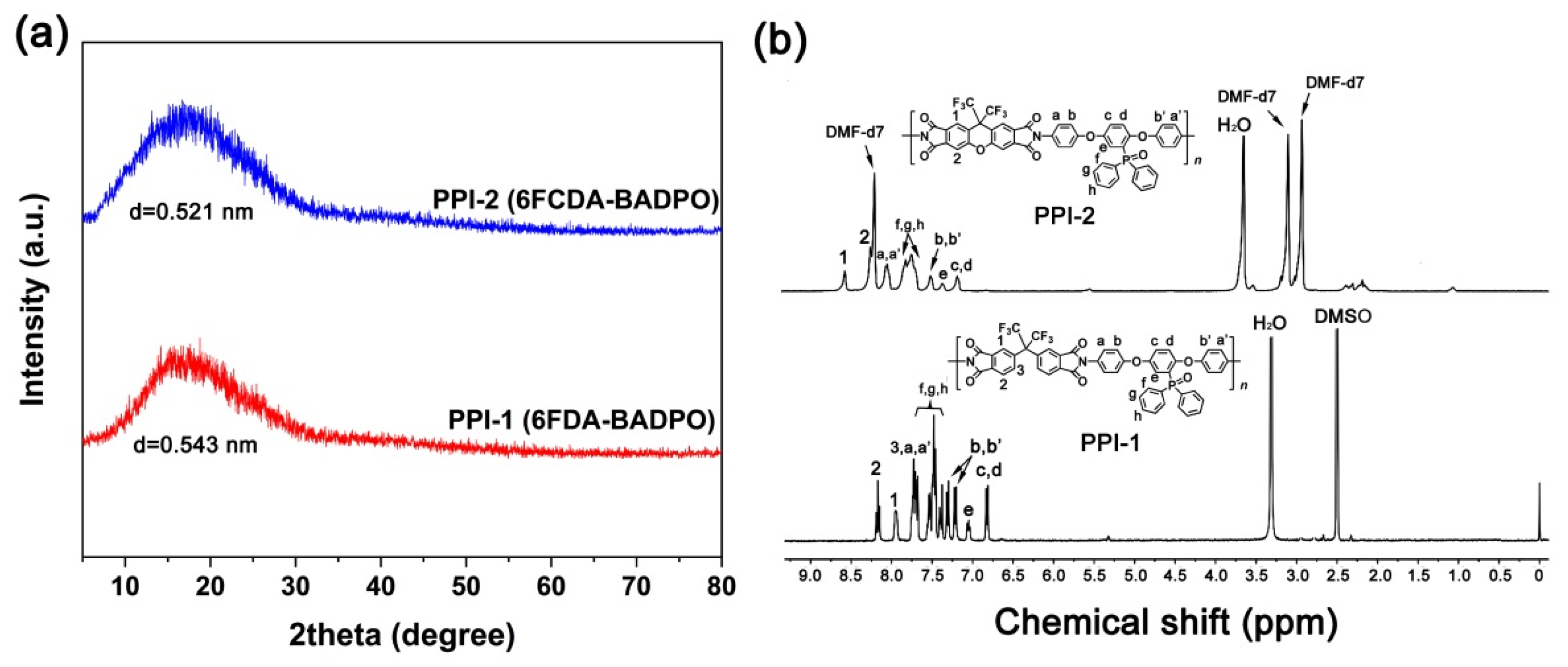
3.1. PPI Resins Synthesis and Films Preparation
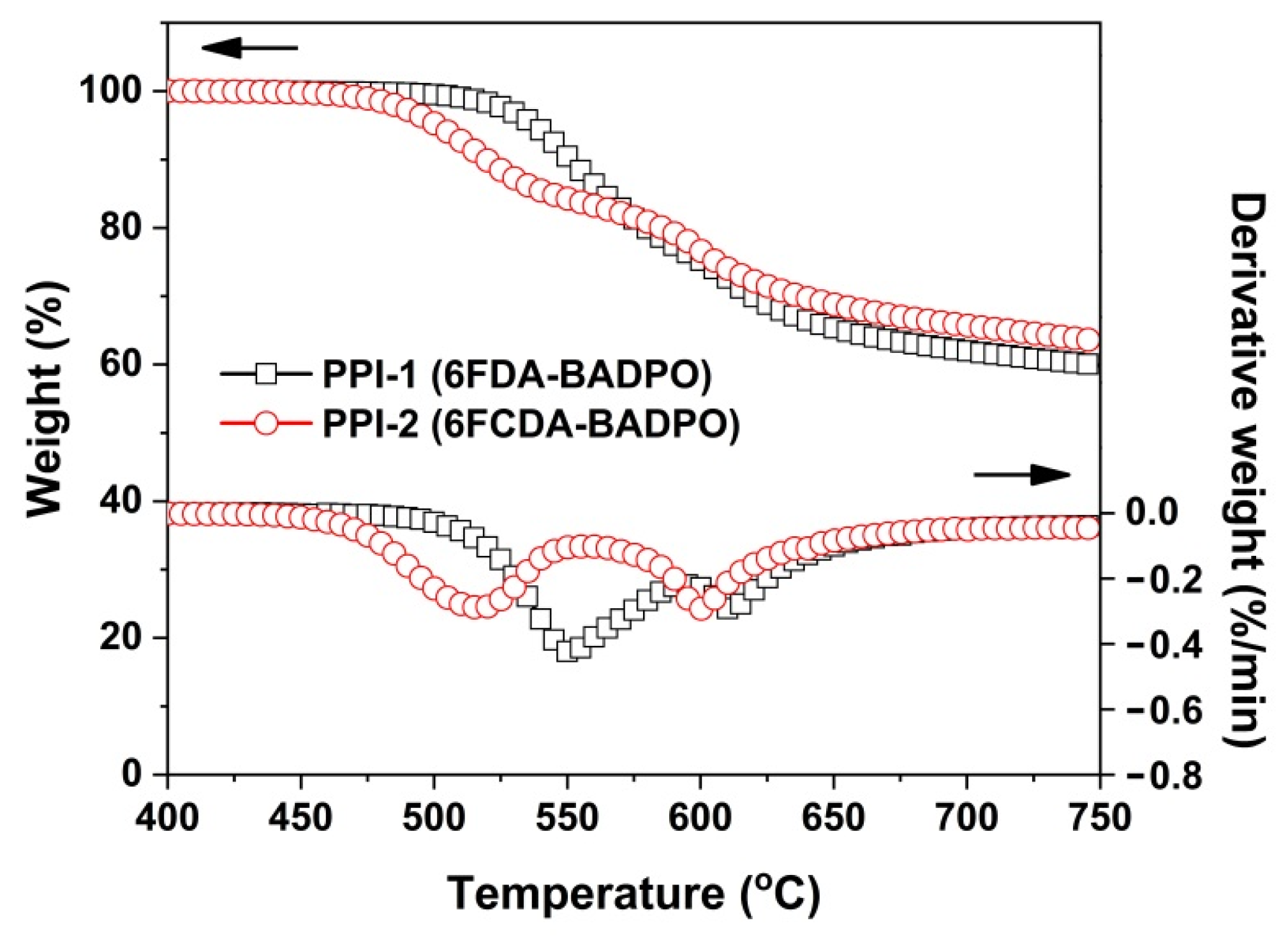
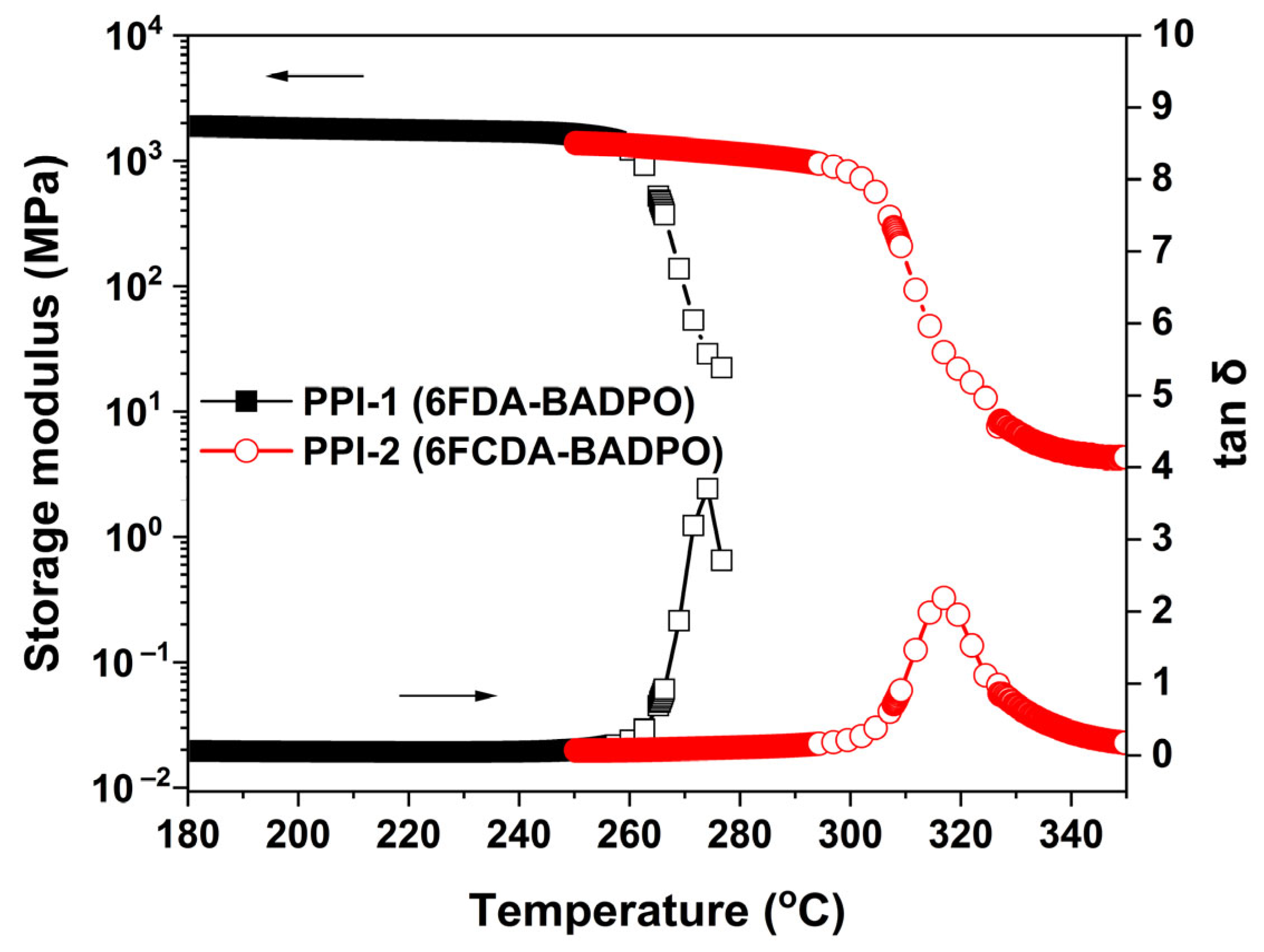
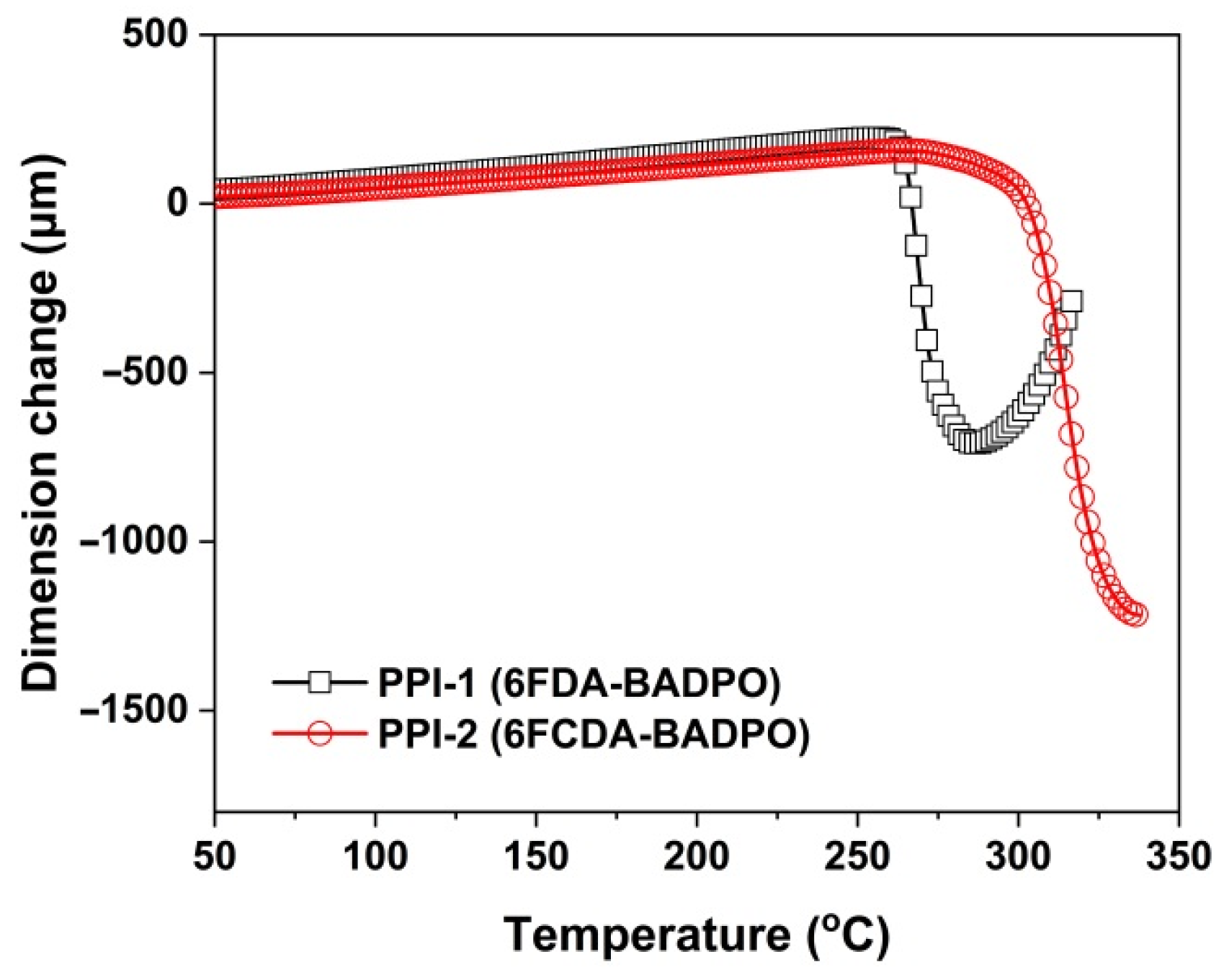
3.2. Thermal Properties
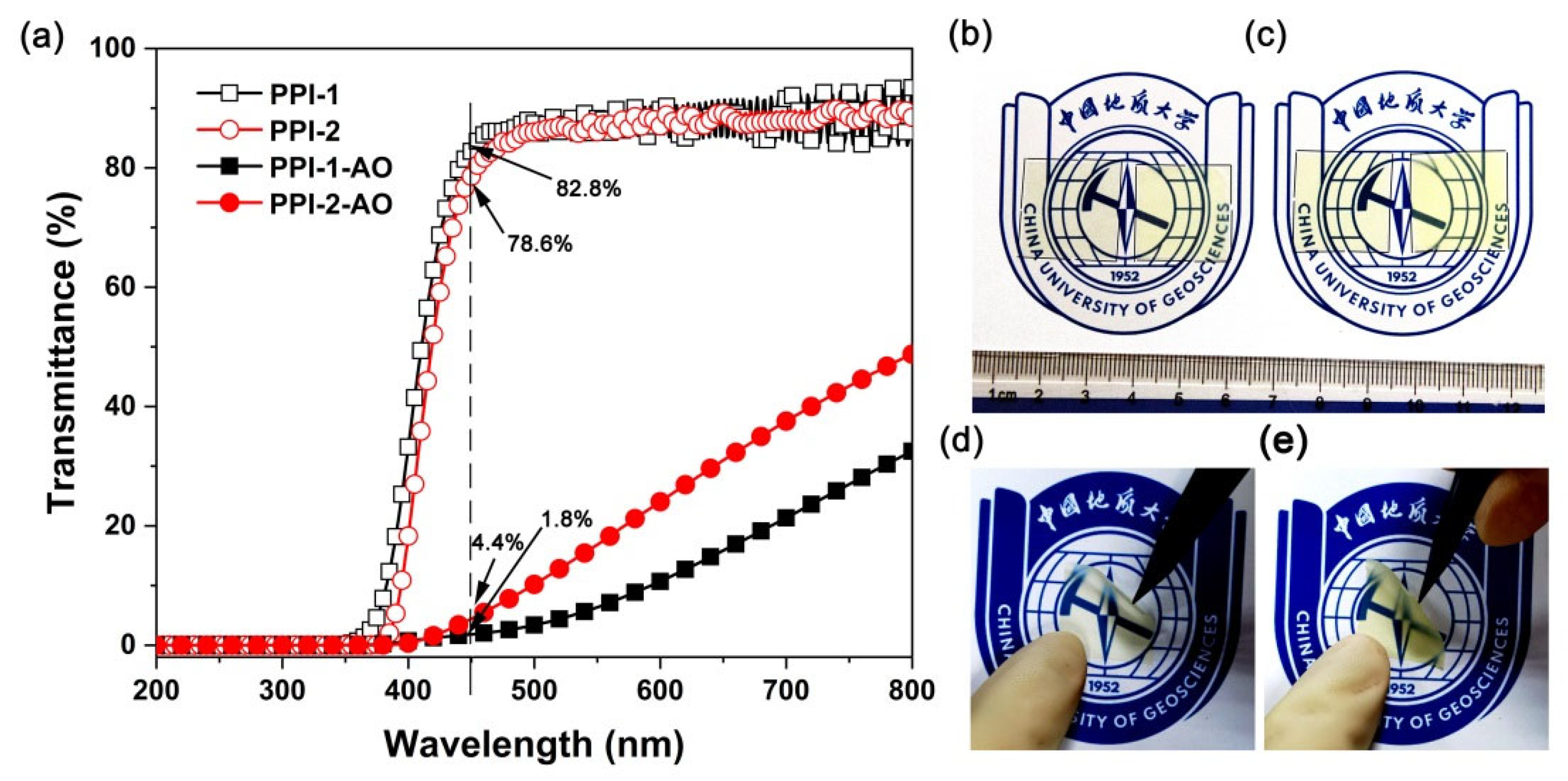
3.3. Optical and Dielectric Properties
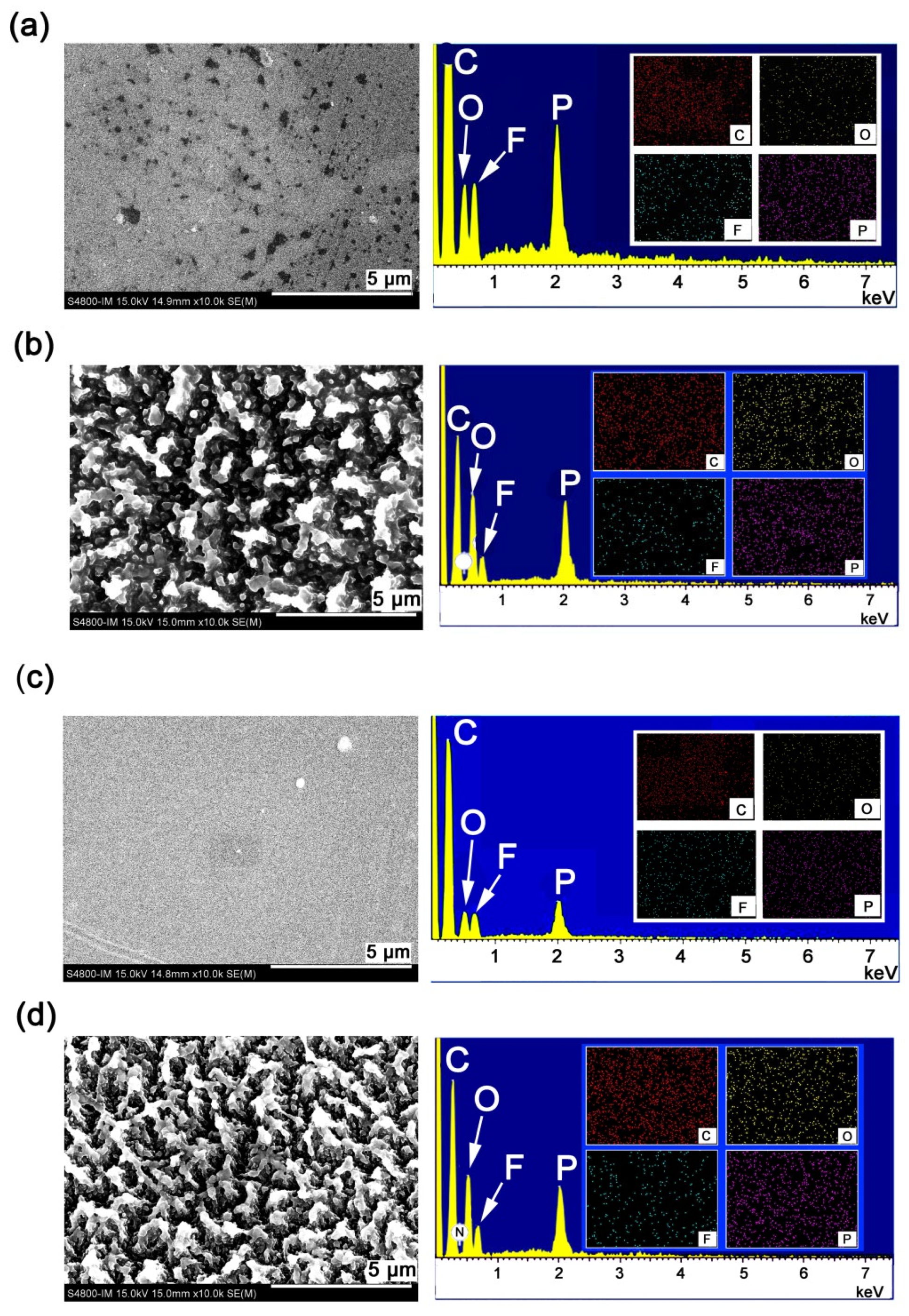
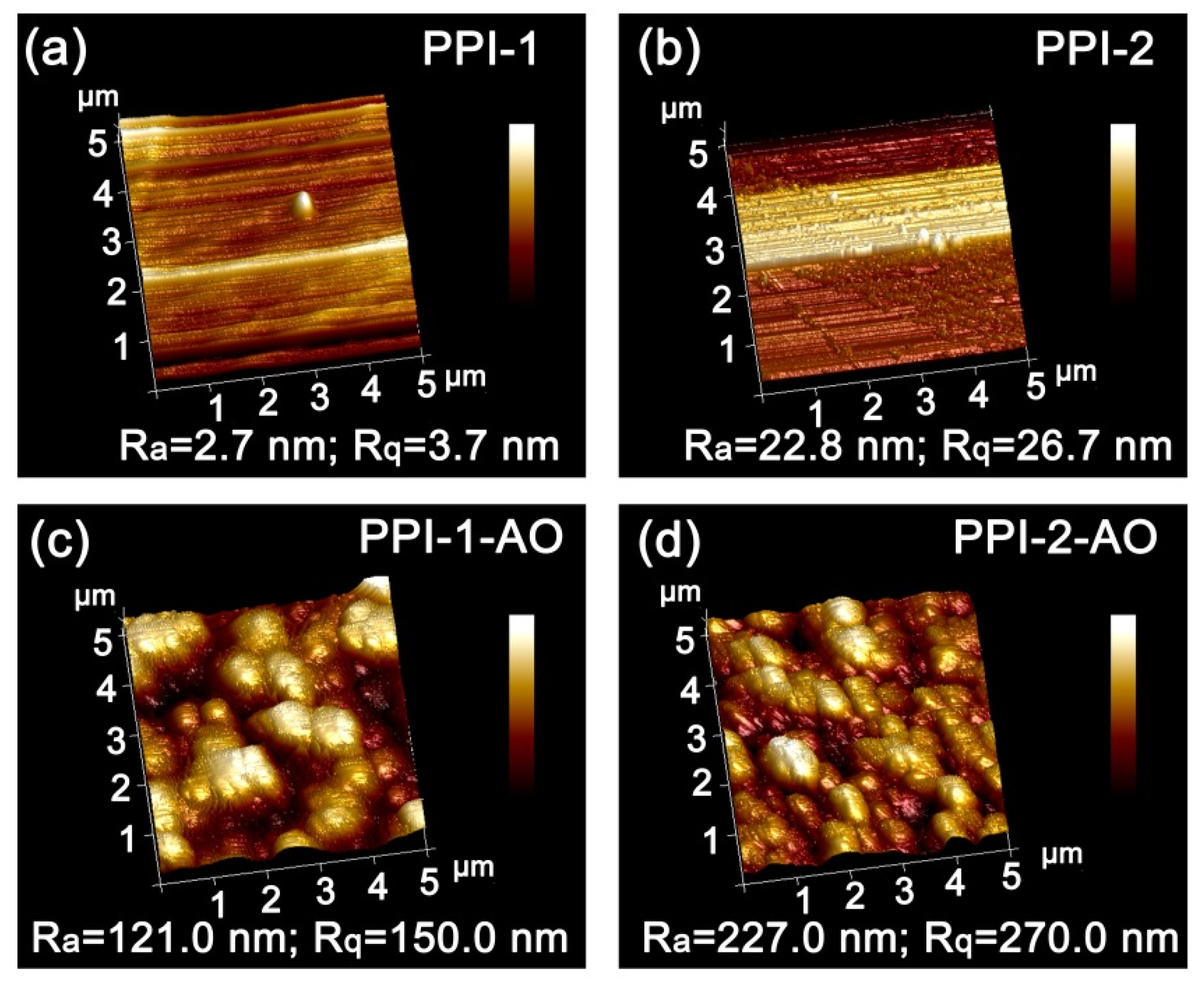
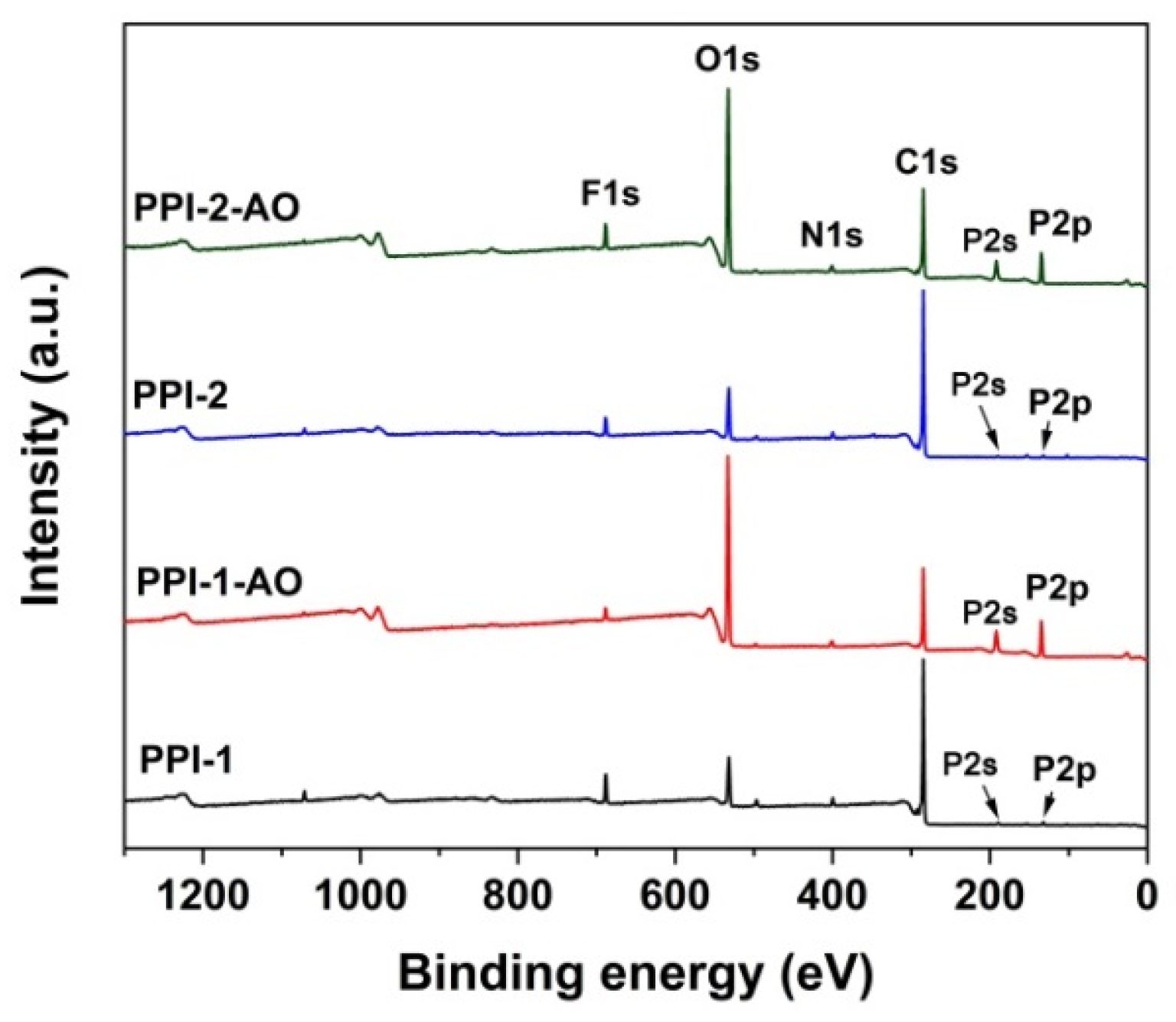
3.4. AO Erosion Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ghidini, T. Materials for space exploration and settlement. Nat. Mater. 2018, 17, 846–850. [Google Scholar] [CrossRef] [PubMed]
- Toor, Z.S. Space applications of composite materials. J. Space Technol. 2018, 8, 65–70. [Google Scholar]
- Naser, M.Z.; Chehab, A.I. Materials and design concepts for space-resilient structures. Prog. Aerosp. Sci. 2018, 98, 74–90. [Google Scholar] [CrossRef]
- Pernigoni, L.; Lafont, U.; Grande, A.M. Self-healing materials for space applications: Overview of present development and major limitations. CEAS Space J. 2021, 13, 341–352. [Google Scholar] [CrossRef]
- Chen, J.; Ding, N.; Li, Z.; Wang, W. Organic polymer materials in the space environment. Prog. Aerosp. Sci. 2016, 83, 37–56. [Google Scholar] [CrossRef]
- Xu, Z.; Croft, Z.L.; Guo, D.; Cao, K.; Liu, G. Recent development of polyimides: Synthesis, processing, and application in gas separation. J. Polym. Sci. 2021, 59, 943–962. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, X.Y.; Sun, Y.G.; Li, W.Z.; Zhang, X.S.; Luan, J. Synthesis and applications of low dielectric polyimide. Res. Chem. Mater. 2023, 2, 49–62. [Google Scholar] [CrossRef]
- Yi, C.; Li, W.; Shi, S.; He, K.; Ma, P.; Chen, M.; Yang, C. High-temperature-resistant and colorless polyimide: Preparations, properties, and applications. Sol. Energy 2020, 195, 340–354. [Google Scholar] [CrossRef]
- Hicyilmaz, A.S.; Bedeloglu, A.C. Applications of polyimide coatings: A review. SN Appl. Sci. 2021, 3, 363. [Google Scholar] [CrossRef]
- Chang, J.H. Equibiaxially stretchable colorless and transparent polyimides for flexible display substrates. Rev. Adv. Mater. Sci. 2020, 59, 1–9. [Google Scholar] [CrossRef]
- Pater, R.H.; Curto, P.A. Advanced materials for space applications. Acta Astronaut. 2007, 61, 1121–1129. [Google Scholar] [CrossRef]
- Gouzman, I.; Grossman, E.; Verker, R.; Atar, N.; Bolker, A.; Eliaz, N. Advances in polyimide-based materials for space applications. Adv. Mater. 2019, 31, 1807738. [Google Scholar] [CrossRef]
- Ke, F.; Song, N.; Liang, D.; Xu, H. A method to break charge transfer complex of polyimide: A study on solution behavior. J. Appl. Polym. Sci. 2013, 127, 797–803. [Google Scholar] [CrossRef]
- Luo, L.; Yao, J.; Wang, X.; Li, K.; Huang, J.; Li, B.; Wang, H.; Yan, F.; Liu, X. The evolution of macromolecular packing and sudden crystallization in rigid-rod polyimide via effect of multiple H-bonding on charge transfer (CT) interactions. Polymer 2014, 55, 4258–4269. [Google Scholar] [CrossRef]
- Hasegawa, M.; Horie, K. Photophysics, photochemistry, and optical properties of polyimides. Prog. Polym. Sci. 2001, 26, 259–335. [Google Scholar] [CrossRef]
- Lei, X.F.; Chen, Y.; Zhang, H.P.; Li, X.J.; Yao, P.; Zhang, Q.Y. Space survivable polyimides with excellent optical transparency and self-healing properties derived from hyperbranched polysiloxane. ACS Appl. Mater. Interfaces 2013, 5, 10207–10220. [Google Scholar] [CrossRef] [PubMed]
- Shimamura, H.; Nakamura, T. Mechanical properties degradation of polyimide films irradiated by atomic oxygen. Polym. Degrad. Stab. 2009, 94, 1389–1396. [Google Scholar] [CrossRef]
- Ni, H.; Xing, Y.; Dai, X.; Zhang, D.; Li, J.; Liu, J.; Yang, S.; Chen, X. Intrinsically heat-sealable polyimide films with atomic oxygen resistance: Synthesis and characterization. High Perform. Polym. 2020, 32, 902–913. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, X.; Shen, Z.; Zhang, X. Preparation of two-component hybrid polyimide film for atomic oxygen erosion resistance. Mater. Today Commun. 2021, 27, 102141. [Google Scholar] [CrossRef]
- Wright, J.S.; Jones, A.; Farmer, B.; Rodman, D.L.; Minton, T.K. POSS-enhanced colorless organic/inorganic nanocomposites (CORIN®) for atomic oxygen resistance in low earth orbit. CEAS Space J. 2021, 13, 399–413. [Google Scholar] [CrossRef]
- St Clair, A.K.; St Clair, T.L. Process for Preparing Highly Optically Transparent/Colorless Aromatic Polyimide Film. US Patent 4603061, 29 July 1986. [Google Scholar]
- St Clair, A.K.; St Clair, T.L. Process for Preparing Essentially Colorless Polyimide Film Containing Phenoxy-Linked Diamines. US Patent 4595548, 17 June 1986. [Google Scholar]
- Khot, A.; Razdan, M.; Parekh, B.; Debies, T.; Takacs, G.A. XPS surface characterization of UV photo-oxidized colorless polyimides. J. Adhes. Sci. Technol. 2012, 26, 2291–2300. [Google Scholar] [CrossRef]
- Fay, C.C.; Stoakley, D.M.; St Clair, A.K. Molecularly oriented films for space applications. High Perform. Polym. 1999, 11, 145–156. [Google Scholar] [CrossRef]
- Trofimenko, S. A new class of fluorinated rigid monomers for polyimides. In Advances in Polyimide Science and Technology; Feger, C., Khojasteh, M.M., Htoo, M.S., Eds.; Technomic Publishing Company: Lancaster, UK, 1993; pp. 3–14. [Google Scholar]
- Trofimenko, S.; Auman, B.C. Polyimides based on 9,9-disubstituted xanthene dianhydrides. Macromolecules 1994, 27, 1136–1146. [Google Scholar] [CrossRef]
- Feiring, A.E.; Auman, B.C.; Wonchoba, E.R. Synthesis and properties of fluorinated polyimides from novel 2,2′-bis(fluoroalkoxy)benzidines. Macromolecules 1993, 26, 2779–2784. [Google Scholar] [CrossRef]
- Coburn, J.C.; Soper, P.D.; Auman, B.C. Relaxation behavior of polyimides based on 2,2ʹ-disubstituted benzidines. Macromolecules 1995, 28, 3253–3260. [Google Scholar] [CrossRef]
- Li, Z.; Liu, J.; Gao, Z.; Yin, Z.; Fan, L.; Yang, S. Organo-soluble and transparent polyimides containing phenylphosphine oxide and trifluoromethyl moiety: Synthesis and characterization. Eur. Polym. J. 2009, 45, 1139–1148. [Google Scholar] [CrossRef]
- Wu, B.; Zhang, Y.; Yang, D.; Yang, Y.; Yu, Q.; Che, L.; Liu, J. Self-healing anti-atomic-oxygen phosphorus-containing polyimide film via molecular level incorporation of nanocage trisilanolphenyl POSS: Preparation and characterization. Polymers 2019, 11, 1013. [Google Scholar] [CrossRef]
- Susa, A.; Bijleveld, J.; Santana, M.H.; Garcia, S.J. Understanding the effect of the dianhydride structure on the properties of semiaromatic polyimides containing a biobased fatty diamine. ACS Sustain. Chem. Eng. 2018, 6, 668–678. [Google Scholar] [CrossRef]
- Furutani, H.; Tsuji, H.; Sogabe, K. Synthesis and properties of polyester imides based on 2,2-bis(4-hydeoxyphenyl)propanedibenzoate-3,3,4,4-tetracarboxylic acid dianhydride. Polym. J. 2017, 49, 587–591. [Google Scholar] [CrossRef]
- Colquhoun, H.M.; O’Mahoney, C.A.; Williams, D.J.; Askari, A.; Mayo, R. Single crystal X-ray studies of aromatic oligomers: Structural models for polyimides derived from hexafluoroisopropylidene-and sulfone-bridged dianhydrides. Polymer 1994, 35, 2265–2271. [Google Scholar] [CrossRef]
- Barton, B.; McCleland, C.W.; Caira, M.R.; de Jager, L.; Hosten, E.C. Crystal X-ray diffraction and molecular modeling considerations elucidate the factors responsible for the opposing host behavior of two isostructural xanthenyl- and thioxanthenyl-derived host compounds. Cryst. Growth Des. 2019, 19, 2396–2418. [Google Scholar] [CrossRef]
- Yu, H.C.; Jung, J.W.; Choi, J.Y.; Oh, S.Y.; Chung, C.M. Structure-property relationship study of partially aliphatic copolyimides for preparation of flexible and transparent polyimide films. J. Macromol. Sci. Part A Pure Appl. Chem. 2017, 54, 97–104. [Google Scholar] [CrossRef]
- Chen, H.X.; Zhang, E.S.; Hong, M.; Liu, W.; Dai, X.M.; Chen, Q.; Qiu, X.P.; Ji, X.L. Molecular weight dependence of associative behavior in polyimide/DMF solutions. Chin. J. Polym. Sci. 2020, 38, 629–637. [Google Scholar] [CrossRef]
- Shu, C.; Wu, X.; Zhong, M.; Yan, D.; Huang, W. The atomic oxygen resistant study of a transparent polyimide film containing phosphorus and fluorine. Appl. Surf. Sci. 2023, 631, 157562. [Google Scholar] [CrossRef]
- Jeong, K.U.; Kim, J.J.; Yoon, T.H. Synthesis and characterization of novel polyimides containing fluorine and phosphine oxide moieties. Polymer 2001, 42, 6019–6030. [Google Scholar] [CrossRef]
- Ando, S.; Sekiguchi, K.; Mizoroki, M.; Okada, T.; Ishige, R. Anisotropic linear and volumetric thermal-expansion behaviors of self-standing polyimide films analyzed by thermomechanical analysis (TMA) and optical interferometry. Macromol. Chem. Phys. 2018, 219, 1700354. [Google Scholar] [CrossRef]
- He, X.; Zhang, S.; Zhou, Y.; Zheng, F.; Lu, Q. The “fluorine impact” on dielectric constant of polyimides: A molecular simulation study. Polymer 2022, 254, 125073. [Google Scholar] [CrossRef]
- Butnaru, I.; Bruma, M.; Gaan, S. Phosphine oxide based polyimides: Structure-property relationship. RSC Adv. 2017, 7, 50508–50518. [Google Scholar] [CrossRef]
- Xiao, F.; Wang, K.; Zhan, M.S. Atomic oxygen resistant phosphorus-containing polyimides for LEO environment. J. Mater. Sci. 2012, 47, 4904–4913. [Google Scholar] [CrossRef]
- Zhao, Y.; Dong, Z.; Li, G.; Dai, X.; Liu, F.; Ma, X.; Qiu, X. Atomic oxygen resistance of polyimide fibers with phosphorus-containing side chains. RSC Adv. 2017, 7, 5437–5444. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, G.M.; Dai, X.M.; Liu, F.F.; Dong, Z.X.; Qiu, X.P. AO-resistant properties of polyimide fibers containing phosphorus groups in main chains. Chin. J. Polym. Sci. 2016, 34, 1469–1478. [Google Scholar] [CrossRef]
- Ji, H.W.; Zhao, X.G.; Li, Q.M.; Shafiq, U.; Zhou, H.W.; Dang, G.D.; Chen, C.H. Atomic oxygen resistant phosphorus-containing copolyimides derived from bis[4-(3-aminophenoxy)phenyl]phenylphosphine oxide. Polym. Sci. Ser. B 2014, 56, 788–798. [Google Scholar]
- Connell, J.W.; Smith, J.G., Jr.; Hergenrother, P.M. Oxygen plasma-resistant phenylphosphine oxide-containing polyimides and poly(arylene ether heterocycle)s: 1. Polymer 1995, 36, 5–11. [Google Scholar] [CrossRef]




| PI | 6FDA a (g, mol) | 6FCDA a (g, mol) | BADPO a (g, mol) | NMP a (g) | Ac2O a (g) | Pyridine (g) |
|---|---|---|---|---|---|---|
| PPI-1 | 22.2120, 0.05 | NA b | 24.6250, 0.05 | 140.5 | 30.6 | 19.8 |
| PPI-2 | NA | 22.9110, 0.05 | 24.6250, 0.05 | 142.6 | 30.6 | 19.8 |
| PI | [η]inh a (dL/g) | Molecular Weight b | Solubility c | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mn (×104 g/mol) | Mw (×104 g/mol) | PDI | NMP | DMAc | DMF | DMSO | CPA | ||
| PPI-1 | 1.38 | 26.74 | 33.74 | 1.26 | ++ | ++ | ++ | ++ | ++ |
| PPI-2 | 1.21 | 9.61 | 15.27 | 1.59 | ++ | ++ | ++ | + | − |
| Samples | Thermal Properties a | Tensile Properties b | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tg, DSC (°C) | Tg, DMA (°C) | T5% (°C) | Tmax1 (°C) | Tmax2 (°C) | Rw750 (%) | CTE (×10−6/K) | TS (MPa) | TM (GPa) | ε (%) | |
| PPI-1 | 264.3 | 274.2 | 537.6 | 550.5 | 611.1 | 59.9 | 49.2 | 115.1 | 3.54 | 6.4 |
| PPI-2 | 311.0 | 316.9 | 501.3 | 516.0 | 600.1 | 63.4 | 41.7 | 125.5 | 4.05 | 9.9 |
| Samples | Optical Properties a | Dielectric Properties b | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| λ (nm) | T450 (%) | nTE | nTM | nav | Δn | L* | a* | b* | Haze (%) | Dk | Df | |
| PPI-1 | 349 | 82.8 | 1.6194 | 1.6068 | 1.6152 | 0.0126 | 95.39 | −2.42 | 5.77 | 0.74 | 3.26 | 0.0233 |
| PPI-2 | 376 | 78.6 | 1.6384 | 1.6069 | 1.6280 | 0.0315 | 94.87 | −3.39 | 9.04 | 1.16 | 3.44 | 0.0304 |
| Samples | W1 a (mg) | W2 a (mg) | ΔW a (mg) | Ey a (10−25 cm3/atom) |
|---|---|---|---|---|
| PPI-1 | 12.68 | 10.95 | 1.74 | 6.99 |
| PPI-2 | 16.96 | 15.31 | 1.65 | 7.23 |
| Kapton® | − | − | − | 30.0 |
| Samples | Relative Atomic Concentration (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Unexposed Samples | AO Exposed Samples | |||||||||
| C1s | N1s | O1s | F1s | P2p | C1s | N1s | O1s | F1s | P2p | |
| PPI-1 | 75.83 | 2.87 | 12.55 | 5.35 | 1.22 | 35.46 | 2.50 | 46.57 | 2.55 | 12.17 |
| PPI-2 | 76.37 | 3.20 | 13.03 | 3.65 | 0.91 | 40.59 | 2.37 | 42.18 | 4.46 | 10.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Ren, X.; Zhang, Y.; Yang, C.; Han, S.; Qi, Y.; Liu, J. Preparation and Properties of Atomic-Oxygen Resistant Polyimide Films Based on Multi-Ring Fluoro-Containing Dianhydride and Phosphorus-Containing Diamine. Polymers 2024, 16, 343. https://doi.org/10.3390/polym16030343
Wang Z, Ren X, Zhang Y, Yang C, Han S, Qi Y, Liu J. Preparation and Properties of Atomic-Oxygen Resistant Polyimide Films Based on Multi-Ring Fluoro-Containing Dianhydride and Phosphorus-Containing Diamine. Polymers. 2024; 16(3):343. https://doi.org/10.3390/polym16030343
Chicago/Turabian StyleWang, Zhenzhong, Xi Ren, Yan Zhang, Changxu Yang, Shujun Han, Yuexin Qi, and Jingang Liu. 2024. "Preparation and Properties of Atomic-Oxygen Resistant Polyimide Films Based on Multi-Ring Fluoro-Containing Dianhydride and Phosphorus-Containing Diamine" Polymers 16, no. 3: 343. https://doi.org/10.3390/polym16030343
APA StyleWang, Z., Ren, X., Zhang, Y., Yang, C., Han, S., Qi, Y., & Liu, J. (2024). Preparation and Properties of Atomic-Oxygen Resistant Polyimide Films Based on Multi-Ring Fluoro-Containing Dianhydride and Phosphorus-Containing Diamine. Polymers, 16(3), 343. https://doi.org/10.3390/polym16030343







